Abstract
Introduction
Foods rich in polyphenols have been positively correlated to a reduced risk of several noncommunicable diseases, including Alzheimer's disease (AD). The aim of this systematic review was to collect and evaluate all the relevant studies on the beneficial effects of polyphenols on AD.
Methods
Studies have been collected through a systematic search on two databases: PubMed and Web of Science. Both randomized controlled trials (RCTs) and observational studies with human subjects were included.
Results
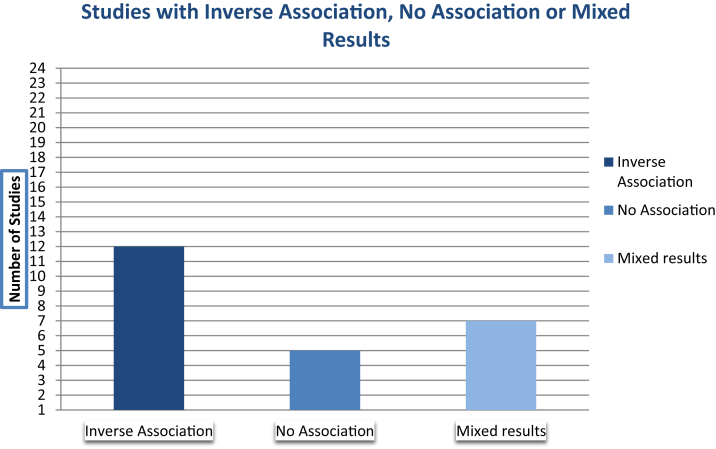
A total of 24 studies were included in this review. Twelve studies found a positive correlation with reduced cognitive decline. Five studies did not find any correlation and seven studies reported mixed results. No conclusive evidence was found for phenolic acids and flavonoids.
Discussion
This systematic review did not find sufficient evidence to confirm that polyphenols have beneficial effects against AD. Further RCTs of human subjects would be necessary to complete the results drawn from this research.
Keywords: Alzheimer's disease, Dementia, Cognitive decline, Polyphenols, Flavonoids, Nutrition, Systematic review, Prevention
1. Introduction
Aging is a very complex process that alters an individual's normal functioning over a long period of time [1]. It involves deterioration of biological functions, especially brain and cognitive functions. However, when this deterioration goes beyond what is expected from normal aging, it affects memory, thinking, orientation, comprehension, calculation, learning capacity, language, and judgment. This is what happens in individuals with Alzheimer's disease (AD). The condition worsens over the years, to the point that individuals affected by it experience physiological and behavioral changes. Dementia and AD are a growing public health problem, with about 10 million new cases every year worldwide [2].
Because of the increase in the number of people growing older and older, AD is at the center of the most recent researches. Many studies have been conducted about its possible causes and risk factors. However, much still remains unknown about the mechanisms through which AD develops. Currently there is no cure, and all treatment strategies are focused on slowing down symptoms and managing behavioral changes [3]. Thus, most research is focused on finding potentially modifiable risk factors, such as diets, which could play a role in the development of AD. Because nutrients are important for everyday systemic functions, they are being largely researched to find a link with the neurodegeneration that occurs in the brain of individuals affected by AD. In fact, many nutrients play a role in biochemical reactions and also operate as an energy source for activity [4]. For this reason, diet alterations directly affect the central nervous system, with implications for the amyloid precursor protein (APP) metabolism and neurodegeneration [4]. Factors related to dietary patterns considered significant are calorie restrictions, antioxidants, hyperhomocysteinemia (hyper-tHcy), and the link between apolipoprotein APOEɛ4 genotype and cholesterol [4].
Researchers have studied the role of nutrition on AD from the macroscopic to the microscopic perspective, from the general level of nutrition to specific diets and nutrients. On a macroscopic level, the main finding on which scientists agree on is that both malnutrition and obesity have an impact on AD [5]. In fact, patients with AD are often reported to be malnourished, with a mean prevalence of 5% of malnourished patients among those living at home [5]. They also have a lower nutritional status at baseline (including significant weight loss and muscle depletion), which is thought to indicate the progression of the disease [6]. At the same time, obesity has been regarded as a determinant factor for the development of AD [5]. A study with 6583 individuals showed that the participants with the largest body waist had a three-fold risk to develop AD, compared with participants with the smallest abdominal diameter [7]. Another cohort study found that the risk ratio for dementia was significantly high in obese subjects aged 30 to 39, whereas it decreased steadily in subjects whose obesity decreased as they aged [7].
Moreover, specific nutrients and vitamins have been linked to the progression of AD. The effects of nutrients on AD have been researched by several studies, which have highlighted the importance of vitamins A, B, C, and E [5]. However, reviews on the topic have showed that only multivitamin combinations produced protective effects, whereas supplements of one vitamin type alone rarely produced any noticeable impact [8]. In fact, all these vitamins are characterized by being natural antioxidants, and therefore reducing oxidative stress and amyloid beta (Aβ)-peptide accumulations, which are thought to be the main causes of aging and AD's neurodegeneration [5]. However, such effects seem to be significant only when a group of vitamins is taken altogether [8].
Polyphenols also have antioxidant properties and several animal studies have been conducted to assess the association between foods rich in polyphenols and amyloid accumulation and reduction of oxidative stress in patients with AD [5]. A study by Hartman et al. [9] found that when given as supplements to a group of mice, pomegranates, which are very rich in polyphenols, reduced the risk for AD of about 50%, compared with the mice control group. A study by Kim et al. [10] based on a transgenic mouse model found that resveratrol extracted from grapes reduced hippocampal neurodegeneration and learning impairment.
The beneficial effects of polyphenols on the development of AD are the focus of this study. This research is a systematic review on the protective properties of polyphenols on the development of AD. The aim of this research is to collect all the relevant studies on the association between polyphenols and AD, and evaluate their evidence. Several reviews have been conducted on the role of nutrition on AD and the effects of certain diets and specific polyphenolic compounds on the progression of this disease. However, these reviews included also animal studies or were focused on a specific nutrient-containing polyphenols, whereas no review has been conducted on the overall effect of polyphenols on the base of human studies only. Because systematic reviews provide some of the highest level of evidence on a subject, presenting a conclusive work on this topic will be useful to guide further research on the prevention and treatment of AD.
Therefore, this article aims to answer the following research question: “Is there sufficient evidence that polyphenols have beneficial effects for the prevention and treatment of Alzheimer's disease?” To answer the research question, this article will first dive in the epidemiology and etiology of AD, the role of polyphenols, and what is currently known about them in Section 1. In Section 2, it will present the methodology based on which the studies included in the review will be selected and analyzed. This will be followed by a descriptive summary of the results in Section 3 and a discussion in Section 4 where the findings are explained and evaluated in terms of strengths and limitations.
1.1. Alzheimer's disease
AD is a progressive neurodegenerative disorder, which is known to develop clinically only in humans and unknown in other mammalian species. It is one of the most common forms of dementia accounting for about 60% to 70% of all cases, and it causes interference with memory, thinking, and behavior. AD is a chronic disease, which progresses and worsens over several years. People living with AD can live on average 8 years after the detection of symptoms, but their survival rate may differ, reaching up to 20 years, depending on an individual's age and other health conditions [11].
1.2. Stages and symptoms
Typically, three stages of AD are distinguished, although the progression of the disease may be faster or slower depending on the individual's health history and personality before becoming ill [2], [3]. Early symptoms are short-term memory loss, meaning difficulty remembering recent events, general forgetfulness, and feeling lost in unfamiliar places [11]. In later stages of the disease, more problems arise that can seriously interfere with an individual's life [3]. For instance, middle stage of AD usually presents symptoms such as feeling lost at home, needing help with personal care, forgetting family names, increasing difficulty with communication, experiencing repetition, and continuous questioning [2], [11]. Late phase of AD includes symptoms such as becoming unaware of time and place, difficulties carrying out logical conversations, interact appropriately with the physical and social environment, not recognizing family members or remembering their names, difficulties walking, and escalated behavior, which might also include aggressive behavior [2], [11].
1.3. Epidemiology and risk factors
AD is estimated to affect nearly 15 million people worldwide [12]. New cases increase steadily from 0.5% per year at age 65 years to about 8% per year after age 85 years [12]. Because the survival rate of the disease is quite high, the prevalence also increases from 3% at age 65 years to nearly 40% after age 85 years [12]. Aging is the greatest risk factor for AD, and most people affected by the disease are older than 65 years [2]. However, although aging is the strongest known risk factor, AD is not an inevitable consequence of aging [2], [3]. In fact, about 9% of cases happen before the age of 65 years and are classified as early onsets of AD [2], [3].
In terms of risk factors, some studies have shown a relationship between the development of AD and other lifestyle factors present in most noncommunicable diseases [12]. Shared risk factors are thought to be physical inactivity, obesity, unhealthy diets, tobacco use and harmful use of alcohol, diabetes, hypertension, and heart disease [12]. Yet, evidence for these relationships is not homogeneous, and further studies are needed to establish a clear connection between specific lifestyle-related risk factors and the development of AD [12]. In terms of genetics, individuals who had a first-degree relative affected by AD seem to be more likely to develop the disease [3]. The APOE gene is thought to be involved in this process [13]. One form of this gene, APOEɛ4, increases a person's risk of developing the disease and is also associated with an earlier age of onset [13]. However, carrying the gene does not necessarily mean developing the disease [3], [13]. In fact, most genetic mechanisms of AD among families has yet remained largely unexplained. Finally, people with Down syndrome are more likely to develop the disease, which usually happens earlier than for most patients with AD [3].
Additional potentially modifiable risk factors include depression, low educational attainment, social isolation, and cognitive inactivity [3], [13]. In addition, people who have had a severe head trauma or head injury seem to have a greater risk of AD [13]. Finally, women seem to be more likely to develop AD, although this might also be because of the fact that women generally live longer than men [3], [13].
1.4. Etiology
Studies have shown that the primary contribution to aging is a result of oxidative stress experienced during normal metabolism. Oxidative stress that results from normal metabolism is often accelerated or amplified by the interaction with toxins in the environment, infections, and metabolic processing of ingested food products. The accumulated by-products generated by such processes can damage many classes of molecules, and their potential impact is especially evident on brain tissue. Therefore, these findings suggest that oxidative stress constitutes the most important overall mechanism in cellular aging [1]. Chronic inflammation is also known to be important for the aging process and for the etiology of AD [1].
Currently, scientists do not yet fully understand the specific causes of AD [13]. There is agreement, however, that it is caused by a complex interplay between genetic and environmental factors. In less than 0.5% of cases, AD is caused by mutations in three genes, APP, presenilin 1 (PSEN1), and PSEN2. In this case, we talk about familial AD (fAD), which usually has an earlier onset than for most individuals, developing symptoms at age between 30 and 50 years. There are two main hypotheses that explain the cause of AD, which are the amyloid hypothesis and tau hypothesis [13]. These two hypotheses relate to two specific hallmarks of the disease that develop abnormally when the brain is affected by AD. These are plaques and tangles [3], [13]. Plaques are a group of protein called Aβ, which damages and destroys brain cells [14]. For this reason, they are thought to constitute one of the main causes of brain-cell death in individuals with AD [14]. Abnormal tangles are, instead, the result of a twist in the threads of tau protein, which is at the head of an internal support and transportation system that carry nutrients throughout the brain cells [14].
1.4.1. Amyloid hypothesis
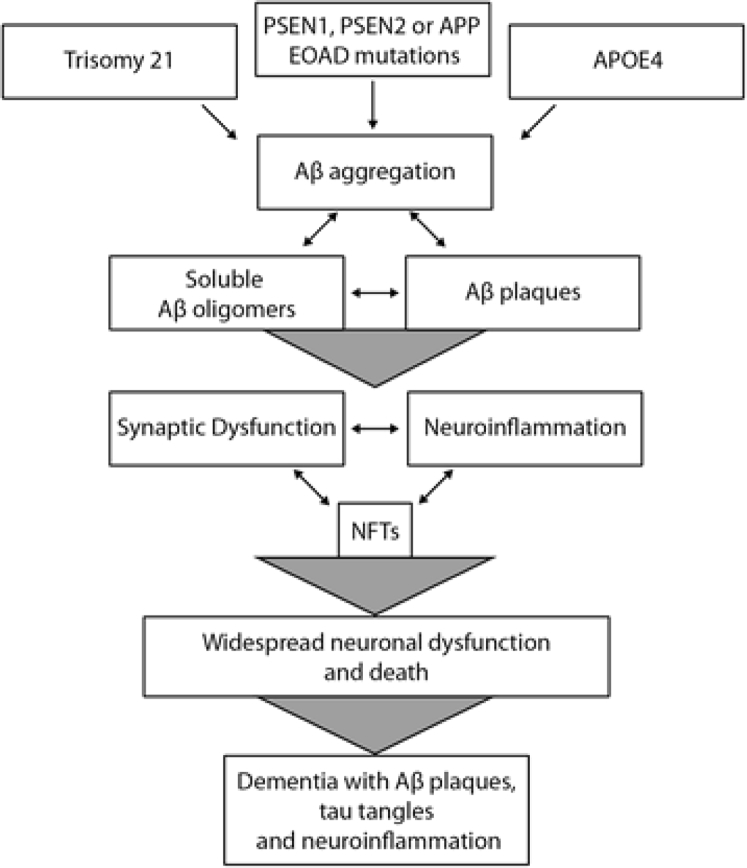
Amyloid plaques are extracellular accumulations, mostly composed of abnormally folded Aβ with 40 or 42 amino acids (Aβ40 and Aβ42), two by-products of APP metabolism [13]. Because of mutations in the APP or PSEN1 or PSEN2 genes, there is increased production of Aβ42 throughout life. The imbalance between Aβ production and Aβ clearance is therefore thought to be the main pathologic process. Aβ oligomers have been reported to be the primary cause of synaptic dysfunction, damage to the dendritic spines, and ultimately to cause neuronal death [13]. Fig. 1 represents a visual summary of the amyloid hypothesis.
Fig. 1.
Summary of the amyloid hypothesis [15]. Abbreviations: APOEɛ4, APOE genotype; APP, amyloid precursor protein; Aβ, amyloid beta; PSEN1, presenilin; PSEN2, presenilin 2; EOAD, early-onset Alzheimer's disease; NFTs, neurofibrilliary tangles.
1.4.2. Tau hypothesis
The tau hypothesis claims that threads of tau protein twist and tangle together, starting this way the neurodegenerative process of AD [13], [14]. When tangles are formed inside the bodies of nerve cells, the microtubules disintegrate, destroying the structure of the cells [13]. This eventually leads to the collapse of the support and transportation system, which tau protein threads are responsible for. This causes interference and malfunction in the communication between neurons and later it leads to brain-cell death [13].
Most current reviews suggest that tau protein alone does not cause AD and that both Aβ and tau protein abnormalities are required to provide an accurate diagnosis of the disease [13]. Several studies have explored the progression and interaction of these two pathologies in vivo, and have provided further evidence that amyloid pathology develops many years before clinical symptoms, whereas tau pathology develops later on leading to clinical symptoms [13].
1.5. Polyphenols and AD
Polyphenols are chemical compounds, a group of phytochemicals found in several drinks such as green and black teas and red wine, and several foods such as fruits, vegetables, chocolate, olive oil, and plants [16]. Some polyphenols are specific to one food group, such as isoflavones, which are specific to soya, whereas others, such as quercetin, are found in all plant products [16]. Several molecules with a polyphenol structure have been found in thousands of plants. These compounds may be classified into different groups based on the function of the number of phenol rings that they contain and on the structural elements that bind these rings to one another. Accordingly, they are distinguished into the following groups: phenolic acids, flavonoids, stilbenes, and lignans [17]. Flavonoids themselves may be divided into six subclasses depending on the type of ring structure: flavonols, flavones, isoflavones, flavanones, anthocyanidins, and flavanols (catechins and proanthocyanidins) [17]. They are considered beneficial to the development of AD through several biological mechanisms [5]. These include interaction with transition metals, inactivation of free radicals, inhibition of inflammatory response, modulation in the activity of different enzymes, and effects on intracellular signaling pathways and gene expression [5], [16]. They are natural antioxidants and are thought to play a role in the prevention and treatment of several diverse conditions [18]. Some of these conditions include obesity, diabetes, osteoporosis, kidney and liver degeneration, cardiopathies, and geriatric diseases including AD [18]. The strongest evidence of health-protective effects is for cardiovascular diseases [19]. On the contrary, the epidemiologic evidence of health gains of polyphenols against cancer is still limited and controversial [19]. There are two main processes typical of polyphenols that interest AD's development: antioxidation and anti-inflammation mechanisms, which are the two main processes involved in the aging mechanism [1]. Polyphenols are considered to have a protective effect against inflammatory mechanisms and as such have been linked to AD, as to many other chronic diseases including diabetes, metabolic syndrome, and atherosclerosis [18].
2. Methodology
This systematic review included articles from two databases, Pubmed and Web of Science. The systematic review was performed in the Netherlands, at Leiden University College The Hague.
2.1. Search terms
From the research question three main themes were individualized: “Alzheimer's disease,” “polyphenols,” and a type of response of the latter on the first one. Synonyms of the terms Alzheimer's disease, “dementia,” and polyphenols were all included to formulate a search strategy that is applied in all the searches on the databases. The final search strategy used to select the articles can be seen in Appendix A. Studies have been first screened by title, then by abstract, and finally by reading of the full text.
2.2. Inclusion and exclusion criteria
Besides the search terms, articles were selected based on certain inclusion and exclusion criteria. First of all, only peer-reviewed journal articles were being included, whereas proceeding articles, reviews, editorial material, meeting abstracts, letters, retracted publications, and book chapters were excluded. In terms of study designs, both observational and interventional studies were included. To be incorporated, the studies had to explore and assess at least one type of polyphenol on at least one factor of the pathology of AD. The language of the articles included was only English, so that data extraction could be as reliable as possible. Moreover, for the research to be feasible, only articles from the past 10 years were included. Finally, for the review to provide greater level of evidence, only studies on human subjects were included.
2.3. Quality evaluation
The quality of the studies selected for the review was assessed in a systematic manner. The scale used was adjusted based on the one developed by Dr Jessica Kiefte-de Jong, as adaptation from the study by Carter et al. and the study conducted by the National Collaborating Center for Methods and Tools [20], [21]. The quality score is composed of five items, and each item is allocated 0, 1, or 2 points for each of the following categories: study design, sample size, exposure, outcome, and adjustments. In total, each study can be awarded a maximum of 10 points. The main adjustments were made for the exposure and outcome sections. The exposure in this research relates to the measurement of dietary intake in observational studies and to whether an intervention was not blinded, single-blinded, or double-blinded in interventional studies. The outcomes in this research relate to assessment of cognitive decline and AD, thus the scale was adjusted to include the assessment methods used for these diagnoses. The evaluation sheet with the scoring criteria for each section can be found in . The final quality evaluation for each study can be seen in Table 2 of Appendix C.
2.4. Data extraction
The following quantitative and qualitative data were extracted from the included publications: authors, year of publication, country of origin, population age and population size, study design (if observational), and duration of study. Moreover, in case of clinical trials also type and amount of supplementation were extracted from the studies. In case of observational studies, the type of measurement used (for baseline and follow-up) was included. Furthermore, information about the objectives and main findings of each study were extracted. To clarify and quantify the main findings, P values and confidence intervals were included, to show whether findings were statistically significant. P values were preferred to other statistical measurements, such as odds ratio and relative risk.
3. Results
3.1. Search results
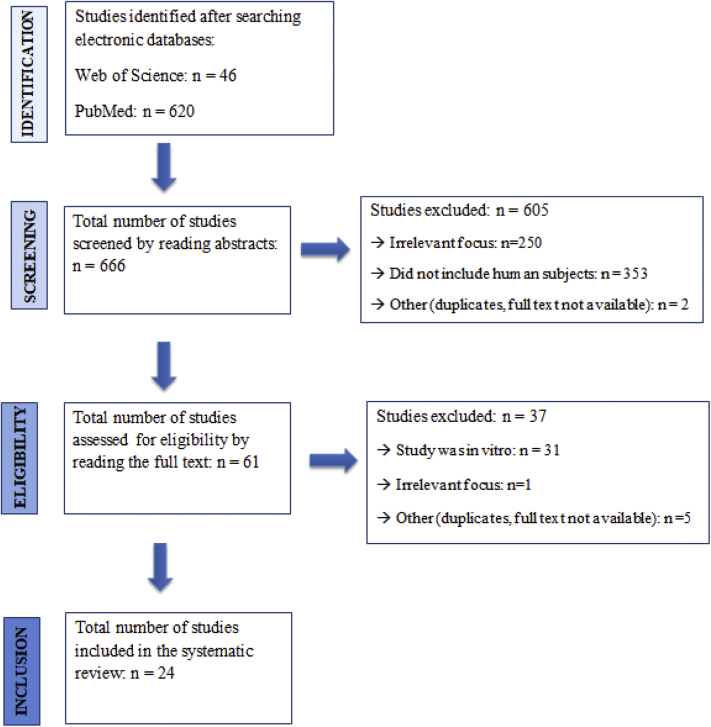
The search in the databases resulted in 666 studies. After thorough screening of the studies, 24 final studies were included. The study flow diagram with the exclusion and inclusion criteria can be visualized in Fig. 2. Of the final studies included, 18 studies were clinical trials, and six studies were observational, of which one was cross-sectional and five were prospective cohort studies. All studies were in English and published between 2008 and 2018.
Fig. 2.
Flowchart of studies' selection.
3.2. Study characteristics
All clinical trials assessed the effects of at least one polyphenol on certain factors of the pathology of AD. Eight randomized controlled trials (RCTs) assessed cognitive decline with the use of certain biomarkers. The biomarkers included are Aβ40 and Aβ42 accumulations, presence of APOEɛ4 genotype, brain volume, tHcy levels, cerebral blood flow, oxidative stress levels, dental gyrus function, aldehydes malondialdehyde levels [22], [23], [24], [25], [26], [27], [28]. Sixteen studies assessed changes in cognition with the use of different cognitive tests, depending on the specific cognitive outcome measured [22], [25], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]. More information on this is available in the data extraction tables in Appendix D. To assess dietary intake, five of six observational studies used either Frequency Food Questionnaire or Semi-quantative Frequency Food Questionnaire [29], [30], [31], [32], [33]. One study used 24-hour dietary record [34], and one study also carried home interviews besides the questionnaires [30]. More information on study characteristics can be found in Tables 3.1 and 4.1 of Appendix D.
Fourteen studies assessed a class of flavonoids (flavonols, flavones, isoflavones, flavanones, anthocyanidins, or catechins). Specifically, four studies assessed green tea consumption [22], [24], [28], [35], two studies looked at soy isoflavones [36], [42], four studies investigated consumption of certain fruit juices (orange, cherry, and blueberry) fortified with polyphenols [25], [26], [37], [43]. Two studies examined cocoa flavonol [38], [44], and one study examined the role of Pycnogenol [39]. One study examined the role of Scutellaria baicalensis and Acacia catechu containing free-B-ring flavonoids (UP326) [40], and one investigated the effects of Bacopa monnieri [41].
One study assessed resveratrol, belonging to the group of stilbenes [27]. Two studies assessed curcumin consumption [23], [45], belonging to the group of phenolic acids, and one study assessed the role of lignans [33].
Three observational studies assessed different types of flavonoids specifically [29], [31], [32]. One assessed flavonoids and lignans [33]. Two other observational studies, instead, examined large classes of polyphenols through dietary questionnaires [30], [34]. Therefore, their results cannot be reduced to one single class of polyphenols. Table 1 shows a summary of the classes of polyphenols assessed by each study.
Table 1.
Summary of the classes of polyphenols assessed by each study
| Phenolic acids | Stilbenes | Lignans | Flavonoids |
All flavonoids | All polyophenols | ||||
|---|---|---|---|---|---|---|---|---|---|
| Catechins | Flavonols | Isoflavones | Flavanones | Anthocyanidins | |||||
| Baum et al. [23] | Turner et al. [27] | Nooyens et al. [33] | Arab et al. [22] | Desideri et al. [38] | Gleason et al. [42] | Alharbi et al. [43] | Kent et al. [25] | Butchart et al. [29] | Devore et al. [30] |
| Ringman et al. [45] | Ide et al. [35] | Henderson et al. [36] | Brickman et al. [44] | Krikorian et al. [37] | Devore et al. [31] | Kesse-Guyot et al. [34] | |||
| Downey et al. [41] | Ide et al. [24] | Morillas-Ruiz et al. [26] | Nooyens et al. [33] | ||||||
| Ryan et al. [39] | Yimam et al. [40] | ||||||||
| Root et al. [32] | |||||||||
In terms of population size, the samples differed greatly between interventional and observational studies. The first group had a population as small as 12 individuals, up until 350 people. The observational studies had a population size ranging from 1091 to 16,010 individuals. The shortest study duration in clinical trials was 21 days and the longest was 4 years. The study duration in observational studies ranged from 2 to 21 years.
With respect to population ages, these were also variable across studies. However, participants were generally older than 49 years. There were few exceptions: one study had a sample consisting of individuals aged between 18 and 30 years [28]. Three studies had an overall population sample ranging between 30 and 65 participants [34], [42], [40].
3.3. Main findings
3.3.1. Randomized controlled trials
3.3.1.1. Fortified fruit juice consumption
The trials assessing fortified fruit juice consumption reported a positive association with improved cognitive performance compared with the placebo groups. Specifically, improved executive function (P < .05), psychomotor speed (P < .05), verbal fluency (P = .014), short-term (P = .014) and long-term memory (P ≤ .001), and improved associate learning (P = .009) [25], [37], [43]. However, they did not identify significant alterations for markers of inflammation. Morillas-Ruiz et al. [26] found an attenuation of tHcy plasmatic levels in patients with AD (P < .05). Yimam et al. [40] also reported an increase in speed and accuracy of processing complex information, compared with the placebo group (P < .05).
3.3.1.2. Green tea consumption
Studies assessing the effects of green tea had conflicting results. On the one hand, the studies showing positive correlation found improved performance on cognitive tests (P = .03) and increased antioxidant capacity of plasma (P = .000) in patients with AD or cognitive dysfunction, compared with the placebo groups [22], [35]. On the other hand, Wightman et al. [28] and Ide et al. [24] did not find any significant association with changes in cognitive performance, mood, or cognitive dysfunction (P = .59) [95% CI −2.97 to 1.74]. However, they did find that it prevents an increase in oxidative stress and reduces cerebral blood flow in the frontal cortex. However, Wightman et al. carried the trial in healthy adults, whereas Ide et al. carried the trial with home residents with cognitive dysfunction [24], [28].
3.3.1.3. Soy consumption
Two studies assessing benefits of soy isoflavones did not find significant differences between the control and treatment groups (P = .15; P ≤ .11) [35], [36]. Gleason et al. [42] assessed isoflavones in patients with AD, whereas Henderson et al. [36] assessed isoflavones in health postmenopausal women. However, Henderson et al. found in secondary analyses a greater improvement on visual memory in the isoflavone group compared with the placebo group. No association was reported for other cognitive factors or test scores [95% CI 0.13–0.35].
3.3.1.4. Cocoa consumption
Desideri et al. [38] and Brickman et al. [44] assessed the role of cocoa flavanol. The first study found that time required to complete cognitive and verbal tests in subjects with mild cognitive impairment was significantly lower in the treatment group compared with the placebo group (P < .05) [38]. The second study found that high-flavonol intake enhanced dentate gyrus' functioning in healthy adults, compared with the control group with low dietary intake of cocoa flavanol (P = .038) [44].
3.3.1.5. Other polyphenolic compounds
The study by Ryan et al. [39] also reported conflicting results. On the one hand, statistically significant interactions were identified for memory-based cognitive variables and consumption of antioxidant Pycnogenol (P < .01). On the other hand, no change was evident for other aspects of cognitive performance, such as concentration or psychomotor abilities in a sample of 101 healthy adults.
Downey et al. [41] found improved cognitive performance (P < .05) in a sample of healthy adults taking 320 or 640 mg of B. monnieri, compared with the placebo group. The study by Turner et al. [27] found that resveratrol was well tolerated by participants and did alter some AD biomarkers (P = .002) in individuals with mild to moderate AD, compared with the placebo group. Finally, Baum et al. [23] and Ringman et al. [45] did not find any significant evidence of the efficacy of curcumin in patients with AD, compared with the placebo groups (P = .36; P = .41). More information on findings from clinical trials can be seen in Table 3.2 of Appendix D.
3.3.2. Observational studies
Devore et al. [31] reported that greater intake of flavonoids was positively correlated with slower rates of cognitive decline [95% CI 0.00–0.07]. However, another study by Devore et al. [30] found a correlation with reduced risk of AD only with vitamin E consumption, and not with intake of vitamin C, β-carotene, and total flavonoids intake [95% CI 0.59–0.95]. Other prospective studies also reported conflicting results. Kesse-Guyot et al. [34] reported that high total polyphenol intake was associated with verbal memory (P = .01), but not with executive functioning (P = .09). Nooyens et al. [33] found that higher lignan intake was associated with speed processing (P = .04), memory (P < .01), and less decline in global cognitive function (P = .01). Higher flavonoid intake was also correlated with cognitive flexibility (P = .01). However, intake of other antioxidants was not associated with cognitive decline. Similarly, Root et al. [32] reported that flavonols intake was associated with preserved cognitive function (P < .001). A study by Butchart et al. [29] did not find any correlation with any of the outcomes observed, including overall cognitive decline, after adjusting for confounders. More information on findings from observational studies can be seen in Table 4.2 of Appendix D. A summary of the findings of studies can be seen in Fig. 3.
Fig. 3.
Summary of findings per number of studies.
3.4. Quality assessment
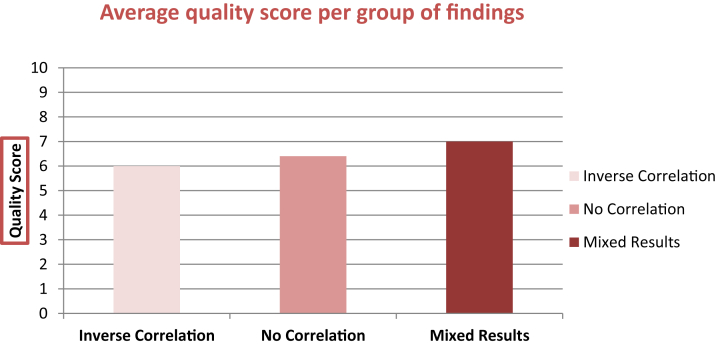
The quality assessment for each study was based on the evaluation sheet available in Appendix B. The overall average score across all studies was 6.3/10. When accounting for outliers [44], [39], [40], the average score was 6.4. RCTs scored on average 6.27, whereas observational studies had an average score of 6.5. The studies which reported a correlation between exposure to polyphenol and reduction of factors of AD pathology had an average score of 6/10, those that found a negative association scored on average 6.4/10, and those that had mixed results had a score of 7/10 on average. These data can be visualized in Fig. 4. Overall, the average score of studies that identified at least one inverse association is 6.31. Finally, it can be concluded that approximately 83% of all studies scored at least 6/10 or higher. Of these studies, approximately 41% scored at least 7/10 or higher. Although not optimal, these scores indicate a sufficient level of quality to consider the findings adequately valid.
Fig. 4.
Quality scores per group of studies, which found either inverse correlation, no correlation, or mixed results.
3.5. Covariates
The main findings from each study may have been affected by whether they were controlled for confounders. On the basis of the quality evaluation, it is possible to establish the following: four of 24 studies did not account for any confounder [24], [26], [28], [44]; 12 of 24 studies accounted for at least key confounders, such as gender, family history, baseline diet, obesity, diabetes, and smoking [23], [27], [30], [31], [32], [33], [34], [35], [36], [37], [43], [38], [40], [41], [45]; eight of 24 studies account for additional confounders such as ethnicity and socioeconomic background [22], [25], [27], [29], [31], [32], [33], [39]. Other potential covariates will be addressed in the following section.
4. Discussion
4.1. Summary of results
The overall objective of this research was to systematically review the evidence on the effects of polyphenols on the development of AD. The research included both RCTs and observational prospective studies looking at the level of association among cognition, behavior, and dietary intake, exclusively on the base of human subjects. Although some negative or insignificant results were reported, 19 of 24 studies had at least one inverse correlation between exposure to one type of polyphenol and one outcome of AD. However, the average quality score for studies, which found an inverse correlation, was lower compared with both studies with negative and mixed findings. Moreover, the validity coming from the high quality score of the studies with negative findings entails that the results from such studies cannot be neglected, even if lower in number. This limits the extent to which this review can conclusively state that polyphenols can systematically reduce the effects of AD.
Furthermore, it is important to draw conclusions about the specific classes of polyphenols tested in the included studies: no evidence was found for phenolic acids (curcumin), and no conclusive evidence was observed for flavonoids, the biggest class of polyphenols assessed in this review. In fact, eight of 15 studies reported an inverse association, three had no correlation, and four had mixed findings. Thus, results are conflicting and it is not possible to consider the evidence coming from these studies complete. The study by Turner et al. [27] presented positive results for resveratrol, belonging to stilbenes; however, this was the only study that assessed a polyphenol from this class. Similarly, Nooyens et al. [33] was the only study to find positive results for lignans.
4.2. Explanation of results
The studies included differ on the exposure and outcome measurements, meaning that they assessed different types of polyphenols and used a diverse range of cognitive tests and/or biomarkers to assess cognitive decline, dementia, and AD development in general. Thus, it is understandable that there are no consistent results across all studies. Of the studies that found at least one positive result (n = 19), 12 assessed outcomes through cognitive tests, whereas seven assessed outcomes by looking at changes in specific biomarkers for AD, such as Aβ40, tHcy levels, or the presence or absence of the APOEε4 genotype. Although both cognitive tests and biomarkers are used for AD diagnosis, biomarkers tend to be less subject to confounders than cognitive tests, and are generally considered reliable in assessing physiological changes and rates of cognitive decline [46]. The fact that only a small number of studies evaluated biomarkers is significant for the level of evidence resulting from this review. Moreover, only two of the six studies evaluating biomarkers found antioxidant capacities of the compound they assessed, and thus reduced oxidative stress [22], [24]. This is relevant to the fact that reduction of oxidative stress is one of the primary reasons why polyphenols are thought to have health benefits, especially in relation to neurodegenerative diseases [47].
Furthermore, dietary polyphenols vary on molecular structure and properties. They range from simple molecules (monomers and oligomers) to polymers [48]. Higher molecular-weight structures are particularly important because of their wide distribution in plants and their contribution to major food qualities [48]. The complexity of dietary polyphenol composition is worsened by their high instability and the capability to transform into various reaction products when the plant cells are damaged, for instance, by food processing or by the extraction process and subsequent biochemical reactions of plant polyphenols [48]. Thus, properties of polyphenols are affected by their structure, and also by degradation and interaction with other constituents of the food matrix [48]. When studies do not account for such transformations, they might result in misleading findings [48]. Some studies also suggest that the kinetics and extent of polyphenol absorption among adults differ depending on whether they have been provided as pure compound, plant extract, or whole food/beverage [49]. This factor may have skewed the findings provided in this review as well. Because polyphenols were treated differently among studies, similar results coming from different polyphenols might not be comparable, if they differed on the type of administration of the polyphenol [49].
However, an interesting factor is that even when assessing the same type of polyphenol, some studies resulted in different findings. This is most likely because of the study design used in these studies. For instance, Ide et al. [24], [35] performed two similar research studies looking at green tea consumption and cognitive dysfunction. Both trials were carried in Japan and both prescribed 2 g per day of green tea [24], [35]. The trial carried in 2014 lasted 3 months and found a positive correlation, but when conducting the same trial in 2016 for the duration of 12 months, no significant correlation was identified with cognitive dysfunction. It could be argued that not only the duration but also the small population sizes of both trials (12 and 33, respectively) might have influenced the results and their level of generalizability.
Furthermore, several confounders and biases present across all studies might have played a role in the final results. First, baseline diets could have skewed the findings, especially considering that the studies were carried out in countries with different diets, thus the population samples might have had a greater or lower intake of polyphenols before the beginning of the exposure. Both the Mediterranean diet and the Japanese diet have been found to decrease the risk and mortality from dementia and AD [5]. This is relevant especially because of the fact that this review included studies carried in Italy, France, Spain, and Japan, in which the populations included for the studies were likely to be following traditional diets. The Mediterranean diet is usually characterized by a high consumption of fruits, vegetables, cereals, bread, potatoes, poultry, beans, nuts, olive oil, and fish; a moderate consumption of alcohol; and a lower consumption of red meat and dairy products [5]. All these foods have been associated with lower risk for AD, although the biological pathways through which this process operates remain mostly unclear [5]. Similarly, the Japanese diet, characterized by high consumption of fish, vegetables, and beans was found to reduce the risk for AD [5]. Not surprisingly, the studies carried in these countries reported a positive correlation with consumption of polyphenols [26], [34], [35], [38]. Western diets, instead, where processed foods and red meat consumption are more prevalent, were found to increase the chances of developing AD [5]. Indeed, except one of the studies by Ide et al. [24] taking place in Japan, the studies that reported a negative correlation with polyphenols conducted were three in the United States, two in Australia, two in the Netherlands, one in the United Kingdom, and one in Scotland [25], [28], [29], [30], [33], [36], [42], [39], [45].
Second, observational studies used food questionnaires and interviews to assess dietary intake. This method is known to be subject of certain recall or report biases among the participants, which might have altered the baseline dietary intake assessment [50]. Similarly, cognitive performance during tests could have been altered by the setting, type of test and scoring method, pressure or overthinking by the participants, and overinterpretation of the results [50]. Socioeconomic status (SES) is also a relevant confounder, because socioeconomic background is considered to be related to access to healthy foods, such as fruits and vegetables rich in polyphenols [51], [52]. It is important to note that only eight of 24 studies accounted for SES, meaning that the findings from the remaining studies might have been skewed because of it [22], [25], [27], [29], [31], [32], [33], [39]. In addition, four of 24 studies did not account for any confounder, thus their findings might be weakened by this factor [24], [26], [28], [44].
4.3. Comparisons with other studies
The studies in this review only included human subjects, resulting in a small number of articles. In contrast, animal studies and in vitro studies tend to be more prevalent. The evidence coming from such studies is much more conclusive and consistent, and reports positive results more frequently than human-based trials. For instance, an in vitro study by Scholey et al. [53] found that epigallocatechin gallate increased α, β, and θ activity, self-rated calmness, and reduced self-reported stress. When compared with the study by Wightman et al. [28], no positive findings on the effects of epigallocatechin gallate were reported in a RCT of human subjects. Similarly, a study by Henry-Vitrac et al. [54] stated that isoflavones could inhibit Aβ-peptide fibril formation in vitro and thus contribute to the prevention of AD. However, these findings contradict with what was found in this research based on human trials. In fact, Gleason et al. [42] and Henderson et al. [36] did not find any significant result for the effects of isoflavones on cognitive development among patients with AD.
Results from animal studies often do not match results from RCTs either. Kim et al. [55] researched the role of curcumin, a nonflavonoid polyphenol found abundantly in Curcuma longa. They discovered that this turmeric plays a beneficial role in patients with AD and in animal models of neurodegenerative disease [55]. Its antioxidant activity protected rat cells from Aβ-induced toxicity [55]. This review included two RCTs, which assessed the effects of curcumin in patients with AD; however, both Baum et al. [23] and Ringman et al. [45] did not find any statically significant result on this relationship. Again, animal models of AD cannot often reproduce the actual physical conditions of living human beings, and this might explain why animal studies did not show convergence with human-based studies included in this review.
Finally, other systematic reviews have been conducted on this topic. However, to the best of our knowledge, this is the only review about all classes of polyphenols including solely human subjects. Other reviews either included in vitro and animal studies or included only human subjects but assessed the effects of one single polyphenolic compound. These latter types of systematic reviews found more comparable results to those reported in this review. For instance, Pase et al. [56] reviewed the effects of B. monnieri based on human trials. Although research on this compound is still at early stages, they found some evidence that it improves memory and potentially also other cognitive abilities. Similarly, the study by Downey et al. [41], present in this review, on improved cognitive performance by B. monnieri reported comparable results with improved performance at first, second, and fourth repetition after dosing.
On the contrary, when comparing the results from other reviews that included animal and cell studies, it is noticeable that there is again a clear gap in translating results to a human sample. In fact, although the validity of such studies is usually recognized, their generalizability to human subjects is much more under discussion. A systematic review by Ebrahimi and Schluesener [57] grouped the findings from animal and cellular studies on the effects of polyphenols on neurodegenerative disorders. They found that polyphenols act as antioxidant, prevent neurotoxicity, and affect amyloidogenic pathway. Although these findings do match some of the results from the human studies included in this research, the overall outcome of this review is much more fragmented and inconclusive. This leads to the hypothesis that the evidence coming from in vitro and animal studies is not enough to suggest the use of polyphenols for the prevention or treatment of AD, but rather it provides substantial data to justify further testing of polyphenols on humans.
4.4. Strengths and limitations
The strength of this systematic review is that it is a comprehensive work on the effects of all polyphenols on the development of AD. Although other studies already reviewed the evidence of the effects of a certain compound containing polyphenols, this review attempted to collect all recent evidence on all classes of polyphenols. This is important because it enables further research on the role of polyphenols in the development of AD and the aging process. Another important strength of this research is that, compared with other systematic reviews on the topic, it included only studies with human subjects. Moreover, this research only included studies published in the last 10 years, which means that their findings are up to date and in line with current knowledge about AD and the etiology of the disease. Finally, 18 of 24 studies are RCTs, which are considered to be the study design with the highest level of evidence. In fact, they are conducted in controlled and documented settings, which increase their reliability and replicability levels compared with other study designs.
However, this research has also several limitations. The studies included provided findings that are difficult to generalize. In fact, the RCTs had small or very small sample populations. Furthermore, the samples came from different countries; therefore, their results might not apply to the world population. This is especially true when comparing results from studies carried in developed and in the developing countries, which might create a baseline difference among the populations. Moreover, even when more studies researched the same exposure measurement, they measured outcomes differently, either with biomarkers or cognitive tests. Because there are several biomarkers to test for AD and different cognitive tests to measure thresholds of cognitive decline, it is hard to determine whether similar findings resulting from different outcome measurements can be considered comparable. Another limitation consists of the study design of this research: only two databases were included, PubMed and Web of Science, because of time constraints; if this research was to be carried by a team, it would be feasible to include more databases and thus potentially include more lines of findings as well. Finally, this research included only studies in English, which might not represent all the evidence, and thus create a language bias possibly leading to erroneous conclusions [58]. A publication bias might have skewed results as well, because studies reporting positive results tend to get published more often than studies with negative results [59].
5. Recommendations
This review concluded that the findings were incomplete to determine with certainty that there is sufficient evidence of the benefits of polyphenols on AD. Therefore, more research is necessary to establish this connection. Currently, the small samples of the yielded studies and the great variety of polyphenolic compounds assessed limit their validity and generalizability. To increase evidence of the beneficial effects of polyphenols on AD, it would be recommended to conduct more clinical trials with bigger population samples. This would increase the generalizability of potential positive findings. Furthermore, none of the studies included in this review made comparisons between different types of polyphenols, but each looked at a different one. Such study design would add an interesting source of evidence on whether different types of polyphenols can have significant differences on the outcomes measured.
In terms of public health recommendations, at the current state of knowledge, natural polyphenols are considered safe to use and well tolerated by patients. Therefore, if polyphenols were found to significantly reduce the risk for AD, it could be recommended for people older than 65 years to take supplements or include them in their everyday diets. If further confirmed by RCTs, supplementation could be considered a valid option as a prevention measure for people at risk of AD and dementia, as well as potential additional treatment to slow down the progression of disease.
6. Conclusion
This review attempted to systematically collect current evidence on the protective effects of polyphenols on the development of AD. A total of 24 studies were included in this review, of which 18 studies were clinical trials and six studies were observational. The findings suggest that the studies included in this review provide inconclusive evidence. Although most studies did find at least one positive result between exposure and outcome, the quality of the studies with positive results was lower compared with the quality of studies with either negative or mixed results. This is an important factor to take into account when evaluating the evidence provided by this review. Another reason, which suggests inclusiveness of the findings, is the great variety of polyphenols assessed, outcome measurements, and study characteristics that made it difficult to generalize and compare the data. Furthermore, several potential confounders present in the study designs and when performing tests are severe limitations to the studies included in this research. The most important confounders discussed in this review are baseline diets, SES, report, and recall biases, which might have interfered with the findings from each study, and thus with the overall results from this review. Finally, this review recommends to conduct further research on polyphenols and AD, specifically by carrying more RCTs and possibly with larger human samples.
Research in Context.
-
1.
Systematic review: Studies have been collected through a systematic search on two databases, PubMed and Web of Science. Only studies with human subjects were included, because they present a higher evidence level. Both randomized controlled trials and observational studies were included. Studies were then evaluated using an adaptation of a previously published quality score.
-
2.
Interpretation: The aim of this systematic review was to collect and evaluate all the relevant studies on the beneficial effects of polyphenols on Alzheimer's disease (AD). The main objective was to assess the current state of evidence on this relationship, only on the basis of studies with human subjects.
-
3.
Future directions: To increase evidence of the beneficial effects of polyphenols on AD, it would be recommended to conduct more clinical trials with larger population samples. This would increase the generalizability of potential positive findings. Furthermore, none of the studies included in this review made comparisons between different types of polyphenols, but each looked at a different one. Such study design would add an interesting source of evidence on whether different types of polyphenols can have significant differences on the outcomes measured. In terms of public health recommendations, if further confirmed by randomized controlled trials, supplementation could be considered a valid option as a prevention measure for people older than 65 years at risk of AD and dementia, as well as potential additional treatment to slow down the progression of disease.
Footnotes
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.trci.2018.09.002.
Supplementary data
References
- 1.Queen B.L., Tollefsbol T.O. Polyphenols and aging. Curr Aging Sci. 2010;3:34–42. doi: 10.2174/1874609811003010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dementia [online]. World Health Organization. 2018. http://www.who.int/mediacentre/factsheets/fs362/en/ Available at: Accessed January 5, 2018.
- 3.Alzheimer's Disease Fact Sheet [online]. National Institute on Aging. 2018. https://www.nia.nih.gov/alzheimers/publication/alzheimers-disease-fact-sheet Available at: Accessed January 5, 2018.
- 4.Dosunmu R., Wu J., Basha M.R., Zawia N.H. Environmental and dietary risk factors in Alzheimer's disease. Expert Rev Neurother. 2007;7:887–900. doi: 10.1586/14737175.7.7.887. [DOI] [PubMed] [Google Scholar]
- 5.Hu N., Yu J., Tan L., Wang Y., Sun L., Tan L. Nutrition and the risk of Alzheimer's disease. Biomed Res Int. 2013;2013:1–12. doi: 10.1155/2013/524820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saragat B., Buffa R., Mereu E., Succa V., Cabras S., Mereu R.M. Nutritional and psycho-functional status in elderly patients with Alzheimer's disease. J Nutr Health Aging. 2012;16:231–236. doi: 10.1007/s12603-011-0347-3. [DOI] [PubMed] [Google Scholar]
- 7.Pugazhenthi S., Qin L., Reddy P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer's disease. Biochim Biophys Acta. 2016;1863:1037–1045. doi: 10.1016/j.bbadis.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah R. The role of nutrition and diet in Alzheimer's disease: a systematic review. J Am Med Dir Assoc. 2013;14:398–402. doi: 10.1016/j.jamda.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Hartman R., Shah A., Fagan A., Schwetye K., Parsadanian M., Schulman R. Pomegranate juice decreases amyloid load and improves behaviour in a mouse model of Alzheimer's disease. Neurobiol Dis. 2006;24:506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Kim D., Nguyen M., Dobbin M., Fischer A., Sananbenesi F., Rodgers J. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry G. IOS Press BV; Amsterdam: 2013. Alzheimer's disease: advances for a new century. [Google Scholar]
- 12.Mayeux R., Yaakov S. Epidemiology of Alzheimer's disease. Cold Spring Harb Perspect Med. 2012;2:a006239. doi: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane C.A., Hardy J., Schott J.M. Alzheimer's disease. Eur J Neurol. 2018;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 14.Alzheimer's Disease [online]. Mayo Clinic. 2018. https://www.mayoclinic.org/diseases-conditions/alzheimers-disease/symptoms-causes/syc-20350447 Available at: Accessed January 5, 2018.
- 15.Morris G.P., Clark I.A., Vissel B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer's disease. Acta Neuropathol Commun. 2014;2:1–21. doi: 10.1186/s40478-014-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zibadi S., Preedy V.R., Watson R.R. Academic Press; London: 2014. Polyphenols in human health and disease. [Google Scholar]
- 17.Manach C., Scalbert A., Morand C., Rémésy C., Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 18.Schluesener J.K., Schluesener H. Plant polyphenols in the treatment of age-associated diseases: revealing the pleiotropic effects of icariin by network analysis. Mol Nutr Food Res. 2014;58:60–69. doi: 10.1002/mnfr.201300409. [DOI] [PubMed] [Google Scholar]
- 19.Zamora-Ros R., Touillaud M., Rothwell J., Romieu I., Scalbert A. Measuring exposure to the polyphenol metabolome in observational epidemiologic studies: current tools and applications and their limits. Am J Clin Nutr. 2014;100:11–26. doi: 10.3945/ajcn.113.077743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter P., Gray L., Troughton J., Khunti K., Davies M. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ. 2010;341:c4229. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Collaborating Centre for Methods and Tools. McMaster University; Hamilton, ON: 2008. http://www.nccmt.ca/resources/search/14 [updated 03 October 2017]. Available at. Accessed January 5, 2018. [Google Scholar]
- 22.Arab H., Mahjoub S., Hajian-Tilaki K., Moghadasi M. The effect of green tea consumption on oxidative stress markers and cognitive function in patients with Alzheimer's disease: a prospective intervention study. Caspian J Intern Med. 2016;7:188–194. [PMC free article] [PubMed] [Google Scholar]
- 23.Baum L., Wai Kei Lam C., Cheung S.K., Kwok T., Lui V., Tsoh J. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 24.Ide K., Yamada H., Takuma, Kawasaki Y., Harada S., Nakase J. Effects of green tea consumption on cognitive dysfunction in an elderly population: a randomized placebo controlled study. Nutr J. 2016;15:1–9. doi: 10.1186/s12937-016-0168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kent K., Charlton K., Roodenrys S., Batterham M., Potter J., Traynor V. Consumption of anthocyanin rich cherry juice for 12 weeks improves memory and cognition in older adults with mild to moderate dementia. Eur J Nutr. 2017;56:333–341. doi: 10.1007/s00394-015-1083-y. [DOI] [PubMed] [Google Scholar]
- 26.Morillas-Ruiz J.M., Rubio-Perez J.M., Albaladejo M.D., Zafrilla P., Parra S., Vidal-Guevara M.L. Effect of an antioxidant drink on homocysteine levels in Alzheimer's patients. J Neurol Sci. 2010;299:175–178. doi: 10.1016/j.jns.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 27.Turner R.S., Thomas R.G., Craft S., van Dyck C.H., Mintzer J., Reynolds B.A. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85:1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wightman E.L., Haskell C.F., Forster J.S., Veasey R.C., Kennedy D.O. Epigallocatechin gallate, cerebral blood flow parameters, cognitive performance and mood in healthy humans: a double-blind, placebo-controlled, crossover investigation. Hum Psychopharmacol Clin Exp. 2012;27:177–186. doi: 10.1002/hup.1263. [DOI] [PubMed] [Google Scholar]
- 29.Butchart C., Kyle J., McNeill G., Corley J., Gow A.J., Starr J.M. Flavonoid intake in relation to cognitive function in later life in the Lothian Birth Cohort 1936. Br J Nutr. 2011;106:141–148. doi: 10.1017/S0007114510005738. [DOI] [PubMed] [Google Scholar]
- 30.Devore E.E., Grodstein F., van Rooij F.J.A., Hofman A., Stampfer M.J., Witteman J.C.M. Dietary antioxidants and long-term risk of dementia. Arch Neurol. 2010;67:819–825. doi: 10.1001/archneurol.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devore E.E., Kang J.H., Breteler M.M.B., Grodstein F. Dietary intake of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012;72:135–143. doi: 10.1002/ana.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Root M., Ravine E., Harper A. Flavonol intake and cognitive decline in middle-aged adults. J Med Food. 2015;18:1327–1332. doi: 10.1089/jmf.2015.0010. [DOI] [PubMed] [Google Scholar]
- 33.Nooyens A.C.J., Milder I.E.J., van Gelder B.M., Bueno-de-Mesquita H.B., van Boxtel M.P.J., Verschuren W.M.M. Diet and cognitive decline at middle age: the role of antioxidants. Br J Nutr. 2015;113:1410–1417. doi: 10.1017/S0007114515000720. [DOI] [PubMed] [Google Scholar]
- 34.Kesse-Guyot E., Fezeu L., Andreeva V.A., Touvier M., Scalbert A., Hercberg S. Total and specific polyphenol intakes in midlife are associated with cognitive function measured 13 years later. J Nutr. 2011;142:76–83. doi: 10.3945/jn.111.144428. [DOI] [PubMed] [Google Scholar]
- 35.Ide K., Yamada H., Takuma N., Park M., Wakamiya N., Nakase J. Green tea consumption affects cognitive dysfunction in the elderly: a pilot study. Nutrients. 2014;6:4032–4042. doi: 10.3390/nu6104032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson V.W., St. John J.A., Hodis H.N., Kono N., McCleary C.A., Franke A.A. Long-term soy isoflavone supplementation and cognition in women: a randomized, controlled trial. Neurology. 2012;78:1841–1848. doi: 10.1212/WNL.0b013e318258f822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krikorian R., Shidler M.D., Nash T.A., Kalt W., Vinqvist-Tymchuk M.R., Shukitt-Hale B. Blueberry supplementation improves memory in older adults. J Agric Food Chem. 2010;58:3996–4000. doi: 10.1021/jf9029332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desideri G., Kwik-Uribe C., Grassi D., Necozione S., Ghiadoni L., Mastroiacovo D. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the cocoa, cognition, and aging (CoCoA) study. Hypertension. 2012;60:794–801. doi: 10.1161/HYPERTENSIONAHA.112.193060. [DOI] [PubMed] [Google Scholar]
- 39.Ryan J., Croft K., Spong J., Downey L., Kure C., Lloyd J. An examination of the effects of the antioxidant Pycnogenol on cognitive performance, serum lipid profile, endocrinological and oxidative stress biomarkers in an elderly population. J Psychopharmacol. 2008;22:553–562. doi: 10.1177/0269881108091584. [DOI] [PubMed] [Google Scholar]
- 40.Yimam M., Burnett B.P., Brownell L., Jia Q. Clinical and preclinical cognitive function improvement after oral treatment of a botanical composition composed of extracts from Scutellaria baicalensis and Acacia catechu. Behav Neurol. 2016;2016:1–9. doi: 10.1155/2016/7240802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Downey L.A., Kean J., Nemeh F., Lau A., Poll A., Gregory R. An acute, double-blind, placebo-controlled crossover study of 320 mg and 640 mg doses of a special extract of Bacopa monnieri (CDRI 08) on sustained cognitive performance. Phytother Res. 2013;27:1407–1413. doi: 10.1002/ptr.4864. [DOI] [PubMed] [Google Scholar]
- 42.Gleason C.E., Fischer B.L., Dowling N.M., Setchell K.D.R., Atwood C.S., Carlsson C.M. Cognitive effects of soy isoflavones in patients with Alzheimer's disease. J Alzheimers Dis. 2015;47:1009–1019. doi: 10.3233/JAD-142958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alharbi M.H., Lamport D.J., Dodd G.F., Saunders C., Harkness L., Butler L.T. Flavonoid rich orange juice is associated with acute improvements in cognitive function in healthy middle aged males. Eur J Nutr. 2016;55:2021–2029. doi: 10.1007/s00394-015-1016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brickman A.M., Khan U.A., Provenzano F.A., Yeung L.K., Suzuki W., Schroeter H. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci. 2014;17:1798–1803. doi: 10.1038/nn.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ringman J.M., Frautschy S.A., Teng E. Oral curcumin for Alzheimer's disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res Ther. 2012;4:1–8. doi: 10.1186/alzrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huynh R.A., Mohan C. Alzheimer's disease: biomarkers in the genome, blood, and cerebrospinal fluid. Front Neurol. 2017;8:1–15. doi: 10.3389/fneur.2017.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scalbert A., Johnson I.T., Saltmarsh M. Polyphenols: antioxidants and beyond. Am J Clin Nutr. 2005;81:215S–217S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- 48.Cheynier V. Polyphenols in foods are more complex than often thought. Am J Clin Nutr. 2005;81:223S–229S. doi: 10.1093/ajcn/81.1.223S. [DOI] [PubMed] [Google Scholar]
- 49.Manach C., Williamson G., Morand C., Scalbert A., Rémésy C. Bioavailability and bioefficacy of polyphenols in humans: review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 50.Webb P., Bain C. 2nd ed. Cambridge University Press; Cambridge: 2011. Essential epidemiology: an introduction for students and health professionals. [Google Scholar]
- 51.Pechey R., Jebb S.A., Kelly M.P., Almiron-Roig E., Conde S., Nakamura R. Socioeconomic differences in purchases of more vs. less healthy foods and beverages: analysis of over 25,000 British households in 2010. Soc Sci Med. 2013;92:22e26. doi: 10.1016/j.socscimed.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giskes K., Avendano M., Brug J., Kunst A.E. A systematic review of studies on socioeconomic inequalities in dietary intakes associated with weight gain and overweight/obesity conducted among European adults. Obes Rev. 2010;11:413–429. doi: 10.1111/j.1467-789X.2009.00658.x. [DOI] [PubMed] [Google Scholar]
- 53.Scholey A., Downey L.A., Ciorciari J., Pipingas A., Nolidin K., Finn M. Acute neurocognitive effects of epigallocatechin gallate (EGCG) Appetite. 2012;58:767–770. doi: 10.1016/j.appet.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 54.Henry-Vitrac C., Berbille H., Mérillon J.M., Vitrac X. Soy isoflavones as potential inhibitors of Alzheimer ß-amyloid fibril aggregation in vitro. Food Res Int. 2010;43:2176–2178. [Google Scholar]
- 55.Kim D.S., Park S.Y., Kim J.K. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from βA(1-42) insult. Neurosci Lett. 2006;303:57–61. doi: 10.1016/s0304-3940(01)01677-9. [DOI] [PubMed] [Google Scholar]
- 56.Pase M.P., Kean J., Sarris J., Neale C., Scholey A.B., Stough C. The cognitive-enhancing effects of Bacopa monnieri: a systematic review of randomized, controlled human clinical trials. J Altern Complement Med. 2012;18:647–652. doi: 10.1089/acm.2011.0367. [DOI] [PubMed] [Google Scholar]
- 57.Ebrahimi A., Schluesener H. Natural polyphenols against neurodegenerative disorders: potentials and pitfalls. Ageing Res Rev. 2012;11:329–345. doi: 10.1016/j.arr.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Morrison A., Polisena J., Husereau D., Moulton K., Clark M., Fiander M. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28:138–144. doi: 10.1017/S0266462312000086. [DOI] [PubMed] [Google Scholar]
- 59.Joober R., Schmitz N., Annable L., Boksa P. Publication bias: what are the challenges and can they be overcome? J Psychiatry Neurosci. 2012;37:149–152. doi: 10.1503/jpn.120065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.