Abstract
Chronic pain in patients with Alzheimer's disease or dementia is a complex issue in the medical field; these patients suffer from the common causes of chronic pain, especially in geriatric medicine. To ensure the correct type and level of given treatment, medical care should be taken to avoid the contribution of chronic pain and cognitive impairment in the elderly population. Acetylcholinesterase inhibitors (AChE-Is) have been proven as an efficient therapeutic resource for significant improvement in dementia of Alzheimer's disease and chronic pain due to the fact that cholinergic deficit is considered as an early finding in cognitive impairment and persisting pain. Some AChE-Is are investigated here in terms of treatment of dementia and chronic pain management. Neostigmine has been used as an adjunct analgesic in the postoperative period and in combination with other analgesic medications in an intrathecal approach. Rivastigmine has, over the past ten years, become the approved agent for the management of dementia of mild to moderate Alzheimer's disease and has gained approval for treating different types of non-Alzheimer's dementia. In this review, we will focus on the two types of AChE-Is (rivastigmine and neostigmine) in the development of their clinical use and their respective mechanisms of actions on improving cognitive function and managing chronic pain.
Keywords: ChE-Is, Neostigmine, Rivastigmine, Cognitive, Chronic pain
1. Introduction
Pain is defined as an unpleasant emotional or sensory experience related to tissue damage [1] and is categorized as either acute or chronic. In this literature, just chronic pain term will be discussed. Chronic pain is known as pain which exceeds the injury duration period or persists for at least six months continuous or intermittent [2]. In a telephone survey study conducted in Canada, overall pain prevalence was 31% for women and 27% for men, and also, the study has shown that chronic pain positively correlates with older age [3]. Owing to the rise in the prevalence of chronic pain in an aging population with increasing associated annual costs [4], [5], many people with chronic pain do not receive adequate pain management. Consequently, that is contributing to the disease burden [6], [7].
As a complex multidimensional experience, chronic pain has a marked impact on several features in people's daily life regarding physical activities [8]. Indeed, a literature review has found that chronic pain affects mood, productivity, social life, participation in leisure activities, and sleep [9]. Another review of clinical and preclinical research has reported that chronic pain is frequently positively correlated with cognitive impairment [10]. That might return to neural systems related to chronic pain, and cognitive processing is strictly linked, and also, they may modulate each other alternately [11]. Generally, cognition is a term for the mental processes which involve a human ability to process, comprehend, and gain knowledge, encompassing attention, processing speed, memory, judgment, planning, problem-solving, language, perception, imagination, and executive functioning [12]. However, chronic pain might not have a direct impact that causes cognitive impairment, but it might have the association with comorbid factors (emotional distress, depressive symptoms, and anxiety) which relate to cognitive impairment [1]. Owing to the increase in the prevalence of chronic pain and cognitive impairment with age with dependence level in long-term care [3], [13], medical care should be taken to avoid the contribution of chronic pain and cognitive impairment in elderly population, especially the elderly population (aged 65 years and over) increasingly represents 15.6% of the Canadian population in 2014 [14].
1.1. Chronic pain and acetylcholine
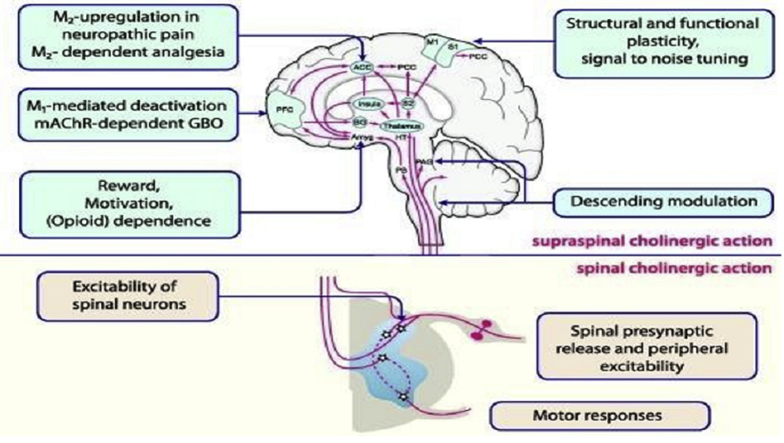
As a multiple neurotransmitter, acetylcholine (ACh) modulates processing of pain in the spinal cord [15]. In response to physiological stimuli (pain) and pharmacological stimuli (α2-adrenergic receptor stimulation in the spinal cord and opioid receptor stimulation in the brain stem), ACh is released [16]. Cholinergic receptors exist in the spinal cord in the superficial and deep dorsal horn, and they are involved in transmission and modulation of pain [17]. The mechanism of ACh modulation of pain in the central nervous system is shown in Fig. 1. Also, there is evidence that has shown that ACh has effects on peripheral antinociceptive [19].
Fig. 1.
Pain pathways that are subject to cholinergic modulation. Muscarinic and nicotinic acetylcholine (ACh) receptors regulate cholinergic modulation on both spinal (lower portion) and supraspinal (upper portion) levels [18].
In aging and/or some age-related disorders, the cholinergic system (including nicotinic and muscarinic ACh receptors, vesicular ACh transporter, butyrylcholinesterase [BuChE], acetylcholinesterase [AChE], and choline acetyltransferase) is modified [20]. Consequently, secretion of cholinesterase enzyme is increased in aging, which causes break down of the ACh, leading to pain deterioration. Therefore, AChE-Is inhibit cholinesterase and improve ACh action in terms of pain modulation.
1.2. Neurodegeneration
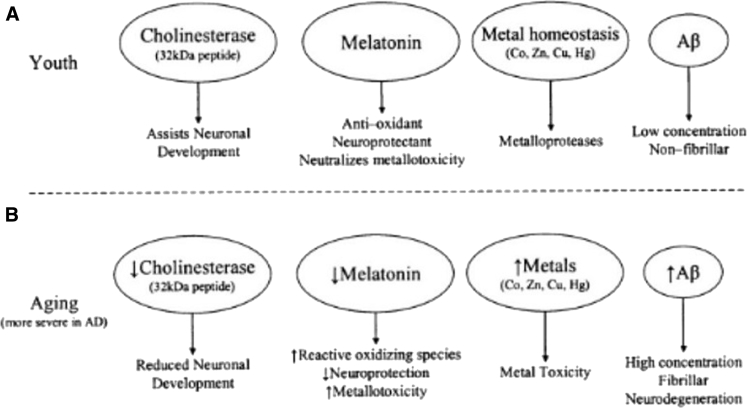
Usually, amyloid-beta (Aβ) peptide presents in a nonfibrillar form in low concentration [20]. Indeed, melatonin, a hormone that is released by the pineal gland in the brain, acts as a neuroprotectant and antioxidant to keep metal homeostasis and to neutralize metal toxicity such as mercury in neuronal cells [20]. In contrast, the cholinergic system (includes nicotinic and muscarinic ACh receptors, vesicular ACh transporter, BuChE, AChE, and choline acetyltransferase) is modified in aging and/or some age-related disorders, such as Alzheimer's disease (AD) and Parkinson's disease (PD) [20]. Moreover, melatonin concentration is decreased, which leads to an increase in reactive oxidizing species, a higher level of metal toxicity, and reduction in neuroprotection [20]. Metal homeostasis tends to be unbalanced and produces metal toxicity, and Aβ levels increase which become fibrillar and form amyloid plaques [20] (Fig. 2). Eventually, this leads to neurodegeneration [20]. Aβ fragment irreversibly opens the mitochondrial pores and induces pathologic stimulation of permeability transition of the mitochondria pores, which usually control ions and peptides transportation [21]. Consequently, this mechanism unbalances calcium homeostasis in the cell and causes cell apoptosis, which mainly impacts the development of neurodegeneration and neurotoxicity [20].
Fig. 2.
Age-related changes in neuronal agents and conditions [20].
Interestingly, in vivo and in vitro studies have reported that AChE, a senile plaque component, stimulates amyloid fibril assembly and formats highly toxic Aβ–AChE complexes which have a neurotoxic effect higher than Aβ peptide's neurotoxicity alone [22]. Moreover, even though the underlying mechanism is still uncovered, AChE peptide is identified as an inducer of apoptotic and necrotic neuronal cell death in an in vivo study [23]. As a result, substances that inhibit AChE's peptides should have potential of neuroprotective agents, including cholinesterase inhibitors (ChE-Is).
1.3. Cholinesterase inhibitors
ChE-Is are a class of medications that block cholinesterase enzymes (AChE and/or butyrylcholinesterase [BChE]) from breaking down ACh, which is the primary neurotransmitter in the central nervous system and the peripheral nervous system [24]. As a result, ChE-Is' effect increases ACh levels in the synaptic cleft. To date, ChE-Is have been approved by the Food and Drug Administration (FDA) and US Food as the most effective medications for treating the underlies symptoms of AD such as associated behavioral abnormalities and cognitive deficits [24], [25]. AD is a progressive chronic neurodegenerative disease that is resulted from the deterioration of cholinergic neurons for different biological reasons over time [26]. The pathological processes of AD include the extracellular precipitation of β-amyloid protein in senile plaques, intracellular creation of neurofibrillary tangles, and loss of cholinergic synaptic connections in brain areas related to memory, learning, behavior and emotional reacts, and executive functioning [27]. However, the loss of cholinergic synaptic connections is indicated to be central of AD symptoms, so the effective treatment strategy of choice is ChE-Is that evaluate ACh levels in the brain [27]. Even though ChE-Is are known as a management treatment of Alzheimer's dementia, they are prescribed for non-Alzheimer's dementia (e.g., Lewy body dementia), psychiatric disorders (attention-deficit hyperactivity disorder and cognitive impairment in schizophrenia patients [28]), and neurological diseases (e.g., blunt head injury) [29]. As an agent that inhibits both AChE and BuChE, rivastigmine provides efficient benefits for patients with dementia [30], [31]. Moreover, a double-blind clinical trial has found that the administration of ChE-Is decreases the nociception pain response and generates analgesic action [32]. The longer-acting anticholinesterase neostigmine lacks neurotoxicity side effects associated with other systemically administrated ChE-Is when it is administrated subarachnoid, probably because of its disability to cross blood-brain barriers (BBBs) [33]. Consequently, the longer action and lack of neurotoxicity make neostigmine an efficient choice to manage chronic pain. Such wider use will lead to more ChE-Is prescriptions, so this review will summarize ChE-Is’ effect on cognitive function and chronic pain.
1.4. Neostigmine
Neostigmine is a parasympathomimetic agent, and it is referred to as carbamate inhibitor because it is considered a carbamic acid ester derivative [34] (Fig. 3). It is commonly used as an anesthetic to reverse nondepolarizing neuromuscular block [36]. Generally, it reversibly inhibits AChE enzyme and leads to more ACh at sites of cholinergic transmission [37]. Neostigmine indirectly stimulates nicotinic and muscarinic receptors [37]. Muscarinic receptors are found in cholinergic interneurons of the dorsal horn in the substantia gelatinosa in laminae III and V of the spinal cord [38]. The nicotinic subunits α3, α4, α5, α7, β2, β3, and β4 are expressed on primary afferent terminals and inhibitory interneurons, as well as descending noradrenergic fibers [38] in the dorsal root ganglion [39] and microglias [40]. Unlike other local anesthetic drugs, neostigmine does not implicate in nonspecific axonal blockade [41]. Moreover, neostigmine has protein binding to the serum albumin ranges between 15 to 25%, and it has half-life ranged between 42 to 60 minutes [42]. Normally, neostigmine is hydrolyzed by cholinesterase and metabolized by hepatic microsomal enzymes [42]. Also, it is poorly absorbed through the gastrointestinal tract when it is taken orally [42]. Significantly, neostigmine does not cross the BBB, partly because it is a quaternary amide and the permeability-glycoprotein (P-gp) on the capillary side of the BBB maintains neostigmine outside the BBB [43].
Fig. 3.
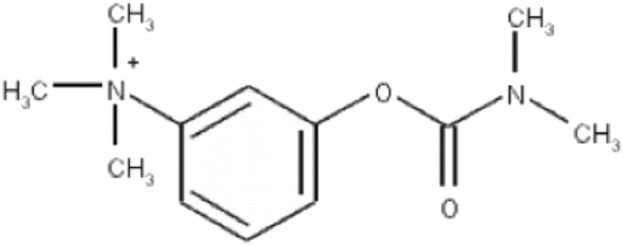
Structural formulas of neostigmine [35].
1.5. Mechanism of action
As an AChE inhibitor, neostigmine reversibly inhibits AChE in the active site by carbamylation of the serine [34] Fig. 4, at mechanism of action. Therefore, it improves cholinergic action by facilitating impulse transmission through neuromuscular junctions [44]. Eventually, neostigmine is hydrolyzed by cholinesterase and metabolized by hepatic microsomal enzymes [44]. Neostigmine has both peripheral (local delivery) and central (spinal and epidural delivery) analgesic activities [45]. Although the central administration of neostigmine has adverse effects such as pruritus caused by the rostral spinal spread of the drug and nausea [41], it might be useful in chronic pain [45]. As antinociceptive, central administration of neostigmine potentially decreases pain by suppression of several afferent fiber systems (Aβ, C, Aδ fibers) [45] (Table 1). Peripherally, neostigmine can not cross the BBB, so it does not cause central side effects when it is administrated locally [47]. However, the central administration of neostigmine has a greater analgesic efficacy in chronic pain than peripheral administration [47].
Fig. 4.
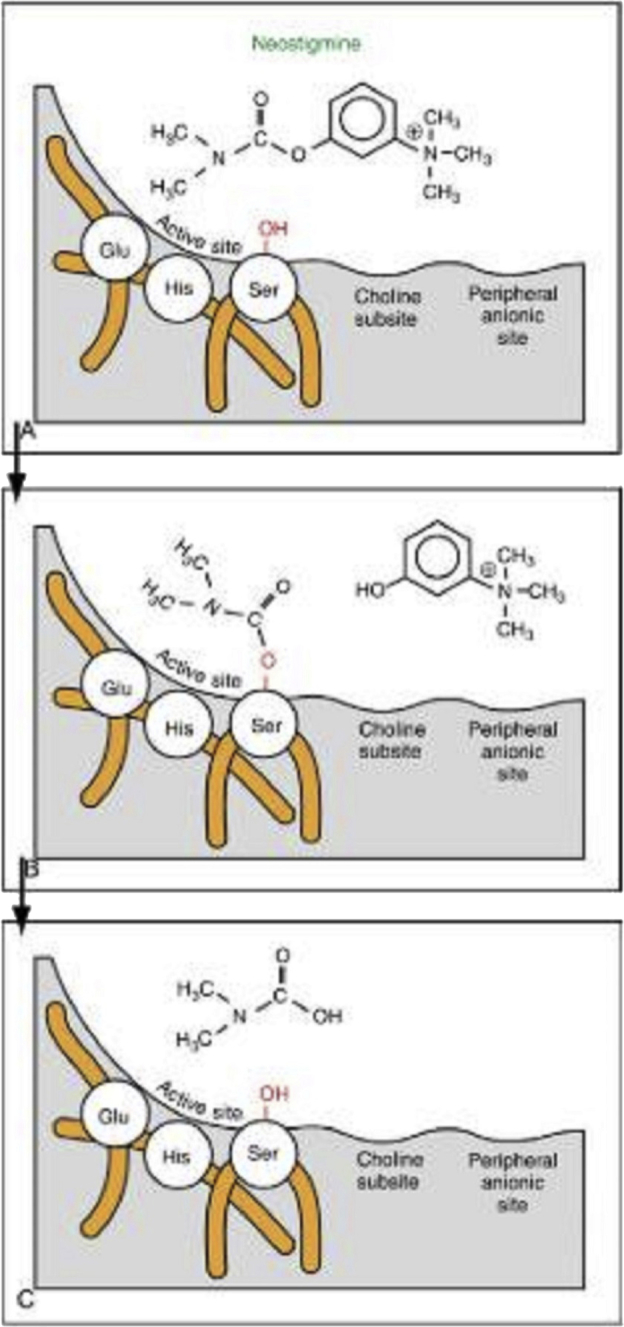
The interaction of neostigmine with acetylcholinesterase in the active site [34].
Table 1.
The nociceptive afferent fibers and their stimulation [46]
| Stimuli types | Aβ | Aδ | C |
|---|---|---|---|
| Nociceptive stimulation | Light touch, proprioception | Temperature, nociception (mechanical, thermal) | Nociception (mechanical, thermal, and chemical) |
1.6. Therapeutic potential
In a randomized, prospective clinical study, intrathecal neostigmine has postoperative analgesic effects longer than intrathecal fentanyl with better hemodynamic stability and less adverse effects [48]. However, different administration routes of neostigmine can provide various degrees of success [15]. In a literature review, intrathecal administration has been limited due to a high incidence of side effects such as vomiting and nausea [15]; therefore, decreasing the dose of intrathecal neostigmine might cover the unwanted side effects. On another hand, epidural administration is well effective and tolerated for both labor analgesia and postoperative analgesia [15]. Also, caudal administration has a useful analgesic effect in postoperative pain, but some studies have found it is associated with some side effects such as vomiting [15]. Interestingly, intra-articular administration with a dose of 500 μg is effective as postoperative analgesic without a serious increase in side effects [15].
In an in vivo study, intrathecal administration of neostigmine has relieved cold allodynia in a dose-related fashion in a rat model [49]. Intrathecal neostigmine has a synergistic antiallodynic effect with other analgesic medications that makes intrathecal neostigmine administration in low dose possible without unwanted adverse effects [49]. In a literature review, trace dose of intrathecal neostigmine with a combination of other analgesic drugs (morphine, ketamine, bupivacaine, naloxone, and clonidine) might have a useful analgesic effect in chronic pain with fewer side effects [50].
1.7. Rivastigmine
Rivastigmine is a ChEI which has a different structural formula than the most used ChE-Is [51] (Fig. 5). It is considered a pseudo-irreversible agent because it has long inhibition action on both AChE and BChE of up to ten hours [51]. Rivastigmine has central nervous system selectivity rather than peripheral, so this makes it to have less peripheral side effects than other ChE-Is such as physostigmine which has more side effects relating to heart and muscle [52]. Rivastigmine has mainly gastrointestinal symptoms as side effects [51]. It is rapidly absorbed when it is taken orally, and it has a bioavailability of 0.355 with low protein binding around 40% [51]. Also, it has a half-life of elimination of less than 2 hours [51]. Indeed, it is turned into an inactive metabolite at the action's location, omitting the hepatic metabolism [51]. Significantly, rivastigmine has dose-dependent inhibition effects on AChE [51]. Rivastigmine is considered a therapeutic agent clinically to treat underlying dementia of AD [51].
Fig. 5.
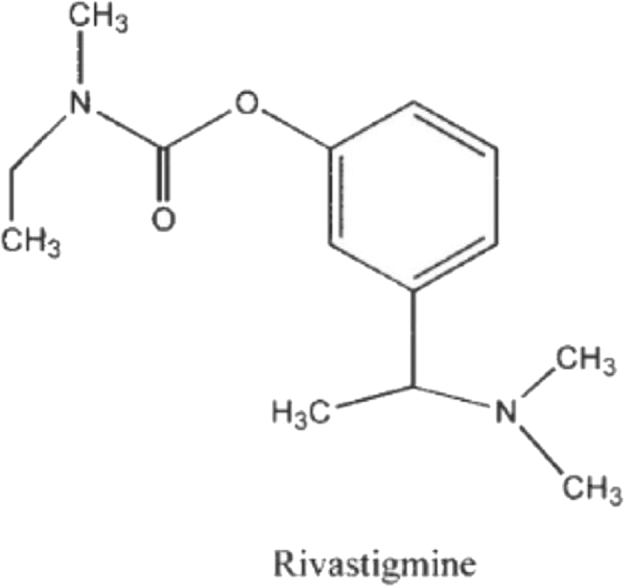
Structural formulas of rivastigmine [51].
1.8. Mechanism of action
Generally, AChE, the main cholinesterase enzyme, breaks down ACh by binding ACh in two sites, namely an anionic site and esteratic site [51]. However, rivastigmine carbamylates the AChE's esteratic site by the carbamate moiety (NH2COOH) for 10 hours, causing long inhibition effect [53]. Unlike some other ChE-Is such as tacrine and donepezil, rivastigmine leaves the esteratic site of ChE carbamylated even after it is hydrolyzed [53]. Then, the phenolic derivative is eliminated from the body [53]. Finally, AChE in carbamylated form hinders any further hydrolysis of ACh [51]. Moreover, AChE exists in two forms, namely tetrameric (G4) and monomeric (G1) [54]. Unlike tacrine and physostigmine, rivastigmine preferentially tends to inhibit G1 four to six times more than G4 form [55]. G4 form is dramatically decreased in the cortex and hippocampus in patients with AD while G1 form remains intact [51]. G4 form involves with ACh regulation while G1 involves with ACh degradation unlinked to ACh release [51]. As a result, rivastigmine's selectivity to inhibit G1 form of AChE could be beneficial to treat dementia of AD [51]. Significantly, rivastigmine has equal central inhibition ratio for both BChE and AChE, which both have pathophysiologic findings (neuritic plaques and neurofibrillary tangles) in patients with AD [56], [57].
Recently, rivastigmine can treat cognitive impairment through inhibition of ChE (cholinergic effect), and also, it has noncholinergic effects by influence Aβ brain level as neuroprotective [58]. Rivastigmine is expected to increase the hepatic clearance of Aβ and prevents Aβ from crossing the BBB [59]. Moreover, rivastigmine upregulates P-gp and lipoprotein receptor-related protein 1, which are decreased in aging and age-related diseases and cause high Aβ brain level [59].
1.9. Therapeutic potential
In a double-blind, randomized controlled trial, rivastigmine has statistically significant benefits in terms of improving cognitive function, enhancing of daily living activities, and changing in Clinician's Interview-Based Impression of Change with caregiver input (CIBIC-Plus) in patients with AD [60]. In a literature review, rivastigmine has potential efficacy in AD dementia and less side effects regardless of its administration dose or routes [61]. However, transdermal rivastigmine, especially high transdermal dose, might give more improvement in AD dementia over oral rivastigmine because of the few reported incidents of forgetting doses in case of oral administration [62]. In an open-label extension study conducted for 24 weeks, there were not any significant clinical side effects while using rivastigmine in both low and high dose of transdermal rivastigmine in patients with mild to moderately severe AD [63]. However, the high transdermal dose has shown more positive impact on cognitive function in patients with AD, and it might be useful in the treatment of dementia of severe AD [63].
A placebo-controlled study has found that although rivastigmine is not related with the improvement in the global impression of improvement in mild cognitive impairment of PD, it significantly improves the clinical rating of global cognitive improvement, the performance-based measure of cognitive functioning, and the disease-related health status and anxiety [64]. In addition, rivastigmine has the ability to improve several elements of postural control in dementia of patients with PD, namely a validated measure of fall risk and the mean velocity of the center of pressure [65]. Indeed, rivastigmine clinically enhances cognitive and neuropsychiatric symptoms and reduces hallucination and behavioral symptoms in patients suffering from dementia with Lewy bodies disease [66]. In a case study, rivastigmine administration rapidly recovers behavioral disturbances and sleep patterns in women with dementia with Lewy bodies [67]. As seen, rivastigmine has a significant role in improving cognitive function in aging and age-related diseases.
2. Discussion
The number of patients with dementia, who have painful medical conditions, has gradually increased. Usually, they suffer from persistent pain lasting for more than six months [68]. Chronic pain in the cognitively impaired is a growing concern due to global aging populations. However, despite the high prevalence of pain in affected individuals, particularly the elderly, the assessment and management of perceived pain is quite difficult because of the frequent loss of cognitive and communicative abilities [15]. This raises a significant question of exactly how patients with dementia and other chronic cognitive neurodegenerative disorders perceive pain [69]. In 2016, a study concluded there are no official guidelines for pain assessment and treatment addressing people with dementia living in nursing homes The efficacy of analgesic medications is used on pain or neuropsychiatric behavior related to dementia has not been adequately studied [70]. This review adds to the evidence of the management of pain and impaired cognitive function and has found some benefit of rivastigmine interventions in the treatment of mild cognitive impairment in age-related diseases [71] and neostigmine in the treatment of chronic pain. Many pharmacologic studies have evaluated the efficacy and the safety of AChE-Is by clinically examining the effect of rivastigmine with other medications, such as galantamine and donepezil, to show a small clinical significance to cognition [72].
In several studies, AChE-Is have proved helpful effects in restoring the cognitive process and chronic pain. It is not surprising that AChE-Is, such as rivastigmine, are used in the symptomatic management of mild to moderate AD. In addition, rivastigmine offers dual inhibition of AChE and BuChE and displays preferential inhibition of the G1 form of AChE, [73]. Also, rivastigmine can improve cognitive functioning in patients with PD and DLB. It was confirmed by previous results in the literature that attention is a crucial domain of functioning in these patients [74]. Furthermore, rivastigmine has been seen to enhance memory functioning, in particular, the accuracy of secondary memory. Therefore, the study demonstrates the importance of evaluating all aspects of cognitive function by using sensitive and reliable indicators to prove acceptability to patients [74]. Neostigmine, on another hand, has been clinically approved to manage chronic pain with a concomitant cognitive disorder, especially in older people. Neostigmine can be administered by different routes, but intrathecal or epidural administrations are the most common routes for patients with chronic pain [15]. Neostigmine has an additive effect and possibly even has a synergistic action on pain-control when coadministered with analgesic medications, such as morphine or clonidine, thereby providing more extended postoperative analgesia than either medication alone [15]. Also, the epidural administration of neostigmine, both for postoperative analgesia and labor analgesia, is safe, efficient, and well tolerated [75]. These findings are based on the clinical observation that neostigmine is effective against postoperative pain [15]. Unfortunately, higher doses of central administration of neostigmine might cause some adverse effects, e.g., nausea.
When considering the finding of the literature review, it is significant to note several issues such as the size of the studied population and the study duration time for each reviewed article. Most of the clinical studies that were reviewed are short studies with a small population. However, some of them have a long follow-up period. This variation of the parameters between the studies might lead to dissimilar results. Despite this limitation, this review uses a scientific method to eliminate bias in the research. Significantly, current therapies for managing cognitive impairment and chronic pain and high-quality research are needed to support these conditions, especially in the aging population. To manage cognitive dysfunction and chronic pain, Dr Gilbert Blaise and his team are currently reviewing and working on a novel combination of five medications (GBM-5), with doses and therapeutic approaches based on the patient's condition [50], [76]. The aim of GBM-5 research, which began in 2015, is to provide a small-dose, long-acting (several weeks) medical product that reduces chronic pain and analgesic drug intake, enhances cognitive function, and improves quality of life [50], [76].
3. Conclusion
ChE-Is have been used in the management of various human diseases over the decades, most frequently based on a common mechanism of action initiated by the inhibition of AChE. Rivastigmine has a dual inhibition of both AChE and BuChE in managing mild to moderate dementias, especially those associated with AD, PD, and Lewy bodies' disease. Neostigmine is a parasympathomimetic agent, which produces cholinergic-mediated analgesia, and has been used as an adjunct analgesic with different routes of administration and varying degrees of success. However, IT neostigmine would preferably be used in selected patients with chronic pain with dose-dependent analgesia to limit a high incidence of adverse effects, particularly nausea and vomiting.
Research in Context.
-
1.
Systematic review: The authors searched Google scholar using the MeSH terms “cholinesterase inhibitors”, “rivastigmine”, “neostigmine”, “dementia”, “cognitive”, and “chronic pain”. Cholinesterase inhibitors such as rivastigmine and neostigmine have been proven as an efficient therapeutic resource for significant improvement in dementia of Alzheimer's disease and chronic pain, respectively. The literature was evaluated to focus on the two types of cholinesterase inhibitors (rivastigmine and neostigmine) in the development of their clinical use and their respective mechanisms of actions on improving cognitive function and managing chronic pain.
-
2.
Interpretation: Data were collected from clinical studies and some other in vivo and in vitro studies for studying the mechanism of action and the effect of rivastigmine and neostigmine on cognitive impairment and chronic pain, respectively. There was substantial evidence that explains the efficiency of both medications.
-
3.
Future directions: This review adds to the evidence of the management of pain and impaired cognitive function and has found some benefit of rivastigmine interventions in the treatment of mild cognitive impairment in age-related diseases and neostigmine in the treatment of chronic pain. Most of the reviewed clinical studies that were conducted to study rivastigmine and neostigmine are short studies with a small population, so it is critical to conduct a clinical study for a long period with big population size to assess the effect of the medications in the long term.
Footnotes
Conflict of interest: None.
Funding: None.
References
- 1.Merskey H., Bogduk N., editors. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. IASP Press; Seattle, Wash: 1994. [Google Scholar]
- 2.Moulin D.E., Clark A.J., Speechley M., Morley-Forster P.K. Chronic pain in Canada-prevalence, treatment, impact and the role of opioid analgesia. Pain Res Manag. 2002;7:179–184. doi: 10.1155/2002/323085. [DOI] [PubMed] [Google Scholar]
- 3.Schopflocher D., Taenzer P., Jovey R. The prevalence of chronic pain in Canada. Pain Res Manag. 2011;16:445–450. doi: 10.1155/2011/876306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johannes C.B., Le T.K., Zhou X., Johnston J.A., Dworkin R.H. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Harstall C., Ospina M. How prevalent is chronic pain. Pain Clin Updates. 2003;11:1–4. [Google Scholar]
- 6.Breivik H., Collett B., Ventafridda V., Cohen R., Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Treede R.D., Rief W., Barke A., Aziz Q., Bennett M.I., Benoliel R. A classification of chronic pain for ICD-11. Pain. 2015;156:1003. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart R.P., Wade J.B., Martelli M.F. Cognitive impairment in patients with chronic pain: the significance of stress. Curr Pain Headache Rep. 2003;7:116–126. doi: 10.1007/s11916-003-0021-5. [DOI] [PubMed] [Google Scholar]
- 9.Prefontaine K., Rochette A. A literature review on chronic pain: the daily overcoming of a complex problem. Br J Occup Ther. 2013;76:280–286. [Google Scholar]
- 10.Moriarty O., McGuire B.E., Finn D.P. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93:385–404. doi: 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Magni G., Marchetti M., Moreschi C., Merskey H., Luchini S.R. Chronic musculoskeletal pain and depressive symptoms in the National Health and Nutrition Examination I. Epidemiologic follow-up study. Pain. 1993;53:163–168. doi: 10.1016/0304-3959(93)90076-2. [DOI] [PubMed] [Google Scholar]
- 12.Nadar M.S., Jasem Z., Manee F.S. The cognitive functions in adults with chronic pain: a comparative study. Pain Res Manag. 2016;2016 doi: 10.1155/2016/5719380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham J.E., Rockwood K., Beattie B.L., Eastwood R., Gauthier S., Tuokko H. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 14.Social Development Canada Government of Canada - Action for Seniors report. Canada.ca. Innovation, Science and Economic Development Canada. 2018. https://www.canada.ca/en/employment-social-development/programs/seniors-action-report.html Available at: Accessed March 11, 2019.
- 15.Habib A.S., Gan T.J. Use of neostigmine in the management of acute postoperative pain and labour pain. CNS Drugs. 2006;20:821–839. doi: 10.2165/00023210-200620100-00004. [DOI] [PubMed] [Google Scholar]
- 16.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606–617. doi: 10.1093/bja/78.5.606. [DOI] [PubMed] [Google Scholar]
- 17.Eisenach J.C. Muscarinic-mediated analgesia. Life Sci. 1999;64:549–554. doi: 10.1016/s0024-3205(98)00600-6. [DOI] [PubMed] [Google Scholar]
- 18.Naser P.V., Kuner R. Molecular, cellular and circuit basis of cholinergic modulation of pain. Neuroscience. 2018;387:135–148. doi: 10.1016/j.neuroscience.2017.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borges L.F., Iversen S.D. Topography of choline acetyltransferase immunoreactive neurons and fibers in the rat spinal cord. Brain Res. 1986;362:140–148. doi: 10.1016/0006-8993(86)91407-1. [DOI] [PubMed] [Google Scholar]
- 20.Lahiri D.K., Chen D.M., Lahiri P., Bondy S., Greig N.H. Amyloid, cholinesterase, melatonin, and metals and their roles in aging and neurodegenerative diseases. Ann New York Acad Sci. 2005;1056:430–449. doi: 10.1196/annals.1352.008. [DOI] [PubMed] [Google Scholar]
- 21.Bachurin S.O., Shevtsova E.P., Kireeva E.G., Oxenkrug G.F., Sablin S.O. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann New York Acad Sci. 2003;993:334–344. doi: 10.1111/j.1749-6632.2003.tb07541.x. [DOI] [PubMed] [Google Scholar]
- 22.Inestrosa N.C., Urra S., Colombres M. Acetylcholinesterase (AChE)-amyloid-β-peptide complexes in Alzheimer's disease. The Wnt signaling pathway. Curr Alzheimer Res. 2004;1:249–254. doi: 10.2174/1567205043332063. [DOI] [PubMed] [Google Scholar]
- 23.Day T., Greenfield S.A. A peptide derived from acetylcholinesterase induces neuronal cell death: characterisation of possible mechanisms. Exp Brain Res. 2003;153:334–342. doi: 10.1007/s00221-003-1567-5. [DOI] [PubMed] [Google Scholar]
- 24.Jann M.W., Shirley K.L., Small G.W. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet. 2002;41:719–739. doi: 10.2165/00003088-200241100-00003. [DOI] [PubMed] [Google Scholar]
- 25.Weinstock M. Selectivity of cholinesterase inhibition. CNS drugs. 1999;12:307–323. [Google Scholar]
- 26.Small G.W., Rabins P.V., Barry P.P., Buckholtz N.S., DeKosky S.T., Ferris S.H. Diagnosis and treatment of Alzheimer disease and related disorders: consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer's Association, and the American Geriatrics Society. JAMA. 1997;278:1363–1371. [PubMed] [Google Scholar]
- 27.Mayeux R., Sano M. Treatment of Alzheimer's disease. N Engl J Med. 1999;341:1670–1679. doi: 10.1056/NEJM199911253412207. [DOI] [PubMed] [Google Scholar]
- 28.Howard A.K., Thornton A.E., Altman S., Honer W.G. Donepezil for memory dysfunction in schizophrenia. J Psychopharmacol. 2002;16:267–270. doi: 10.1177/026988110201600313. [DOI] [PubMed] [Google Scholar]
- 29.Bentué-Ferrer D., Tribut O., Polard E., Allain H. Clinically significant drug interactions with cholinesterase inhibitors. CNS Drugs. 2003;17:947–963. doi: 10.2165/00023210-200317130-00002. [DOI] [PubMed] [Google Scholar]
- 30.Ballard C.G. Advances in the treatment of Alzheimer’s disease: benefits of dual cholinesterase inhibition. Eur Neurol. 2002;47:64–70. doi: 10.1159/000047952. [DOI] [PubMed] [Google Scholar]
- 31.Greig N.H., Utsuki T., Yu Q.S., Zhu X., Holloway H.W., Perry T. A new therapeutic target in Alzheimer's disease treatment: attention to butyrylcholinesterase. Curr Med Res Opin. 2001;17:159–165. doi: 10.1185/0300799039117057. [DOI] [PubMed] [Google Scholar]
- 32.Petersson J., Gordh T.E., Hartvig P., Wiklund L. A double-blind trial of the analgesic properties of physostigmine in postoperative patients. Acta Anaesthesiol Scand. 1986;30:283–288. doi: 10.1111/j.1399-6576.1986.tb02414.x. [DOI] [PubMed] [Google Scholar]
- 33.Taylor P. 12th ed. Macmillan; New York: 2011. Anticholinesterase agents. Goodman & Gilman’s The Pharmacological Basis of Therapeutics; pp. 239–254. [Google Scholar]
- 34.Wecker L. Elsevier Health Sciences; Philadelphia, PA: 2018. Brody's Human Pharmacology: Mechanism-based Therapeutics. [Google Scholar]
- 35.Prostigmin (Neostigmine): Side Effects, Interactions, Warning, Dosage & Uses. RxList. 2016. https://www.rxlist.com/prostigmin-drug.htm Available at: Accessed March 17, 2019.
- 36.Calvey T.N., Wareing M., Williams N.E., Chan K. Pharmacokinetics and pharmacological effects of neostigmine in man. Br J Clin Pharmacol. 1979;7:149–155. doi: 10.1111/j.1365-2125.1979.tb00915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neostigmine methylsulfate. National Center for Biotechnology Information, Bethesda, MD. 2017. https://pubchem.ncbi.nlm.nih.gov/compound/5824 Available at: Accessed March 16, 2019.
- 38.Vincler M., Eisenach J.C. Plasticity of spinal nicotinic acetylcholine receptors following spinal nerve ligation. Neurosci Res. 2004;48:139–145. doi: 10.1016/j.neures.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Genzen J.R., Van Cleve W., McGehee D.S. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J Neurophysiol. 2001;86:1773–1782. doi: 10.1152/jn.2001.86.4.1773. [DOI] [PubMed] [Google Scholar]
- 40.Thomsen M.S., Mikkelsen J.D. The α7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-α release from microglia. J Neuroimmunol. 2012;251:65–72. doi: 10.1016/j.jneuroim.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Hood D.D., Eisenach J.C., Tuttle R. Phase I safety assessment of intrathecal neostigmine methylsulfate in humans. Anesthesiology. 1995;82:331–343. doi: 10.1097/00000542-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Neostigmine. DrugBank. 2019. https://www.drugbank.ca/drugs/DB01400 Available at: Accessed March 19, 2019.
- 43.Breivik H. The burden of central anticholinergic drugs increases pain and cognitive dysfunction. More knowledge about drug-interactions needed. Scand J pain. 2017;17:186–188. doi: 10.1016/j.sjpain.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 44.Trevisani G.T., Hyman N.H., Church J.M. Neostigmine: safe and effective treatment for acute colonic pseudo-obstruction. Dis Colon Rectum. 2000;43:599–603. doi: 10.1007/BF02235569. [DOI] [PubMed] [Google Scholar]
- 45.Buerkle H., Boschin M., Marcus M.A., Brodner G., Wusten R., Van Aken H. Central and peripheral analgesia mediated by the acetylcholinesterase-inhibitor neostigmine in the rat inflamed knee joint model. Anesth Analg. 1998;86:1027–1032. doi: 10.1097/00000539-199805000-00023. [DOI] [PubMed] [Google Scholar]
- 46.Marchand S. The physiology of pain mechanisms: from the periphery to the brain. Rheum Dis Clin N Am. 2008;34:285–309. doi: 10.1016/j.rdc.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Lauretti G.R., de Oliveira R., Perez M.V., Paccola C.A. Postoperative analgesia by intraarticular and epidural neostigmine following knee surgery. J Clin Anesth. 2000;12:444–448. doi: 10.1016/s0952-8180(00)00189-6. [DOI] [PubMed] [Google Scholar]
- 48.Shakya M.L., Yadav A.S., Dwivedi S. Comparative evaluation of intrathecal neostigmine with intrathecal fentanyl for post-operative pain relief. Int J Sci Study. 2016;4:168–171. [Google Scholar]
- 49.Kroin J.S., Buvanendran A., Kari M., Jain L.A., Tuman K.J. Anti-allodynic effect of intrathecal pregabalin and neostigmine in rats with neuropathic pain. Anesthesiology. 2008;109:A599. [Google Scholar]
- 50.Eldufani J. Role of the multidrug-based approach to control chronic pain and cognitive impairment in people with chronic refractory pain: literature review, Master Thesis from University of Montreal.
- 51.Jann M.W. Rivastigmine, a new-generation cholinesterase inhibitor for the treatment of Alzheimer's disease. Pharmacotherapy. 2000;20:1–2. doi: 10.1592/phco.20.1.1.34664. [DOI] [PubMed] [Google Scholar]
- 52.Weinstock M. Advances in Research on Neurodegeneration. Springer; Vienna: 1997. Possible role of the cholinergic system and disease models; pp. 93–102. [DOI] [PubMed] [Google Scholar]
- 53.Enz A., Floersheim P. Alzheimer Disease. Birkhäuser; Boston: 1997. Cholinesterase inhibitors: an overview of their mechanisms of action; pp. 211–215. [Google Scholar]
- 54.Anand R. Efficacy and safety results of the early phase studies with Excelon (ENA-713) in Alzheimer's disease; an overview. J Drug Devel Clin Prac. 1996;8:109–116. [Google Scholar]
- 55.Enz A., Amstutz R., Boddeke H., Gmelin G., Malanowski J. Vol. 98. Elsevier Science Publishers B.V.; Amsterdam, The Netherlands: 1993. Brain selective inhibition of acetylcholinesterase: a novel approach to therapy for Alzheimer's disease; pp. 431–438. (Progress in brain research). [DOI] [PubMed] [Google Scholar]
- 56.Giacobini E. Cholinergic basis for Alzheimer therapy. Birkhäuser; Boston, MA: 1991. The second generation of cholinesterase inhibitors: pharmacological aspects; pp. 247–262. [Google Scholar]
- 57.Cutler N.R., Polinsky R.J., Srarnek J.J., Enz A., Jhee S.S., Mancione L. Dose-dependent CSF acetylcholinesterase inhibition by SDZ ENA 713 in Alzheimer's disease. Acta Neurol Scand. 1998;97:244–250. doi: 10.1111/j.1600-0404.1998.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 58.Kim H.G., Moon M., Choi J.G., Park G., Kim A.J., Hur J. Donepezil inhibits the amyloid-beta oligomer-induced microglial activation in vitro and in vivo. Neurotoxicology. 2014;40:23–32. doi: 10.1016/j.neuro.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Mohamed L.A., Qosa H., Kaddoumi A. Age-related decline in brain and hepatic clearance of amyloid-beta is rectified by the cholinesterase inhibitors donepezil and rivastigmine in rats. ACS Chem Neurosci. 2015;6:725–736. doi: 10.1021/acschemneuro.5b00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Birks J.S., Evans J.G. Rivastigmine for Alzheimer's disease. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD001191.pub3. [DOI] [PubMed] [Google Scholar]
- 61.Sarwary M. Comparison of cognitive decline medications of Alzheimerś disease: Efficacy and safety of Donepezil, Galantamine, Rivastigmine and Memantine, Degree Thesis in Pharmacy from Umeå University.
- 62.Reñé R., Ricart J., Hernández B. From high doses of oral rivastigmine to transdermal rivastigmine patches: user experience and satisfaction among caregivers of patients with mild to moderate Alzheimer disease. Neurología. 2014;29:86–93. doi: 10.1016/j.nrl.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 63.Farlow M.R., Grossberg G.T., Sadowsky C.H., Meng X., Velting D.M. A 24-week, open-label extension study to investigate the long-term safety, tolerability, and efficacy of 13.3 mg/24 h rivastigmine patch in patients with severe Alzheimer disease. Alzheimer Dis Associated Disord. 2015;29:110–116. doi: 10.1097/WAD.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 64.Mamikonyan E., Xie S.X., Melvin E., Weintraub D. Rivastigmine for mild cognitive impairment in Parkinson disease: A placebo-controlled study. Movement Disord. 2015 Jun;30:912–918. doi: 10.1002/mds.26236. [DOI] [PubMed] [Google Scholar]
- 65.McDonald J., Pourcher E., Nadeau A., Corbeil P. A randomized trial of oral and transdermal rivastigmine for postural instability in Parkinson disease dementia. Clin Neuropharmacol. 2018;41:87–93. doi: 10.1097/WNF.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 66.Madson K.A., Brown S. Rivastigmine: Dementia with lewy bodies. Hosp Pharm. 2016;51:129–131. doi: 10.1310/hpj5102-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Terzaghi M., Rustioni V., Manni R., Pacchetti C., Zangaglia R., Ossola M. Agrypnia with nocturnal confusional behaviors in dementia with Lewy bodies: immediate efficacy of rivastigmine. Movement Disord. 2010;25:647–649. doi: 10.1002/mds.22726. [DOI] [PubMed] [Google Scholar]
- 68.Pickering G., Jourdan D., Dubray C. Acute versus chronic pain treatment in Alzheimer’s disease. Eur J Pain. 2006;10:379–384. doi: 10.1016/j.ejpain.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 69.Horgas A.L., Elliott A.F. Pain assessment and management in persons with dementia. Nurs Clin North Am. 2004;39:593–606. doi: 10.1016/j.cnur.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Husebo B.S., Achterberg W., Flo E. Identifying and managing pain in people with Alzheimer’s disease and other types of dementia: a systematic review. CNS Drugs. 2016;30:481–497. doi: 10.1007/s40263-016-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feldman H.H., Ferris S., Winblad B., Sfikas N., Mancione L., He Y. Effect of rivastigmine on delay to diagnosis of Alzheimer's disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6:501–512. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- 72.Doody R.S., Ferris S.H., Salloway S., Sun Y., Goldman R., Watkins W.E. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009;72:1555–1561. doi: 10.1212/01.wnl.0000344650.95823.03. [DOI] [PubMed] [Google Scholar]
- 73.Bhattacharya S., Montag D. Acetylcholinesterase inhibitor modifications: a promising strategy to delay the progression of Alzheimer's disease. Neural Regen Res. 2015;10:43–45. doi: 10.4103/1673-5374.150648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wesnes K.A., McKeith I.G., Ferrara R., Emre M., Del Ser T., Spano P.F. Effects of rivastigmine on cognitive function in dementia with lewy bodies: a randomised placebo-controlled international study using the cognitive drug research computerised assessment system. Demen Geriatr Cogn Disord. 2002;13:183–192. doi: 10.1159/000048651. [DOI] [PubMed] [Google Scholar]
- 75.Prado W., Goncalves A. Antinociceptive effect of intrathecal neostigmine evaluated in rats by two different pain models. Braz J Med Biol Res. 1997;30:1225–1231. doi: 10.1590/s0100-879x1997001000014. [DOI] [PubMed] [Google Scholar]
- 76.Abdolmohammadi S., Hétu P.O., Néron A., Blaise G. Efficacy of an intrathecal multidrug infusion for pain control in older adults and in end-stage malignancies: a report of three cases. Pain Res Manag. 2015;20:118–122. doi: 10.1155/2015/405630. [DOI] [PMC free article] [PubMed] [Google Scholar]