Abstract
Background
Hepatic surgery is appropriate for selected patients with colorectal liver metastases (CRLM). Advances in chemotherapy have led to modification of management, particularly when metastases disappear. Treatment should address all initial CRLM sites based on pretherapeutic cross‐sectional imaging. This study aimed to evaluate pretherapeutic fiducial marker placement to optimize CRLM treatment.
Methods
This pilot investigation included patients with CRLM who were considered for potentially curative treatment between 2009 and 2016. According to a multidisciplinary team decision, lesions smaller than 25 mm in diameter that were more than 10 mm deep in the hepatic parenchyma and located outside the field of a planned resection were marked. Complication rates and clinicopathological data were analysed.
Results
Some 76 metastases were marked in 43 patients among 217 patients with CRLM treated with curative intent. Of these, 23 marked CRLM (30 per cent), with a mean(s.d.) size of 11·0(3·4) mm, disappeared with preoperative chemotherapy. There were four complications associated with marking: two intrahepatic haematomas, one fiducial migration and one misplacement. After a median follow‐up of 47·7 (range 18·1–144·9) months, no needle‐track seeding was noted. Of four disappearing CRLM that were marked and resected, two presented with persistent active disease. Other missing lesions were treated with thermoablation.
Conclusion
Pretherapeutic fiducial marker placement appears useful for the curative management of CRLM.
Introduction
Colorectal liver metastases (CRLM) occur in about 60 per cent of patients with colorectal cancer1. Surgery, often in association with thermoablation2, offers these patients the best chance of prolonged survival3, 4, with 5‐year survival rates of over 50 per cent and a curative rate of 17 per cent4. However, only 25 per cent of CRLM are resectable5, 6 at the time of diagnosis, although up to 20 per cent of CRLM initially assessed as unresectable become resectable after chemotherapy7, 8, 9.
Preoperative chemotherapy is the treatment of choice in patients with borderline resectable or unresectable CRLM, especially when the carcinoembryonic antigen level is raised and performance status is preserved10, 11, 12. It has been estimated13, 14 that in 9–37 per cent of patients treated with preoperative chemotherapy at least one CRLM will disappear after preoperative chemotherapy, challenging the curative intent strategy. Despite a complete radiological response, a complete pathological response is estimated to occur in anything from 17 to 80 per cent of these patients13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24. A previous study described two types of vanishing lesion: disappearing lesions, defined as CRLM that disappear from the future resected liver; and missing metastases (MM), defined as CRLM that disappear but remain part of the future liver remnant15.
According to a recent consensus statement16, the goal of hepatic resection should be to remove all original sites of disease with a technique of maximum parenchymal sparing. The efficacy of this approach is jeopardized by MM17, 18. Marking those lesions at risk of disappearing using a fiducial marker was proposed to facilitate the elective treatment of these areas25. This pilot investigation was aimed to evaluate this strategy, focusing on marking‐related complications.
Methods
From August 2009 to December 2016, all patients who presented with pathologically proven potentially curative CRLM and who were considered for preoperative chemotherapy at the tertiary University Hospital of Lyon (Hospices Civils de Lyon) were registered in the study. Approximately 100 patients with CRLM are treated there each year. This pilot investigation was performed according to the Declaration of Helsinki. Patients signed a dedicated informed consent before the marking during a preinterventional consultation (ethical considerations are detailed in Appendix S1, supporting information).
Patient selection
An initial comprehensive radiological assessment was performed in all patients and ideally included at least a body scan by enhanced multidetector CT (MDCT), along with gadolinium‐enhanced MRI with diffusion‐weighted imaging (DWI) (Fig. 1). Before starting systemic chemotherapy, each patient was evaluated in a multidisciplinary team (MDT) meeting that included hepatobiliary surgeons, interventional radiologists and medical oncologists. After reassessment of cross‐sectional imaging, the therapeutic strategy was defined and potentially resectable patients were then determined. All liver lesions that remained uncertain after imaging, and thus not deemed to be assessed as CRLM, were biopsied using contrast‐enhanced ultrasound imaging or CT guidance. CRLM were deemed resectable when a hepatectomy could achieve a negative margin while preserving more than 30 per cent of the total estimated liver volume, sparing two continuous liver segments, and maintaining vascular inflow/outflow and biliary drainage20, 26. Thermoablation was considered as an alternative to surgery for deep lesions (more than 1 cm into the liver capsule) smaller than 2 cm.
Figure 1.
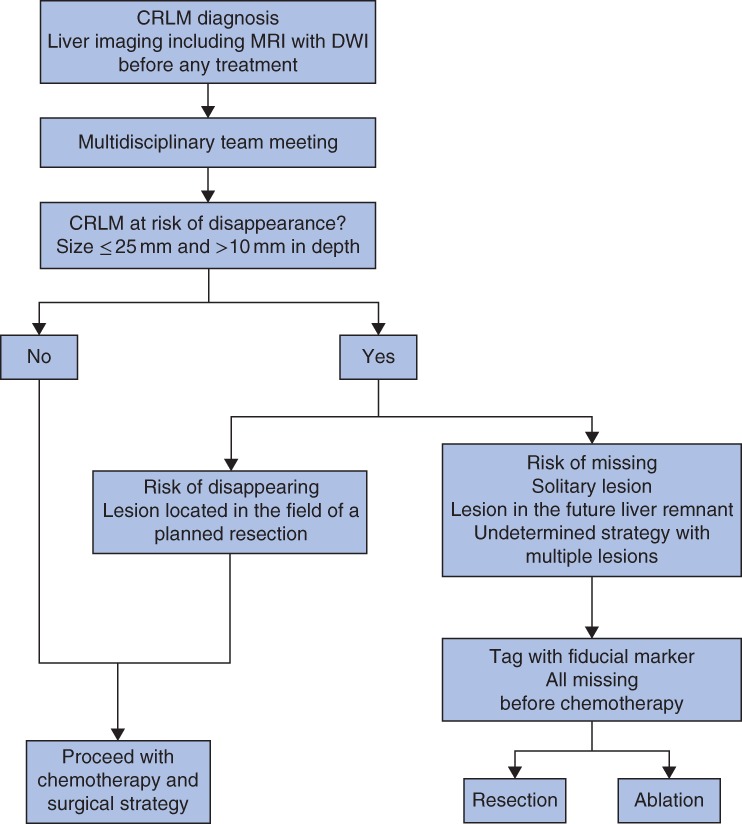
Strategy for fiducial marker placement for lesions at risk of disappearance. CRLM, colorectal liver metastases; DWI, diffusion‐weighted imaging
Patients received preoperative chemotherapy according to French guidelines, and the regimen was determined during an MDT meeting27. The response to chemotherapy was evaluated radiologically with MDCT and/or MRI, every four cycles, according to the Response Evaluation Criteria in Solid Tumours28, as well as morphologically29. Lesions at risk of disappearance were defined as lesions smaller than 25 mm and more than 10 mm deep in the liver parenchyma.
Metastases at risk were classified into two groups: ‘disappearing’, when the lesion was included in the field of the planned hepatectomy; and ‘missing’, when the lesion was in the future liver remnant or a potential resection would compromise a large amount of the normal liver. When lesions at risk of becoming missing were assigned to ablative treatment, patients were assigned to radiological marking to evaluate the response to chemotherapy.
Marking technique
Procedures were performed with local anaesthetic (lidocaine 1 per cent) or conscious sedation when more than one lesion required marking. After checking the coagulation profile, a 3‐mm ring precharged in a 137‐mm 18‐G puncture needle (O‐Twist‐Marker®; BIP Medical, Türkenfeld, Germany) was used. Using ultrasonographic guidance, the tip of the needle was placed to deliver the clip at the margin of the lesion, usually at the posterior edge, rather than inside the lesion, to minimize the risk of tumour seeding. In each case, the position of the clip relative to the lesion was described precisely. If lesions could not be visualized by conventional ultrasound imaging, contrast‐enhanced ultrasonography (SonoVue®; Bracco International, Amsterdam, the Netherlands), CT guidance, or the real‐time fusion of ultrasonography and MRI (diffusion weighted or 3D T1 Gd‐enhanced sequences) images were used to localize the metastases. CT and liver MRI were performed before the operation.
All complications following fiducial placements were graded according to the Clavien–Dindo classification30, with particular attention to hepatic parenchymal haematoma, fiducial marker migration, needle‐track dissemination and in situ recurrence. Fiducial markers located at a distance more than 10 mm from the lesion at the follow‐up imaging were judged to be misplaced.
Treatment
Surgery was performed under general anaesthesia after abdominal exploration, liver mobilization and intraoperative ultrasonography (IOUS). Each resected specimen was submitted to pathological examination to evaluate the pathological response5. Surgery was performed 4–6 weeks after the last chemotherapy session. The goal of surgery was to obtain complete resection of all CRLM with clear margins.
Thermoablation (radiofrequency or microwave ablation) was performed as described previously2, either during surgery when associated with liver resection or percutaneously with real‐time ultrasound imaging or CT or fusion imaging guidance, under general anaesthesia.
Follow‐up
After surgery, all patients were followed up by clinical examination and radiological assessment via MRI or MDCT every 3 months for 2 years, then every 6 months for 3 years, then annually.
Statistical analysis
Continuous variables are expressed as median (range) or mean(s.d.). The complication rate was assessed by reporting the ratio of the number of complications to the number of procedures. Duration of follow‐up started from the marking placement. Data were analysed according to procedures, patients, marked lesions, disappearing lesions and lesions of less than 5 mm at the time of treatment, considering that the quality of elective treatment would be worse for lesions smaller than this.
Results
Between August 2009 and December 2016, 217 patients with CRLM were treated with curative intent (Fig. 2). Among this consecutive group, 43 patients (19·8 per cent) had 76 CRLM that met the criteria for fiducial marking. Patient characteristics are summarized in Table 1. Twenty‐one patients (49 per cent) presented with initially unresectable disease. All 43 patients underwent MRI before marking. MRI, MDCT and contrast‐enhanced ultrasound imaging were performed initially in 32 patients (74 per cent), and on the day before liver‐directed treatment in 34 patients (79 per cent). The main drug regimens used were FOLFOX (27 patients), FOLFIRI (10) and FOLFIRINOX (2) with or without a biological agent. The mean(s.d.) number of cycles between the diagnosis and radical treatment was 4·6(1·4) in the 37 patients with no disease progression.
Figure 2.
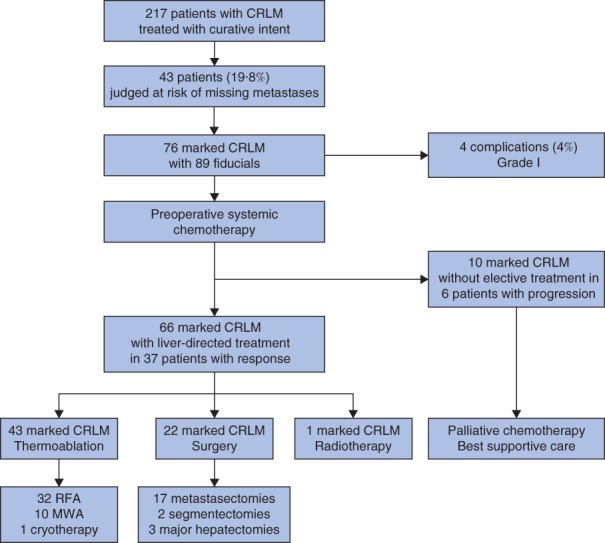
Overview of patient selection and treatment of marked colorectal liver metastases. CRLM, colorectal liver metastases; RFA, radiofrequency ablation; MWA, microwave ablation
Table 1.
Patient characteristics
| No. of patients (n = 43) | |
|---|---|
| Age at diagnosis (years)* | 64·6(8·9) |
| Sex ratio (M : F) | 31 : 12 |
| Site of primary tumour | |
| Colon | 31 |
| Rectum | 12 |
| Liver metastases | |
| No. per patient* | 6·6(5·4) |
| Synchronous | 30 |
| Metachronous | 13 |
| Solitary | 9 |
| Bilobar | 30 |
| Initially resectable disease | 22 |
| Initially unresectable disease | 21 |
| Portal vein embolization | 9 |
| Concurrent extrahepatic metastasis | 9 |
| Initial imaging evaluation | |
| MRI + CT + (CE)US + PET | 2 |
| MRI + CT + (CE)US | 30 |
| MRI + CT | 6 |
| CT + (CE)US | 5 |
| Preoperative chemotherapy regimen | |
| FOLFOX | 15 |
| FOLFOX + bevacizumab | 8 |
| FOLFOX + anti‐EGFR | 4 |
| FOLFIRI + bevacizumab | 3 |
| FOLFIRI + anti‐EGFR | 6 |
| FOLFIRI | 1 |
| FOLFIRINOX | 2 |
| Capecitabine | 4 |
| No. of cycles before hepatic treatment† | 4·6(1·4) |
Values are *mean(s.d.) and †mean(s.d.) based on 37 patients (6 patients with 14 marked metastases were excluded because of progression). (CE)US, (contrast‐enhanced) ultrasonography; PET, positron emission tomography; EGFR, epidermal growth factor receptor.
Disease progression occurred after marking in six patients (14 per cent) with ten marked metastases. These patients did not undergo the planned hepatic treatment. Of 28 patients who underwent surgery, four developed new CRLM at IOUS not previously identified by preoperative imaging. Fifteen patients had surgery as the sole hepatic treatment, and 21 had a combination of surgery and ablative techniques. In one patient the marked CRLM were treated with radiotherapy. The median duration of follow‐up was 47·7 (range 18·1–144·9) months.
Marked metastases
Characteristics of the marked metastases are summarized in Table 2. Some 76 metastases were marked with 89 fiducial markers. Ultrasound guidance was sufficient to mark 42 CRLM (55 per cent). The mean(s.d.) diameter of the marked lesions was 13·6(5·6) mm at diagnosis and 7·6(7·2) mm at time of specific treatment.
Table 2.
Characteristics of marked metastases
| No. of marked metastases (n = 76) | |
|---|---|
| Size of metastases (mm) | |
| At diagnosis* | 13·6(5·6) |
| At specific treatment† | 7·6(7·2) |
| Marking techniques | |
| Total no. of fiducial markers | 89 |
| Local anaesthesia (no. of patients) | 24 |
| Guiding technique | |
| US | |
| No. of CRLM | 42 |
| No. of fiducial markers | 50 |
| CEUS | |
| No. of CRLM | 13 |
| No. of fiducial markers | 15 |
| CT | |
| No. of CRLM | 13 |
| No. of fiducial markers | 16 |
| US–MRI | |
| No. of CRLM | 3 |
| No. of fiducial markers | 3 |
| US–CT | |
| No. of CRLM | 5 |
| No. of fiducial markers | 5 |
| Complications‡ | 4 (4) |
| Treatment of marked metastases | |
| Thermoablation (21 patients) | 43 (57) |
| RFA | 32 |
| MWA | 10 |
| Cryotherapy | 1 |
| Surgery (15 patients) | 22 (29) |
| Metastasectomy | 17 |
| Segmentectomy | 2 |
| Major hepatectomy | 3 |
| Size at diagnosis (mm)* | 15·8(6·9) |
| Size at surgery (mm)* | 8·9(8·3) |
| Histological size (mm)* | 11·9(7·1) |
| Pathological response (% viable cells)* | 32(25) |
| Radiotherapy (1 patient) | 1 (1) |
| No specific treatment (progression) (6 patients) | 10 (13) |
Values in parentheses are percentages unless indicated otherwise; values are
mean(s.d.) and
mean(s.d.) based on 37 patients (6 patients with 14 marked metastases were excluded because of progression).
Total number of complications for total number of fiducial markers placed. CRLM, colorectal liver metastases; US, ultrasonography; CEUS, contrast‐enhanced ultrasonography; RFA, radiofrequency ablation; MWA, microwave ablation.
Of the marked lesions, 43 (57 per cent) were treated using an ablative technique (6 before surgery). Liver resection was performed for 22 of the 76 marked lesions (29 per cent). Parenchymal‐sparing hepatectomy (PSH, metastasectomy) was performed for 17 of these 22 lesions, and five were treated by anatomical resection (2 segmentectomies and 3 major hepatectomies). The mean(s.d.) radiological size of the marked metastases treated by surgery was 15·8(6·9) mm at diagnosis and 8·9(8·3) mm at surgery, with a mean(s.d.) remaining viable cell rate of 32 (25) per cent. At the time of the liver‐directed treatment, 21 of the 43 patients (49 per cent) had 31 marked CRLM smaller than 5 mm. Of these lesions seven (23 per cent) were resected, six using a PSH approach, and ten metastases were not treated because of progression.
Missing metastases
Twenty‐three of the 76 marked lesions (30 per cent) in 16 patients disappeared (Table 3). The mean(s.d.) size of these lesions at diagnosis was 11·0(3·4) mm. All 23 of the MM were treatable: four by surgery, 18 by thermoablation and one by stereotactic radiotherapy. Of the four MM surgically resected, two contained viable residual tumour cells and the two other lesions demonstrated complete histological responses.
Table 3.
Characteristics of missing metastases and lesions smaller than 5 mm at elective treatment
| No. of marked metastases (n = 76) | |
|---|---|
| Missing marked metastases | 23 (30) |
| No. of patients | 16 |
| Size at diagnosis (mm)* | 11·0(3·4) |
| Treatment | |
| Surgery | 4 |
| Metastasectomy | 3 |
| Major hepatectomy | 1 |
| Lesion visible on IOUS | 0 |
| Pathological response (% viable cells) | 30, 30, 0, 0† |
| Thermoablation | 18 |
| RFA | 17 |
| MWA | 1 |
| Radiotherapy | 1 |
| Marked lesions < 5 mm at elective treatment | 31 (41) |
| No. of patients | 21 |
| Size at diagnosis (mm)* | 11·5(3·5) |
| Treatment | |
| Surgery | 7 |
| Metastasectomy | 6 |
| Major hepatectomy | 1 |
| Thermoablation | 23 |
| RFA | 21 |
| MWA | 1 |
| Cryotherapy | 1 |
| Radiotherapy | 1 |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.).
Individual pathological responses for the four patients who had surgery. IOUS, intraoperative ultrasonography; RFA, radiofrequency ablation; MWA, microwave ablation.
Marking‐related complications
Four marking‐related complications occurred in 89 procedures: two hepatic parenchymal haematomas, one fiducial marker migration and one fiducial marker misplacement. All complications were grade 1; none required any specific treatment, with no delay in planned chemotherapy. Fiducial marker migration occurred during the marking of a 15‐mm CRLM close to the median hepatic vein. The marker migrated through the hepatic vein to the pulmonary parenchyma, with no clinical consequences (Fig. 3 a,b). The two hepatic haematomas were smaller than 2 cm, with no clinical or biological effects. Both of these involved ultrasound‐guided marking (Fig. 3 c–f). The misplacement involved a lesion close to the portal branch of segment III in a patient who had undergone previous right hepatectomy and two metastasectomies from segments II and III. It was not possible to access the new lesion in the remnant of segment III. The fiducial marker was placed 17 mm from the lesion.
Figure 3.
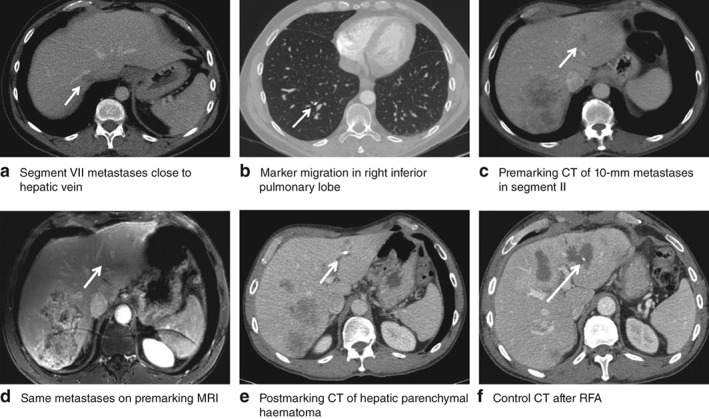
Fiducial marker placement‐related complications. a,b Fiducial marker migration: a premarking CT scan with metastases of segment VII close to the hepatic vein; b postmarking CT scan with fiducial marker migration in the subsegmental branch of the posterobasal segment from the inferior right lobe with no evidence of pulmonary embolism. c–f Hepatic parenchymal haematoma: c premarking CT and d premarking MRI scans showing a 10‐mm metastasis in segment III (white arrow); e control CT scan the day after marking showing a hepatic parenchymal haematoma; f control CT scan after radiofrequency ablation (RFA)
At the initial diagnosis, six patients among the study population (14 per cent) presented with peritoneal metastases. Five others developed peritoneal metastases after fiducial placement, with a median time from diagnosis of 18·2 (range 12·2–23·0) months. Of these 11 patients, seven were treated with curative intent with cytoreduction and hyperthermic intraperitoneal chemotherapy. At operation, the location of the peritoneal nodules did not correlate with the needle track. In the other four patients, cross‐sectional imaging showed no sign of perihepatic needle track‐related metastasis.
Discussion
Fiducial marker placement allowed the elective treatment of all 23 patients with MM, 18 by thermoablation, four with surgery and one with radiotherapy. Two of the four resected patients with MM still had viable malignant cells on pathological analysis. This rate varies widely between 20 and 83 per cent13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, with an in situ MM recurrence rate reported to occur in 33–74 per cent of lesions31, emphasizing the importance of dealing with these lesions in an attempted curative treatment strategy. Strategies to achieve comprehensive hepatic clearance are evolving rapidly, and include PSH32, 33, which can be combined with ablation to optimize treatment and limit morbidity34, 35. In retrospective series, the oncological outcomes of these combined strategies are associated with lower progression‐free survival but similar overall survival compared with that achieved by liver resection without ablation2, 34, 35. Although liver‐sparing resection would be the optimal treatment for CRLM after MDT reassessment, thermoablation was adopted for 43 of the 76 marked metastases of the present series, allowing a follow‐up response to chemotherapy.
Fiducial placement improved the targeting of small lesions in chemotherapy‐altered liver parenchyma, without impairing further treatments. ‘Difficult to localize’ lesions were those that responded to chemotherapy and almost disappeared. The marker enabled these to be targetable (Fig. 4).
Figure 4.
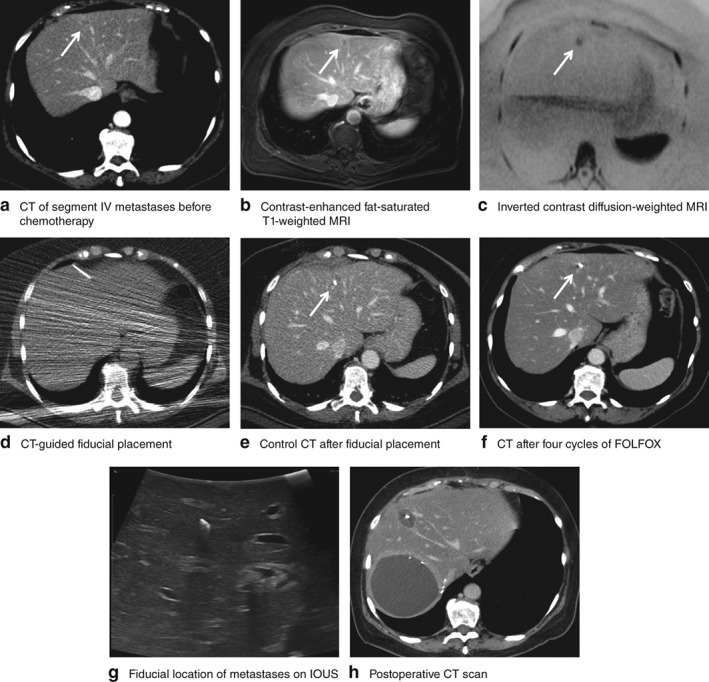
Left colonic adenocarcinoma with four liver metastases in segments IV, VI, VII and VIII. Before chemotherapy, cross‐sectional images from a CT, b MRI contrast‐enhanced fat‐saturated T1‐weighted image and c MRI inverted contrast diffusion‐weighted image showed liver metastasis at risk of being missed (white arrow) in segment IV. After CT‐guided marking (d) a control CT scan confirmed good fiducial marker placement (e). f After four cycles of FOLFOX–bevacizumab, the marked lesion disappeared from the CT scan. g The fiducial marker allowed the location of the missing metastasis to be identified easily by intraoperative ultrasonography (IOUS), allowing radiofrequency ablation in addition to a right hepatectomy. h A postoperative control CT scan confirmed the good targeting of the ablation
Fiducial marker migration is a well recognized phenomenon. For example, a previous study36 reported a patient in whom a 2‐mm spherical gold marker dropped from the liver through the vena cava to the hip vein with no adverse reaction after 19 months. Another study37 described embolization at the junction of the vena cava and right atrium of a 4‐mm platinum marker implanted for hepatic stereotactic body radiotherapy. The fiducial marker was easily removed with an endovascular procedure, and the patient was asymptomatic. Kitamura and colleagues38 studied fiducial migration inside the liver and estimated it to be less than 2·5 mm from the basal position of the marker.
In the present study, a risk factor for migration was the proximity of a lesion to a major hepatic vein. In such cases, the risk may be mitigated by determining the location of regional veins on preimplantation images and planning a safer approach37, 39.
Misplacement of markers is another clinical issue. This has been estimated previously to affect about 10 per cent of placements, where lesions were located high in the liver (segments VII and VIII) or proximal to major portal pedicles39. The fiducial marker misplaced in the present series occurred in a lesion difficult to reach, given previous liver surgery, despite a CT‐guided approach. Hepatic haematomas and fiducial marker migrations were probably due to the proximity to hepatic vessels. These might be avoided by placing the marker at the lesion edge opposite the vessel, by excluding patients on anticoagulant medications or by using a thinner needle with microcoil placement.
Another major concern was the risk of metastatic track seeding. Track dissemination has been well described for hepatocellular carcinoma and is thought to occur in 2·3 per cent of patients40, but has not been investigated widely in the field of CRLM41, 42. In a large analysis of CRLM biopsies performed with 20‐ or 22‐G needles, it was found that 10 per cent had implantation metastases. Accordingly, the authors concluded that these metastases negatively impacted survival in 80 per cent of these patients43. In another retrospective study44, CRLM‐resected patients who underwent biopsy before referral were compared with those who did not undergo biopsy. Among biopsied patients, 19 per cent had evidence of needle‐track deposits. Operative mortality and morbidity rates in the two groups were similar, but the 4‐year survival rate after liver resection was worse in the group that had a preoperative biopsy: mean(s.e.m.) 32.5(5.5) versus 46.7(2.8) per cent (P = 0·008)44. It has been recommended that biopsies should not be performed in potentially resectable patients43, 44, 45. Despite consistent follow‐up and regular cross‐sectional imaging, there was no evidence of track seeding in the present series. This might reflect the use of effective chemotherapy regimens or technical factors, as the preferred method was to mark the posterior edge of the tumour rather than the tumour itself25.
The present study suffers from limitations due to its retrospective analysis. The small number of patients precluded survival analyses. As a result, the impact of fiducial placement strategy on prognosis and in situ recurrence remains undetermined. The proportion of patients at risk of MM and the incidence of MM were low, but the management of these patients was challenging, highlighting the importance of a multidisciplinary approach to treat lesions at risk of disappearance.
Disclosure
The authors declare no conflict of interest.
Supporting information
Appendix S1 Ethical considerations.
Funding information No funding
References
- 1. Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G et al; European Colorectal Metastases Treatment Group . Towards a pan‐European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer 2006; 42: 2212–2221. [DOI] [PubMed] [Google Scholar]
- 2. Imai K, Allard MA, Castro Benitez C, Vibert E, Sa Cunha A, Cherqui D et al Long‐term outcomes of radiofrequency ablation combined with hepatectomy compared with hepatectomy alone for colorectal liver metastases. Br J Surg 2017; 104: 570–579. [DOI] [PubMed] [Google Scholar]
- 3. Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW et al Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27: 3677–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M et al Actual 10‐year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007; 25: 4575–4580. [DOI] [PubMed] [Google Scholar]
- 5. Blazer DG III, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ et al Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 2008; 26: 5344–5351. [DOI] [PubMed] [Google Scholar]
- 6. Adam R, Wicherts DA, de Haas RJ, Aloia T, Lévi F, Paule B et al Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol 2008; 26: 1635–1641. [DOI] [PubMed] [Google Scholar]
- 7. Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D et al Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long‐term survival. Ann Surg 2004; 240: 644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leonard GD, Brenner B, Kemeny NE. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol 2005; 23: 2038–2048. [DOI] [PubMed] [Google Scholar]
- 9. Masi G, Cupini S, Marcucci L, Cerri E, Loupakis F, Allegrini G et al Treatment with 5‐fluorouracil/folinic acid, oxaliplatin, and irinotecan enables surgical resection of metastases in patients with initially unresectable metastatic colorectal cancer. Ann Surg Oncol 2006; 13: 58–65. [DOI] [PubMed] [Google Scholar]
- 10. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P et al; EORTC Gastro‐Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und‐tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM‐CAO); Australasian Gastro‐Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD) . Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008; 371: 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sorbye H, Mauer M, Gruenberger T, Glimelius B, Poston GJ, Schlag PM et al; EORTC Gastro‐Intestinal Tract Cancer Group; Cancer Research UK (CRUK); Arbeitsgruppe Lebermetastasen und‐tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM‐CAO); Australasian Gastro‐Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD) . Predictive factors for the benefit of perioperative FOLFOX for resectable liver metastasis in colorectal cancer patients (EORTC Intergroup Trial 40983). Ann Surg 2012; 255: 534–539. [DOI] [PubMed] [Google Scholar]
- 12. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P et al; EORTC Gastro‐Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM‐CAO); Australasian Gastro‐Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD) . Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long‐term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013; 14: 1208–1215. [DOI] [PubMed] [Google Scholar]
- 13. Auer RC, White RR, Kemeny NE, Schwartz LH, Shia J, Blumgart LH et al Predictors of a true complete response among disappearing liver metastases from colorectal cancer after chemotherapy. Cancer 2010; 116: 1502–1509. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka K, Takakura H, Takeda K, Matsuo K, Nagano Y, Endo I. Importance of complete pathologic response to prehepatectomy chemotherapy in treating colorectal cancer metastases. Ann Surg 2009; 250: 935–942. [DOI] [PubMed] [Google Scholar]
- 15. Elias D, Youssef O, Sideris L, Dromain C, Baton O, Boige V et al Evolution of missing colorectal liver metastases following inductive chemotherapy and hepatectomy. J Surg Oncol 2004; 86: 4–9. [DOI] [PubMed] [Google Scholar]
- 16. Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN; Americas Hepato‐Pancreato‐Biliary Association; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract . Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford) 2013; 15: 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bischof DA, Clary BM, Maithel SK, Pawlik TM. Surgical management of disappearing colorectal liver metastases. Br J Surg 2013; 100: 1414–1420. [DOI] [PubMed] [Google Scholar]
- 18. Ramia‐Angel JM, la Plaza RD, Quiñones JE. Complete clinical response of liver metastasis after chemotherapy: to resect or not? World J Gastrointest Oncol 2011; 3: 107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benoist S, Brouquet A, Penna C, Julié C, El Hajjam M, Chagnon S et al Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol 2006; 24: 3939–3945. [DOI] [PubMed] [Google Scholar]
- 20. Elias D, Goere D, Boige V, Kohneh‐Sharhi N, Malka D, Tomasic G et al Outcome of posthepatectomy‐missing colorectal liver metastases after complete response to chemotherapy: impact of adjuvant intra‐arterial hepatic oxaliplatin. Ann Surg Oncol 2007; 14: 3188–3194. [DOI] [PubMed] [Google Scholar]
- 21. van Vledder MG, de Jong MC, Pawlik TM, Schulick RD, Diaz LA, Choti MA. Disappearing colorectal liver metastases after chemotherapy: should we be concerned? J Gastrointest Surg 2010; 14: 1691–1700. [DOI] [PubMed] [Google Scholar]
- 22. Goèré D, Gaujoux S, Deschamp F, Dumont F, Souadka A, Dromain C et al Patients operated on for initially unresectable colorectal liver metastases with missing metastases experience a favorable long‐term outcome. Ann Surg 2011; 254: 114–118. [DOI] [PubMed] [Google Scholar]
- 23. Ferrero A, Langella S, Russolillo N, Vigano' L, Lo Tesoriere R, Capussotti L. Intraoperative detection of disappearing colorectal liver metastases as a predictor of residual disease. J Gastrointest Surg 2012; 16: 806–814. [DOI] [PubMed] [Google Scholar]
- 24. Owen JW, Fowler KJ, Doyle MB, Saad NE, Linehan DC, Chapman WC. Colorectal liver metastases: disappearing lesions in the era of Eovist hepatobiliary magnetic resonance imaging. HPB (Oxford) 2016; 18: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zalinski S, Abdalla EK, Mahvash A, Vauthey JN. A marking technique for intraoperative localization of small liver metastases before systemic chemotherapy. Ann Surg Oncol 2009; 16: 1208–1211. [DOI] [PubMed] [Google Scholar]
- 26. Ychou M, Viret F, Kramar A, Desseigne F, Mitry E, Guimbaud R et al Tritherapy with fluorouracil/leucovorin, irinotecan and oxaliplatin (FOLFIRINOX): a phase II study in colorectal cancer patients with non‐resectable liver metastases. Cancer Chemother Pharmacol 2008; 62: 195–201. [DOI] [PubMed] [Google Scholar]
- 27. Phelip JM, Bouché O, Conroy T. Thésaurus National de Cancérologie Digestive: Cancer Colorectal Métastatique; January 2016. https://www.snfge.org/tncd [accessed 7 February 2019].
- 28. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–216. [DOI] [PubMed] [Google Scholar]
- 29. Shindoh J, Loyer EM, Kopetz S, Boonsirikamchai P, Maru DM, Chun YS et al Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol 2012; 30: 4566–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuhlmann K, van Hilst J, Fisher S, Poston G. Management of disappearing colorectal liver metastases. Eur J Surg Oncol 2016; 42: 1798–1805. [DOI] [PubMed] [Google Scholar]
- 32. Desjardin M, Desolneux G, Brouste V, Degrandi O, Bonhomme B, Fonck M et al Parenchymal sparing surgery for colorectal liver metastases: the need for a common definition. Eur J Surg Oncol 2017; 43: 2285–2291. [DOI] [PubMed] [Google Scholar]
- 33. Moris D, Dimitroulis D, Vernadakis S, Papalampros A, Spartalis E, Petrou A et al Parenchymal‐sparing hepatectomy as the new doctrine in the treatment of liver‐metastatic colorectal disease: beyond oncological outcomes. Anticancer Res 2017; 37: 9–14. [DOI] [PubMed] [Google Scholar]
- 34. Dupré A, Jones RP, Diaz‐Nieto R, Fenwick SW, Poston GJ, Malik HZ. Curative‐intent treatment of recurrent colorectal liver metastases: a comparison between ablation and resection. Eur J Surg Oncol 2017; 43: 1901–1907. [DOI] [PubMed] [Google Scholar]
- 35. Evrard S, Poston G, Kissmeyer‐Nielsen P, Diallo A, Desolneux G, Brouste V et al Combined ablation and resection (CARe) as an effective parenchymal sparing treatment for extensive colorectal liver metastases. PLoS One 2014; 9: e114404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shirato H, Harada T, Harabayashi T, Hida K, Endo H, Kitamura K et al Feasibility of insertion/implantation of 2·0‐mm‐diameter gold internal fiducial markers for precise setup and real‐time tumor tracking in radiotherapy. Int J Radiat Oncol Biol Phys 2003; 56: 240–247. [DOI] [PubMed] [Google Scholar]
- 37. Hennessey H, Valenti D, Cabrera T, Panet‐Raymond V, Roberge D. Cardiac embolization of an implanted fiducial marker for hepatic stereotactic body radiotherapy: a case report. J Med Case Rep 2009; 3: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kitamura K, Shirato H, Shimizu S, Shinohara N, Harabayashi T, Shimizu T et al Registration accuracy and possible migration of internal fiducial gold marker implanted in prostate and liver treated with real‐time tumor‐tracking radiation therapy (RTRT). Radiother Oncol 2002; 62: 275–281. [DOI] [PubMed] [Google Scholar]
- 39. Passot G, Odisio BC, Zorzi D, Mahvash A, Gupta S, Wallace MJ et al Eradication of missing liver metastases after fiducial placement. J Gastrointest Surg 2016; 20: 1173–1178. [DOI] [PubMed] [Google Scholar]
- 40. Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev 2007; 33: 437–447. [DOI] [PubMed] [Google Scholar]
- 41. Robertson EG, Baxter G. Tumour seeding following percutaneous needle biopsy: the real story! Clin Radiol 2011; 66: 1007–1014. [DOI] [PubMed] [Google Scholar]
- 42. Rodgers MS, Collinson R, Desai S, Stubbs RS, McCall JL. Risk of dissemination with biopsy of colorectal liver metastases. Dis Colon Rectum 2003; 46: 454–458. [DOI] [PubMed] [Google Scholar]
- 43. Ohlsson B, Nilsson J, Stenram U, Akerman M, Tranberg KG. Percutaneous fine‐needle aspiration cytology in the diagnosis and management of liver tumours. Br J Surg 2002; 89: 757–762. [DOI] [PubMed] [Google Scholar]
- 44. Jones OM, Rees M, John TG, Bygrave S, Plant G. Biopsy of resectable colorectal liver metastases causes tumour dissemination and adversely affects survival after liver resection. Br J Surg 2005; 92: 1165–1168. [DOI] [PubMed] [Google Scholar]
- 45. Metcalfe MS, Bridgewater FH, Mullin EJ, Maddern GJ. Useless and dangerous – fine needle aspiration of hepatic colorectal metastases. BMJ 2004; 328: 507–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Ethical considerations.


