Abstract
Atypical chronic myeloid leukemia (aCML), BCR-ABL1 negative is a rare myelodysplastic syndrome/myeloproliferative neoplasm for which no current standard of care exists. The blood smear of patients with aCML showed prominent immature granulocytosis, and granulocytic dysplasia. We admitted a 58-year-old man with splenomegaly, hyperleukocytosis, anemia, and thrombocytopenia; then cytology, cytogenetic and molecular biology analysis of bone morrow were performed and the diagnosis of aCML was made according to 2016 World Health Organization diagnostic criteria. The patient was initially treated by chemotherapy; the patient achieved an aggravation of anemia. This motivated the change of treatment.
Key Words: Atypical chronic myeloid leukemia, World Health Organization, Myelodysplastic syndroms/myeloproliferative syndroms, BCR-ABL1, Philadelphia chromosome, Cytology, Karyotype, Molecular biology
1. Introduction
Atypical chronic myeloid leukemia (aCML) is a subtype of myelodysplastic syndroms/myeloproliferative syndroms diseases (MDS/MPN), the incidence rate about 1/100,000 or less [1]. Patients present in the 7th or 8th decade of life with leukocytosis, splenomegaly, frequent anemia and variable platelet counts. The aCML is defined largely by morphologic criteria by circulating myeloid precursors, obvious dysgranulopoiesis, cytogenetic and molecular studies are negative for Ph1 and BCR-ABL A fusion gene. Overall, aCML is generally associated staggeringly with poor prognosis: median survival less is than 2 years, with 20–40% chance of evolving to acute myeloid leukemia [2]. The challenges of aCML relate to its heterogeneous clinical and genetic features, high rate of transformation to acute myeloid leukemia, and historically poor survival. In this report we summarize our experience with a patient diagnosed with aCML revealed by dermohypodermitis.
2. Case report
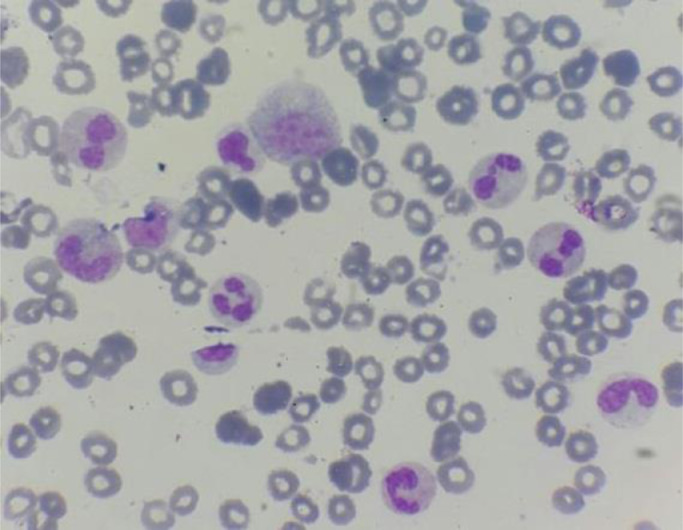
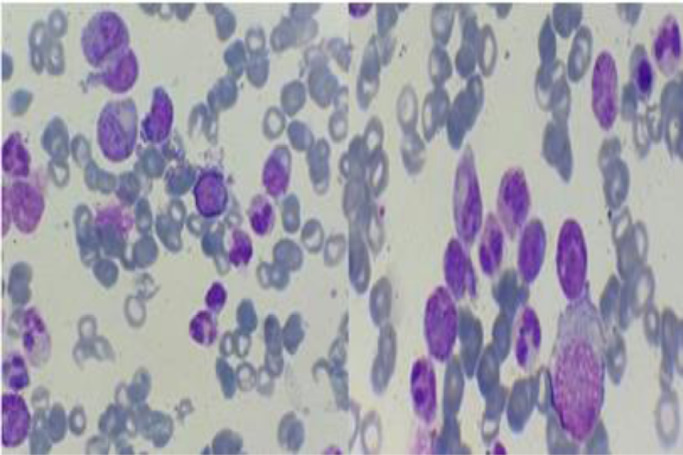
We present a case about a man in 58 year-old, with a negative past medical history. In the last 2 months, the patient reported increasing fatigue, and describes inflammatory skin lesion on left temple. Physical examination revealed pallor and mild splenomegaly, without tumor syndrom. Laboratory examinations showed hyperleukocytosis with Wight Blood Cell WBC 56.8 × 109/L (reference range, 4–10 × 109/L), thrombocytopenia with Platelets 112 × 109/L (reference range, 120–400 × 109/L), and hemoglobin of 93 g/L (reference range, 130–180 g/L). Manual differential count revealed 60% neutrophiles, 16% metamyelocytes, 8% myelocytes, 2% promyelocytes; 1% Blasts, 3% monocytes, 1% basophilic granulocytes, 2% Eosinophilic granulocytes, 7% lymphocytes, with dysgranulopoisesis showed abnormal nuclear segmentation: hypolobation nuclear, hypersegmentation nuclear, Pseudo Pelger-Huet and hypogranular granulocytes (Fig. 1). Then the Bone Marrow Aspirate (BMA) was performed: morphology of BMA showed a hypercellular marrow, with myeloid hyperplasia to 73%, including: myeloblasts 3%, promyelocytes 5%, myelocytes 12%, metamyelocytes 20%, neutrophilic granulocytes 28%, eosinophils 5%, basilophiles 0%, and 4% monocytes, with subtle dysgranulopoiesis (Fig. 2). Cytogenetic analysis revealed a normal karyotype: 46, XY. Molecular biology analysis of BCR/ABL fusion gene negative. The histopathological study of skin lesion from the left temple showed polymorphic dermohypodermitis, with negative immunohistochemical complement: CD3 and CD 20. The patient was diagnosed with aCML and treated with hydroxurea (2 capsules of 500 mg/j × 21 days). The evolution was marked by the fall of the white blood cells (57 G/L to 24 G/L), and the moderate reduction of the size of the spleen. However, there was an aggravation of anemia (from 9.5 g/dL to 8 g/dL) which motivated the change of treatment and the patient was put on azacytidine 75 mg/m2/j × 1 to 7 days;every 28 days. HLA typing is also underway to search for potential donors for hematopoietic stem cell transplantation (HSCT).
Fig. 1.
Blood smear of the case report- dysgranulopoiesis: hypogranular granulocytes, hypolobation nuclear, and Pseudo Pelger-Huet.
Fig. 2.
Spinal smear of the case report.
3. Discussion
Atypical chronic myeloid leukemia is a clonal hematopoietic disorder characterized by both dysplastic and proliferative features, including persistent granulocytosis with left shift, bone marrow hypercellularity with dysplastic hematopoiesis, and myeloid preponderance; aCML is a disorder of older adults with apparently no sex predominance [2]. The aCML is a rare myeloid neoplasm with an incidence of 1–2 cases for every 100 cases of Philadelphia-positive CML. Clinical manifestations, similar to those of myeloproliferative syndrom, are related to anemia, thrombocytopenia, and splenomegaly. The etiology and pathogenesis of aCML are not known. The 2016 WHO diagnostic criteria for aCML relies on: 1)Peripheral blood leukocytosis (WBC ≥ 13 × 109/L) due to increased number of neutrophils and their precursors ≥ 10%, 2) Dysgranulopoiesis (abnormal chromatin clumbing). 3) Basophils < 2% of leukocytes, No or minimal absolute basophilia 4) Monocytes < 10% of leukocytes, No or minimal absolute monocytosis 5) Hypercellular BM with granulocytic proliferation and granulocytic, dysplasia, with or without dysplasia in the erythroid and megakaryocytic lineages 6) <20% blasts in the blood and BM; 7) No Philadelphia chromosome or BCR-ABL fusion gene. 8) No rearrangement of PDGFRA, PDGFRB, FGFR1 rearrangement or JAK2 [3]. This patient meets the aCML diagnostic criteria. He has mild splenomegaly and normal chromosomes, negative BCR-ABL fusion gene, peripheral blood monocytes <1.0 × 109/L, myeloid precursor in the peripheral blood is 30%, with 60% neutrophils, therefore, it can be excluded the diagnosis of Chronic Myeloid Leukemia (CML), Chronic Myelomonocytic Leukemia (CMML) and chronic neutrophilic leukemia (CNL). So the diagnosis of aCML was correct.
No specific chromosomal changes correlations with disease features were found in aCML patients. The most common karyotypic changes reported in aCML include trisomy 8 and del (20q), abnormalities involving other chromosomes such as 12, 13, 14, 17, 19 and 21 have also been described [4]. The etiology and pathogenesis of aCML are not known, many gene mutations have been found in aCML, such as, NRAS/KRAS (33% patients), TET2 (33% patients) CBL (10% patients), E2H2 (13% patients) and SETBP1 (25% patients) and so on [5]. In this case, the patient has normal chromosome. The syndrome of abnormal chromatin clumping, which has long been recognized as representing a hematologic neoplasm with both myelodysplastic and myeloproliferative features is, in most cases, simply a morphologic variant of aCML; the risk of leukemic transformation is approximately 30% [6].The majority of patients die of BM failure.
Atypical CML patients showed poor prognosis when treated with conventional chemotherapy, the median survival is only 24 to 25 months [7]. A retrospective study in MD Anderson Cancer Center found that: Age (>65 years), anemia (hemoglobin < 10 g/dL), white blood cell count (WBC > 50 × 109/L) are three independent factors in aCML [7]. Treatment options such as AML induction type chemotherapy followed by an allogeneic stem cell transplantation are an option for only a minority of patients, but are the only modality that offers a cure. Koldehoff et al. [8] confirmed that allogeneic hematopoietic stem cell transplantation (HSCT) evaluated the outcome of aCML. Our candidate have included Hydroxyurea, that is converted to a free radical at the active site of the M2 protein subunit of ribonucleotide reductase, inactivating the enzyme, and DNA synthesis is selectively inhibited, producing cell death in S phase and synchronization of the fraction of cells that survive [9]. Our patient has an aggravation of anemia which the changement of treatment was motivated. 5-Azacytidine, in particular, has been shown to be effective in all subtypes of MDS to prolong the time to disease progression and is the only drug in this group that has been licensed by the US Food and Drug Administration (FDA) for all subtypes of MDS [10].
4. Conclusion
In conclusion, aCML is difficult to distinguish from other subtypes of MPS (CML, CNL and CMML), which is easy to misdiagnosis clinically. The presence of a granulocytic proliferation associated with marked dysgranulopoiesis and the absence of BCR-ABL1 translocation are the defining features of aCML. The success of azacytidine in improving overall survival of high-risk patients with MDS makes it worth studying in aCML cases. The only known curative therapy involves stem cell transplantation for aCML, but that carries high risks of toxicity and morbidity.
CRediT authorship contribution statement
Jihane Belkhair: Validation, Writing - original draft, Supervision. Abderahim Raissi: Validation, Writing - original draft, Supervision. Hicham Elyahyaoui: Validation, Writing - original draft, Supervision. Mustapha Ait Ameur: Validation, Writing - original draft, Supervision. Mohamed Chakour: Validation, Writing - original draft, Supervision.
Declaration of Competing Interest
The authors declare no competing financial interests.
Acknowledgments
Acknowledgment
We thank Dr A. Raissi for her cooperation to drafting the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lrr.2019.100172.
Appendix. Supplementary materials
References
- 1.Cazzola M., Malcovati L., Invernizzi R. Myelodysplastic/myeloproliferative neoplasms. hematology. Am. Soc. Hematol. Educ. Program. 2011;2011(1):264–272. doi: 10.1182/asheducation-2011.1.264. [DOI] [PubMed] [Google Scholar]
- 2.Faramarz N. first ed. Academic presse; Cambridge, Massachusetts: 2013. Atlas of Hematopathology Morphology, Immunophenotype, Cytogenetics, and Molecular Approaches. [Google Scholar]
- 3.Arber D.A., Orazi A., Hasserjian R. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood J. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 4.Efferi A., Gilliland G. Classification of chronic myeloid disorders: from Dameshek towards a semi-molecular system. Best Pract. Res. Clin. Haematol. 2006;19(3):365–385. doi: 10.1016/j.beha.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Piazza R., Valletta S., Winkelmann N., Redaelli S., Spinelli R. Recurrent SETBP1 mutations in atypical chronic myeloid leukemia. Nat. Genet. 2013;45(1):18–24. doi: 10.1038/ng.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czader M., Orazi A. second ed. Churchill Livingstone; London: 2011. Blood and Bone Marrow Pathology. [Google Scholar]
- 7.Onida F., Ball G., Kantarjian H.M., Smith T.L., Glassman A. Characteristics and outcome of patients with Philadelphia chromosome negative, bcr/abl negative chronic myelogenous leukemia. Cancer. 2002;95(October, 8):1673–1684. doi: 10.1002/cncr.10832. [DOI] [PubMed] [Google Scholar]
- 8.Koldehoff M., Beelen D.W., Trenschel R., Steckel N.K., Peceny R. Outcome of hematopoietic stem cell transplantation in patients with atypical chronic myeloid leukemia. Bone Marrow Transpl. 2004;34(December, 12):1047–1050. doi: 10.1038/sj.bmt.1704686. [DOI] [PubMed] [Google Scholar]
- 9.Yarbro J.W. Mechanism of action of hydroxyurea. Semin. Oncol. 1992;19(June, 3 Suppl 9):1. - [PubMed] [Google Scholar]
- 10.Kaminskas E., Farrell A.T., Wang Y.C., Sridhara R., Pazdur R. FDA drug approval summary: azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist. 2005;10(. March, 3):176–182. doi: 10.1634/theoncologist.10-3-176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.