Abstract
Background
Small bowel obstruction is a common surgical emergency, and is associated with high levels of morbidity and mortality across the world. The literature provides little information on the conservatively managed group. The aim of this study was to describe the burden of small bowel obstruction in the UK.
Methods
This prospective cohort study was conducted in 131 acute hospitals in the UK between January and April 2017, delivered by trainee research collaboratives. Adult patients with a diagnosis of mechanical small bowel obstruction were included. The primary outcome was in‐hospital mortality. Secondary outcomes included complications, unplanned intensive care admission and readmission within 30 days of discharge. Practice measures, including use of radiological investigations, water soluble contrast, operative and nutritional interventions, were collected.
Results
Of 2341 patients identified, 693 (29·6 per cent) underwent immediate surgery (within 24 h of admission), 500 (21·4 per cent) had delayed surgery after initial conservative management, and 1148 (49·0 per cent) were managed non‐operatively. The mortality rate was 6·6 per cent (6·4 per cent for non‐operative management, 6·8 per cent for immediate surgery, 6·8 per cent for delayed surgery; P = 0·911). The major complication rate was 14·4 per cent overall, affecting 19·0 per cent in the immediate surgery, 23·6 per cent in the delayed surgery and 7·7 per cent in the non‐operative management groups (P < 0·001). Cox regression found hernia or malignant aetiology and malnutrition to be associated with higher rates of death. Malignant aetiology, operative intervention, acute kidney injury and malnutrition were associated with increased risk of major complication.
Conclusion
Small bowel obstruction represents a significant healthcare burden. Patient‐level factors such as timing of surgery, acute kidney injury and nutritional status are factors that might be modified to improve outcomes.
Introduction
Small bowel obstruction (SBO) is a common surgical condition, accounting for 50 per cent of emergency laparotomies each year in the UK1 and over 300 000 admissions annually in the USA2. It is common in low‐ and middle‐income countries, accounting for 1·8 deaths per 100 000 population per year3. The condition is associated with a mortality rate of approximately 10 per cent and high rates of morbidity among survivors4, 5. The causes of SBO are diverse, and, depending on suspected cause and patient factors, surgery may be indicated. SBO interrupts enteral feeding and gut homeostasis. In the absence of bowel ischaemia, a trial of non‐operative management may be adopted, with the option of delayed surgery in patients whose obstruction typically fails to resolve within 2 days. Longer periods of conservative management may be attempted for patients with adhesional obstruction or in patients with co‐morbidities who are considered initially unsuitable for operative management6. Surgery may involve resection of non‐viable intestine7, 8.
There is global attention on outcomes in emergency surgery9. The USA has taken steps to improve outcomes through the National Surgical Quality Improvement Program (NSQIP). This primarily captures data on patients undergoing surgical procedures and lacks information on the large cohort managed by emergency general surgeons without surgical intervention10. The UK National Emergency Laparotomy Audit (NELA)1 focused on improving standards of care for patients undergoing emergency laparotomy, and provides information on timing of diagnostics, assessments, interventions and outcomes. It also lacks information on the group who do not undergo surgery. Identification of patient groups where non‐operative management is unlikely to be successful, and outcomes from conservative management of SBO have been poorly studied to date. Better data on management of patients with SBO is needed to determine current practice, define outcomes across the condition and identify areas where care may be improved.
The aims of this study were to establish current practice in the management of patients admitted acutely with SBO, describe outcomes following treatment and identify clinical factors associated with poor outcome.
Methods
This study was conducted in line with a predefined protocol11, and reported in line with STROBE12 and SAMPL13 guidelines.
Public and patient involvement
This study was conceived based on public and patient research priority setting by the Association of Coloproctology of Great Britain and Ireland14. A patient representative identified through the Bowel Disease Research Foundation provided feedback and input on study design, attended all steering group meetings, and advised on interpretation of findings. The patient representative was involved in the preparation of this manuscript and is a co‐author.
Funding and role of funders
Funding and non‐financial support was received from the Bowel Disease Research Foundation, Association of Surgeons of Great Britain and Ireland, Association of Coloproctology of Great Britain and Ireland, Association of Upper Gastrointestinal Surgeons, British Association for Parenteral and Enteral Nutrition, British Society of Gastroenterology, Royal College of Surgeons of England, Royal College of Surgeons of Edinburgh, Royal College of Anaesthetists, British Association for Surgical Oncology and National Emergency Laparotomy Audit during the conduct of the study. Funding bodies provided financial support for administrative and statistical support, and for dissemination materials. As all funders had dual roles as specialty associations, Royal Colleges or charities, they were invited to have representation on the steering group. Analysis and sharing of findings was undertaken independently of the funding bodies.
Transparency
This manuscript is an honest, accurate and transparent account of the study being reported. No important aspects of the study have been omitted, and any discrepancies from the study as originally planned have been explained. M.J.L. and N.S.F. are guarantors.
Research collaborative network
The National Audit of Small Bowel Obstruction (NASBO) was designed and delivered by surgical trainee research collaboratives15 with the support of Royal Colleges, professional specialty associations and the Bowel Disease Research Foundation. NASBO was established to provide prospectively determined high‐quality information on patients treated for mechanical SBO of all causes, including non‐operative management, use of diagnostic tools and supportive strategies including nutrition.
Collaborators provided input into the design, including conducting the pilot study, data collection and validation, and review of final manuscript before submission. Each participating site included oversight by a designated consultant surgeon, with data collection undertaken by trainee surgeons or allied health professionals. An independent team member who was not involved in primary data collection undertook a validation exercise. Roles are presented in Appendix S3 (supporting information), in line with trainee research collaborative principles16.
Ethical approval and governance
Assessment by the South‐East Scotland Research and Ethics committee confirmed that this study did not require ethical approval (reference NR/1610AB10). Sites secured local audit and Caldicott Guardian permissions to participate and were not permitted to collect data without confirmation of approvals. The audit was registered with the Healthcare Quality Improvement Partnership.
Patient identification and eligibility
All UK‐based sites offering emergency surgery were eligible to participate in this study, and invited through targeted e‐mails from specialty associations and through social media. The audit also appeared on the Healthcare Quality Improvement Partnership database, and some sites enrolled proactively. Patients were identified across an 8‐week period from 16 January to 13 March 2017 and followed up for 30 days after discharge. Patients were screened for eligibility at referral to the surgical team. Patients were eligible for inclusion in the study if they had a diagnosis of SBO and were aged 18 years or older. A clinical diagnosis of SBO had to be made or confirmed by a consultant or a specialty trainee with 3 or more years of postgraduate surgical experience. Patients subsequently found to have non‐mechanical SBO, left colonic obstruction causing SBO, or who were managed with palliative intent from the time of admission were excluded from analysis.
Data and definitions
Data on route of referral to the surgical team, baseline demographics (age, sex, height, weight), co‐morbidity (Charlson Co‐morbidity Index, CCI17) and admission parameters (such as presence of acute kidney injury (AKI), white cell count) were captured. The time spent nil by mouth before referral, and duration of any preceding hospital stay (for example, on a medical ward before referral) was documented. Use and timing of abdominal radiography, CT and use of water‐soluble contrast agents were recorded. Data on operative interventions included timing, approach and key components of the operation (such as small bowel resection, stoma formation). Nutritional data, including BMI, interval between last and reintroduction of enteral nutrition, and use of nutritional support interventions, were recorded. Nutritional Risk Index (NRI) was calculated using ideal bodyweight, current bodyweight and admission albumin level.
Data were entered into a secure, fully audited REDCap database18 housed at the University of Sheffield. All records were pseudo‐anonymized, and accessible only to the local team and research team database administrators.
Patients were classified into three treatment groups: an immediate surgery group, where a decision to operate was made within 24 h of surgical review; a delayed surgery group that included patients who were managed initially with non‐operative intent but subsequently required surgical intervention; and a non‐operative group comprising patients who did not undergo surgery at any time point.
Outcomes
The primary outcome was in‐hospital mortality. Secondary outcomes included a composite of major complications (in‐hospital mortality, unplanned intensive care admission, 30‐day readmissions), pneumonia, cardiac complications and surgical‐site infections. The case report form is presented in Appendix S4 (supporting information). Definitions of recorded outcomes are available in Appendix S5 (supporting information).
Validation
To ensure data accuracy and case ascertainment, validation of key fields of 25 per cent of all patient records was undertaken by an independent investigator at each site who had not been involved in primary data collection. Records were identified for sampling by using a random number generator at the coordinating site, and validation was completed within a predetermined 30‐day time window. Categorical fields were deemed accurate when there was exact agreement between responses. Continuous variables were considered accurate with a perfect match, or rounding error of less than 0·5 of the reported value. Unit data were excluded if the validation process was incomplete. Accuracy was calculated as a percentage of matching fields in each record.
Statistical analysis
A sample of the population over 8 weeks was planned. Using data from a 2‐week pilot in eight hospitals, it was anticipated that 80 hospitals would generate a sample size of 2000 patients, with which it would be possible to detect a difference in primary outcomes from 5 to 10 per cent at 99 per cent power with an α value of 0·050, with an allocation ratio of 1 : 3 between immediate surgery, delayed surgery and non‐operative groups. It was conducted in line with the published protocol11.
For the outcomes survival and complications, models were constructed to adjust for clinically plausible variables, including age, CCI19 and the NRI20. CCI scores were stratified into no comorbidity (0), mild co‐morbidity (1–10) and significant comorbidity (11 and over). NRI was categorized into low (more than 97·5), moderate (83·5–97·5) and high (below 83·5) risk. For survival analyses, Cox proportional hazards models were constructed to adjust for clinically plausible variables. Models were clustered by centre to adjust for hospital‐level effects. Effect sizes are presented as hazards ratios (HRs) with 95 per cent confidence intervals.
For the binary outcomes in‐hospital complications and 30‐day readmission, multilevel mixed‐effects logistic regression models were constructed to adjust for patient‐level (level 1 fixed effects) and hospital‐level (level 2 random effects) factors. Effect sizes for these models are presented as odds ratios (ORs) with 95 per cent confidence intervals. Model fit was guided by clinical plausibility, the Akaike information criterion and goodness of fit (measured using adjusted R 2). To investigate the relationship between time to surgery and mortality, adjusted binary logistic regression was used to predict the risk of death, which was subsequently plotted against time to surgery in the form of a restricted cubic spline. To provide more information on the effects of co‐morbidities and age, splines were stratified by age and CCI. For all tests, statistical significance was set at P ≤ 0·050 a priori. All analyses were performed in R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria), using the finalfit, lme4, survival, splines and tidyverse packages.
Results
A total of 2604 patients from 131 hospitals were entered into the study; 152 patients were subsequently excluded by collaborators following diagnostic test results. Before statistical analysis, 18 records were excluded as they did not meet the study inclusion criteria and a further 20 records were excluded owing to missing outcome data. As this analysis focused on outcomes following treatment with curative intent, data on 73 patients who received end‐of‐life care were excluded, leaving 2341 patients in the analysis (Fig. 1). The independent validation study confirmed data accuracy at 92·4 per cent.
Figure 1.
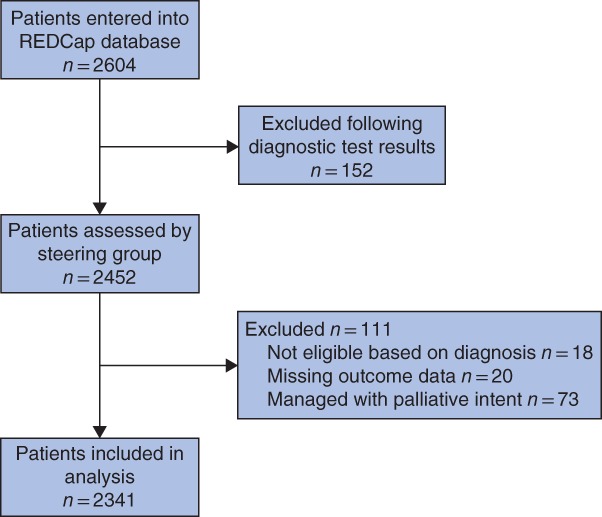
Flow chart showing identification of patients for analysis
Patient demographics and characteristics
Patients included in the final analysis had a mean(s.d.) age of 67·1(16·9) years and 54·8 per cent were women. The mean CCI score was 3·4(6·2). Characteristics according to management strategy are presented in Table 1 and a summary of SBO aetiology is provided in Table S1 (supporting information). Histograms of aetiology and management strategy by age (as malignancy becomes more common) are shown in Fig. 2. Adhesive SBO affected all ages. Hernia and malignancy were common causes of SBO in older patients. Of 1193 patients (51·0 per cent) who had an operation, 693 (29·6 per cent of all included patients) did so immediately, with a further 500 (21·4 per cent) requiring delayed surgery. AKI was documented in 21·6 per cent of patients at admission. It was more common in patients who underwent immediate surgery (27·7 per cent) than among those who underwent delayed (23·4 per cent) or no (17·2 per cent) surgery.
Table 1.
Patient characteristics
| Non‐operative (n = 1148) | Immediate surgery (n = 693) | Delayed surgery (n = 500) | P † | ||
|---|---|---|---|---|---|
| Age at admission to study (years)* | 66·9(16·9) | 67·4(16·5) | 67·1(17·4) | 0·738‡ | |
| Sex | M | 541 (47·1) | 316 (45·6) | 199 (39·8) | 0·027 |
| F | 604 (52·6) | 377 (54·4) | 301 (60·2) | ||
| Missing | 3 (0·3) | 0 (0) | 0 (0) | ||
| CCI score* | 3·6(6·4) | 3·3(6) | 3·1(5·8) | 0·353‡ | |
| Nutritional Risk Index | Low risk | 604 (52·6) | 339 (48·9) | 270 (54·0) | 0·005 |
| Moderate risk | 327 (28·5) | 246 (35·5) | 165 (33·0) | ||
| Severe risk | 60 (5·2) | 37 (5·3) | 21 (4·2) | ||
| Missing | 157 (13·7) | 71 (10·2) | 44 (8·8) | ||
| Accommodation before admission | Own home | 1114 (97·0) | 681 (98·3) | 492 (98·4) | 0·208 |
| Residential home | 8 (0·7) | 4 (0·6) | 3 (0·6) | ||
| Nursing home | 25 (2·2) | 6 (0·9) | 5 (1·0) | ||
| Missing | 1 (0·1) | 2 (0·3) | 0 (0) | ||
| Source of referral | Emergency department | 807 (70·3) | 480 (69·3) | 312 (62·4) | 0·018 |
| General practice | 197 (17·2) | 132 (19·0) | 106 (21·2) | ||
| Clinic admission | 12 (1·0) | 14 (2·0) | 11 (2·2) | ||
| Referral from inpatient team | 132 (11·5) | 66 (9·5) | 71 (14·2) | ||
| Missing | 0 (0) | 1 (0·1) | 0 (0) | ||
| AKI on admission | No | 951 (82·8) | 500 (72·2) | 382 (76·4) | < 0·001 |
| Yes | 197 (17·2) | 192 (27·7) | 117 (23·4) | ||
| Missing | 0 (0) | 1 (0·1) | 1 (0·2) | ||
| Admission white cell count (×109/l) | < 11·9 | 665 (57·9) | 363 (52·4) | 302 (60·4) | 0·052 |
| 12·0–15·9 | 283 (24·7) | 193 (27·8) | 126 (25·2) | ||
| > 16·0 | 200 (17·4) | 136 (19·6) | 72 (14·4) | ||
| Missing | 0 (0) | 1 (0·1) | 0 (0) | ||
| Radiology performed | No imaging | 7 (0·6) | 17 (2·5) | 4 (0·8) | < 0·001 |
| AXR only | 297 (25·9) | 98 (14·1) | 31 (6·2) | ||
| CT only | 136 (11·8) | 148 (21·4) | 54 (10·8) | ||
| CT and AXR | 708 (61·7) | 429 (61·9) | 411 (82·2) | ||
| Missing | 0 (0) | 1 (0·1) | 0 (0) | ||
| Oral or rectal water‐soluble contrast agent | No | 822 (71·6) | 653 (94·2) | 358 (71·6) | < 0·001 |
| Yes | 324 (28·2) | 39 (5·6) | 142 (28·4) | ||
| Missing | 2 (0·2) | 1 (0·1) | 0 (0) | ||
| Operative approach | Laparoscopic | – | 57 (8·2) | 39 (7·8) | < 0·001§ |
| Laparoscopic converted to open | – | 41 (5·9) | 37 (7·4) | ||
| Open – groin | – | 85 (12·3) | 11 (2·2) | ||
| Open – midline | – | 451 (65·1) | 389 (77·8) | ||
| Open – other | – | 42 (6·1) | 22 (4·4) | ||
| Missing | – | 17 (2·5) | 2 (0·4) |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.). CCI, Charlson Co‐morbidity Index; AKI, acute kidney injury; AXR, abdominal X‐ray.
χ2 test, except
Kruskal–Wallis test and
two‐sample χ2 test across operated groups only.
Figure 2.

Histograms showing causes of small bowel obstruction grouped by management strategy. a Non‐operative, b immediate surgery and c delayed surgery
Clinical outcomes
The overall mortality rate was 6·6 per cent (154 of 2341), with no significant differences between treatment groups (Table 2). The relationship between time to surgery and death, stratified by age and co‐morbidity, is presented in Fig. 3. The overall rate of unplanned high‐dependency unit or ICU admission was 9·9 per cent (232 of 2431). This was highest in the group undergoing delayed surgery (14·7 per cent for immediate versus 20·6 per cent for delayed). The rate of major complications (unplanned critical care admission, reoperation or death) was 14·4 per cent overall, 19·0 per cent in the immediate surgery, 23·6 per cent in the delayed surgery and 7·7 per cent in non‐operative management groups (P < 0·001). Patients in the delayed surgery group fared worse overall, with higher rates of infective, surgical and other complications than the other treatment groups. They had a significantly longer duration of hospital stay compared with the immediate surgery and non‐operative groups (mean(s.d.) 18·6(15·0), 12·7(12·1) and 7·3(10·5) days respectively) (Table 2).
Table 2.
Outcomes after small bowel obstruction
| Non‐operative (n = 1148) | Immediate surgery (n = 693) | Delayed surgery (n = 500) | P † | ||
|---|---|---|---|---|---|
| Major complications | |||||
| 30‐day mortality | No | 1071 (93·3) | 644 (92·9) | 463 (92·6) | 0·911 |
| Yes | 73 (6·4) | 47 (6·8) | 34 (6·8) | ||
| Missing | 4 (0·3) | 2 (0·3) | 3 (0·6) | ||
| Any major complication | No | 1050 (91·5) | 554 (79·9) | 377 (75·4) | < 0·001 |
| Yes | 88 (7·7) | 132 (19·0) | 118 (23·6) | ||
| Missing | 10 (0·9) | 7 (1·0) | 5 (1·0) | ||
| Reoperation | No | – | 652 (94·1) | 467 (93·4) | 0·613‡ |
| Yes | – | 33 (4·8) | 29 (5·8) | ||
| Missing | – | 8 (1·2) | 4 (0·8) | ||
| Unplanned high‐dependency or ICU admission | No | 1114 (97·0) | 582 (84·0) | 392 (78·4) | < 0·001 |
| ICU | 17 (1·5) | 60 (8·7) | 58 (11·6) | ||
| High‐dependency care | 10 (0·9) | 42 (6·1) | 45 (9·0) | ||
| Missing | 7 (0·6) | 9 (1·3) | 5 (1·0) | ||
| Infectious complications | |||||
| Urinary tract infection | No | 1105 (96·3) | 648 (93·5) | 452 (90·4) | < 0·001 |
| Not urinary catheter‐associated | 31 (2·7) | 19 (2·7) | 26 (5·2) | ||
| Urinary catheter‐associated | 10 (0·9) | 18 (2·6) | 18 (3·6) | ||
| Missing | 2 (0·2) | 8 (1·2) | 4 (0·8) | ||
| Lower respiratory tract infection | No | 1046 (91·1) | 592 (85·4) | 413 (82·6) | < 0·001 |
| Yes | 100 (8·7) | 94 (13·6) | 84 (16·8) | ||
| Missing | 2 (0·2) | 7 (1·0) | 3 (0·6) | ||
| Surgical complications/events | |||||
| Deep surgical‐site infection | No | – | 650 (93·8) | 459 (91·8) | < 0·001‡ |
| Yes | – | 35 (5·1) | 38 (7·6) | ||
| Missing | – | 8 (1·2) | 3 (0·6) | ||
| Superficial surgical‐site infection | No | – | 616 (88·9) | 426 (85·2) | 0·053‡ |
| Yes | – | 69 (10·0) | 71 (14·2) | ||
| Missing | – | 8 (1·2) | 3 (0·6) | ||
| Abdominal wall dehiscence | No | – | 671 (96·8) | 472 (94·4) | 0·039‡ |
| Yes | – | 14 (2·0) | 23 (4·6) | ||
| Missing | – | 8 (1·2) | 5 (1·0) | ||
| Anastomotic leak | No | – | 678 (97·8) | 485 (97·0) | 0·051‡ |
| Yes | – | 6 (0·9) | 12 (2·4) | ||
| Missing | – | 9 (1·3) | 3 (0·6) | ||
| Small bowel resection | No small bowel resection | – | 434 (62·6) | 407 (81·4) | < 0·001‡ |
| Small bowel resection | – | 242 (34·9) | 89 (17·8) | ||
| Missing | – | 17 (2·5) | 4 (0·8) | ||
| Other complications | |||||
| Venous thromboembolism (PE or DVT) | No | 1138 (99·1) | 676 (97·5) | 485 (97·0) | 0·002 |
| Yes | 7 (0·6) | 8 (1·2) | 12 (2·4) | ||
| Missing | 3 (0·3) | 9 (1·3) | 3 (0·6) | ||
| Radiologically guided drainage | No | 1135 (98·9) | 674 (97·3) | 485 (97·0) | 0·018 |
| Yes | 10 (0·9) | 11 (1·6) | 12 (2·4) | ||
| Missing | 3 (0·3) | 8 (1·2) | 3 (0·6) | ||
| Delirium | No | 1118 (97·4) | 639 (92·2) | 458 (91·6) | < 0·001 |
| Yes | 27 (2·4) | 47 (6·8) | 39 (7·8) | ||
| Missing | 3 (0·3) | 7 (1·0) | 3 (0·6) | ||
| Cardiovascular event (MI, new heart block, stroke, TIA) | No | 1101 (95·9) | 636 (91·8) | 453 (90·6) | < 0·001 |
| Yes | 42 (3·7) | 49 (7·1) | 44 (8·8) | ||
| Missing | 5 (0·4) | 8 (1·2) | 3 (0·6) | ||
| Readmission within 30 days | No | 940 (81·9) | 590 (85·1) | 426 (85·2) | 0·001 |
| Yes | 187 (16·3) | 77 (11·1) | 56 (11·2) | ||
| Missing | 21 (1·8) | 26 (3·8) | 18 (3·6) | ||
| Duration of hospital stay (days)* | 7·3(10·5) | 12·7(12·1) | 18·6(15·0) | < 0·001§ | |
| Time with no enteral intake (days) | < 5 | 808 (70·4) | 378 (54·5) | 106 (21·2) | < 0·001 |
| ≥ 5 | 249 (21·7) | 267 (38·5) | 351 (70·2) | ||
| Missing | 91 (7·9) | 48 (6·9) | 43 (8·6) |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.). PE, pulmonary embolism; DVT, deep vein thrombosis; MI, myocardial infarction; TIA, transient ischaemic attack.
χ2 test, except
two‐sample χ2 test across operated groups only and
Kruskal–Wallis test.
Figure 3.

Survival by time to surgery stratified by Charlson Co‐morbidity Index score. a Charlson Co‐morbidity Index (CCI) score 0, b CCI score 1–10 and c CCI score 11 and over
Surgical intervention
The median time to surgery was 1 (i.q.r. 0–1) in the immediate surgery group and 3 (2–6) days in the delayed surgery group. A summary of procedures performed is reported in Table S2 (supporting information). Small bowel resection was more frequent in the immediate surgery group (34·8 versus 17·8 per cent; P < 0·001). A groin approach was more common in the immediate surgery group (12·3 versus 2·2 per cent) as hernias were more frequent in this category. Laparoscopic intervention was attempted in 14·1 per cent of immediate operations and 15·1 per cent of delayed operations, of which 42 and 49 per cent respectively were converted to open procedures.
Use of diagnostic tests
All but 28 patients underwent diagnostic imaging; 80·6 per cent underwent abdominal CT, and 66·1 per cent had both abdominal X‐ray and CT (Table 1). The mean(s.d.) interval from CT to surgery was 2·4(13·2) days. CT was used less in the non‐operative group than the immediate and delayed surgery groups (73·5, 83·3 and 93·0 respectively). Water‐soluble contrast studies (independent of CT) were used in 21·6 per cent of patients overall, with differences observed between aetiologies: they were used in 356 of 1150 patients (31·0 per cent) with adhesive obstruction, 35 of 415 (8·4 per cent) with hernia and 20 of 167 (12·0 per cent) with malignant obstruction.
Nutritional management
At the time of admission, 36·6 per cent of patients were stratified as being at moderate or severe risk of malnutrition according to the NRI (Table 1). Overall, 331 patients (14·1 per cent) received parenteral nutrition, although this was significantly more likely in those who underwent surgery (immediate surgery: 107 of 693, 15·4 per cent; delayed surgery: 162 of 500, 32·4 per cent; non‐operative: 62 of 1148, 5·4 per cent; P < 0·001). Patients in the delayed operation group were significantly more likely not to receive enteral nutrition for more than 5 days than those in the other treatment groups (delayed surgery: 351 of 500, 70·2 per cent; immediate surgery: 267 of 693, 38·5 per cent; non‐operative: 249 of 1148, 21·7 per cent; P < 0·001).
Factors associated with mortality
In multivariable analysis, operative management was associated with a significantly lower hazard of death, regardless of whether surgery was immediate or delayed (Table 3). An unadjusted survival curve showed comparable survival up to day 10 (Fig. 4). Beyond this point, patients in the non‐operative group were significantly more likely to die (P < 0·001). The hazard of death rose with age (Fig. 3), and this persisted when adjusted for other variables (Table 3). Patients treated for hernia (HR 1·96, 95 per cent c.i. 1·16 to 3·31; P = 0·012) or malignancy (HR 2·54, 1·46 to 4·41; P = 0·001) had significantly worse survival than patients with adhesional SBO (reference group). Patients with poor nutritional status were significantly less likely to survive, even after adjustment (moderate nutritional risk: adjusted HR 1·55, 1·01 to 2·39, P = 0·045; severe nutritional risk: adjusted HR 2·13, 1·16 to 3·91; P = 0·015). Timing of CT, AKI at admission, accommodation before admission, sex and CCI were not adversely associated with survival following multivariable adjustment.
Table 3.
Predictors of survival after small bowel obstruction
| Univariable analysis* | Multivariable analysis* | |||
|---|---|---|---|---|
| Hazard ratio | P | Hazard ratio | P | |
| Final treatment group | ||||
| Non‐operative | 1·00 (reference) | 1·00 (reference) | ||
| Immediate surgery | 0·60 (0·42, 0·88) | 0·008 | 0·54 (0·35, 0·85) | 0·008 |
| Delayed surgery | 0·37 (0·24, 0·57) | < 0·001 | 0·37 (0·23, 0·59) | < 0·001 |
| Age at admission to study (per year) | 1·05 (1·03, 1·06) | < 0·001 | 1·05 (1·03, 1·06) | < 0·001 |
| Sex | ||||
| M | 1·00 (reference) | 1·00 (reference) | ||
| F | 0·89 (0·64, 1·22) | 0·465 | 1·10 (0·74, 1·64) | 0·637 |
| CCI score (per point) | 1·04 (1·02, 1·07) | < 0·001 | 1·01 (0·99, 1·04) | 0·316 |
| Admission white cell count (×109/l) | ||||
| < 11·9 | 1·00 (reference) | 1·00 (reference) | ||
| 12·0–15·9 | 1·03 (0·68, 1·55) | 0·900 | 1·06 (0·63, 1·78) | 0·832 |
| > 16·0 | 1·82 (1·24, 2·67) | 0·002 | 1·67 (1·04, 2·67) | 0·033 |
| Accommodation before admission | ||||
| Own home | 1·00 (reference) | 1·00 (reference) | ||
| Residential home | 1·31 (0·32, 5·28) | 0·708 | 0·89 (0·16, 5·09) | 0·898 |
| Nursing home | 1·46 (0·54, 3·96) | 0·452 | 0·55 (0·16, 1·90) | 0·346 |
| Aetiology | ||||
| Adhesions | 1·00 (reference) | 1·00 (reference) | ||
| Crohn's disease | 0·18 (0·02, 1·30) | 0·089 | 0·47 (0·06, 3·61) | 0·467 |
| Hernia | 1·94 (1·28, 2·95) | 0·002 | 1·96 (1·16, 3·31) | 0·012 |
| Malignancy | 2·22 (1·39, 3·57) | 0·001 | 2·54 (1·46, 4·41) | 0·001 |
| Other | 1·01 (0·61, 1·67) | 0·968 | 0·88 (0·51, 1·52) | 0·650 |
| Timing of CT (h after admission) | ||||
| No CT | 1·00 (reference) | 1·00 (reference) | ||
| < 24 | 0·91 (0·54, 1·53) | 0·727 | 1·07 (0·62, 1·85) | 0·810 |
| 24–48 | 0·51 (0·20, 1·29) | 0·155 | 0·80 (0·32, 2·00) | 0·627 |
| > 48 | 1·10 (0·61, 2·00) | 0·746 | 1·17 (0·63, 2·17) | 0·629 |
| Nutritional Risk Index | ||||
| Low risk | 1·00 (reference) | 1·00 (reference) | ||
| Moderate risk | 1·81 (1·24, 2·65) | 0·002 | 1·55 (1·01, 2·39) | 0·045 |
| Severe risk | 2·26 (1·29, 3·96) | 0·004 | 2·13 (1·16, 3·91) | 0·015 |
| AKI on admission | ||||
| No | 1·00 (reference) | 1·00 (reference) | ||
| Yes | 1·72 (1·23, 2·39) | 0·001 | 1·38 (0·89, 2·13) | 0·145 |
| Source of referral | ||||
| Emergency department | 1·00 (reference) | – | ||
| General practice | 0·89 (0·57, 1·39) | 0·609 | – | |
| Clinic admission | 0·35 (0·05, 2·50) | 0·294 | – | |
| Referral from inpatient team | 1·12 (0·74, 1·69) | 0·601 | – | |
Values in parentheses are 95 per cent confidence intervals. CCI, Charlson Co‐morbidity Index; AKI, acute kidney injury.
Cox proportional hazards analysis.
Figure 4.

Unadjusted survival curve by management strategy. P < 0·001 (log rank test)
Table 4.
Predictors of major complications after small bowel obstruction
| Major complications | Univariable analysis‡ | Multivariable analysis§ | ||||
|---|---|---|---|---|---|---|
| No | Yes | Odds ratio* | P | Odds ratio* | P | |
| Final treatment group | ||||||
| Non‐operative | 1050 (53·0) | 88 (26·0) | 1·00 (reference) | 1·00 (reference) | ||
| Immediate surgery | 554 (28·0) | 132 (39·1) | 2·84 (2·13, 3·80) | < 0·001 | 3·25 (2·19, 4·82) | < 0·001 |
| Delayed surgery | 377 (19·0) | 118 (34·9) | 3·73 (2·77, 5·05) | < 0·001 | 3·32 (2·25, 4·89) | < 0·001 |
| Age at admission to study (years) | 65·9(17·0)† | 73·4(14·6)† | 1·03 (1·02, 1·04) | < 0·001 | 1·03 (1·02, 1·04) | < 0·001 |
| Sex | ||||||
| M | 899 (45·4) | 148 (43·8) | 1·00 (reference) | 1·00 (reference) | ||
| F | 1079 (54·6) | 190 (56·2) | 1·07 (0·85, 1·35) | 0·570 | 1·07 (0·79, 1·44) | 0·664 |
| CCI score | 3·2(5·9)† | 4·8(7·2)† | 1·04 (1·02, 1·06) | < 0·001 | 1·02 (1·00, 1·04) | 0·117 |
| Admission white cell count (×109/l) | ||||||
| < 11·9 | 1126 (56·8) | 191 (56·5) | 1·00 (reference) | 1·00 (reference) | ||
| 12·0–15·9 | 510 (25·7) | 86 (25·4) | 0·99 (0·75, 1·30) | 0·966 | 0·96 (0·67, 1·36) | 0·808 |
| > 16·0 | 345 (17·4) | 61 (18·0) | 1·04 (0·76, 1·42) | 0·795 | 0·96 (0·64, 1·45) | 0·847 |
| Accommodation before admission | ||||||
| Own home | 1938 (97·9) | 328 (97·0) | 1·00 (reference) | – | ||
| Residential home | 12 (0·6) | 3 (0·9) | 1·48 (0·34, 4·68) | 0·547 | – | |
| Nursing home | 29 (1·5) | 7 (2·1) | 1·43 (0·57, 3·10) | 0·404 | – | |
| Aetiology | ||||||
| Adhesions | 1015 (54·3) | 126 (40·6) | 1·00 (reference) | 1·00 (reference) | ||
| Crohn's disease | 98 (5·2) | 6 (1·9) | 0·49 (0·19, 1·06) | 0·101 | 0·82 (0·32, 2·11) | 0·675 |
| Hernia | 346 (18·5) | 66 (21·3) | 1·54 (1·11, 2·11) | 0·009 | 0·91 (0·59, 1·40) | 0·661 |
| Malignancy | 130 (7·0) | 38 (12·3) | 2·35 (1·55, 3·51) | < 0·001 | 1·69 (1·03, 2·78) | 0·039 |
| Other | 281 (15·0) | 74 (23·9) | 2·12 (1·54, 2·90) | < 0·001 | 1·20 (0·80, 1·81) | 0·374 |
| Timing of CT (h after admission) | ||||||
| No CT | 421 (21·7) | 27 (8·1) | 1·00 (reference) | 1·00 (reference) | ||
| < 24 | 1229 (63·5) | 230 (69·1) | 2·92 (1·96, 4·51) | < 0·001 | 1·91 (1·14, 3·20) | 0·014 |
| 24–48 | 108 (5·6) | 15 (4·5) | 2·17 (1·09, 4·16) | 0·023 | 1·44 (0·62, 3·34) | 0·396 |
| > 48 | 178 (9·2) | 61 (18·3) | 5·34 (3·32, 8·80) | < 0·001 | 3·47 (1·88, 6·38) | < 0·001 |
| Nutritional Risk Index | ||||||
| Low risk | 1076 (61·3) | 129 (43·0) | 1·00 (reference) | 1·00 (reference) | ||
| Moderate risk | 596 (33·9) | 137 (45·7) | 1·92 (1·48, 2·49) | < 0·001 | 1·91 (1·37, 2·66) | < 0·001 |
| Severe risk | 84 (4·8) | 34 (11·3) | 3·38 (2·16, 5·19) | < 0·001 | 3·41 (1·95, 5·98) | < 0·001 |
| AKI on admission | ||||||
| No | 1601 (80·8) | 215 (63·8) | 1·00 (reference) | 1·00 (reference) | ||
| Yes | 380 (19·2) | 122 (36·2) | 2·39 (1·86, 3·06) | < 0·001 | 1·41 (1·00, 1·97) | 0·048 |
| Source of referral | ||||||
| Emergency department | 1362 (68·8) | 224 (66·3) | 1·00 (reference) | – | ||
| General practice | 379 (19·1) | 52 (15·4) | 0·83 (0·60, 1·14) | 0·271 | – | |
| Clinic admission | 34 (1·7) | 3 (0·9) | 0·54 (0·13, 1·51) | 0·304 | – | |
| Referral from inpatient team | 206 (10·4) | 59 (17·5) | 1·74 (1·25, 2·39) | 0·001 | – | |
Values in parentheses are percentages unless indicated otherwise;
values in parentheses are 95 per cent confidence intervals and
values are mean(s.d.). CCI, Charlson Co‐morbidity Index; AKI, acute kidney injury.
Logistic regression;
multilevel mixed‐effects logistic regression. Odds ratios for age and CCI score are shown per year and per point respectively.
Factors associated with major complications
Major complications (unplanned critical care admission, reoperation or death) were reported in 14·4 per cent of patients. Factors associated with a higher rate of major complications included any form of operative management (immediate surgery: adjusted OR 3·25, 95 per cent c.i. 2·19 to 4·82; P < 0·001; delayed surgery: adjusted OR 3·32, 2·25 to 3·89; P < 0·001). Patients with SBO secondary to malignancy were more likely to develop major complications than patients with other causes of SBO (malignancy versus adhesions: univariable OR 2·35, 1·55 to 3·51; P < 0·001). This effect persisted after adjustment (multivariable OR 1·69, 1·03 to 2·78; P = 0·039). A similar effect was noted with the presence of AKI on admission (adjusted OR 1·41, 1·00 to 1·97; P = 0·048). Poor nutritional status was significantly associated with higher odds of major complications (moderate risk of malnutrition: adjusted OR 1·91, 1·37 to 2·66; P < 0·001; severe risk: adjusted OR 3·41, 1·95 to 5·98; P < 0·001).
Discussion
Acute admission with SBO was associated with substantial mortality (6·6 per cent) and a considerable risk of major complications (14·4 per cent). Patients had high rates of AKI (21·6 per cent) and many were at risk of moderate or severe malnutrition (36·6 per cent). Another key finding was substantial variation in the use of diagnostic imaging and nutritional interventions, as well as in the timing of surgery where this took place. Morbidity rates were higher in patients undergoing either immediate or delayed surgery owing to the absence of surgery‐associated complications in patients managed conservatively. Factors associated with adverse outcomes included increasing age, AKI, moderate to severe risk of malnutrition on admission, and hernia or malignancy as causes of SBO.
NASBO reports data captured in a prospectively developed database, with independent validation on a national cohort of patients with SBO. The study covered all sizes and types of hospital in the UK National Health Service that provided acute surgical services. In contrast to other reports, the present study provides comparable data on patients who did not undergo surgery. Inclusion of data on nutritional status and interventions represented a novel addition. The study was strengthened by high data accuracy at 92·4 per cent.
The major limitation of the present study is its observational design, and associations identified cannot imply causative relationships. The timing of the snapshot may not be representative of year‐round practice, and data may be lacking on patients with SBO managed by services other than surgery. Participation in NASBO was voluntary. Although coverage was broad, omission of data from the few non‐participating hospitals may also have introduced bias. A less obvious but important limitation was the lack of information on resources that may influence service provision and patient outcomes. This information was collected but is not reported in this patient‐level analysis. Survey work on decision‐making has been investigated as part of NASBO and by others21, 22. This may be particularly relevant where surgery was deliberately deferred in an effort to avoid operation in a particularly high‐risk patient. Furthermore, the need to combine heterogeneous data into categories for analysis requires careful consideration when interpreting the data. For example, malignant causes of SBO included patients with potentially curable obstructing primary tumours and those with multilevel obstruction from disseminated cancer.
The present study shares the national reach of NELA but, unlike NELA, provided comprehensive coverage of patients who did not undergo surgery. Recent analysis of NELA outcomes in patients undergoing surgical intervention for adhesive SBO reported 30‐day mortality at a very similar level of 7·2 per cent, with risk of death associated with increasing age, co‐morbidity, outcome prediction score and degree of contamination at surgery. It also demonstrated the negative association between survival and delayed surgery23.
The present findings indicate higher rates of mortality than those from two major recent international retrospective cohort studies looking at both operated and conservatively managed patients. An NSQIP study10 that included a subset of patients with all‐cause SBO at 13 voluntary USA and Canadian institutions reported lower unadjusted mortality rates of 3·3 per cent in operated patients and 4·5 per cent in the non‐operative group10. Another recent multinational retrospective study24 limited to four centres reported a mortality rate of just 2 per cent across both operated and non‐operated patients with adhesive SBO; however, the primary objective of that study was retrospective validation of a proposed risk prediction tool and it may have been subject to selection bias24. Both studies had non‐surgery rates exceeding 60 per cent10, 24.
Around half of patients with SBO (49·0 per cent) were managed successfully with a conservative approach, with comparable mortality rates to the operated groups and fewer short‐term complications. Patients in the immediate surgery group had evidence of higher levels of organ failure on admission and a trend towards greater systemic inflammatory response, suggesting a sicker patient group. This group appeared to have better outcomes than the less unwell group who underwent delayed surgery for non‐resolving SBO. Potential explanations for these observations are complex. Early intervention and source control in the immediate surgery group, deteriorating physiological and nutritional status in the delayed surgery group, and clinical bias in deferring surgery in unfit patients may all have contributed21. This complexity has important implications for both surgical decision‐making and patient counselling, making a strong case for involvement of perioperative anaesthetic, critical care and elderly care clinicians in best advising high‐risk patients.
This study suggests that delayed surgery is associated with worse outcomes for patients with SBO23, 25. There are mitigating factors that may result in deferral of surgery, including need for preoperative resuscitation, likelihood of successful conservative management, anticipated complexity of surgery (such adhesiolysis within a hostile abdomen or in association with complex abdominal wall hernias), significant pre‐existing co‐morbidity or frailty, need for prolonged critical care admission, minimal chances of discharge from hospital and/or need for long‐term escalation in social care needs. None of these are captured as part of this study and all may contribute to outcome.
There is scope for improvement in care throughout the patient pathway. Approximately one in ten patients was referred from an inpatient hospital ward. It is important that placement within the appropriate specialty occurs promptly to facilitate early expert review5. There was also considerable variation in the use of imaging, with many patients assessed with both abdominal radiography and CT. Consultant surgeons emphasize the need for assessment CT (radiation dose 7·9 mSv in a modern multislice CT scanner26) to guide treatment decisions and timing of interventions22, with CT rendering an additional abdominal radiograph (potentially an additional radiation exposure of around 0·7 mSV) redundant27. Patients undergoing initial conservative management of adhesive SBO could receive water‐soluble contrast agents to stratify whether or not the obstruction is likely to resolve without surgery28. Although many surgeons report that they would consider this test22, only 21·6 per cent of patients received it in the present study. Barriers to the adoption of water‐soluble contrast in managing patients with adhesive SBO require further investigation.
Optimization of the patient with SBO should also consider strategies to prevent or mitigate complications29. Due recognition should be given to the fact that patients with SBO already have gut failure30, and prevention of further compromise is essential as each additional organ failure adds 5–10 per cent to mortality rates31. One in five patients had evidence of AKI at the point of admission, a rate similar to that in the overall intensive care population32.
Patients with SBO in this cohort were at a high risk of malnutrition. National Institute for Health and Care Excellence guidelines33 suggest initiation of parenteral nutrition in patients who have lacked, or are likely to lack, oral intake by mouth for 5 or more days. At present, there is no high‐quality evidence to guide nutritional strategy, but poor nutrition in this cohort was associated with an increased risk of major complications and death. This suggests that addressing nutritional issues in patients with SBO might improve overall patient outcomes.
Given the combination of the high incidence of SBO as a reason for acute admission and the need for emergency laparotomy with appreciable morbidity and mortality, there is a need for research interventions to improve outcomes. Characterization of patients at risk of poor outcomes, with risk assessment including co‐morbidity, aetiology, malnutrition risk and presence of AKI, is likely to facilitate the value of specific interventions. An early stratification tool might identify those unlikely to recover with non‐operative management as well as those unlikely to survive surgical intervention.
Disclosure
The authors declare no conflict of interest.
Supporting information
Appendix S1 NASBO Steering Group
Appendix S2 NASBO Collaborators
Appendix S3 Collaborator contributions
Appendix S4 Data fields captured
Appendix S5 Definition of complications
Table S1 Aetiologies of Small Bowel Obstruction
Table S2 Procedures performed for small bowel obstruction
Presented to meetings of the Association of Coloproctology of Great Britain and Ireland, Bournemouth, UK, July 2017, British Association for Parenteral and Enteral Nutrition, Birmingham, UK, November 2017, British Association for Surgical Oncology, Glasgow, UK, December 2017, Association of Surgeons of Great Britain and Ireland, Liverpool, UK, May 2018, World Society of Emergency Surgery, Bertinoro, Italy, June 2018, and British Society of Gastroenterology, Liverpool, UK, November 2018
Funding information
Association of Coloproctology of Great Britain and Ireland
Association of Surgeons of Great Britain and Ireland
Association of Upper Gastrointestinal Surgeons
Bowel Disease Research Foundation
British Association for Parenteral and Enteral Nutrition
British Association for Surgical Oncology
British Society of Gastroenterology
National Emergency Laparotomy Audit
Royal College Of Anaesthetists
Royal College of Surgeons of Edinburgh
Royal College of Surgeons of England
Contributor Information
M. J. Lee, Email: m.j.lee@sheffield.ac.uk.
on behalf of NASBO steering group and NASBO collaborators:
John Abercrombie, Austin Acheson, Derek Alderson, Iain Anderson, Michael Davies, Zaed Hamady, Marianne Hollyman, Sarah Hare, Ellen Lee, John Northover, Christopher Lewis, Malcolm McFall, Aravinth Murugananthan, David Murray, Pritam Singh, Gillian Tierney, Ciaran Walsh, Jonathan Wild, Timothy Wilson, S Abbott, Y Abdulaal, S Afshar, J Ah‐Chuen, T Ahmed, M Akhtar, F Akram, E Aldred, A Ali, M Aly, A Amajuoyi, V Amin, D Anderson, O Anderson, A Andreou, A Ansari, S Appleton, R Ardley, F Arshad, O Ashour, A Asour, A Athem, M Athersmith, F Ayoub, H Azeem, B Azhar, T Badenoch, C Baillie, D Bandyopadhyay, J Barker, S Barker, B Barkham, R Baron, J Barrie, E Barry‐Yarrow, G Bashir, N Battersby, G Bazoua, N Behar, S Bellam, C Berger, S Bhandari, S Bhasin, S Biggs, C Bisset, L Blake, N Blencowe, T Boam, A Boddy, C Boereboom, M Bogdan, R Bogle, P Bohra, M Boland, H Bolkan, C Borg, R Boulton, G Bouras, M Boyer, J Boyle, G Branagan, H Brewer, C Briggs, J Broadhurst, E Brown, J Brown, L Brown, O Brown, K Burns, K Butcher, M Butler, B Byrne, L Campbell, C Capper, M Cartmell, T Cash, S Chan, N Chandratreya, J Chapman, S Chapman, A Charalabopoulos, C Cheek, S Chok, W Choong, M Chow, J Chowdhury, P Coe, P Conaghan, G Conn, N Cook, T Cook, S Cooper, J Cornish, D Cotton, C Cox, P Coyne, R Crook, J Crozier, G Cuffolo, P Cunha, N Curtis, J Cutting, K Da Costa, L da Silva, B Das, M Davenport, J Davies, T Davies, A Day, S Dayal, S Dean, G Demetriou, F Dengu, R Dennis, H Dent, P Dent, M Deputy, L Devoto, G Di Benedetto, S Dindyal, E Donnelly, P Doody, E Douka, C Downham, H Dowson, H Edent, K Edgerton, N Ekpete, M El Farran, O Elamin, M Eljaafari, N Elsaid, M El‐Sharif, J Evans, M Evans, R Ewe, A Ewing, K Exarchou, R Fallaize, M Faoury, S Farag, E Farinella, G Faulkner, H Ferguson, O Fisher, J Fletcher, A Forouzanfar, A Foster, R Fox, N Francis, V Fretwell, D Fung, E Gammeri, J Garnham, A Geraghty, A Gilbert, C Gill, M Gill, M Gillespie, P Giordano, J Glasbey, M Goh, A Golder, N Green, T Gregoir, T Grey, E Groundwater, T Grove, S Growcott, S Gunasekaran, H Habib, J Haddow, V Halahakoon, C Halkias, C Hall, A Hampson, L Hancock, T Hanna, J Hannay, A Harikrishnan, R Harries, G Harris, J Hartley, K Harvey, P Hawkin, J Hawkins, R Healy, R Heard, R Heartshorne, S Heller, L Hendra, P Herrod, N Heywood, G Hicks, B Hobson, S Holtham, S Holtham, C Hope, P Hopley, T Hossain, S Hossaini, F Howse, T Hubbard, A Humphreys, H Ikram, M Ioannis, M Iqbal, N Iqbal, R Jain, J Jatania, P Jenkinson, S Jokhan, A Jones, C Jones, L Jones, H Joshi, K Joshi, M Joy, P Jull, G Kakaniaris, G Kakaniaris, R Kallam, E Kane, P Kang, R Kanitkar, S Kauser, F Kazmi, M Kedrzycki, S Kelly, J Kendall, M Khan, T Khan, G King, A Kisiel, C Kitsis, I Kolawole, S Korambayil, S Kosasih, A Kosti, A Kotb, S Kouris, K Kshatriya, S Kumar, G Lafaurie, R Lal, A Lau, T Lazim, T Lazim, A Lazzaro, K Lee, R Lefroy, D Leinhardt, D Leinhardt, H Lennon, K Leong, B Levy, E Lim, J Lim, S Lindley, D Liu, P Lloyd, D Locker, S Lockwood, C Lowe, J Lund, R Lunevicius, A Lunt, S Lutfi, A Luther, S Luwemba, P Mahankali‐Rao, S Mahroof, D Mai, S Majid, A Malik, K Malik, K Mann, S Mansour, N Manu, R Mapara, C Martin, J Martin, R Martin, C Mason, L Massey, J Mathias, P Mathur, K Maude, D McArthur, S McCain, S McCluney, B McIlroy, S McKay, N McKinley, A McNair, D McWhirter, P Mekhail, K Mellor, J Merchant, L Merker, D Messenger, A Miles, S Mir, A Mishra, P Mistry, V Miu, M Moat, K Mockford, E Mohamed, I Mohamed, M Mondragon‐Pritchard, N Moore, L Moretti, H Morris, T Morrison, V Morrison‐Jones, J Moss, S Moug, D Mountford, R Moynihan, K Muhammad, D Muldoon‐Smith, J Mulholland, M Mullan, E Murgitroyd, K Murugaiyan, A Myers, I Mykoniatis, G Nana, T Nash, A Nassar, R Newton, C Ng, P Ng, P Ng, K Nguyen, K Nguyen, F Nicholas, M Noor, J Nowers, C Nugent, A Nunn, R Nunn, N Obeid, J O'Callaghan, R O'Hara, O Oke, J Olivier, A O'Neill, S O'Neill, D Osei‐Bordom, L Osgood, S Panagiotopoulos, B Panchasara, R Parks, H Patel, P Patel, R Patel, S Patel, K Pawelec, C Payne, K Pearson, G Perin, I Peristerakis, B Petronio, L Phelan, J Phillips, C Pisaneschi, J Pitt, K Plunkett‐Reed, L Ponchietti, A Pouzi, M Pouzi, A Powell, A Powell‐Chandler, N Pranesh, V Proctor, S Pywell, A Qureshi, N Qureshi, M Rahman, Z Rai, S Ramcharan, K Rangarajan, M Rashid, H Reader, A Rehman, S Rehman, C Rengifo, E Richards, N Richardson, A Robinson, D Robinson, B Rossi, F Rutherford, I Sadien, T Saghir, K Sahnan, G Salahia, J Sarveswaran, M Saunders, B Scott, K Scott, A Seager, S Seal, E Sezen, F Shaban, P Shah, P Shah, M Shahmohammadi, A Shamsiddinova, S Shankar, A Sharpe, V Shatkar, A Sheel, T Shields, M Shinkwin, J Shurmer, A Siddika, S Siddiqui, R Simson, P Sinclair, B Singh, S Singh, J Sivaraj, P Skaife, B Skelly, A Skinner, N Slim, C Smart, N Smart, F Smith, I Smith, R Smith, G Spence, A Sreedhar, J Steinke, L Stevenson, E Stewart‐Parker, M Stott, B Stubbs, B Stubbs, N Stylianides, S Subramonia, M Swinkin, M Swinscoe, N Symons, W Tahir, T Taj, K Takacs, J Tam, K Tan, S Tani, N Tanner, D Tao, M Taylor, B Thava, K Thippeswamy, C Thomas, E Thompson, R Thompson, C Thompson‐Reil, C Thorn, F Tongo, G Toth, A Turnbull, J Turnbull, C Valero, G van Boxel, M Varcada, M Venn, N Ventham, M Venza, D Vimalachandran, I Virlos, T Wade, A Wafi, K Waite, M Walker, N Walker, T Walker, U Walsh, S Wardle, R Warner, J Watfah, N Watson, J Watt, J Watts, J Wayman, C Weegenaar, H West, M West, L Whitehurst, M Whyler, M Wiggans, S Wijeyekoon, G Williams, R Williams, A Williamson, J Williamson, J Wilson, A Winter, L Wolpert, J Wong, E Yeap, T Yeong, S Zaman, B Zappa, D Zosimas, S Abbott, Y Abdulaal, S Afshar, M Akhtar, D Anderson, S Appleton, D Bandyopadhyay, G Bashir, N Behar, S Bhandari, G Branagan, R Boulton, C Borg, G Bouras, J Boyle, H Brewer, L Brown, C Briggs, M Cartmell, S Chan, N Chandratreya, P Conaghan, J Cornish, D Cotton, P Coyne, J Crozier, T Cook, P Cunha, N Curtis, A Day, S Dayal, R Dennis, P Dent, H Dowson, R Fallaize, S Farag, M El Farran, G Faulkner, P Giordano, T Grey, V Halahakoon, J Hannay, A Harikrishnan, S Holtham, P Hawkin, C Hall, L Hancock, J Hartley, F Howse, R Kallam, G Kakaniaris, S Kelly, S Lockwood, D Leinhardt, B Levy, R Lal, T Lazim, J Lund, R Lunevicius, P Mathur, K Maude, D McArthur, B McIlroy, A Miles, M Moug S Mondragon‐Pritchard, D Messenger, M Mullan, A Myers, K Muhammad, C Mason, J Sarveswaran, V Shatkar, B Singh, B Skelly, S Subramonia, M Swinscoe, B Thava, C Thorn, S Panagiotopoulos, P Patel, J Phillips, I Peristerakis, A Qureshi, M Saunders, P Shah, A Sheel, S Siddiqui, P Skaife, N Smart, I Smith, L Stevenson, N Stylianides, J Steinke, B Stubbs, R Thompson, M Varcada, D Vimalachandran, I Virlos, J Watfah, N Watson, M Walker, N Ventham, H West, J Wilson, S Wijeyekoon, J Ah‐Chuen, T Ahmed, F Akram, E Aldred, A Ali, M Aly, A Amajuoyi, V Amin, A Andreou, A Ansari, R Ardley, F Arshad, O Ashour, A Asour, F Ayoub, H Azeem, B Azhar, C Baillie, J Barker, B Barkham, R Baron, J Barrie, E Barry‐Yarrow, N Battersby, G Bazoua, C Berger, S Bhasin, S Biggs, C Bisset, N Blencowe, A Boddy, C Boereboom, M Bogdan, R Bogle, P Bohra, H Bolkan, M Boyer, J Broadhurst, E Brown, J Brown, K Burns, K Butcher, C Capper, T Cash, J Chapman, S Chapman, A Charalabopoulos, C Cheek, S Chok, W Choong, J Chowdhury, P Coe, G Conn, N Cook, S Cooper, C Cox, R Crook, G Cuffolo, L da Silva, B Das, M Davenport, J Davies, T Davies, S Dean, G Demetriou, F Dengu, H Dent, G Di Benedetto, S Dindyal, E Donnelly, E Douka, C Downham, H Edent, K Edgerton, M El‐Sharif, O Elamin, N Elsaid, J Evans, M Evans, R Ewe, A Ewing, H Ferguson, O Fisher, J Fletcher, A Forouzanfar, A Foster, R Fox, N Francis, V Fretwell, D Fung, E Gammeri, J Garnham, A Geraghty, A Gilbert, M Gill, M Gillespie, J Glasbey, A Golder, N Green, E Groundwater, T Grove, H Habib, J Haddow, C Halkias, A Hampson, T Hanna, R Harries, K Harvey, J Hawkins, R Healy, R Heartshorne, S Heller, L Hendra, P Herrod, N Heywood, G Hicks, P Ng, S Holtham, C Hope, P Hopley, T Hossain, S Hossaini, T Hubbard, A Humphreys, H Ikram, M Ioannis, M Iqbal, J Jatania, P Jenkinson, S Jokhan, A Jones, C Jones, L Jones, H Joshi, K Joshi, M Joy, P Jull, G Kakaniaris, E Kane, R Kanitkar, S Kauser, F Kazmi, M Kedrzycki, J Kendall, T Khan, G King, A Kisiel, C Kitsis, I Kolawole, S Kosasih, A Kosti, A Kotb, A Lau, G Lafaurie, T Lazim, A Lazzaro, R Lefroy, D Leinhardt, H Lennon, K Leong, E Lim, J Lim, S Lindley, D Liu, P Lloyd, D Locker, C Lowe, A Lunt, S Lutfi, A Luther, S Luwemba, P Mahankali‐Rao, D Mai, S Majid, A Malik, N Manu, R Mapara, C Martin, J Martin, L Massey, J Mathias, S McCain, S McCluney, A McNair, P Mekhail, J Merchant, L Merker, S Mir, P Mistry, V Miu, M Moat, E Mohamed, I Mohamed, N Moore, L Moretti, H Morris, T Morrison, J Moss, D Mountford, R Moynihan, D Muldoon‐Smith, J Mulholland, E Murgitroyd, K Murugaiyan, I Mykoniatis, G Nana, T Nash, A Nassar, R Newton, P Ng, K Nguyen, K Nguyen, F Nicholas, M Noor, J Nowers, C Nugent, A Nunn, J O'Callaghan, R O'Hara, A O'Neill, J Olivier, D Osei‐Bordom, L Osgood, B Panchasara, R Parks, H Patel, K Pawelec, C Payne, K Pearson, G Perin, B Petronio, L Phelan, C Pisaneschi, J Pitt, L Ponchietti, A Powell, A Powell‐Chandler, N Pranesh, V Proctor, N Qureshi, M Rahman, Z Rai, S Ramcharan, K Rangarajan, M Rashid, H Reader, A Rehman, S Rehan, C Rengifo, N Richardson, A Robinson, D Robinson, B Rossi, F Rutherford, I Sadien, T Saghir, K Sahnan, G Salahia, B Scott, K Scott, A Seager, S Seal, E Sezen, F Shaban, P Shah, M Shahmohammadi, A Shamsiddinova, S Shankar, A Sharpe, T Shields, M Shinkwin, J Shurmer, A Siddika, R Simson, S Singh, J Sivaraj, A Skinner, C Smart, F Smith, R Smith, A Sreedhar, E Stewart‐Parker, M Stott, B Stubbs, N Symons, T Taj, J Tam, K Tan, S Tani, D Tao, K Thippeswamy, C Thomas, E Thompson, C Thompson‐Reil, F Tongo, G Toth, A Turnbull, J Turnbull, T Wade, A Wafi, K Waite, N Walker, T Walker, U Walsh, S Wardle, R Warner, J Watt, J Watts, J Wayman, C Weegenaar, M West, M Whyler, L Whitehurst, M Wiggans, G Williams, R Williams, A Williamson, J Williamson, A Winter, L Wolpert, J Wong, G van Boxel, E Yeap, S Zaman, B Zappa, D Zosimas, O Anderson, A Athem, M Athersmith, T Badenoch, S Barker, S Bellam, T Boam, M Boland, L Blake, O Brown, M Butler, B Byrne, L Campbell, M Chow, K Da Costa, J Cutting, M Deputy, L Devoto, P Doody, N Ekpete, M Eljaafari, K Exarchou, M Faoury, E Farinella, C Gill, M Goh, T Gregoir, S Growcott, S Gunasekaran, G Harris, R Heard, B Hobson, N Iqbal, R Jain, P Kang, M Khan, S Korambayil, S Kouris, K Kshatriya, S Kumar, K Lee, S Mahroof, K Malik, K Mann, S Mansour, R Martin, S McKay, N McKinley, D McWhirter, K Mellor, A Mishra, K Mockford, V Morrison‐Jones, C Ng, R Nunn, S O'Neill, O Oke, N Obeid, R Patel, S Patel, K Plunkett‐Reed, M Pouzi, S Pywell, E Richards, P Sinclair, N Slim, G Spence, M Swinkin, W Tahir, K Takacs, N Tanner, M Taylor, C Valero, M Venn, M Venza, and T Yeong
References
- 1. NELA Project Team . Third Patient Report of the National Emergency Laparotomy Audit (NELA). RCoA: London, 2017. [Google Scholar]
- 2. Ray NF, Denton WG, Thamer M, Henderson SC, Perry S. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg 1998; 186: 1–9. [DOI] [PubMed] [Google Scholar]
- 3. Stewart B, Khanduri P, McCord C, Ohene‐Yeboah M, Uranues S, Vega Rivera F et al Global disease burden of conditions requiring emergency surgery. Br J Surg 2014; 101: e9–e22. [DOI] [PubMed] [Google Scholar]
- 4. Scott JW, Olufajo OA, Brat GA, Rose JA, Zogg CK, Haider AH et al Use of national burden to define operative emergency general surgery. JAMA Surg 2016; 151: e160480. [DOI] [PubMed] [Google Scholar]
- 5. Aquina CT, Becerra AZ, Probst CP, Xu Z, Hensley BJ, Iannuzzi JC et al Patients with adhesive small bowel obstruction should be primarily managed by a surgical team. Ann Surg 2016; 264: 437–447. [DOI] [PubMed] [Google Scholar]
- 6. Association of Surgeons of Great Britain and Ireland (ASGBI), Royal College of Surgeons of England . Commissioning Guide: Emergency General Surgery. ASGBI: London, 2014. [Google Scholar]
- 7. Di Saverio S, Coccolini F, Galati M, Smerieri N, Biffl WL, Ansaloni L et al Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2013 update of the evidence‐based guidelines from the world society of emergency surgery ASBO working group. World J Emerg Surg 2013; 8: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maung AA, Johnson DC, Piper GL, Barbosa RR, Rowell SE, Bokhari F et al; Eastern Association for the Surgery of Trauma . Evaluation and management of small‐bowel obstruction: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg 2012; 73(Suppl 4): S362–S369. [DOI] [PubMed] [Google Scholar]
- 9. GlobalSurg Collaborative . Mortality of emergency abdominal surgery in high‐, middle‐ and low‐income countries. Br J Surg 2016; 103: 971–988. [DOI] [PubMed] [Google Scholar]
- 10. Wandling MW, Ko CY, Bankey PE, Cribari C, Cryer HG, Diaz JJ et al Expanding the scope of quality measurement in surgery to include nonoperative care: results from the American College of Surgeons National Surgical Quality Improvement Program emergency general surgery pilot. J Trauma Acute Care Surg 2017; 83: 837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee MJ, Sayers AE, Drake TM, Hollyman M, Bradburn M, Hind D et al; NASBO Steering Group . UK‐based, multisite, prospective cohort study of small bowel obstruction in acute surgical services: National Audit of Small Bowel Obstruction (NASBO) protocol. BMJ Open 2017; 7: e016796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014; 12: 1495–1499. [DOI] [PubMed] [Google Scholar]
- 13. Lang TA, Altman DG. Basic statistical reporting for articles published in biomedical journals: the ‘Statistical Analyses and Methods in the Published Literature’ or the SAMPL guidelines. Int J Nurs Stud 2015; 52: 5–9. [DOI] [PubMed] [Google Scholar]
- 14. McNair AG, Heywood N, Tiernan J, Verjee A, Bach SP, Fearnhead NS; ORACLE Collaboration . A national patient and public colorectal research agenda: integration of consumer perspectives in bowel disease through early consultation. Colorectal Dis 2017; 19: O75–O85. [DOI] [PubMed] [Google Scholar]
- 15. Nepogodiev D, Chapman SJ, Kolias AG, Fitzgerald JE, Lee M, Blencowe NS; National Surgical Research Collaborative . The effect of trainee research collaboratives in the UK. Lancet Gastroenterol Hepatol 2017; 2: 247–248. [DOI] [PubMed] [Google Scholar]
- 16. National Research Collaborative and Association of Surgeons in Training Collaborative Consensus Group . Recognising contributions to work in research collaboratives: guidelines for standardising reporting of authorship in collaborative research. Int J Surg 2018; 52: 355–360. [DOI] [PubMed] [Google Scholar]
- 17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 20. Veterans Affairs Total Parenteral Nutrition Cooperative Study Group . Perioperative total parenteral nutrition in surgical patients. N Engl J Med 1991; 325: 525–532. [DOI] [PubMed] [Google Scholar]
- 21. Thornblade LW, Truitt AR, Davidson GH, Flum DR, Lavallee DC. Surgeon attitudes and practice patterns in managing small bowel obstruction: a qualitative analysis. J Surg Res 2017; 219: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee MJ, Sayers AE, Wilson TR, Acheson AG, Anderson ID, Fearnhead NS; NASBO Steering Group . Current management of small bowel obstruction in the UK: results from the National Audit of Small Bowel Obstruction clinical practice survey. Colorectal Dis 2018; 20: 623–630. [DOI] [PubMed] [Google Scholar]
- 23. Peacock O, Bassett MG, Kuryba A, Walker K, Davies E, Anderson I et al; National Emergency Laparotomy Audit (NELA) Project Team . Thirty‐day mortality in patients undergoing laparotomy for small bowel obstruction. Br J Surg 2018; 105: 1006–1013. [DOI] [PubMed] [Google Scholar]
- 24. Hernandez MC, Birindelli A, Bruce JL, Buitendag JJP, Kong VY, Beuran M et al Application of the AAST EGS grade for adhesive small bowel obstruction to a multi‐national patient population. World J Surg 2018; 42: 3581‐3588. [DOI] [PubMed] [Google Scholar]
- 25. Playforth RH, Holloway JB, Griffen WO Jr. Mechanical small bowel obstruction: a plea for earlier surgical intervention. Ann Surg 1970; 171: 783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Public Health England . Medical Radiation: Patient Dose Information: Guidance; 2008. https://www.gov.uk/government/publications/medical‐radiation‐patient‐doses/patient‐dose‐information‐guidance [accessed 10 January 2018].
- 27. Pantos I, Thalassinou S, Argentos S, Kelekis NL, Panayiotakis G, Efstathopoulos EP. Adult patient radiation doses from non‐cardiac CT examinations: a review of published results. Br J Radiol 2011; 84: 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ceresoli M, Coccolini F, Catena F, Montori G, Di Saverio S, Sartelli M et al Water‐soluble contrast agent in adhesive small bowel obstruction: a systematic review and meta‐analysis of diagnostic and therapeutic value. Am J Surg 2016; 211: 1114–1125. [DOI] [PubMed] [Google Scholar]
- 29. Dimick JB, Pronovost PJ, Cowan JA, Lipsett PA. Complications and costs after high‐risk surgery: where should we focus quality improvement initiatives? J Am Coll Surg 2003; 196: 671–678. [DOI] [PubMed] [Google Scholar]
- 30. ESCP Intestinal Failure Group , Vaizey CJ, Maeda Y, Barbosa E, Bozzetti F, Calvo J et al European Society of Coloproctology consensus on the surgical management of intestinal failure in adults. Colorectal Dis 2016; 18: 535–548. [DOI] [PubMed] [Google Scholar]
- 31. Bingold TM, Lefering R, Zacharowski K, Meybohm P, Waydhas C, Rosenberger P et al; DIVI Intensive Care Registry Group . Individual organ failure and concomitant risk of mortality differs according to the type of admission to ICU – a retrospective study of SOFA score of 23 795 patients. PLoS One 2015; 10: e0134329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nfor TK, Walsh TS, Prescott RJ. The impact of organ failures and their relationship with outcome in intensive care: analysis of a prospective multicentre database of adult admissions. Anaesthesia 2006; 61: 731–738. [DOI] [PubMed] [Google Scholar]
- 33. National Institute for Health and Care Excellence (NICE) . Nutrition Support for Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition. NICE: London, 2006. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 NASBO Steering Group
Appendix S2 NASBO Collaborators
Appendix S3 Collaborator contributions
Appendix S4 Data fields captured
Appendix S5 Definition of complications
Table S1 Aetiologies of Small Bowel Obstruction
Table S2 Procedures performed for small bowel obstruction


