Abstract
Background
It is not clear whether laparoscopic transcystic exploration (LTCE) laparoscopic choledochotomy (LCD) is superior in the management of choledocholithiasis. In this meta‐analysis, the success of LTCE versus LCD was evaluated.
Methods
Cochrane Central Register of Controlled Trials, Web of Science, Trip, PubMed, Ovid and Embase databases were searched systematically for relevant literature up to May 2017. Studies that compared the success rate of LTCE and LCD in patients with choledocholithiasis were included. PRISMA guidelines were followed. Multiple independent reviewers contributed on a cloud‐based platform. Random‐effects model was used to calculate odds ratios (ORs) or standardized mean differences (MDs) with 95 per cent confidence intervals. An a priori hypothesis was generated based on clinical experience that LTCE is as successful as LCD.
Results
Of 3533 screened articles, 25 studies comprising 4224 patients were included. LTCE achieved a lower duct clearance rate than LCD (OR 0.38, 95 per cent c.i. 0·24 to 0·59). It was associated with a shorter duration of surgery (MD −0·86, 95 per cent c.i. −0·97 to −0·77), lower bile leak (OR 0·46, 0·23 to 0·93) and shorter hospital stay (MD −0·78, −1·14 to −0·42) than LCD. There was no statistically significant difference in conversion, stricture formation or reintervention rate.
Conclusion
LCD has a higher rate of successful duct clearance, but is associated with a longer duration of surgery and hospital stay, and a higher bile leak rate.
Introduction
Concomitant common bile duct (CBD) stones are present in 3–15 per cent of patients with symptomatic cholelithiasis in the Western world1. In approximately 2 per cent of patients these stones are considered clinically significant2. These patients require CBD stone extraction for symptomatic relief and to prevent serious associated complications, including cholangitis, hepatic abscess and acute pancreatitis3.
Advances in preoperative imaging, endoscopic and laparoscopic surgical techniques have led to less invasive methods of extracting CBD stones4, and there is now a range of potential management options5. In the early era of laparoscopic cholecystectomy, patients with suspected CBD stones were commonly referred for endoscopic retrograde cholangiopancreatography (ERCP) and sphincterotomy. Although still a valid management option, this approach has the disadvantage of being a two‐stage procedure with potential increased costs and morbidity6. With increased laparoscopic experience, single‐stage laparoscopic cholecystectomy and CBD exploration have become an increasingly popular alternative7. There are two main approaches to laparoscopic common bile duct exploration (LCBDE): laparoscopic transcystic exploration (LTCE), reaching the CBD via the cystic duct, and laparoscopic choledochotomy (LCD), exploring the CBD directly via a choledochotomy.
Several high‐quality comparisons between ERCP and LCBDE have been performed8. With the trend of primary CBD closure and reduced morbidity of this procedure9, as opposed to the resultant morbidity from ERCP10, there is a movement towards single‐stage surgical management of gallstone disease. Little attention, however, has been paid to comparing the two different approaches of LCDBE. Frequently, data for both approaches are reported together as combined figures, limiting direct comparison of success rate and safety. Moreover, patients undergoing either of the approaches are treated differently in the postoperative period11. The standard surgical approach has been LCD. With increased experience, a trend is observed towards LTCE assuming lower morbidity. Currently, evidence is limited on whether LTCE results in at least a similar clearance rate of the CBD stones. The aim of this study was to compare both approaches in terms of clearance rate and other relevant outcomes from the available literature.
Methods
This systematic review and meta‐analysis was conducted according to PRISMA guidelines12. The study protocol was registered with PROSPERO, the international prospective register of systematic reviews (registration number CRD42017079458).
Eligibility criteria and outcomes
The criteria for considering studies for inclusion in this review were defined using the Population, Intervention, Comparison and Outcome (PICO) strategy. The study population comprised adults who presented with CBD stones diagnosed via imaging, with no previous cholecystectomy. The type of intervention was LTCE for the treatment of CBD stones, and the comparator was the standard LCD approach. The primary outcome of the study was the success rate of the approach, identified by the rate of complete clearance without conversion from transcystic to transcholedochal or from either to an open approach. The reported incidence of retained CBD stones was used to validate the clearance rate. Secondary outcomes included duration of surgery, length of hospital stay, conversion to open procedure, and intraoperative or postoperative complications (early and late).
Interventional and observational studies comparing the LTCE approach with LCD were evaluated for inclusion. Studies were excluded if they did not meet the above criteria or if there was no statement in the article on ethical approval. Articles that did not report outcomes for both arms, review articles or meta‐analyses, editorials and animal studies were excluded.
The search was primarily for articles in English. Studies presented in other languages were, however, considered for inclusion based on the inclusion and exclusion criteria and the presence of an abstract in English, French or Italian. There was no publication date restriction.
Literature search
Cochrane Central Register of Controlled Trials, Web of Science, Trip, PubMed, Ovid and Embase databases were searched systematically to identify relevant articles published up to May 2017. Citation alerts were set up for potentially missed or recent articles published during the manuscript synthesis. Google and Google Scholar were used to find non‐indexed publications, to reduce the risk of publications bias.
Study selection and data extraction
Four authors conducted their database search independently. They screened titles and abstracts first. Duplicates were handled in Mendeley® (Elsevier, London, UK)12. If more than one paper was published by the same group, their most recent publication was selected if the number included was larger than their earlier publication and there was no clear indication that the recent study did not include patients from the earlier publication. Articles found suitable for inclusion were then cross‐referenced to ensure inclusion of all eligible studies. Articles that could not be obtained from the internet with multi‐institutional access were sought via the library service. Detailed search strategies, Boolean operators, different search techniques, filters and limits were documented (Table S1, supporting information).
Platforms used for collaborative work
The independently short‐listed search results from across the databases were exported to cloud‐based shared tables (Google sheets: access https://docs.google.com/spreadsheets/d/1uswlPUDrrX9BQj_VpSEHpiQKk91y6Gq1av4SGZ4JYEg/edit?usp=sharing) for further selection and conflict resolution. Two authors decided on conflicts in inclusion or exclusion. Included studies were further exported to Mendeley® citation manager for citations. The initial manuscript draft was produced on Google Docs (Google, Mountain View, California, USA) for live collaborative editing, then exported to Word® processor (Microsoft, Redmond, Washington, USA) for final editing.
Quality assessment
The median quality score for the RCTs was judged based on the Cochrane Handbook13. The quality assessment stratifies the current evidence and projects the need for further research on the topic based on the quality of the available evidence into: high‐quality evidence where further research is not expected to change the current confidence in the estimate of the effect size, moderate‐quality evidence if further research is likely to influence confidence in the estimated effect and may change it; low‐quality evidence if further research is very likely to influence confidence in the estimate of effect and is likely to change it; and very low‐quality evidence when there is no certainty about the estimated effect. The median quality score for the non‐randomized studies was based on the West suggestion14. Quality grading is dependent on the rigor of the research methodology.
Statistical analysis
Statistical analysis was performed using Comprehensive Meta‐Analysis software for Windows® version 2 (Biostat, Englewood, New Jersey, USA). This software was used to generate all forest plots. Heterogeneity was calculated with the χ2 test. An I 2 value above 30 per cent or P < 0·050 was considered an indicator of observed heterogeneity15. In case of significant heterogeneity, the random‐effects model was used rather than the fixed‐effect model. Results for dichotomous data were stated as odds ratios (ORs), and those for continuous data as standardized mean differences (MDs). Both were provided with their 95 per cent confidence intervals. The random‐effect models was applied for the estimated pooled effect size, given the observed heterogeneity and an adequate number of the included studies16.
When data were summarized as median (range) rather than mean(s.d.), these values were converted to mean(s.d.) when necessary, as described previously17.
Results
Of 4381 citations screened, some 25 studies comprising 4224 patients were included (Fig. 1 a). Of these patients, 2320 (54·9 per cent) and 1904 (45·1 per cent) underwent LTCE and LCD respectively. Three studies were RCTs; all others were retrospective studies. The publication date of the studies ranged from 1995 to 2016. The regional origin of the studies is displayed in Fig. 1 b. These studies are summarized in Table S2 (supporting information)18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42. The mean age of patients in the studies ranged from 38 to 68 years. The majority of studies (18 of 25) did not provide a breakdown of mean age per treatment arm. Only two studies18, 19 provided a breakdown of the male to female ratio for each treatment arm. None of the 25 studies reported mean BMI. Only one study20 reported the preoperative laboratory investigation (median (range) bilirubin concentration for LTCE 20 (6–74) μmol/l and for LCD 20 (7–89) μmol/l). Biliary colic was the most common patient presentation.
Figure 1.
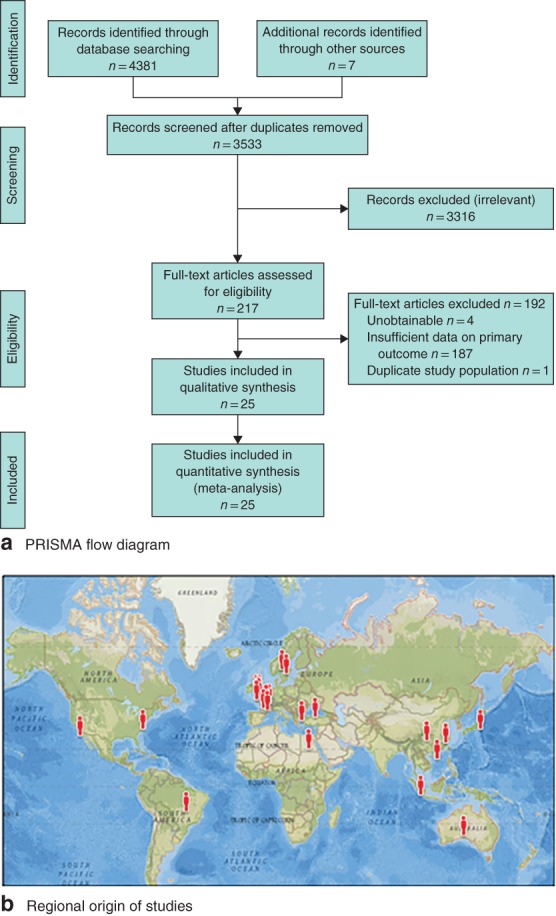
PRISMA flow diagram for the systematic review and regional origin of the studies. a PRISMA diagram; b map of regional origin of studies.
The median quality score for the RCTs was 8 (range 7–16) of 30, and that for the non‐randomized studies14 was 15 (range 10–27) of 40. Details of the quality scorings are provided in Tables S3 and S4 (supporting information).
There was considerable heterogeneity regarding the primary outcome and the secondary outcomes of mean duration of surgery and hospital stay (Figs 2 and 3). The random‐effects model was therefore used for these outcome measures. There was non‐significant heterogeneity regarding conversion to an open procedure, stricture, bile leak and reintervention, yet, given the nature of the included studies, the random‐effects model was used43.
Figure 2.
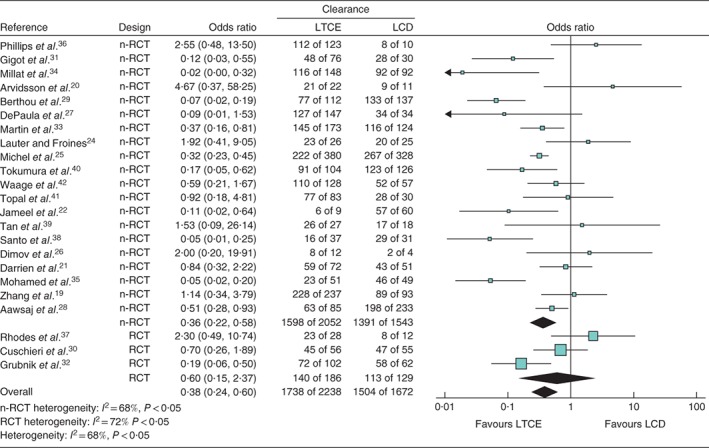
Forest plot for successful duct clearance in patients with choledocholithiasis undergoing a laparoscopic transcystic or transcholedochal approach. Studies that had 100 per cent success in both arms18, 23 were not included in the analysis, so calculation of an odds ratio was not possible in the pooled analysis. LTCE, laparoscopic transcystic exploration; LCD, laparoscopic choledochotomy; nRCT, non‐randomized clinical trial. A random‐effects model was used for meta‐analysis. Odds ratios are shown with 95 per cent confidence intervals.
Figure 3.
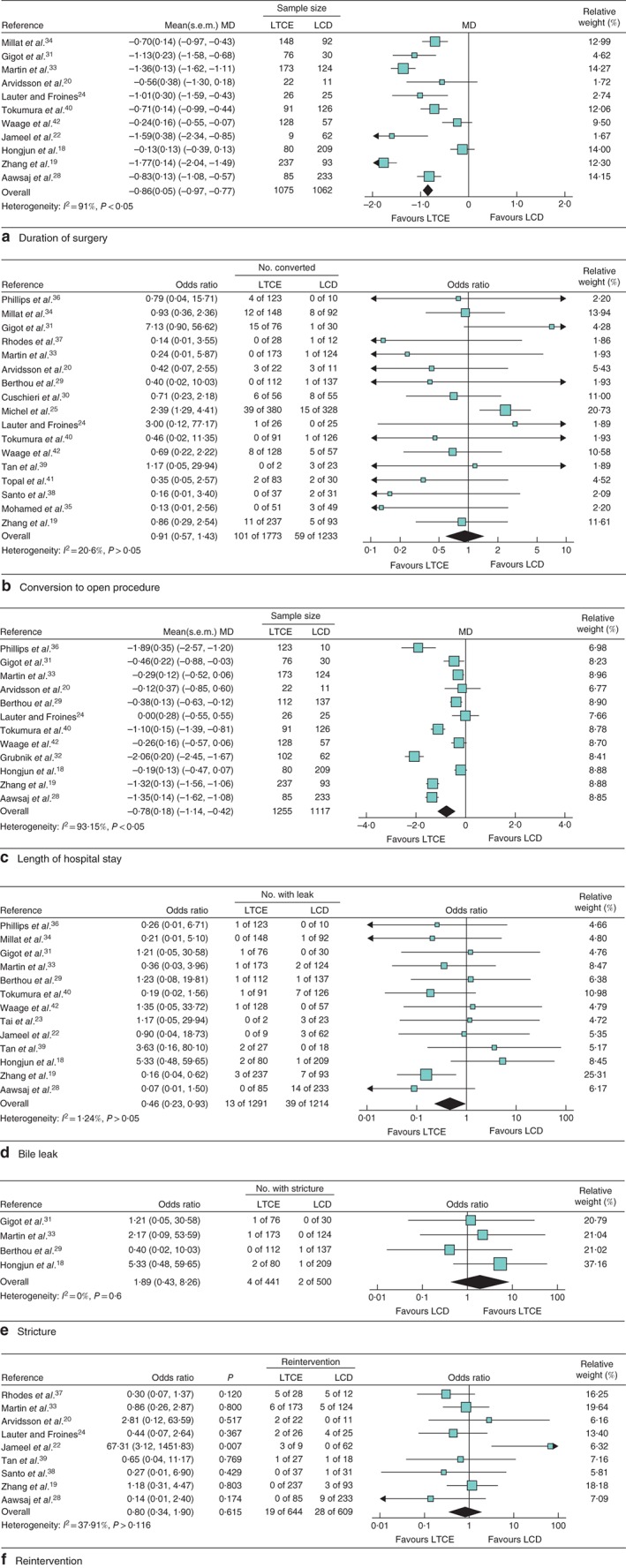
Forest plots for duration of surgery, conversion to open procedure, length of hospital stay, bile leak, stricture and reintervention in patients with choledocholithiasis undergoing a laparoscopic transcystic or transcholedochal approach. a Duration of surgery, showing an approximately 45 min longer operating time in the laparoscopic choledochotomy (LCD) group; b Conversion to open procedure; c length of hospital stay; d bile leak (as studies that had no leaks in either arm20, 24, 25, 26 were not included in generation of the forest plot, the software did not permit calculation of the odds ratio); e stricture; f reintervention (studies that reported no reinterventions18, 26, 27 were not included in the analysis, so calculation of an odds ratio was not possible). Random‐effects models were used for meta‐analysis. LTCE, laparoscopic transcystic exploration. a,c Standardized mean differences (MDs) and b,d–f odds ratios are shown with 95 per cent confidence intervals.
Success rate
In all studies success was defined as complete duct clearance. The use of completion cholangiography to confirm duct clearance for both groups was clearly stated in eight of the 25 studies. One further study30 clearly stated that completion cholangiography was used in the LCD group but did not mention whether it was used in the LTCE group. Four other studies19, 20, 33, 34 stated that postoperative cholangiography was performed in patients undergoing biliary drainage to confirm clearance before removal of the T‐tube. Performance of completion cholangiography was not recorded in 11 studies. No significant association between instruments used and success was observed for either approach (Table S5, supporting information).
The odds of successful duct clearance were lower for LTCE than for LCD (OR 0·38, 95 per cent c.i. 0·24 to 0·59). No difference between the two approaches (OR 0·60, 0·15 to 2·37) was observed in RCTs, whereas the pooled estimate for non‐randomized trials showed significantly higher odds in favour of LCD (OR 0·36, 0·22 to 0·58).
Cumulative analysis – temporal trend
The effect of time on outcome is shown in Fig. S1 (supporting information). From 1995 to 1999, no significant difference was observed between the two approaches. From 2000 onwards the studies consistently showed a higher rate of successful duct clearance with LCD compared with LTCE.
Surgical data and morbidity
A shorter mean(s.d.) duration of surgery for LTCE compared with LCD (129(59) versus 175(61) min respectively; MD −0·86, 95 per cent c.i. −0·97 to −0·77) was observed (Fig. 3 a). No significant difference in conversion rate was found between the two approaches (Fig. 3 b). A significantly shorter mean hospital stay was seen for LTCE compared with LCD (MD −0·78, −1·14 to −0·42 (Fig. 3 c).
LTCE resulted in significantly fewer bile leaks than LCD (OR 0·46, 95 per cent c.i. 0·23 to 0·93) (Fig. 3 d). None of the RCTs reported on the incidence of bile leak in both arms. The incidence of biliary stricture did not significantly differ between the groups (Fig. 3 e).
No difference was seen in the pooled effect estimate for reintervention following for LTCE compared with LCD (OR 0·80, 95 per cent c.i. 0·34 to 1·90) (Fig. 3 f). Table S6 (supporting information) summarizes the types of procedure in each group after the primary intervention. From the aspect of patient selection, extracted data relating to the diameter of each duct, and the number and size of stones in each group were not informative (Table S7, supporting information).
Publication bias
The classical fail‐safe N test of bias was significant (Z = −7·6, P < 0·001). The identified number of studies required for the P value to fall above α − α = 0·050 was 326 studies. A funnel plot demonstrating the distribution of standard error by the log odds ratio is shown in Fig. S2 (supporting information).
Discussion
Successful duct clearance occurred more often with LCD than with LTCE. LCD was, however, associated with a longer duration of surgery and hospital stay. This was probably a result of the additional time required for sutured closure of the CBD and the higher risk of bile leak respectively. Bile duct suturing is a challenging task and has a significant learning curve44. Clipping the cystic duct stump is easier. No significant difference in conversion rate, bile duct stricture or reoperation was observed.
The included studies have recognized obstacles to successful LTCE, including an inability to negotiate the cystic duct (in particular due to long, tortuous cystic ducts with low insertions), multiple small stones in the non‐dilated CBD, and some stones being too large to be removed by LTCE21. It can be difficult and time‐consuming to remove multiple small stones using LTCE, with a significant risk of displacing some stones into the proximal CBD that cannot then be retrieved.
A temporal trend over time was observed. Studies published from 2000 all showed consistently higher odds of successful duct clearance with LCD. This was probably associated with improved technology, including the widespread use of high‐definition cameras and dedicated instruments. The refinement in surgical techniques and the learning curve could also have been a factor44, yet none of the included studies reported this being an issue. Before 2000 completion cholangiography was typically reserved for patients requiring biliary drainage, whereas after 2000 completion cholangiography appears to have been used more liberally to confirm stone clearance. It is possible that this may, in part, help explain the observed temporal trend in clearance rates.
The temporal trend observed does not negate the need for further studies to address this issue. Most studies included were not of high quality, with only three RCTs with significant heterogeneity. Scrutinizing the types and combination of instruments used did not reveal any clear pattern or difference between the two arms that may have accounted for the temporal trend seen before and after 2000.
In a single previous meta‐analysis45 on this subject, no significant differences between LTCE or LCD in the analysis of rates of stone clearance, conversion to open procedures, total morbidity, operating time or blood loss were observed. The authors observed a reduction in biliary complications in the LTCE group and concluded that this route was safer than the LCD approach. These results and conclusions are different to findings in the present study. This may be a result of the inclusion of studies with comparisons other than LCD versus LTCE and possible confusion in defining transcystic bile duct exploration.
Unfortunately, insufficient data were available in the included studies to make inferences about the impact on stricture formation of the relationship between duct diameter and the approach used. The authors are concerned, however, that there might be selection bias, with larger stones tending to be approached via the CBD. More recent trends towards primary closure without a T‐tube22 are often limited by the diameter of the bile duct as a risk factor for the leak46. The leak, however, is usually of little clinical importance compared with the added morbidity associated with T‐tube or stent insertion47.
There are limitations to this study. The full text of four relevant articles48, 49, 50, 51 could not be obtained, and there was not enough information in the abstracts on the primary outcome. By design, this study was liable for publication bias52. In an attempt to minimize this risk of bias, the inclusion of articles was not limited by language or date. In addition, the consistency of reporting of the secondary outcomes in the included papers was highly variable.
Supporting information
Table S1 Search strategies and Boolean characters used across the various databases
Table S2 Baseline demographics of included patients and summary of their presentations
Table S3 Quality assessment of included RCTs according to the Cochrane Handbook of Systematic Reviews 42
Table S4 Quality assessment of non‐RCTs included in the review according to the scoring system of West et al. 43
Table S5 Instrument details and completion cholangiography
Table S6 Summary of reinterventions in each group after the primary exploration and cholecystectomy
Table S7 Number of stones and duct diameter
Fig. S1 Cumulative analysis demonstrating the temporal trend for the primary outcome
Fig. S2 Funnel plot of standard error versus log odds ratio for statistical testing of publication bias
Acknowledgements
R.S. and M.B. contributed equally to this publication.
Disclosure: The authors declare no conflict of interest.
Funding information
No funding
References
- 1. Winder JS, Pauli EM. Common bile duct stones: health care problem and incidence In Multidisciplinary Management of Common Bile Duct Stones, Hazey JW, Conwell DL, Guy GE. (eds). Springer: Cham, 2016, 5–15. [Google Scholar]
- 2. Lee DH, Ahn YJ, Lee HW, Chung JK, Jung IM. Prevalence and characteristics of clinically significant retained common bile duct stones after laparoscopic cholecystectomy for symptomatic cholelithiasis. Ann Surg Treat Res 2016; 91: 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Working Party of the British Society of Gastroenterology; Association of Surgeons of Great Britain and Ireland; Pancreatic Society of Great Britain and Ireland; Association of Upper GI Surgeons of Great Britain and Ireland . UK guidelines for the management of acute pancreatitis. Gut 2005; 54(Suppl 3): iii1–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rocha FG. Surgical Common Bile Duct Exploration https://www.uptodate.com/contents/common‐bile‐duct‐exploration [accessed 26 November 2017].
- 5. Williams EJ, Green J, Beckingham I, Parks R, Martin D, Lombard M. Guidelines on the management of common bile duct stones (CBDS). Gut 2008; 57: 1004–1021. [DOI] [PubMed] [Google Scholar]
- 6. Petelin JB, Mayfield T. 24. Laparoscopic common bile duct exploration: transcystic duct approach In The SAGES Manual, Soper N, Scott‐Conner C. (eds). Springer: New York, 2012; 311–329. [Google Scholar]
- 7. Salama AF, Abd Ellatif ME, Abd Elaziz H, Magdy A, Rizk H, Basheer M et al Preliminary experience with laparoscopic common bile duct exploration. BMC Surg 2017; 17: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rogers SJ, Cello JP, Horn JK, Siperstein AE, Schecter WP, Campbell AR et al Prospective randomized trial of LC+LCBDE vs ERCP/S+LC for common bile duct stone disease. Arch Surg 2010; 145: 28–33. [DOI] [PubMed] [Google Scholar]
- 9. Alhamdani A, Mahmud S, Jameel M, Baker A. Primary closure of choledochotomy after emergency laparoscopic common bile duct exploration. Surg Endosc 2008; 22: 2190–2195. [DOI] [PubMed] [Google Scholar]
- 10. Szary NM, Al‐Kawas FH. Complications of endoscopic retrograde cholangiopancreatography: how to avoid and manage them. Gastroenterol Hepatol (N Y) 2013; 9: 496–504. [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang WJ, Xu GF, Huang Q, Luo KL, Dong ZT, Li JM et al Treatment of gallbladder stone with common bile duct stones in the laparoscopic era. BMC Surg 2015; 15: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JPT, Green S. (eds). Cochrane Handbook for Systematic Reviews of Interventions. Wiley: Chichester, 2008. [Google Scholar]
- 14. West S, King V, Carey TS, Lohr KN, McKoy N, Sutton SF et al Systems to Rate the Strength of Scientific Evidence. Agency for Healthcare Research and Quality, US Department of Health and Human Services: Bethesda, 2002. [PMC free article] [PubMed] [Google Scholar]
- 15. Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta‐analysis: I 2 is not an absolute measure of heterogeneity. Res Synth Methods 2017; 8: 5–18. [DOI] [PubMed] [Google Scholar]
- 16. Jackson D, Turner R. Power analysis for random‐effects meta‐analysis. Res Synth Methods 2017; 8: 290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hongjun H, Yong J, Baoqiang W. Laparoscopic common bile duct exploration: choledochotomy versus transcystic approach? Surg Laparosc Endosc Percutan Tech 2015; 25: 218–222. [DOI] [PubMed] [Google Scholar]
- 19. Zhang WJ, Xu GF, Huang Q, Luo KL, Dong ZT, Li JM et al Treatment of gallbladder stone with common bile duct stones in the laparoscopic era. BMC Surg 2015; 15: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arvidsson D, Haglind E, Leijonmarck CE, Strömberg C. [Laparoscopy possible even in cholelithiasis. Results for 96 patients from three different hospitals are reviewed.] Lakartidningen 1997; 94: 2724–2728. [PubMed] [Google Scholar]
- 21. Darrien JH, Connor K, Janeczko A, Casey JJ, Paterson‐Brown S. The surgical management of concomitant gallbladder and common bile duct stones. HPB Surg 2015; 2015: 165068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jameel M, Darmas B, Baker AL. Trend towards primary closure following laparoscopic exploration of the common bile duct. Ann R Coll Surg Engl 2008; 90: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tai CK, Tang CN, Ha JP, Chau CH, Siu WT, Li MK. Laparoscopic exploration of common bile duct in difficult choledocholithiasis. Surg Endosc 2004; 18: 910–914. [DOI] [PubMed] [Google Scholar]
- 24. Lauter DM, Froines EJ. Laparoscopic common duct exploration in the management of choledocholithiasis. Am J Surg 2000; 179: 372–374. [DOI] [PubMed] [Google Scholar]
- 25. Michel J, Navarro F, Montpeyroux F, Burgel JS, Le Moine MC, Daures JP et al [Treatment of common bile duct stones with laparoscopy. Retrospective multicenter study with 612 patients.] Gastroenterol Clin Biol 2000; 24: 404–408. [PubMed] [Google Scholar]
- 26. Dimov RS, Kantchev RI, Boev BG, Ivanov TI, Apostolov IA, Hinov AG et al Laparoscopic exploration of bile ducts in patients with calculosis. Indications, methods and first results. Folia Med (Plovdiv) 2013; 55: 33–38. [DOI] [PubMed] [Google Scholar]
- 27. DePaula AL, Hashiba K, Bafutto M. Laparoscopic management of choledocholithiasis. Surg Endosc 1994; 8: 1399–1403. [DOI] [PubMed] [Google Scholar]
- 28. Aawsaj Y, Light D, Horgan L. Laparoscopic common bile duct exploration: 15‐year experience in a district general hospital. Surg Endosc 2016; 30: 2563–2566. [DOI] [PubMed] [Google Scholar]
- 29. Berthou JC, Drouard F, Charbonneau P, Moussalier K. Evaluation of laparoscopic management of common bile duct stones in 220 patients. Surg Endosc 1998; 12: 16–22. [DOI] [PubMed] [Google Scholar]
- 30. Cuschieri A, Lezoche E, Morino M, Croce E, Lacy A, Toouli J et al E.A.E.S. multicenter prospective randomized trial comparing two‐stage vs single‐stage management of patients with gallstone disease and ductal calculi. Surg Endosc 1999; 13: 952–957. [DOI] [PubMed] [Google Scholar]
- 31. Gigot JF, Navez B, Etienne J, Cambier E, Jadoul P, Guiot P et al A stratified intraoperative surgical strategy is mandatory during laparoscopic common bile duct exploration for common bile duct stones. Lessons and limits from an initial experience of 92 patients. Surg Endosc 1997; 11: 722–728. [DOI] [PubMed] [Google Scholar]
- 32. Grubnik VV, Tkachenko AI, Ilyashenko VV, Vorotyntseva KO. Laparoscopic common bile duct exploration versus open surgery: comparative prospective randomized trial. Surg Endosc 2012; 26: 2165–2171. [DOI] [PubMed] [Google Scholar]
- 33. Martin IJ, Bailey IS, Rhodes M, O'Rourke N, Nathanson L, Fielding G. Towards T‐tube free laparoscopic bile duct exploration: a methodologic evolution during 300 consecutive procedures. Ann Surg 1998; 228: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Millat B, Atger J, Deleuze A, Briandet H, Fingerhut A, Guillon F et al Laparoscopic treatment for choledocholithiasis: a prospective evaluation in 247 consecutive unselected patients. Hepatogastroenterology 1997; 44: 28–34. [PubMed] [Google Scholar]
- 35. Mohamed MA, Bahram MA, Ammar MS, Nassar AH. One‐session laparoscopic management of combined common bile duct and gallbladder stones versus sequential ERCP followed by laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A 2015; 25: 482–485. [DOI] [PubMed] [Google Scholar]
- 36. Phillips EH, Rosenthal RJ, Carroll BJ, Fallas MJ. Laparoscopic trans‐cystic‐duct common‐bile‐duct exploration. Surg Endosc 1994; 8: 1389–1393. [DOI] [PubMed] [Google Scholar]
- 37. Rhodes M, Sussman L, Cohen L, Lewis MP. Randomised trial of laparoscopic exploration of common bile duct versus postoperative endoscopic retrograde cholangiography for common bile duct stones. Lancet 1998; 351: 159–161. [DOI] [PubMed] [Google Scholar]
- 38. Santo MA, Domene CE, Riccioppo D, Barreira L, Takeda FR, Pinotti HW. Common bile duct stones: analysis of the videolaparoscopic surgical treatment. Arq Gastroenterol 2012; 49: 41–51. [DOI] [PubMed] [Google Scholar]
- 39. Tan KK, Shelat VG, Liau KH, Chan CY, Ho CK. Laparoscopic common bile duct exploration: our first 50 cases. Ann Acad Med Singapore 2010; 39: 136–142. [PubMed] [Google Scholar]
- 40. Tokumura H, Umezawa A, Cao H, Sakamoto N, Imaoka Y, Ouchi A et al Laparoscopic management of common bile duct stones: transcystic approach and choledochotomy. J Hepatobiliary Pancreat Surg 2002; 9: 206–212. [DOI] [PubMed] [Google Scholar]
- 41. Topal B, Aerts R, Penninckx F. Laparoscopic common bile duct stone clearance with flexible choledochoscopy. Surg Endosc 2007; 21: 2317–2321. [DOI] [PubMed] [Google Scholar]
- 42. Waage A, Strömberg C, Leijonmarck CE, Arvidsson D. Long‐term results from laparoscopic common bile duct exploration. Surg Endosc 2003; 17: 1181–1185. [DOI] [PubMed] [Google Scholar]
- 43. Clark TS, Linzer DA. Should I use fixed or random effects? Polit Sci Res Methods 2015; 3: 399–408. [Google Scholar]
- 44. Hong KM, Chai BD, Seo DB, Ku KB, Park KH. The learning curve for laparoscopic common bile duct exploration through a choledochotomy – report on 50 patients. J Minim Invasive Surg 2008; 11: 111–119. [Google Scholar]
- 45. Feng Q, Huang Y, Wang K, Yuan R, Xiong X, Wu L. Laparoscopic transcystic common bile duct exploration: advantages over laparoscopic choledochotomy. PLoS One 2016; 11: e0162885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hua J, Lin S, Qian D, He Z, Zhang T, Song Z. Primary closure and rate of bile leak following laparoscopic common bile duct exploration via choledochotomy. Dig Surg 2015; 32: 1–8. [DOI] [PubMed] [Google Scholar]
- 47. Parra‐Membrives P, Martínez‐Baena D, Lorente‐Herce J, Jiménez‐Riera G. Comparative study of three bile duct closure methods following laparoscopic common bile duct exploration for choledocholithiasis. J Laparoendosc Adv Surg Tech A 2018; 28: 145–151. [DOI] [PubMed] [Google Scholar]
- 48. Chen X, Ding Y, Wang W, Zhang A. The comparative study on two types of laparoscopic common bile duct exploration. J Clin Surg 2007; 17: 231–235. [Google Scholar]
- 49. Yongze T, Dexin C, Haibin L, Andong Z, Jin X. Comparison of transcystic with trans‐duct incision in laparoscopic choledochotomy with primary ductal closure. Chin J Min Inv Surg 2013; 21: 869–872. [Google Scholar]
- 50. Wu S, Zhan S, Qiu H. The clinical study of treatment common bile duct stones by laparoscopic cystic duct approach. Anhui Med J 2014; 11: 685–686. [Google Scholar]
- 51. Huang S. Efficacy comparison of laparoscopic common bile duct through the cystic duct lithotomy and choledocholithotomy surgery. Mod Pract Med 2015; 27: 213–214. [Google Scholar]
- 52. Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in meta‐analyses using individual participant data: a database survey. BMJ 2012; 344: d7762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Search strategies and Boolean characters used across the various databases
Table S2 Baseline demographics of included patients and summary of their presentations
Table S3 Quality assessment of included RCTs according to the Cochrane Handbook of Systematic Reviews 42
Table S4 Quality assessment of non‐RCTs included in the review according to the scoring system of West et al. 43
Table S5 Instrument details and completion cholangiography
Table S6 Summary of reinterventions in each group after the primary exploration and cholecystectomy
Table S7 Number of stones and duct diameter
Fig. S1 Cumulative analysis demonstrating the temporal trend for the primary outcome
Fig. S2 Funnel plot of standard error versus log odds ratio for statistical testing of publication bias


