Abstract
The encapsulation of Ib-M6 antibacterial peptide in pellets of polyvinyl alcohol (PVA) and polyvinyl alcohol-alginate (PVA-Alg) matrices was carried out in order to explore its controlled release and activity against Escherichia coli K-12. The pellets were obtained by combined ice segregation induced self-assembly (ISISA) and freezing-thawing methods and their microstructure was studied by scanning electron microscopy. Bromothymol blue was used as a model compound to study the transport mechanisms and release from pellets. The results show that there is a significant effect of the total concentration of PVA precursor solutions, the mass ratio of PVA of different molecular weights and the addition of alginate on the microstructure and transport properties of pellets. The antibacterial activity of Ib-M6 against Escherichia coli K-12 was not affected by the encapsulation in PVA pellets. However, the release of Ib-M6 from PVA-Alg pellets was not possible, probably due to the electrostatic interaction of positively charged Ib-M6 and negatively alginate structure. Nonetheless, the controlled release of Ib-M6 from polymeric matrices can be fitting by modifying parameters such as the concentration and type of polymer precursors.
Keywords: Biotechnology, Materials chemistry, PVA, ISISA method, Freezing-thawing method, Controlled release, Antibacterial properties
1. Introduction
Antibacterial resistance is one of the biggest challenges in public health worldwide which has generated the need to develop new more efficient antibacterial compounds [1]. In this sense, antimicrobial peptides (AMPs) arise as an alternative to treatment with conventional antibiotics because they have a broad spectrum of action. AMPs exhibit antibacterial activity against gram-negative and gram-positive bacteria, as well as antifungal and antiviral activity [2, 3, 4]. Also, AMPs have low toxicity against human cells and they exhibit different mechanisms of action, which hinders the generation of bacterial resistance [5, 6].
Recently, it was reported an analog of Ib-AMP4 antimicrobial peptide named Ib-M6 (sequence EWGRRMMGWGRGRRMMRRWW-NH2). This peptide is characterized by having a high positive charge (+6) due to R residues and high hydrophobicity by W residues. This leads to exhibit antibacterial activity against Escherichia coli K-12 with IC50 of 1 μM. Also, Ib-M6 did not exhibit hemolytic activity at this concentration [7].
Although AMPs have an important activity in vitro, their properties exhibit a significant decrease in vivo due to factors such as degradation by proteases and the dependence of the concentration of salts in the serum, which hinders its formulation [8]. For this reason, various controlled release systems have been designed to reduce the degradation of AMPs and allow them to keep their activity in the concentration of the therapeutic range [9, 10, 11].
Polymeric matrices have attracted great interest as new systems in the development of controlled release of biologically active molecules due to their versatility, biocompatibility and biodegradability [12]. Pellets are commonly used for this purpose due to the decrease in the exposure time of biomolecules in the gastrointestinal tract and they can be prepared from different polymers [13].
Polyvinyl alcohol (PVA) is a synthetic polymer with protein structure stabilization properties and preservation of the biological activity [14]. It is possible to obtain PVA pellets through different procedures such as ice segregation induced self-assembly (ISISA), in which unidirectional freezing in liquid nitrogen is carried out at a controlled immersion speed [15]. This method allows obtaining microchannels in the pellet by the formation of ice crystals in the freezing direction of PVA aqueous precursor solutions [16]. The type of obtained pore will depend on variables such as impurities in precursor solutions, the total concentration and the degree of hydrolysis of PVA [16]. Furthermore, we previously reported for the first time, to our knowledge, the structure and stability of PVA polymeric matrices are significantly affected by the molecular weight of PVA used as precursors [17].
Another commonly used method is freezing-thawing process which consists of physical cross-linking of PVA for obtaining hydrogels with high elasticity and stability [18]. However, obtaining PVA pellets is difficult due to the presence of hydroxyl groups that give hydrophilic characteristics to the polymer, allowing to trap large amounts of water inside its structure, which decreases its stability [19].
Sodium alginate (Alg) is a natural polymer widely used in biomedical applications. This polymer has been used for the encapsulation of biomolecules as insulin [20, 21, 22] and glucose oxidase [23, 24] and it consists of units of β-D-mannuronic acid and α-L-glucuronic acid with a pKa between 3.38 and 3.65 [25]. This allows controlling its charge at different pH values making it a system of great interest for oral controlled release drug delivery. Sodium alginate form hydrogels with small pores up to 200 nm by ionotropic gelation using divalent ions such as Ca2+ [26]. Despite the above, calcium alginate pellets exhibit low retention capacity of encapsulated molecules [27, 28].
Keeping in mind the above, in this work we report the encapsulation of Ib-M6 peptide in PVA and PVA-Alg pellets. The antibacterial activity of Ib-M6 released from pellets was evaluated against Escherichia coli K-12 used as gram-negative reference bacteria. This constitutes the continuation of exploratory work to determine the spectrum of activity of the Ib-M6 peptide and its potential use in biological systems.
2. Materials and methods
2.1. Materials
The following reagents were used as received without further purification: Polyvinyl alcohol (PVA23, Aldrich, Mw 13000–23000, 98% hydrolysed), polyvinyl alcohol (PVA98, Aldrich, Mw 89000–98000, 99+% hydrolysed), alginic acid sodium salt (Panreac, Mw 10000–600000), calcium chloride dihydrate (ACS reagent), bromothymol blue (Aldrich, 95%, ACS reagent), Müller-Hinton broth (MHB, Scharlau), sodium chloride (USP, Panreac) and deionized water (18 MΩ cm at 25 °C). Ib-M6 (sequence EWGRRMMGWGRGRRMMRRWW-NH2) antibacterial peptide was supplied by Biomatik.
2.2. Preparation of PVA and PVA-Alg pellets
PVA pellets were prepared using an ISISA modified procedure published previously [17]. Briefly, three aqueous solutions of precursor polymers with a total concentration of PVA (PVA23 + PVA98) of 2.5, 5.0 and 7.5% (w/v) were prepared, respectively. The PVA23:PVA98 mass ratio was 1:3 in all cases. The solutions of precursor polymers were dropped into liquid nitrogen using a syringe needle (0.8 mm × 40 mm). Subsequently, the obtained pellets were lyophilized using an Alpha 1–2 LD Plus freeze-drier. The nomenclature assigned to each obtained pellet is shown in Table 1.
Table 1.
Nomenclature assigned to each PVA pellet.
| Total concentration of PVA (% w/v)∗ | Nomenclature assigned to pellets |
|---|---|
| 2.5 | PV25 |
| 5.0 | PV50 |
| 7.5 | PV75 |
PVA23 + PVA98.
PVA-Alg pellets were prepared by ionotropic gelation of alginate followed by a physical cross-linking of PVA precursors by freeze-thaw method [29]. In this case, aqueous solutions of precursor polymers containing simultaneously dissolved PVA23, PVA98 and alginic acid sodium salt were used. The total concentration of PVA was fixed at 5.0% (w/v) (defined by preliminary results of PVA pellets) and alginic acid sodium salt was varied in the range of 0.5–1.5% (w/v). The solutions of precursor polymers were dropped into a CaCl2 1.5M aqueous solution (pH adjusted to 5.6) using a syringe needle (0.8 mm × 40 mm) under continuous magnetic stirring (500 rpm) at 25 °C. The stirring was maintained for 1 h and the obtained pellets were filtered and washed with deionized water. These pellets were frozen at -20 °C for 12h and thawed at 25 °C for 4h. Finally, the pellets were stored at 4 °C. The nomenclature of pellets and the conditions of preparation are shown in Table 2.
Table 2.
Composition of PVA-Alg pellets. These conditions were established in the analysis of PVA pellets.
| Total concentration of polymers (%w/v)∗ | Mass ratio of total PVA:Alginate | Nomenclature assigned to pellets |
|---|---|---|
| 5.5 | 5.0:0.5 | PV50-A05 |
| 6.0 | 5.0:1.0 | PV50-A10 |
| 6.5 | 5.0:1.5 | PV50-A15 |
PVA23 + PVA98 + alginic acid sodium salt.
2.3. Encapsulation in PVA and PVA-Alg pellets
Bromothymol blue (BB) was used as a model compound to evaluate the transport properties of pellets. This compound was chosen because it is possible to modify the charge thereof by varying the pH of the medium due to its pKa (7.1), either encapsulation or release. For the encapsulation, a stock solution of BB was added to the aqueous solution of precursor polymers of PVA and PVA-Alg in such of a way that the final concentration of BB was 89.5 mg/L in all cases.
The experiments of controlled release of BB were carried out in water at pH 7.4 (adjusted with NaOH). For this, three pellets of each preparation were added in separate vessels containing 2.0 mL of water under permanent stirring in a shaker (250 rpm) at 25 °C. The release of BB was monitored by measuring the absorbance at 620 nm using an AvaSpec-2048 fiber optic spectrometer (Avantes).
Ib-M6 antimicrobial peptide was encapsulated in PVA and PVA-Alg pellets following the same procedure used for the encapsulation of BB. In this case, the concentration of Ib-M6 in the aqueous solutions of precursor polymers was 100 μM.
2.4. PVA and PVA-Alg pellets characterization
Scanning electron micrographs of PVA and PVA-Alg pellets were obtained with a Quanta Field Emission Gun microscope (model 650) operated at 20.0 kV. The images were obtained in the secondary electron mode. In all cases, pellets were sputtered with Au (60 s) prior to analyses in order to prevent electric charging.
Fourier-transform infrared (FTIR) measurements of PVA-Alg pellets were performed using a Bruker Tensor II spectrometer equipped with a Platinum ATR cell and a cooled deuterated triglycine sulfate (DTGS) detector. The ATR-FTIR spectra were collected with 16 scans at a resolution of 5 cm−1.
2.5. Antibacterial activity against E. coli
The activity of the Ib-M6 released from biopolymeric pellets was evaluated against Escherichia coli K-12 by the plate count technique. For this, E. coli K-12 was cultured aerobically in Müller-Hinton Broth (MHB) at 37 °C by 24 h. After this time, the culture was diluted with saline solution to achieve an inoculum of approximately 1×108 colony forming units per mL (CFU/mL). Aliquots of 250 μL were added to well of 96-well plate and one pellet of each preparation was used. The growth kinetics of E. coli were determined for 6 h. For this, ten-fold serial dilutions were made of the inoculum of the bacteria and 3 μL of each dilution were seeded in petri dish. Finally, the count of colony forming units (CFU) was made. Negative and positive growth controls were performed by adding only E. coli, pellets without peptide and free Ib-M6 peptide in Tris buffer (10 mM, pH 7.4) at a concentration of 5 μM.
3. Results and discussion
3.1. Structural characterization of pellets
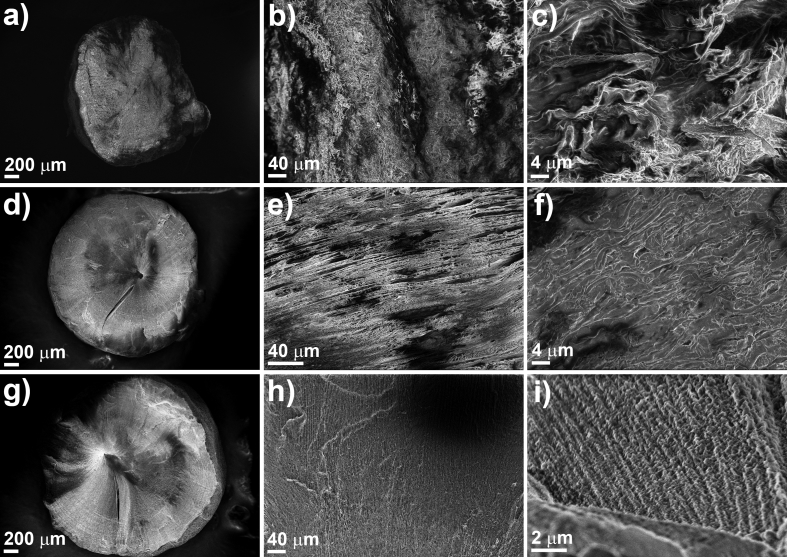
The effect of varying the total concentration of PVA from 2.5 to 7.5% (w/v) on the microstructural properties was evaluated. Scanning electron micrographs (SEM) of PVA pellets are shown in Fig. 1. The obtained pellets have an average diameter from 2.24 to 2.58 mm (Fig. 1a,d,g).
Fig. 1.
Scanning electron micrographs of PV25 (a, b, c), PV50 (d, e, f) and PV75 (g, h, i) pellets.
The morphology and microstructure of pellets are amorphous when a total concentration of PVA of 2.5% (w/v) was used (Fig. 1a-c). This is due to the low amount of polymer produces a more unstable structure that collapses when ice crystals are removed. On the other hand, compact structures with defined channels and aligned in the cooling direction were observed when the total concentration of PVA increases to 5.0 or 7.5% (w/v) (Fig. 1d-i). The polymer concentration defines the amount of ice crystals formed in the freezing process and that are removed by lyophilization process.
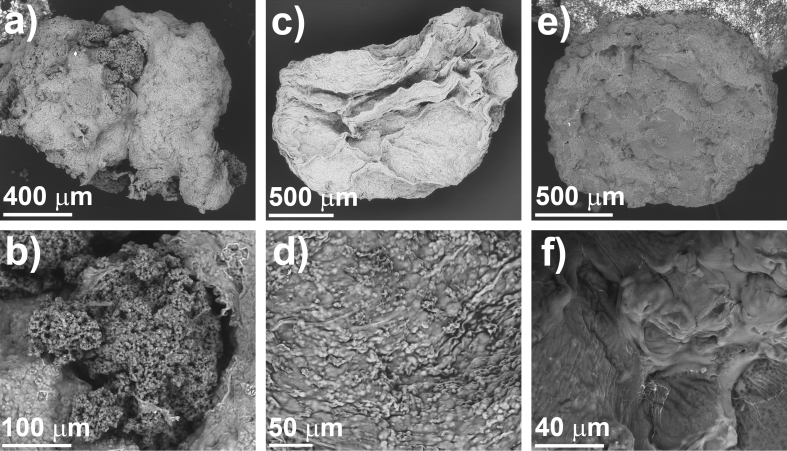
Considering the above, PVA concentration of 5.0% (w/v) was selected to prepare PVA-Alg pellets. These pellets were obtained by ionotropic gelation of alginate followed by physical cross-linking of PVA by freeze-thaw method. In all cases, the PVA concentration was kept constant while alginate was varied from 0.5 to 1.5% (w/v) (see Table 2). The microstructure of PVA-Alg pellets was analyzed by SEM and the results are shown in Fig. 2.
Fig. 2.
SEM micrographs of PVA-Alg pellets: (a, b) PV50-A05 (c, d) PV50-A10 and (e, f) PV50-A15.
The obtained pellets have a mean diameter between 1.39 and 1.53 mm. The results show that the structure of pellets prepared with relative low alginate concentration (0.5 and 1.0%) collapse after physical cross-linking processes. Furthermore, they exhibit amorphous and disordered channels (Fig. 2a-d). Nevertheless, it was observed that the morphology is more defined when the total polymer concentration increases (Fig. 2e).
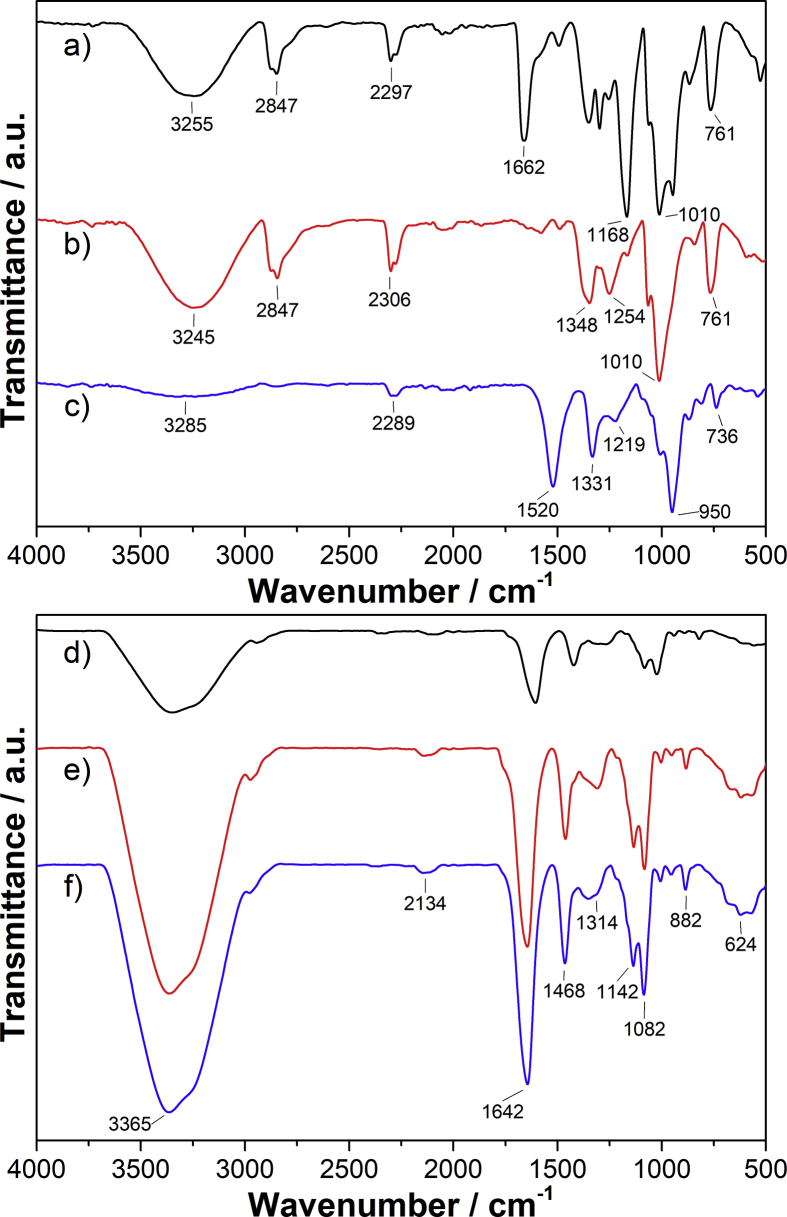
Fourier-transform infrared spectroscopy (FTIR) was used in order to evaluate the interaction between the polymers present in the structure (Fig. 3).
Fig. 3.
FTIR spectra of precursor polymers (top) and PVA-Alg pellets (bottom): (a) PVA23 precursor, (b) PVA98 precursor, (c) alginic acid sodium salt precursor and pellets of (d) PV50-A05, (e) PV50-A10 and (f) PV50-A15.
The OH stretching vibrations of PVA23, PVA98 and NaAlg are observed between 3285 and 3245 cm−1 (Fig. 3a-c). This band appears in 3365 cm−1 for pellets (Fig. 3d-f). The band around 1520 cm−1 in Fig. 3c is assigned to the carbonyl group of NaAlg while band around 1662 cm−1 of PVA23 (Fig. 3a) is due to the stretching of C=O and C–O groups from the unhydrolyzed part in the polymer. This band is absent in Fig. 3b because PVA98 is 99% hydrolyzed. Meanwhile, the stretching C=O of NaAlg in pellets appears in 1642 cm−1.
3.2. Release of BB from PVA and PVA-Alg pellets
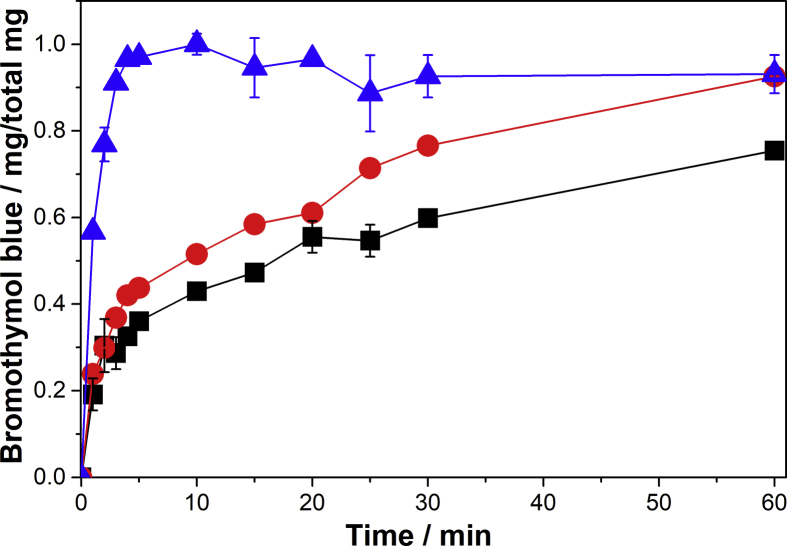
Bromothymol blue (BB) was used as a model compound in order to evaluate the encapsulation-release processes of positively charged molecules in PVA and PVA-Alg pellets. The release kinetic curve of BB from PVA pellets is shown in Fig. 4.
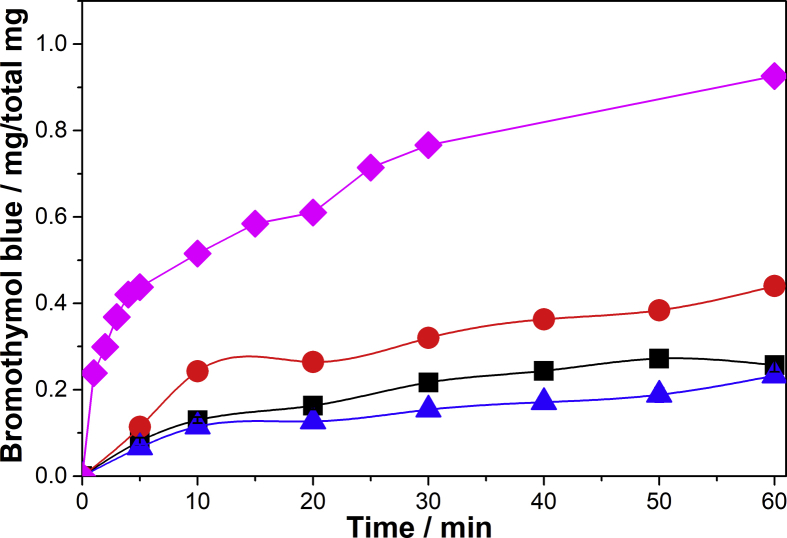
Fig. 4.
Bromothymol blue release from PV25 (■), PV50 (●) and PV75 (▲) pellets.
The increase in the total concentration of PVA favors a fast release of BB. It was previously reported [17] that PV75 pellets experiment a swelling of their structure when they come into contact with water. This is favored by their well-consolidated microstructure and channels. This phenomenon allows the fast release of BB by simple diffusion. On the other hand, PV25 and PV50 pellets do not exhibit a significant difference in kinetic behavior probably due to the release of BB is by an erosion mechanism [17].
The release kinetics of BB from PVA pellets was evaluated using the mathematical model proposed by Korsmeyer-Peppas [30]. The release curves can be parameterized by the following general expression (Eq. 1), which describes the solute release from hydrophilic polymers.
| (1) |
Where Mt/M∞ is the fractional BB released at time t, k is a kinetic constant and n is the diffusional exponent which is related to release mechanisms [31, 32, 33]. The obtained parameters for each curve in Fig. 4 are reported in Table 3. It was not possible to perform the analysis for PV75 because these pellets release more than 60% of BB before 2 min.
Table 3.
Kinetic obtained parameters for each PVA pellet according to Korsmeyer-Peppas model.
| Pellet | k | n | R2 |
|---|---|---|---|
| PV25 | 0.2190 | 0.2900 | 0.94353 |
| PV50 | 0.2561 | 0.3102 | 0.97491 |
| PV75 | N.C. | N.C. | N.C. |
N.C. Not calculated. k is the kinetic constant. n is the diffusional exponent. R is the correlation coefficient.
Considering the correlation coefficient, the adjustment of the obtained results with the proposed model was not satisfactory. This indicates the presence of complex release mechanisms as dye diffusion and swelling, dissolution or erosion of pellets [32]. In spite of the above, it was observed an effect of the total concentration of PVA on the release kinetics of BB. As mentioned above, the microstructure of PV75 pellets favors swelling thereof which results in a fast and uncontrolled release of the dye. On the other hand, the PV25 and PV50 pellets exhibit a gradual release, but the latter has a well-consolidated microstructure. Taking into account the above, the PV50 pellets were selected to be modified with alginate in order to improve the release characteristics.
The release kinetic curve of BB from PVA-Alg pellets is shown in Fig. 5. The results show a considerable control in the dye release when alginate is used. However, the amount of BB released does not reach the amount released by PV50 pellets at the time evaluated. It can be observed that the release kinetic increases when the alginate concentration increases from 0.5 to 1.0%. However, a significant decrease in the release rate of BB is observed at an alginate concentration of 1.5%. This can be due to the fact that the structure of the PV50-A15 pellets is compact, which limits the transport of the dye.
Fig. 5.
Bromothymol blue release from PV50-A05 (■), PV50-A10 (●) and PV50-A15 (▲) pellets. The result of PV50 pellets (◆) was included for comparative purposes.
Resulting parameters from the fitting of each curve according to Korsmeyer-Peppas model are shown in Table 4.
Table 4.
Resulting kinetic obtained parameters for each PVA-Alg pellet.
| Pellet | k | n | R2 |
|---|---|---|---|
| PV50-A05 | 0.04500 | 0.4462 | 0.95428 |
| PV50-A10 | 0.07453 | 0.4288 | 0.94268 |
| PV50-A15 | 0.03450 | 0.4471 | 0.94098 |
k is the kinetic constant. n is the diffusional exponent. R is the correlation coefficient.
As in PVA pellets results, the kinetic model used for PVA-Alg system does not fit to the obtained results and the release mechanisms are complex. Nevertheless, diffusional exponent n > 0.43 indicates a non-Fickian transport mechanism which is consistent with what is reported in the literature for PVA systems [32].
The above results show that the release mechanism of BB from PVA-Alg pellets can be governed by two processes, considering that this dye has a negative charge on the pH of the release medium. First, the charge repulsion between the negatively charged polymer (alginate) and BB causes the last one to be expelled towards outside. Second, the microstructure compaction as a result of the increase in the total alginate concentration will limit the diffusion of molecules from the inside of the sphere to the outside. This allows performing fine control of release kinetics by modifying the microstructural (pore size) and charge (by the addition of negatively charged polymers) properties of pellets.
3.3. Antibacterial activity of Ib-M6 peptide released from biopolymeric pellets
E. coli K-12 was used as a reference bacterium in order to evaluate the activity of the Ib-M6 peptide when it is released from pellets. The growth kinetics of E. coli K-12 in the presence and absence of free and encapsulated Ib-M6 peptide on biopolymeric pellets are shown in Fig. 6. The concentration used for free peptide was 5.0 μM.
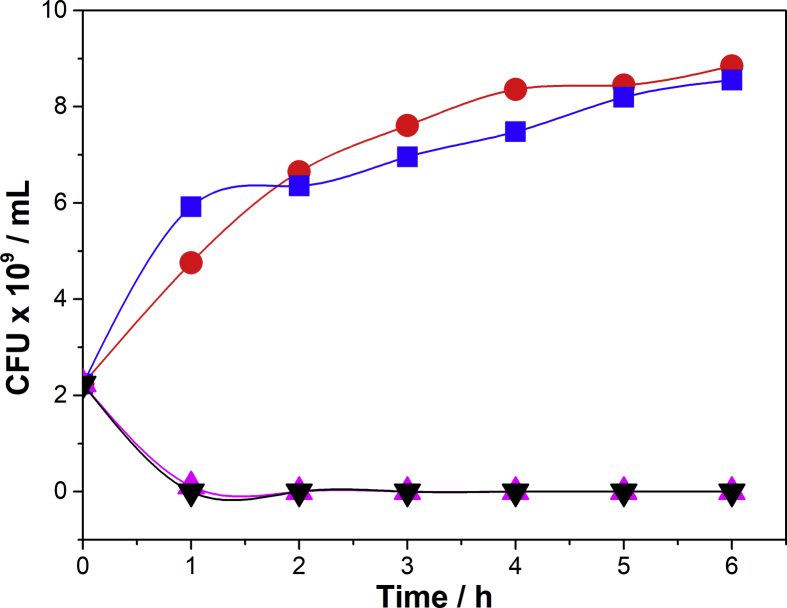
Fig. 6.
Growth kinetics of E. coli K-12 in the absence (●) and presence of free (▲) and encapsulated Ib-M6 peptide on PV50 pellets (▼). Results of E. coli K-12 in presence of PV50 (■) pellets without the addition of Ib-M6 were included for comparative purposes.
It was not observed growth of microorganisms in the presence of Ib-M6 peptide both free and encapsulated in PV50 pellets. This indicates that the activity of this peptide is not affected by the encapsulation in polyvinyl alcohol matrices. Also, it is noteworthy that there is no significant difference in the activity of the peptide when it is encapsulated. On the other hand, it was found that there is no masking effect on the activity of the peptide by the presence of PVA, since no effect on the growth of E. coli was observed. This can be seen in Fig. 6 where it is shown that the growth kinetics of E. coli in the presence of PV50 pellets is similar to the bacteria alone. This is because PVA is a non-toxic and biocompatible polymer that it does not have an effect on the bacteria.
Contrary to expectations, it was not possible to carry out the Ib-M6 peptide release from PVA-Alg system. This can be due to the highly positively charged peptide molecules (6+) that are trapped on the negatively charged alginate network which prevent their release.
4. Conclusions
The ISISA method was useful to control the structure of PVA pellets by modifying the total concentration of PVA in the precursor solution. Amorphous pellets with poorly defined channels were obtained at a total concentration of PVA of 2.5% (w/v), while structures with radially aligned microchannels were obtained at 7.5% (w/v). Moreover, the addition of alginate to the PVA pellets favours the structure compaction.
Variations in the total concentration of PVA, mass ratio of PVA and alginate addition allowed to modify the release rate of bromothymol blue (used as reference) for a time of 60 min. However, it was not possible fitting the dye release kinetics to the model proposed by Korsmeyer-Peppas. The above indicates that it is possible that non-Fickian processes govern the transport mechanisms.
The encapsulation of Ib-M6 peptide in PVA pellets did not affect the antimicrobial activity against Escherichia coli. In addition, polymer masking effect on antimicrobial activity was not observed. Finally, it was not possible to achieve Ib-M6 release from the PVA-Alg pellets probably due to electrostatic attraction between the high positive charge of the peptide and the negative charge of the alginate.
Declarations
Author contribution statement
J.M. Flórez-Castillo, J.L. Ropero-Vega: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mercedes Perullini, Matias Jobbágy: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Universidad de Santander (Colombia) under Grant 068-16. J.L. Ropero-Vega and J.M. Flórez Castillo were supported by COLCIENCIAS in the frame of the program “Convocatoria nacional para estudios a nivel de doctorado en Colombia – año 2009”.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
J.M. Flórez-Castillo, Email: johanna.florez@udes.edu.co.
J.L. Ropero-Vega, Email: jose.ropero@udes.edu.co.
References
- 1.Gibson M.K., Forsberg K.J., Dantas G. Improved annotation of antibiotic resistance determinants reveals microbial resistomes cluster by ecology. ISME J. 2015;9:207–216. doi: 10.1038/ismej.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maturana P., Martinez M., Noguera M.E. Lipid selectivity in novel antimicrobial peptides: implication on antimicrobial and hemolytic activity. Colloids Surfaces B Biointerfaces. 2017;153:152–159. doi: 10.1016/j.colsurfb.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Pane K., Durante L., Crescenzi O. Antimicrobial potency of cationic antimicrobial peptides can be predicted from their amino acid composition: application to the detection of “cryptic” antimicrobial peptides. J. Theor. Biol. 2017;419:254–265. doi: 10.1016/j.jtbi.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Nordström R., Malmsten M. Delivery systems for antimicrobial peptides. Adv. Colloid Interface Sci. 2017;242:17–34. doi: 10.1016/j.cis.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 6.Glukhov E., Stark M., Burrows L.L., Deber C.M. Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J. Biol. Chem. 2005;280:33960–33967. doi: 10.1074/jbc.M507042200. [DOI] [PubMed] [Google Scholar]
- 7.Flórez-Castillo J.M., Perullini M., Jobbágy M., de Jesús Cano Calle H. Enhancing antibacterial activity against Escherichia coli K-12 of peptide ib-AMP4 with synthetic analogues. Int. J. Pept. Res. Ther. 2014;20:365–369. [Google Scholar]
- 8.Marr A., Gooderham W., Hancock R. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006;6:468–472. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Chan H.F., Leong K.W. Advanced materials and processing for drug delivery: the past and the future. Adv. Drug Deliv. Rev. 2013;65:104–120. doi: 10.1016/j.addr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruno B.J., Miller G.D., Lim C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2014;4:1443–1467. doi: 10.4155/tde.13.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel A., Patel M., Yang X., Mitra A. Recent advances in protein and peptide drug delivery: a special emphasis on polymeric nanoparticles. Protein Pept. Lett. 2014;21:1102–1120. doi: 10.2174/0929866521666140807114240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HARRISON K. Introduction to polymeric drug delivery systems. Biomed Polym. 2007:33–56. [Google Scholar]
- 13.Rahman M.A., Ahuja A., Baboota S. Recent advances in pelletization technique for oral drug delivery: a review. Curr. Drug Deliv. 2009;6:122–129. doi: 10.2174/156720109787048339. [DOI] [PubMed] [Google Scholar]
- 14.Alves M.-H., Jensen B.E.B., Smith A.A.A., Zelikin A.N. Poly(Vinyl alcohol) physical hydrogels: new vista on a long serving biomaterial. Macromol. Biosci. 2011;11:1293–1313. doi: 10.1002/mabi.201100145. [DOI] [PubMed] [Google Scholar]
- 15.Qian L., Zhang H. Controlled freezing and freeze drying: a versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2011;86:172–184. [Google Scholar]
- 16.Gutiérrez M.C., García-Carvajal Z.Y., Jobbágy M. Poly(vinyl alcohol) scaffolds with tailored morphologies for drug delivery and controlled release. Adv. Funct. Mater. 2007;17:3505–3513. [Google Scholar]
- 17.Sonego J.M., Flórez-Castillo J.M., Jobbágy M. Highly structured polyvinyl alcohol porous carriers: tuning inherent stability and release kinetics in water. ACS Omega. 2018;3:2390–2395. doi: 10.1021/acsomega.7b01961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricciardi R., Auriemma F., Gaillet C. Investigation of the crystallinity of freeze/thaw poly(vinyl alcohol) hydrogels by different techniques. Macromolecules. 2004;37:9510–9516. [Google Scholar]
- 19.Chee B.S., Goetten de Lima G., Devine D.M., Nugent M.J.D. Investigation of the effects of orientation on freeze/thawed Polyvinyl alcohol hydrogel properties. Mater Today Commun. 2018;17:82–93. [Google Scholar]
- 20.Sarmento B., Ferreira D.C., Jorgensen L., van de Weert M. Probing insulin’s secondary structure after entrapment into alginate/chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2007;65:10–17. doi: 10.1016/j.ejpb.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Sarmento B., Ribeiro A.J., Veiga F. Insulin-loaded nanoparticles are prepared by alginate ionotropic pre-gelation followed by chitosan polyelectrolyte complexation. J. Nanosci. Nanotechnol. 2007;7:2833–2841. doi: 10.1166/jnn.2007.609. [DOI] [PubMed] [Google Scholar]
- 22.Sarmento B., Ribeiro A., Veiga F. Alginate/Chitosan nanoparticles are effective for oral insulin delivery. Pharm. Res. 2007;24:2198–2206. doi: 10.1007/s11095-007-9367-4. [DOI] [PubMed] [Google Scholar]
- 23.Blandino A., Macias M., Cantero D. Immobilization of glucose oxidase within calcium alginate gel capsules. Process Biochem. 2001;36:601–606. [Google Scholar]
- 24.Blandino A., Macías M., Cantero D. Glucose oxidase release from calcium alginate gel capsules. Enzym. Microb. Technol. 2000;27:319–324. doi: 10.1016/s0141-0229(00)00204-0. [DOI] [PubMed] [Google Scholar]
- 25.Xie L., Jiang M., Dong X. Controlled mechanical and swelling properties of poly(vinyl alcohol)/sodium alginate blend hydrogels prepared by freeze-thaw followed by Ca2+ crosslinking. J. Appl. Polym. Sci. 2012;124:823–831. [Google Scholar]
- 26.Leong J.Y., Lam W.H., Ho K.W. Advances in fabricating spherical alginate hydrogels with controlled particle designs by ionotropic gelation as encapsulation systems. Particuology. 2016;24:44–60. [Google Scholar]
- 27.Sachan N.K., Pushkar Seema, Antesh Jha A.B. Sodium alginate : the wonder polymer for controlled drug delivery. J. Pharm. Res. 2009;2:1191–1199. [Google Scholar]
- 28.Cheng B., Li D., Huo Q. Two kinds of ketoprofen enteric gel beads (CA and CS-SA) using biopolymer alginate. Asian J. Pharm. Sci. 2018;13:120–130. doi: 10.1016/j.ajps.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hua S., Ma H., Li X. pH-sensitive sodium alginate/poly(vinyl alcohol) hydrogel beads prepared by combined Ca2+ crosslinking and freeze-thawing cycles for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2010;46:517–523. doi: 10.1016/j.ijbiomac.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Korsmeyer R.W., Gurny R., Doelker E. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983;15:25–35. [Google Scholar]
- 31.Ritger P.L., Peppas N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release. 1987;5:23–36. [PubMed] [Google Scholar]
- 32.Ritger P.L., Peppas N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release. 1987;5:37–42. [PubMed] [Google Scholar]
- 33.Peppas N.A., Sahlin J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989;57:169–172. [Google Scholar]