Abstract
We have conducted a prospective randomized study to determine the effect of intravenous or local administration of tranexamic acid (TXA) in perioperative transfusion rates, hospital stay and overall hospitalization costs in patients underwent total knee (TKA) or total hip (THA) arthroplasty. During 2015-2016, 125 THA and 124 TKA consecutive patients were randomly allocated to receive low dose TXA either intravenously (ivTXA groups) or local administration (locTXA groups) or to serve as controls. Power analysis showed that 41 patients in each group were required in order to have an 80% probability of demonstrating a between surgeries difference of more than 35%. Full blood counts obtained on the first and third postoperative day and the maximum hemoglobin difference was documented in all patients. The costs of hospitalization, transfusions and TXA were retrieved by the hospital financial administration. All groups were homogenic in regards to age and preoperative Hgb levels. In both THA and TKA patients, a statistically significant reduction in the maximum hemoglobin difference was found for both the intravenous (ivTXA) and local application (locTXA) groups compared to controls (P<0.001). The average hospitalization was reduced by 2.2 and 2.9 days in THA and TKA patients in respect. The hospitalization costs for the control groups were higher both in THA (286 € more) and TKA (374 € more) patients. We were able to demonstrate that both intravenous and local administration of TXA can significantly reduce transfusion rate, hospital stay and overall cost in TKA or THA patients.
Key words: tranexamic acid, hip arthroplasty, knee arthroplasty, transfusion rate, hospitalization, cost-effectiveness
Introduction
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) are among the most successful procedures for patients with painful degenerative knee or hip diseases. The amount of these procedures is expected to rise dramatically in the upcoming years as a consequence of ageing in a more demanding population.1 Perioperative blood loss has been associated with increased morbidity and higher risk of allogenic blood transfusion,2,3 ranging to as high as 54% for TKA and 80% for THA.4,5 Allogenic transfusion can result in substantial cost increasing and significant complications, namely adverse immunological reactions, increased risk of infection,6-8 coagulopathies, and acute renal failure.9 The relatively high cost of blood transfusions both in terms of direct and indirect hospital costs as illustrated by the increased length of stay and treating complications have led to an extensive research and protocols for perioperative blood management.
Recently, the use of tranexamic acid (ΤXΑ) has emerged as a safe and effective solution for the control of perioperative blood loss and the reduction in transfusion needs. ΤXΑis a synthetic lysine analog that binds to plasminogen, thereby inhibiting fibrin degradation. 10 Intravenous solutions, topical application and recently, oral ΤXΑ has been utilized to decrease perioperative blood loss with varying degrees of effectiveness.11,12 While the clinical advantages of ΤXΑ in reducing transfusion rates in both THA and TKA have been well documented, there has been considerable variation regarding the most clinically effective regimen for TXA administration (intravenously or local) but also in regards to the economic impact of its use as this is fairly dependent on institutional and governmental guidelines. We have conducted therefore, a prospective controlled randomized clinical study to determine the clinical effect of a low dose protocol of intravenous or local administration of ΤXΑ in THA and TKA patients with regards to transfusion rate and length of hospital stay, as well as to the cost effectiveness of this treatment in a National Health System which is under severe financial restrictions.
Materials and Methods
A standardized protocol for administration of TXA in patients undergoing THA and TKA was initiated to our institution in 2015. For the THA patients the intravenous administration included a single dose of 1gr TXA applied immediately before skin incision. In the local application group 2 gr of TXA was diluted in normal saline and the resulting solution (75 mL) was applied to the tissues just prior to wound closure. Half of the solution was applied to the peri-acetabular capsule and half in the fascia. For the TKA patients, the intravenous administration included a dose of 500 mg TXA before tourniquet inflation and a second dose of 500 mg TXA immediately before tourniquet release. In the local application group 2 gr of TXA was diluted in normal saline and the resulting solution (75 mL) was applied to the tissues after cement hardening. Half of the solution was applied to the knee capsule and half in the fascia. The posterior capsule was infiltrated before components insertion. The technique of application for both TKA and THA patients is illustrated in Figure 1.
Figure 1.
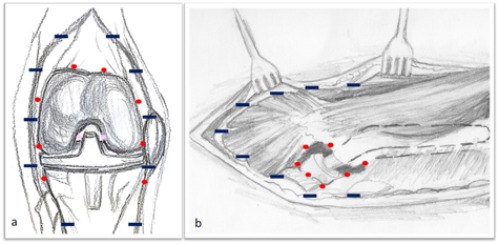
Technique of application for both total a) knee and b) hip arthroplastic patients.
During 2015-2016, 125 THA and 124 TKA consecutive patients with primary osteoarthritis were randomly allocated to receive either intravenous or local administration of TXA or to serve as controls. The primary outcome of the study was to determine the effect of intravenous or local administration of TXA in the perioperative transfusion rates, hospital stay and overall hospitalization costs. Power analysis showed that 41 patients in each group would be sufficient to yield a statistically significant result in regards to blood transfusion rates with a projected difference of 35%. Exclusion criteria of general health status were any patient with a history of angioplasty in the last six months, deep vein thrombosis, pulmonary embolism, stroke, atrial fibrillation, heart failure (NYHA class 3 or 4), myocardial infarction and hematologic or clotting disorders (hemophilia, thrombophilia). Additionally, patients with laboratory values of impaired renal function (creatine >1.5) or abnormal clotting screen (INR>1.5, aPTT >1.5 times normal values, PLT <150000) and patients that had taken anticoagulants up to seven days prior to surgery were also excluded. Disease specific criteria for knee osteoarthritis patients were knee flexion deformity >20°; varus and valgus deformity >20°; post-traumatic arthritis and rheumatoid arthritis. For the hip osteoarthritis patients were any femoral deformity, such in cases of hip dysplasia and severe metaphyseal or acetabular deformity secondary to fracture. Patients with rheumatoid arthritis were also excluded. Patient selection was blinded from the surgical team and was performed in an outpatient basis, 1 to 2 months before admission during standard preoperative evaluation, including orthopedic, cardiological and pulmonary assessment. A Hospital scientific board ethics approval was obtained (AΠ 115/12-02-2015) and all patients were consented for the study. All procedures performed were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration. The patients were randomly allocated to one of three groups for each type of joint replacement: Control group, intravenous application (ivTXA), and local application (locTXA). We used stratified block randomization consisting of a random sequence of blocks of 10 consecutive surgical procedures each. Randomization was performed in the operating theatre, after anesthetic induction and just before incision, using a sequentially numbered opaque sealed envelope. Anesthetic technique was freely chosen by the anesthesiologists to be applied to study participants. All the operations in the study were performed by the senior consultant (GA) in order to reduce variability. The surgical approach used in the THA patients was the modified Hardinge and the selection of stem (press-fit or cemented) was based on surgeon’s preference. Total knee replacements were performed with cemented prostheses (Posterior Stabilized or Posterior Retaining) using pneumatic tourniquet inflated with compressed air with pressure of 150 mmHg above the systolic blood pressure. All patients received prophylaxis for deep venous thrombosis with LMWH the night before the operation and 6 hours postoperatively and for one month thereafter. Data were retrieved by full blood counts obtained on the first and third postoperative day. The maximum hemoglobin difference was documented in all patients. Transfusion triggers were: Hgb <8 and symptomatic patients defined by hypotension, tachycardia or persistent fatigue and inability to perform ADLs postoperatively. The costs of hospitalization, transfusions and TXA were retrieved by the hospital financial administration office in the year 2017 so as to be up-to date. All the patients were clinically followed for evidence of DVT for 6 months postoperatively. Type of anesthesia (general or regional) and types of stem (cemented or press-fit) and TKA component (PCL retained or sacrificed) were also analyzed in terms of hospital stay, Hgb level and transfusion rates.
Statistical analysis
It was calculated that a sample size of 41 patients per group was required in order to have an 80% probability of demonstrating a between surgeries difference of more than 35% (50% control, 15% in ivTXA or locTXA) of transfused patients with a significance of <1.7% (two-tailed test with Bonferroni correction). Data were expressed as mean±SD for quantitative variables and as percentages for qualitative variables. The Kolmogorov-Smirnov test was utilized for normality analysis of the quantitative variables. The homogeneity of patients’ distribution between the 3 groups was checked by means of the X2 test and one-way ANOVA model. Comparison of outcome variables qualitative or quantitative between three groups was analyzed using the One-way ANOVA model, Bonferroni test for pairwise comparisons and X2 test respectively. All tests were two-sided, a P-value of <0.05 was used to denote statistical significance. All analyses were carried out using the statistical package SPSS version 17.00 (Statistical Package for the Social Sciences, SPSS Inc., Chicago, IL, USA).
Results
All three groups were homogenic in regards to age and preoperative Hgb levels (Table 1). The mean age of THA patients was 68.33±10.54 and the mean preoperative Hgb level 13.47±1.26 whereas for the TKA patients the values were 71.06±7.23 and 13.30±1.26 in respect. In THA patients, a statistically significant reduction in the maximum hemoglobin difference was found for both the intravenous (ivTXA) and the local application (locTXA) groups compared to the control group (P<0.001). Pairwise comparisons presented difference between Control and ivTXA and locTXA groups (P<0.001). There was also a statistically significant difference between the groups concerning the transfusion rate (P<0.001). The use of tranexamic acid was beneficial in reducing hospitalization duration as both the ivTXA and locTXA groups displayed a statistically significant decrease in the days of hospitalization with an average of 2.3 and 2.1 days respectively (P<0.001) (Table 2). The results were similar in the TKA group. The drop of hemoglobin was significantly lower in both the locTXA and ivTXA groups (P=0.001), and the same applied to the transfusion rate, where a statistically significant difference between groups was evident (P<0.001). Pairwise comparisons presented difference between Control and ivTXA and locTXA groups (P<0.001). The average hospitalization was reduced by an average of 2,9 days both in the ivTXA and locTXA groups (P<0.001) (Table 2). The use of a press-fit femoral stem in the THA cohort was associated only with a statistically significant decrease in the hospitalization duration (5,8 vs 9.3 days, P<0.001). Implant selection both in terms of posterior retaining or sacrificing TKA had no statistically significant effect on the transfusion rate of patients in this study. The posterior sacrificing knee design was associated with a decrease in the duration of hospitalization that reached statistical significance in all three groups (P values of 0.03, 0.024 and 0.05 for the control, ivTXA and locTXA groups respectively). However, there was no difference in the transfusion rates and hemoglobin decrease among the two designs (Table 3). The type of anesthesia did not seem to have a significant effect on the transfusion rates in any of the studied groups neither for the THA nor for the TKA patients (Table 4). The average cost of hospitalization in our department is 75 € per day, the average cost per unit of blood including the relevant laboratory work-up and materials is approximately 150 € whereas tranexamic acid costs 1 € per gram. The hospitalization costs for the THA patients in the control group were on average 286 € higher than the respective costs for the ivTXA and locTXA groups patients (P<0.001). The difference was greater for the TKA patients where the control group patients had an increase of approximately 374 € compared to the patients in the ivTXA and locTXA groups (P<0.001) (Table 5).
Table 1.
Homogeneity between groups.
| Average | Control | ivTXA | locTXA | P-value | ||
|---|---|---|---|---|---|---|
| THA | Age; mean±SD | 68.33±10.54 | 70.93±9.63 | 67.59±10.15 | 66.14±11.49 | 0.117 |
| Hgb; baseline; mean±SD | 13.47±1.26 | 13.45±1.19 | 13.28±1.29 | 13.70±1.30 | 0.322 | |
| TKA | Age; mean±SD | 71.06±7.23 | 72.71±8.05 | 69.73±6.87 | 70.74±6.55 | 0.166 |
| Hgb baseline; mean±SD | 13.30±1.26 | 13.25±1.26 | 13.39±1.36 | 13.27±1.30 | 0.859 |
TKA, total knee arthroplasty; THA, total hip arthroplasty.
Table 2.
Transfusion, hospitalization (days) and hemoglobin (g/dL) differences between groups.
| Average | Control | ivTXA [n=43] | locTXA [n=41] | P-value [n=41] | |
|---|---|---|---|---|---|
| THA | Number | n=43 | n=41 | n=41 | |
| Transfusion; no/yes, n (%) | 16 (37.2) / 27 (62.8) | 36 (87.8) / 5 (12.2) | 37 (90.2) / 4 (9.8) | <0.001 | |
| Hospitalization days; mean±SD | 7.21±2.58 | 4.90±1.67 | 5.10±1.81 | <0.001 | |
| Hgb max diff; mean±SD | -3.85±1.30 | -3.21±0.97 | -2.90±0.98 | <0.001 | |
| TKA | Number | n=41 | n=41 | n=42 | |
| Transfusion; no/yes, n (%) | 14 (34.1) / 27 (65.9) | 38 (92.7) / 3 (7.3) | 38 (90.5) / 4 (9.5) | <0.001 | |
| Hospitalization days; mean±SD | 7.78±3.11 | 4.83±1.41 | 4.81±1.04 | <0.001 | |
| Hgb max diff; mean±SD | -3.32±1.43 | -2.76±1.07 | -2.35±0.91 | 0.001 |
TKA, total knee arthroplasty; THA, total hip arthroplasty.
Table 3.
Effect of TKA design: posterior stabilized vs posterior retaining.
| TKA design | P-value | |||
|---|---|---|---|---|
| Posterior Stabilized | Posterior Retaining | |||
| Control | Transfusion; no/yes n (%) | 11 (39.3) / 17 (60.7) | 3 (23,1) / 10 (76.9) | 0.481 |
| Days of hospitalization; mean±SD | 8.50±3.39 | 6.23±1.78 | 0.030 | |
| Hgb max diff; mean±SD | -3.42±1.61 | -3.11±0.97 | 0.456 | |
| ivTXA | Transfusion; no/yes n (%) | 13 (86.7) / 2 (13.3) | 25 (96.2) / 1 (3.8) | 0.543 |
| Days of hospitalization; mean±SD | 5.50±1.34 | 4.46±1.33 | 0.024 | |
| Hgb max diff; mean±SD | -2.85±1.23 | -2.71±1.00 | 0.695 | |
| locTXA | Transfusion; no/yes n (%) | 12 (80.0) / 3 (20.0) | 26 (96.3) / 1 (3.7) | 0.122 |
| Days of hospitalization; mean±SD | 5.40±0.74 | 4.48±1.05 | 0.005 | |
| Hgb max diff; mean±SD | -2.41±1.03 | -2.31±0.85 | 0.758 | |
TKA, total knee arthroplasty; THA, total hip arthroplasty.
Table 4.
Effect of type of anesthesia: general versus spinal.
| Tranfusion | Anesthesia | P-value | |||
|---|---|---|---|---|---|
| General | Spinal | ||||
| THA | Control | no/yes, n (%) | 6 (40.0) / 9 (60.0) | 10 (35.7) / 18 (64.3) | 1.000 |
| ivTXA | no/yes, n (%) | 12 (85.7) / 2 (14.3) | 24 (88.9) / 3 (11.1) | 1.000 | |
| locTXA | no/yes, n (%) | 22 (91.7) / 2 (8.3) | 15 (88.2) / 2 (11.8) | 1.000 | |
| TKA | Control | no/yes, n (%) | 2 (40.0) / 3 (60.0) | 12 (33.3) / 24 (66.7) | 1.000 |
| ivTXA | no/yes, n (%) | 12 (100.0) / 0 (0.0) | 26 (89.7) / 3 (10.3) | 0.543 | |
| locTXA | no/yes, n (%) | 9 (100.0) / 0 (0.0) | 29 (87.9) / 4 (12.1) | 0.561 | |
Table 5.
Projected hospitalization costs (implants and operative costs excluded) in Euros.
| Total | Control | IvTXA | locTXA | P-value | |
|---|---|---|---|---|---|
| THA | 125 | n=43 | n=41 | n=41 | |
| 496.06±249.64 | 683.72±289.65 | 397.65±167.91 | 397.65±138.54 | <0.001 | |
| TKA | 124 | n=41 | n=41 | n=42 | |
| 498.28±272.01 | 748.17±321.93 | 373.92±129.35 | 375.75±106.07 | <0.001 |
TKA, total knee arthroplasty; THA, total hip arthroplasty.
Discussion
The effect of TXA in reducing transfusion rates in patients underwent THA or TKA has been extensively studied the last years but, the protocols utilized have shown a great variability in terms of dosage and application. Our data demonstrated the effectiveness of a low dose TXA in reducing blood transfusions in primary TKA and THA patients. In our TKA patients, we used an intravenous protocol consisting of 500 mg TXA prior to tourniquet inflation, followed by a second dose of 500 mg right before deflating the tourniquet; in the local application group we used 2 gr of TXA in 75 mL of normal saline. Most published studies have recommended intravenous administration of TXA in two doses, albeit higher, with the first dose being before deflating the tourniquet and the second postoperatively. We felt that a dose prior to tourniquet inflation would be more effective as previously shown by Tanaka et al.13 The 2 gr dose for local application has been already studied,14 and although higher doses have been proven to be effective and safe,15 we utilized the lowest dose due to safety concerns. In our series the local TXA dose proved to be at least as effective as the ivTXA administration with statistically significant better results than the control group. However, the use of local tranexamic acid was abandoned after the study was completed due to concerns regarding the sterilization process, even though there were no postoperative infections reported in either group. The approach utilized in TKA patients was a standard medial parapatellar approach. We used both posterior retaining and posterior sacrificing components. We found a tendency for shorter length of stay in the posterior sacrificing knee group compared to the posterior retaining group. This result is of uncertain significance as the groups were not equally distributed and the study was not designed to detect this type of difference.
The dosing regimens in THA patients have ranged from 10 mg/Kg up to 20 mg/Kg,12,16 or even 2 gr intravenously given in two doses.17 Our dose is in the lower range as there were concerns about the potential hazards. The dose for the local application was higher (2 gr diluted in a 75 mL solution) which remains on the lower range of the published regimens.18 The effectiveness of local application of TXA in primary THA has been clearly demonstrated, 19 however the majority of studies utilized intravenous regimens. In our center, concerns primarily regarding the sterilization process and secondarily the likelihood of sufficient distribution in THA soft tissues have significantly reduced its usage after the completion of the study. In terms of implant choice, the cemented stem patients showed significant higher length of stay compared to the press fit subgroup. In our opinion this should be attributed to the fact, that in our institution cemented stems are reserved for patients with poor bone quality which are usually frailer.
The type of anesthesia did not seem to have a significant effect on any of the primary and secondary outcomes for either the THA or TKA cohort. This contrasts the findings of Haughom et al.,20 who found a decrease in transfusion rates with neuraxial anesthesia in THA patients in an extensive retrospective analysis of 28,857 THAs. The same was reported in TKA patients, where spinal anesthesia was associated with a lower need for transfusion in a retrospective series of 14.052 cases.21 The length of stay was significantly decreased in all groups and subgroups that received TXA, with a more pronounced effect on the TKA patients, possibly due to the stricter anatomic compartments of the knee that accentuate the effects of a larger postoperative hematoma both in terms of pain and delayed rehabilitation, as the surgeon concern towards increased risk of infection.
The cost effectiveness of TXA has been mostly studied in the US with a variety of projected gains ranging from hundreds to thousands of dollars per patient.15,16,22 To our knowledge this is the first study dealing with the cost-effectiveness of tranexamic acid in the Greek Health System. The average savings of 284 € per THA patient and 386 € per TKA patient are significant when put into context. The current average cost of THA and TKA in the Greek Health System is estimated and reimbursed by the Social Insurance System in the area of 3000 € per case. Effectively, a 10% reduction of cost can be achieved and with the widespread application of TXA and stricter transfusion protocols a great impact on the healthcare costs can be achieved. In our department for the year 2017 the projected savings from the widespread use of TXA were in excess of 85,000 €. We are aware that the fairly small number of patients, the existence of non-quantified triggers for transfusion and the fact that we didn’t evaluate other parameters such as pain control or objective measurement of DVT are some limitations of the current study. We believe however that the prospective nature of our study strengthens our position for the transfusion and budget sparing benefits of global TXA protocol application.
Conclusions
In this study we were able to demonstrate that both intravenous and local administration of TXA can significantly reduce transfusion rate and hospital stay in patients underwent TKA or THA. The use of TXA is also cost effective especially in our country were the availability of allogenic blood is limited and the financial resources significantly reduced the last years. Although the Greek Health System is not the only that struggled for resources, further studies from similar backgrounds will possibly show a magnified effect of such blood sparing protocols.
Funding Statement
Funding: none.
References
- 1.Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am 2014;96:624-30. [DOI] [PubMed] [Google Scholar]
- 2.Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet 1996;348:1055-60. [DOI] [PubMed] [Google Scholar]
- 3.Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology 2010;113:482-95. [DOI] [PubMed] [Google Scholar]
- 4.Shenolikar A, Wareham K, Newington D, et al. Cell salvage auto transfusion in total knee replacement surgery. Transfus Med 1997;7:277-80. [DOI] [PubMed] [Google Scholar]
- 5.Faris PM, Ritter MA, Abels RI. The effects of recombinant human erythropoietin on perioperative transfusion requirements in patients having a major orthopaedic operation. The American Erythropoietin Study Group. J Bone Joint Surg Am 1996;78:62-72. [DOI] [PubMed] [Google Scholar]
- 6.Newman ET, Watters TS, Lewis JS, et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am 2014;96:279-84. [DOI] [PubMed] [Google Scholar]
- 7.Steinitz D, Harvey EJ, Leighton RK, Petrie DP. Is homologous blood transfusion a risk factor for infection after hip replacement? Can J Surg 2001;44:355-8. [PMC free article] [PubMed] [Google Scholar]
- 8.Innerhofer P, Klingler A, Klimmer C, et al. Risk for postoperative infection after transfusion of white blood cell-filtered allogeneic or autologous blood components in orthopedic patients undergoing primary arthroplasty. Transfusion 2005; 45:103-10. [DOI] [PubMed] [Google Scholar]
- 9.Lemaire R. Strategies for blood management in orthopaedic and trauma surgery. J Bone Joint Surg Br 2008;90:1128-36. [DOI] [PubMed] [Google Scholar]
- 10.McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs 2012;72:585-617. [DOI] [PubMed] [Google Scholar]
- 11.Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br 2011;93:39-46. [DOI] [PubMed] [Google Scholar]
- 12.Garneti N, Field J. Bone bleeding during total hip arthroplasty after administration of tranexamic acid. J Arthroplasty 2004;19:488-92. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka N, Sakahashi H, Sato E, et al. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br 2001;83:702-5. [DOI] [PubMed] [Google Scholar]
- 14.Georgiadis AG, Muh SJ, Silverton CD, et al. A prospective double-blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty. J Arthroplasty 2013;8S:78-82. [DOI] [PubMed] [Google Scholar]
- 15.Chimento GF, Huff T, Ochsner JL, et al. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty 2013;8S:74-7. [DOI] [PubMed] [Google Scholar]
- 16.Stoicea N, Moran K, Mahmoud AR, et al. Tranexamic acid use during total hip arthroplasty: A single center retrospective analysis. Medicine (Baltimore) 2018;21:e10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg 2000;120:518-20. [DOI] [PubMed] [Google Scholar]
- 18.Sarzaeem MM, Razi M, Kazemian G, et al. Comparing efficacy of three methods of tranexamic acid administration in reducing hemoglobin drop following total knee arthroplasty. J Arthroplasty 2014;8:1521-4. [DOI] [PubMed] [Google Scholar]
- 19.Yue C, Kang P, Yang P, et al. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty 2014;12:2452-6. [DOI] [PubMed] [Google Scholar]
- 20.Haughom BD, Schairer WW, Nwachukwu BU, et al. Does Neuraxial Anesthesia Decrease Transfusion Rates Following Total Hip Arthroplasty? J Arthroplasty 2015;30:116-20. [DOI] [PubMed] [Google Scholar]
- 21.Pugely AJ, Martin CT, Gao Y, et al. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am 2013;95:193-9. [DOI] [PubMed] [Google Scholar]
- 22.Gillette BP, Maradit Kremers H, Duncan CM, et al. Economic impact of tranexamic acid in healthy patients undergoing primary total hip and knee arthroplasty. J Arthroplasty 2013;8S: 137-9. [DOI] [PubMed] [Google Scholar]


