Abstract
Introduction
Dialysis patients are frequently advised to restrict fruit and vegetable intake due to their high potassium content. This study aimed to evaluate the association between dietary fiber intake and major adverse cardiovascular events (MACE) among dialysis patients.
Methods
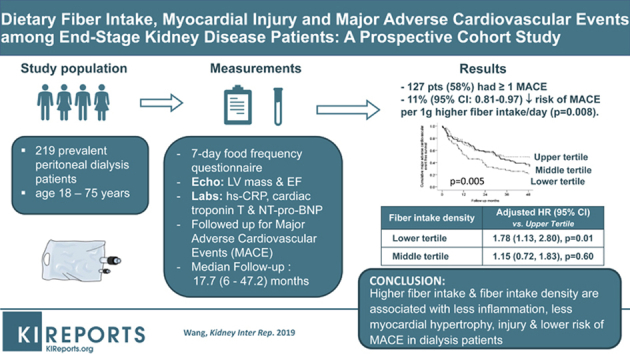
A total of 219 prevalent dialysis patients were prospectively recruited from a major university teaching hospital and regional dialysis center in Hong Kong. Dietary fiber intake estimated using a 7-day locally validated food frequency questionnaire was examined in relation to a primary composite outcome of MACE over a follow-up period of 4 years.
Results
A total of 127 patients were complicated with 1 or more MACE. In the multivariable Cox regression analysis, every 1 g higher fiber intake, and every 1 g/d per 1000 kcal higher fiber intake density were associated with an 11% (95% confidence interval [CI]: 0.81–0.97) and a 13% lower risk of MACE (95% CI: 0.77–0.99), respectively, independent of clinical, demographic, biochemical, hemodynamic, adequacy parameters, dietary protein, energy intake, inflammatory, and cardiac markers. Patients in the lower tertile of fiber intake density showed an increased hazard for MACE (adjusted hazard ratio: 1.78; 95% CI: 1.13–2.80) than those in the upper tertile.
Conclusion
Higher fiber intake and higher fiber intake density may be associated with less inflammation, less myocardial hypertrophy, injury, and lower risk of MACE in dialysis patients. These data form an important basis for a randomized controlled trial to examine fiber supplementation on cardiovascular outcomes in the dialysis population.
Keywords: dialysis, fiber intake, major adverse cardiovascular events
Graphical abstract
Diet has long been implicated in playing an important etiologic role in atherosclerosis and cardiovascular disease in the general population.1, 2 Numerous epidemiologic studies in the general population have reported a strong, inverse relationship between dietary fiber intake and risk of coronary heart disease, with higher fiber intake associated with a 20% to 40% risk reduction in coronary disease.3, 4, 5, 6 Recent data from US general population cohorts showed that increased fiber intake from pre– to post–myocardial infarction was significantly associated with a 31% lower risk of all-cause mortality.7
Dietary fiber intake may modify cardio-metabolic risk profile by delaying absorption of carbohydrates, increasing satiety, and ameliorating postprandial hyperglycemia, enhancing peripheral insulin sensitivity, and lowering low-density lipoprotein-cholesterol.8, 9, 10 However, the importance of fiber intake among patients with chronic kidney disease (CKD) is underrecognized. Patients with advanced CKD are frequently advised to restrict intake of fruits, green leafy vegetables, and whole-grain cereals due to their high potassium content, and risk of hyperkalemia and hyperphosphatemia, respectively. Fiber intake in patients with advanced CKD is therefore generally reduced compared with that of the general population.11 Recent data from the National Health and Nutrition Examination Survey suggested that the relationship between total fiber intake with inflammation and mortality appeared much stronger in those with kidney disease than those without kidney disease,12 providing preliminary evidence that more emphasis should perhaps be placed on encouraging intake of this specific component, “fiber,” in the diet of patients with CKD.
In this study, we aimed to evaluate the association between dietary fiber intake and risk of MACE in a prospective dialysis cohort. We also tested the associations, if any, between fiber intake and various inflammatory and cardiac biomarkers.
Concise Methods
Study Design and Study Participants
We performed a prospective longitudinal cohort study in 219 patients with CKD stage 5D, age between 18 and 75 years, who had received chronic dialysis treatment for at least 3 months from a total peritoneal dialysis population of 240 in a single regional dialysis center and a major university hospital in Hong Kong from 1999 to 2001. Twenty-one patients were excluded based on the exclusion criteria, which included patients with ongoing systemic inflammatory disease, such as systemic lupus erythematosus, patients with underlying malignancies, chronic obstructive pulmonary disease, or active tuberculous infection still undergoing treatment, as these conditions may influence dietary intake. Approval was obtained from the institutional research ethics committee of the university to conduct this study. Informed consent was obtained from all patients before participating in the study. At study baseline, all eligible patients underwent dietary assessment, echocardiographic examination, clinical assessment, blood taking for various biochemical analyses, and assessment of dialysis adequacy. In hospitalized patients, all the assessments, including dietary assessment, were deferred for at least 1 month after complete resolution of the acute complication.
Dietary Assessment
All patients underwent a 7-day food frequency questionnaire that has been validated in our local Chinese population13 to estimate average daily energy, protein, and total fiber intake. In brief, the questionnaire consisted of 253 food items in the following 7 categories: bread/pasta/rice (16 items), vegetables (63 items), fruits (26 items), meat (39 items)/fish (31 items)/eggs (5 items), beverages (37 items), dimsum/snacks (39 items), soups (10 items), and oil/salt/sauces. Items chosen were those most frequently consumed, based on previous local surveys, and overlapped with some items of the questionnaire used in the Australian Chinese Dietary Survey.14 Wherever possible, individuals were told before the visit that a survey on a week’s diet would be carried out and were advised to record at home to help the interview. On the day of the interview, individuals were asked to complete the questionnaire by recording their diet for the week preceding the interview as follows: the food item, the size of the portion, and the number of times of consumption each day and each week. Portion size was explained to individuals with the aid of photographs to illustrate individual food portion size, using bowls with a volume of 240 ml and plates 17.5-cm in diameter. Nutrient quantity was calculated using food tables from McCance and Widdowson (Holland et al.15) and 2 Chinese food tables16, 17 in which validation had been carried out using chemical analysis. Data were cross-checked by examining the dietary pattern (for example, if meals were skipped) to see if it corresponded to the number of times staple foods such as rice or noodles were consumed over the 1-week period. The dietary questionnaire we used in this study has been validated in the Chinese population and the validity of the nutrient quantitation was examined by comparing calculated 24-hour intake of total energy, sodium, and potassium, with estimated value of energy expenditure (basal metabolic rate × 1.4) and 24-hour urinary sodium and potassium outputs. All the food frequency questionnaires were administered by a group of trained research assistants with experience in performing dietary interviews and blinded to clinical and biochemical details of all subjects. Data from the food frequency questionnaire were input into computer format and analyzed. Dietary energy, protein, and total fiber intake were normalized to patients’ actual dry weight and fiber intake density was daily fiber intake normalized per 1000-kcal energy intake.
Echocardiographic Examination
Two-dimensional echocardiography was performed using a GE-VingMed System 5 echocardiographic machine (GE-VingMed Sound AB, Horten, Norway) with a 3.3-mHz multiphase array probe in subjects lying in the left decubitus position by a single experienced cardiologist blinded to all clinical, biochemical, and dietary details of patients. All echocardiographic data were analyzed according to the guidelines of the American Society of Echocardiography.18 Ejection fraction was obtained using a modified biplane Simpson’s method from apical 2- and 4-chamber views.19
Biochemical Analysis
Fasting venous blood samples were collected for measurement of blood hemoglobin, serum urea, creatinine, calcium, phosphate, intact parathyroid hormone, high-sensitivity C-reactive protein (hs-CRP), albumin, cholesterol, triglyceride, fetuin-A, N-terminal pro-brain natriuretic peptide (NT-pro-BNP), and cardiac troponin. Intact parathyroid hormone was measured by Immulite 1000 Analyzer (Siemens Healthcare Diagnostics, Deerfield, IL). hs-CRP was measured using the Tina-quant C-reactive protein latex ultra-sensitive assay. Albumin was measured using the bromcresol purple method, and total cholesterol and triglyceride were measured by the Hitachi 911 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). High-density lipoprotein-cholesterol was measured by the precipitation of Apo B containing lipoproteins with phosphotungstate, and low-density lipoprotein-cholesterol was calculated using the Friedewald formula. Serum fetuin-A was determined using a human fetuin-A enzyme-linked immunosorbent assay kit (Epitope Diagnostics, San Diego, CA). NT-pro-BNP and cardiac troponin T were quantified by electrochemiluminescence immunoassay on the Elecsys 2010 analyzer (Roche Diagnostics, Indianapolis, IN).
Indices of Dialysis Adequacy
Twenty-four–hour urine and dialysate were collected for measurement of total, peritoneal dialysis, and residual kidney clearance of urea and creatinine using standard method.20 Glomerular filtration rate was calculated as an average of 24-hour urinary urea and creatinine clearance.21
Clinical Data Collection
A thorough medical history was taken at the time of study entry to obtain clinical and demographic data, including diabetes, smoking status, details of any previous history of angina, myocardial infarction with or without percutaneous coronary intervention or coronary artery bypass grafting, heart failure, previous stroke or transient ischemic attacks, intermittent claudication or other symptoms suggestive of peripheral vascular disease with or without history of amputation or revascularization, and use of different antihypertensive medications. Systolic and diastolic blood pressures were measured on every follow-up visit at 8-week intervals for 1 year preceding study baseline and were averaged to give the final reading.
Follow-up and Outcome Measures
All patients were prospectively followed up for 4 years from the day of baseline assessments at study entry or until death. No patient was lost to follow-up. The primary outcome examined was first MACE, which was defined a priori and the cohort was purposely assembled to examine this outcome. MACE included angina with electrocardiographically documented changes of myocardial ischemia, acute myocardial infarction, electrocardiographically documented arrhythmia, heart failure, or thromboembolic or hemorrhagic stroke, all of which were defined according to standard clinical criteria, peripheral vascular disease, or sudden cardiac death. Peripheral vascular disease was defined as the presence of intermittent claudication with angiographic or sonographic detection of ≥50% stenosis of the major arteries of the lower limb, with or without revascularization procedures, ischemic leg ulceration, gangrene with or without amputation, and aortic aneurysm. Sudden cardiac death was defined as unexpected natural death within 1 hour from the symptom onset and without any prior condition that would appear fatal.22, 23 The nature of the first MACE was provided by the attending physician and this information was retrieved from the computerized Clinical Management System of the Hong Kong Hospital Authority and the Renal Registry Database that keep detailed records of all hospitalization episodes and causes of death. In case of death out of hospital, family members were interviewed by telephone to ascertain the circumstances surrounding death. For patients who had multiple MACEs, survival analysis was limited to the first event.
Statistical Analysis
Continuous data were expressed as mean ± SD or median (interquartile range) depending on the distribution. Patients were stratified into tertiles according to daily dietary fiber intake and fiber intake densities. Between-group comparisons were done using 1-way analysis of variance or Kruskal-Wallis test for continuous data depending on data distribution and χ2 test for categorical data. Survival curves were generated by means of Kaplan-Meier estimates, and differences in MACE-free survival were compared by the log-rank test. In this analysis, patients who underwent kidney transplantation or were permanently transferred to hemodialysis were censored at the time of transfer to alternative renal replacement therapy. If a patient died within 3 months of transfer to hemodialysis, then he or she was not censored, as the MACE was considered to reflect the health status during the period of failing peritoneal dialysis treatment. To examine the effects of fiber intake and fiber intake density in predicting the first MACE, univariate and multivariable Cox regression models were fitted using covariates with P < 0.1 in the univariate analysis. Age and gender were included in the multivariable Cox regression models irrespective of their statistical significance. Statistical analysis was performed using SPSS version 20.0 for Windows Software (IBM Corp, Chicago, IL). A P value of less than 0.05 was considered statistically significant.
Results
Table 1 presents the baseline characteristics. The average daily fiber intake was 5.0 ± 2.5 g/d and was higher in men than women (5.5 ± 2.6 vs 4.4 ± 2.3 g/d; P = 0.001). However, fiber intake density did not differ between men and women (3.7 ± 1.4 and 3.5 ± 1.5g/1000 kcal/d; P = 0.5). We stratified patients by tertiles of fiber intake. Table 2 compared the characteristics of patients across tertiles of fiber intake density.
Table 1.
Baseline characteristics in relation to tertile stratification of dietary fiber intakea
| Variables | Total (n = 219) | Dietary fiber intake stratified in tertiles |
P for trend | ||
|---|---|---|---|---|---|
| Upper (n = 73) | Middle (n = 73) | Lower (n = 73) | |||
| Clinical and demographic | |||||
| Age (yr) | 556 ± 12 | 53 ± 13 | 56 ± 11 | 58 ± 10 | 0.009 |
| Men, n (%) | 109 (49.5) | 45 (61.6) | 35 (47.9) | 29 (39.7) | 0.3 |
| Diabetes mellitus, n (%) | 66 (30) | 22 (30.1) | 19 (26.0) | 25 (34.2) | 0.6 |
| Positive smoking history, n (%) | 54 (24.5) | 25 (34.2) | 13 (17.8) | 15 (20.5) | 0.5 |
| Body mass index (kg/m2) | 23.0 ± 3.4 | 23.1 ± 4.0 | 23.0 ± 3.0 | 23.0 ± 3.2 | 0.9 |
| Duration of dialysis (mo)b | 26.5 (15, 51) | 24 (12, 37.5) | 26 (16.5, 57.5) | 39 (16, 61.5) | 0.1 |
| Systolic BP (mm Hg) | 147 ± 17.2 | 144 ± 16 | 148 ± 18 | 148 ± 17 | 0.1 |
| Diastolic BP (mm Hg) | 82 ± 9 | 83 ± 10 | 82 ± 9 | 82 ±10 | 0.6 |
| Pulse pressure (mm Hg) | 64 ± 14 | 61 ± 12 | 66 ± 14 | 66 ± 15 | 0.02 |
| Hypertension, n (%) | 189 (85.9) | 61 (83.6) | 63 (86.3) | 64 (87.7) | 0.5 |
| Background AVD, n (%) | 52 (23.6) | 19 (26.0) | 12 (16.4) | 21 (28.8) | 0.7 |
| Coronary artery disease, n (%) | 44 (20) | 16 (21.9) | 11 (15.1) | 17 (23.3) | 0.8 |
| Echocardiographic | |||||
| LV mass index (g/m2) | 224 ± 85 | 207 ± 69 | 217 ± 81 | 247 ± 99 | 0.004 |
| LV volume index (ml/m2) | 66 ± 20 | 67 ± 22 | 63 ± 18 | 67 ± 21 | 0.2 |
| LV ejection fraction (%) | 53 ± 8 | 52 ± 10 | 53 ± 8 | 52 ± 8 | 0.2 |
| Dialysis | |||||
| Total weekly urea clearance | 1.81 ± 0.44 | 1.76 ± 0.46 | 1.87 ± 0.49 | 1.80 ± 0.36 | 0.6 |
| PD urea clearance | 1.53 ± 0.35 | 1.4 ± 0.4 | 1.6 ± 0.3 | 1.6 ± 0.3 | <0.001 |
| Renal urea clearance | 0.28 ± 0.41 | 0.38 ± 0.42 | 0.29 ± 0.50 | 0.16 ± 0.26 | 0.001 |
| Total weekly CrCl (l/wk per 1.73 m2) | 56.3 ± 21.6 | 61.1 ± 26.7 | 56.6 ± 22.9 | 51.2 ± 11.0 | 0.005 |
| PD CrCl (l/wk per 1.73 m2) | 39.8 ± 8.16 | 36.1 ± 7.21 | 40.3 ± 8.03 | 42.8 ± 7.74 | <0.001 |
| Renal CrCl (l/wk per 1.73 m2) | 16.5 ± 23.1 | 25.1 ± 27.9 | 16.3 ± 23.6 | 8.39 ± 12.2 | <0.001 |
| Normalized PCR (g/kg per d)b | 0.94 (0.82, 1.08) | 0.99 (0.85, 1.10) | 0.94 (0.86, 1.09) | 0.88 (0.78, 1.03) | 0.03 |
| % with RRF | 134 (60.9) | 57 (78.1) | 44 (60.3) | 32 (43.8) | <0.001 |
| Residual GFR (ml/min/1.73m2)b | 0.55 (0.00, 1.89) | 0.92 (0.17, 2.75) | 0.44 (0.0, 1.76) | 0.00 (0.00, 1.06) | <0.001 |
| Biochemical | |||||
| Hemoglobin (g/dl) | 9.18 ± 1.71 | 9.77 ± 2.01 | 8.92 ± 1.36 | 8.85 ± 1.54 | 0.001 |
| Serum albumin (g/dl) | 28.6 ± 5.2 | 29.7 ± 5.6 | 28.3 ± 5.4 | 27.7 ± 4.3 | 0.02 |
| Serum potassium (mmol/l) | 4.01 ± 0.62 | 4.16 ± 0.59 | 4.07 ± 0.62 | 3.80 ± 0.58 | 0.001 |
| Plasma calcium (mmol/l) | 2.56 ± 0.23 | 2.55 ± 0.22 | 2.55 ± 0.25 | 2.57 ± 0.21 | 0.7 |
| Plasma phosphate (mmol/l) | 1,67 ± 0.46 | 1.71 ± 0.50 | 1.69 ± 0.43 | 1.61 ± 0.47 | 0.2 |
| Intact PTH (pmol/l)b | 42.4 (18.7, 74.1) | 40.6 (18.9, 67.7) | 36.3 (16.7, 64.7) | 48.9 (19.9, 82.9) | 0.2 |
| Total cholesterol (mmol/l) | 5.39 ± 1.19 | 5.32 ± 1.07 | 5.60 ± 1.27 | 5.25 ± 1.22 | 0.06 |
| HDL-cholesterol (mmol/l) | 1.24 ± 0.42 | 1.19 ± 0.35 | 1.27 ± 0.48 | 1.25 ± 0.43 | 0.4 |
| LDL-cholesterol (mmol/l) | 2.18 ± 0.98 | 3.33 ± 0.90 | 3.41 ± 0.98 | 3.09 ± 1.05 | 0.2 |
| Triglyceride (mmol/l) | 2.08 ± 1.48 | 1.87 ± 0.99 | 2.24 ± 1.90 | 2.14 ± 1.40 | 0.3 |
| Hs-CRP (mg/l)b | 2.90 (0.90, 0.98) | 1.39 (0.77, 5.43) | 3.0 (0.95, 8.08) | 4.28 (1.25, 11.4) | 0.006 |
| Interleukin-6 (pg/ml)b | 9.7 (5.2, 18.1) | 6.9 (4.5, 14.6) | 10.7 (5.4, 18.6) | 10.3 (7.0, 18.80 | 0.05 |
| Fetuin-A (g/l) | 0.31 ± 0.07 | 0.32 ± 0.07 | 0.31 ± 0.06 | 0.29 ± 0.06 | 0.003 |
| Cardiac troponin T (μg/l)b | 0.06 (0.01, 0.15) | 0.03 (0.01, 0.09) | 0.03 (0.01, 0.15) | 0.1 (0.02, 0.18) | 0.002 |
| NT-pro-BNP (pg/ml)b | 5842 (1950, 17,533) | 3730 (1631, 13,304) | 4798 (1717, 13,872) | 9366 (2640, 35,000) | 0.02 |
| Dietary intake | |||||
| Total fiber intake (g/d) | 5.0 ± 2.5 | 7.7 ± 2.0 | 4.8 ± 0.7 | 2.5 ± 0.8 | <0.001 |
| Energy intake (kcal/kg per day) | 24.6 ± 8.8 | 29.9 ± 8.8 | 24.5 ± 7.3 | 19.6 ± 6.9 | <0.001 |
| Protein intake (g/kg per day) | 1.1 ± 0.5 | 1.3 ± 0.5 | 1.1 ± 0.4 | 0.9 ± 0.4 | <0.001 |
AVD, atherosclerotic vascular disease; BP, blood pressure; CrCl, creatinine clearance; GFR, glomerular filtration rate; HDL, high-density lipoprotein; hs-CRP, high sensitive C-reactive protein; LDL, low-density lipoprotein; LV, left ventricular; NT-pro-BNP, N-terminal pro-brain natriuretic peptide; PCR, protein catabolic rate; PD, peritoneal dialysis; PTH, parathyroid hormone; RRF, residual renal function.
Dietary fiber intake >5.88 g/d as upper tertile (reference group), 3.71 g to 5.88 g/d as middle tertile, and <3.71 g/d as lower tertile.
Continuous data are expressed as mean ± SD unless specified otherwise.
Median (interquartile range).
Table 2.
Baseline characteristics in relation to tertile stratification of fiber intake densitya
| Variables | Fiber intake density stratified by tertile |
P for trend | ||
|---|---|---|---|---|
| Upper (n = 73) | Middle (n = 73) | Lowest (n = 73) | ||
| Clinical and demographic | ||||
| Age (yr) | 54.4 ± 12.0 | 55.4 ± 11.9 | 56.8 ± 11.1 | 0.5 |
| Men, n (%) | 36 (51.4) | 38 (50.7) | 35 (47.3) | 0.9 |
| Diabetes mellitus, n (%) | 22 (31.4) | 26 (34.7) | 18 (24.3) | 0.4 |
| Positive smoking history, n (%) | 18 (25.7) | 19 (25.3) | 16 (21.6) | 0.9 |
| Body mass index (kg/m2) | 23.6 ± 4.05 | 22.5 ± 2.87 | 23.1 ± 3.17 | 0.1 |
| Duration of dialysis (mo)b | 24 (13, 27) | 25 (13, 50) | 39 (19, 60) | 0.03 |
| Systolic BP (mm Hg) | 144 ± 16 | 149 ± 18 | 146 ± 17 | 0.3 |
| Diastolic BP (mm Hg) | 81 ± 10 | 84 ± 10 | 81 ± 9 | 0.1 |
| Pulse pressure (mm Hg) | 63 ± 13 | 65 ± 14 | 65 ± 14 | 0.7 |
| Hypertension, n (%) | 56 (80) | 66 (88) | 66 (89.2) | 0.2 |
| Background AVD, n (%) | 18 (25.7) | 19 (25.3) | 15 (20.3) | 0.7 |
| Coronary artery disease, n (%) | 17 (24.3) | 15 (20) | 12 (16.2) | 0.5 |
| Echocardiographic | ||||
| LV mass index (g/m2) | 218 ± 75.6 | 202 ± 74 | 251 ± 97.6 | 0.002 |
| LV volume index (ml/m2) | 63.3 ± 19.4 | 65.4 ± 20.6 | 68.4 ± 21.3 | 0.3 |
| LV ejection fraction (%) | 52.5 ± 8.9 | 52.2 ± 8.38 | 52.4 ± 7.59 | 1.0 |
| Dialysis | ||||
| Total weekly urea clearance | 1.74 ± 0.39 | 1.86 ± 0.40 | 1.83 ± 0.50 | 0.2 |
| PD urea clearance | 1.46 ± 0.34 | 1.55 ± 0.36 | 1.57 ± 0.35 | 0.1 |
| Renal urea clearance | 0.28 ± 0.38 | 0.31 ± 0.35 | 0.25 ± 0.49 | 0.4 |
| Total weekly CrCl (l/wk per 1.73 m2) | 57.8 ± 25.7 | 57.6 ± 19.5 | 53.5 ± 19.3 | 0.4 |
| PD CrCl (l/wk per 1.73 m2) | 38.5 ± 7.18 | 38.9 ± 8.51 | 41.7 ± 8.30 | 0.04 |
| Renal CrCl (l/wk per 1.73 m2) | 19.3 ± 27.1 | 18.7 ± 20.4 | 11.9 ± 21.3 | 0.1 |
| Normalized PCR (g/kg per day)b | 0.91 (0.81, 1.08) | 0.96 (0.87, 1.07) | 0.93 (0.79, 1.09) | 0.5 |
| % with RRF | 44 (62.9) | 55 (73.3) | 34 (45.9) | 0.04 |
| Residual GFR (ml/min per 1.73 m2)b | 0.91 (0.81, 1.08) | 0.96 (0.87, 1.07) | 0.93 (0.79, 1.09) | 0.01 |
| Biochemical | ||||
| Hemoglobin (g/dl) | 9.45 ± 1.90 | 9.20 ± 1.58 | 8.92 ± 1.62 | 0.2 |
| Serum albumin (g/dl) | 28.7 ± 5.10 | 29.1 ± 5.81 | 27.9 ± 4.46 | 0.4 |
| Plasma potassium (mmol/l) | 4.15 ± 0.60 | 4.06 ± 0.61 | 3.82 ± 0.59 | 0.004 |
| Plasma calcium (mmol/l) | 2.56 ± 0.23 | 2.52 ± 0.20 | 2.60 ± 0.24 | 0.08 |
| Plasma phosphate (mmol/l) | 1.73 ± 0.53 | 1.61 ± 0.39 | 1.69 ± 0.46 | 0.3 |
| Intact PTH (pmol/l)b | 42.8 (18.6, 62.8) | 33.6 (13.1, 61.2) | 50.7 (20.5, 53.9) | 0.6 |
| Total cholesterol (mmol/l) | 5.53 ± 1.22 | 5.30 ± 1.16 | 0.36 ± .1.21 | 0.5 |
| HDL-cholesterol (mmol/l) | 1.16 ± 0.35 | 1.25 ± 0.47 | 1.30 ± 0.43 | 0.2 |
| LDL-cholesterol (mmol/l) | 3.50 ± 0.96 | 3.17 ± 0.94 | 3.17 ± 1.03 | 0.08 |
| Triglyceride (mmol/l) | 2.13 ± 1.74 | 2.10 ± 1.46 | 2.03 ± 1.22 | 0.9 |
| hs-CRP (mg/l)b | 1.99 (0.78, 7.91) | 1.75 (0.80, 5.71) | 3.86 (1.24, 11.2) | 0.09 |
| Interleukin-6 (pg/ml)b | 8.05 (4.05, 18.15) | 9.30 (5.30, 15.90) | 10.2 (6.80, 19.20) | 0.1 |
| Fetuin-A (g/l) | 0.32 ± 0.06 | 0.31 ± 0.06 | 0.30 ± 0.08 | 0.6 |
| Troponin (μg/l)b | 0.03 (0.01, 0.14) | 0.03 (0.01, 0.12) | 0.08 (0.03, 0.17) | 0.01 |
| NT-pro-BNP (pg/ml)b | 4455 (1722, 18,249) | 5156 (2165, 13,395) | 7973 (1894, 28,291) | 0.3 |
| Dietary intake | ||||
| Fiber intake (g/d) | 6.94 ± 2.53 | 5.02 ± 1.76 | 3.05 ± 1.48 | <0.001 |
| Fiber intake density (g/d per 1000 kcal energy intake) | 5.27 ± 1.07 | 3.46 ± 0.38 | 2.14 ± 0.55 | <0.001 |
| Energy intake (kcal/kg per day) | 22.9 ± 7.46 | 26.5 ± 9.54 | 24.4 ± 8.83 | 0.05 |
| Protein intake (g/kg per day) | 1.07 ± 0.41 | 1.16 ± 0.49 | 1.08 ± 0.44 | 0.4 |
BP, blood pressure; CrCl, creatinine clearance; GFR, glomerular filtration rate; HDL, high-density lipoprotein; hs-CRP, high sensitive C-reactive protein; LDL, low-density lipoprotein; LV, left ventricular; NT-pro-BNP, N-terminal pro-brain natriuretic peptide; PCR, protein catabolic rate; PD, peritoneal dialysis; PTH, parathyroid hormone; RRF, residual renal function.
Fiber intake density defined as daily fiber intake adjusted per 1000 kcal energy intake.
Fiber intake density >4.10 g/d per 1000 kcal energy intake as upper tertile (reference group), 2.90 to 4.10 and <2.90 g/d per 1000 kcal energy intake as middle and lower tertiles, respectively.
Continuous data are expressed as mean ± SD unless specified otherwise.
Median (interquartile range).
During the median follow-up of 17.7 (interquartile range, 6.0–47.2) months, 127 patients (58%) experienced 1 or more MACE. The nature of first MACE included acute coronary syndrome or acute myocardial infarction in 15 patients (6.8%), ischemic or hemorrhagic cerebrovascular event in 16 patients (7.3%), heart failure in 76 patients (34.7%), peripheral vascular disease in 5 patients (2.3%), electrocardiographically confirmed arrhythmia in 8 patients (3.7%), and sudden cardiac death in 7 patients (3.2%).
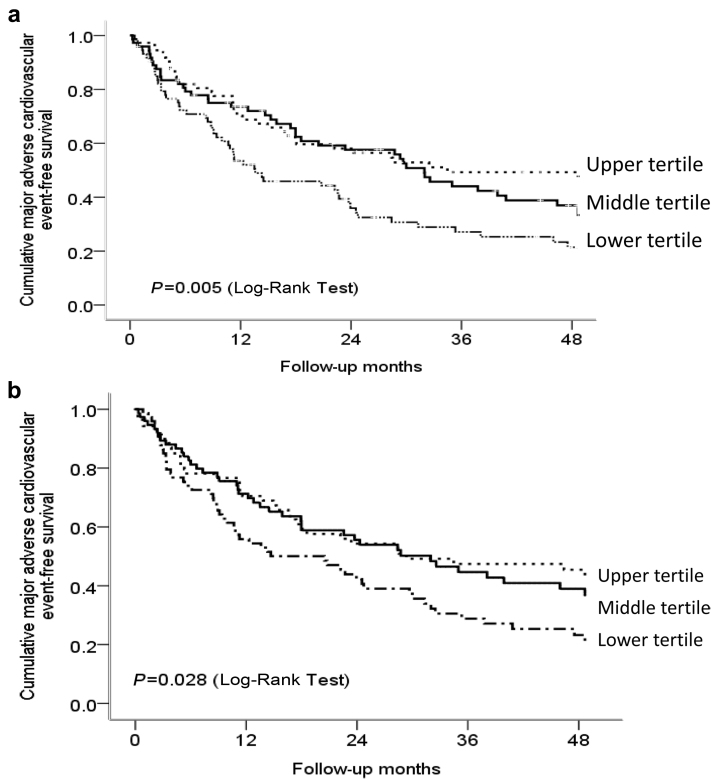
Supplementary Table S1 shows the univariate Cox regression analysis in relation to first MACE. Fiber intake (hazard ratio: 0.85; 95% CI: 0.78–0.92; P < 0.001) and fiber intake density (hazard ratio: 0.85, 95% CI: 0.74–0.97; P = 0.02) were associated with the development of first MACE. Compared with patients in the upper tertile of fiber intake density, those in the lower tertile were at 1.68-fold (95% CI: 1.09–2.58; P = 0.02) increase in the risk of developing first MACE. In the multivariable Cox regression analysis controlling for clinical, biochemical, dialysis, echocardiographic parameters, and cardiac biomarkers, fiber intake was significantly associated with first MACE. Every 1 g/d increase in fiber intake was associated with an 11% reduction (95% CI: 0.81–0.97) in the risk of developing MACE (Table 3). Its significance outweighed other cardiovascular risk factors, such as diabetes, background atherosclerotic vascular disease, left ventricular mass index, pulse pressure, serum albumin, hs-CRP, and residual glomerular filtration rate and was independent of protein and energy intake. Figure 1a shows the Kaplan-Meier estimates of cumulative MACE-free survival probabilities in relation to tertiles of fiber intake. Patients in the lower tertile had the lowest cumulative MACE-free survival probability among the 3 groups (P = 0.005).
Table 3.
Multivariable Cox regression analysis of dietary fiber intake in relation to first major adverse cardiovascular event
| Variables | Unit increase | Hazard ratio (95% confidence intervals) | P |
|---|---|---|---|
| Dietary fiber intake (g/d) | 1 g/d | 0.89 (0.81–0.97) | 0.008 |
| Serum albumin (g/dl) | 1 g/dl | 0.94 (0.89–0.98) | 0.005 |
| Diabetes mellitus | Yes | 1.79 (1.19–2.70) | 0.005 |
| Pulse pressure (mm Hg) | 1 mm Hg | 1.02 (1.01–1.03) | 0.007 |
| Background AVD | Yes | 1.73 (1.16–2.58) | 0.007 |
| Duration of dialysis (mo) | 1 mo | 0.99 (0.99–1.00) | 0.04 |
| Serum fetuin-A (μg/l) | 1 μg/l | 12.9 (0.64–261.6) | 0.09 |
| Cardiac troponin T (μg/l) | 1 μg/l | 2.60 (1.14–5.93) | 0.02 |
| NT-pro-BNP (pg/ml) | 1 pg/ml | 1.00 (1.00–1.00) | <0.001 |
| Left ventricular mass index (g/m2) | 1 g/m2 | 1.00 (1.00–1.01) | 0.06 |
AVD, atherosclerotic vascular disease; NT-pro-BNP, N-terminal-pro-brain natriuretic peptide.
Out of the model including age, gender, residual glomerular filtration rate, high-sensitivity C-reactive protein, interleukin-6, daily energy intake, and protein intake and hemoglobin.
Figure 1.
Cumulative major adverse cardiovascular event-free survival probabilities in relation to (a) dietary fiber intake stratified by tertiles and (b) fiber intake density stratified by tertiles. Dietary fiber intake >5.88 g/d as upper tertile (reference group), 3.71 g to 5.88 g/d as middle tertile, and <3.71 g as lower tertile. Fiber intake density defined as grams of daily fiber intake adjusted per 1000 kcal energy intake. Fiber intake density >4.10 g/d per 1000 kcal energy intake as upper tertile (reference group), 2.90 to 4.10, and <2.90 g/d per 1000 kcal energy intake as middle and lower tertiles, respectively.
Figure 1b shows the Kaplan-Meier estimates of cumulative MACE-free survival probabilities in relation to tertiles of fiber intake density. Those in the lower tertile of fiber intake density had the lowest MACE-free survival probabilities over 4 years as compared with those in the upper tertile (P = 0.028). In the multivariable Cox regression analysis considering the same clinical, biochemical, dialysis, echocardiographic parameters, and cardiac biomarkers, fiber intake density was associated with MACE both as a continuous variable (adjusted hazard ratio: 0.87; 95% CI: 0.77–0.99; P = 0.046) and when stratified into tertiles (Table 4). Compared with patients in the upper tertile of fiber intake density, those in the lower tertile showed an increased hazard (adjusted hazard ratio: 1.78; 95% CI: 1.13–2.80; P = 0.01) for MACE, independent of other cardiovascular risk factors, including diabetes mellitus, background atherosclerotic vascular disease, pulse pressure, serum albumin, hs-CRP, residual glomerular filtration rate, left ventricular mass index, duration of dialysis, cardiac troponin T, and NT-pro-BNP.
Table 4.
Multivariable Cox regression analysis of fiber intake density in relation to first major adverse cardiovascular event
| Variables | Unit increase | Hazard ratio (95% confidence intervals) | P |
|---|---|---|---|
| Serum albumin (g/dl) | 1 g/dl | 0.94 (0.90–0.98) | 0.004 |
| Diabetes mellitus | Yes | 1.89 (1.26–2.83) | 0.002 |
| Pulse pressure (mm Hg) | 1 mm Hg | 1.02 (1.01–1.04) | 0.003 |
| Background AVD | Yes | 1.77 (1.19–2.54) | 0.005 |
| Residual GFR (ml/min per 1.73 m2) | 1 ml/min per 1.73 m2 | 0.90 (0.80–1.00) | 0.06 |
| Duration of dialysis (mo) | 1 mo | 0.99 (0.98–0.99) | 0.02 |
| Cardiac troponin T (μg/l) | 1 μg/l | 2.86 (1.23–6.66) | 0.05 |
| NT-pro-BNP (pg/ml) | 1 pg/ml | 1.000 (1.000–1.000) | 0.003 |
| Left ventricular mass index (g/m2) | 1 g/m2 | 1.0 (1.00–1.01) | 0.08 |
| Fiber intake density (g/d adjusted per 1000 kcal energy intake) | |||
| vs upper tertile (ref group) | Lower tertile | 1.78 (1.13–2.80) | 0.01 |
| vs upper tertile (ref group) | Middle tertile | 1.15 (0.72–1.83) | 0.6 |
AVD, atherosclerotic vascular disease; GFR, glomerular filtration rate; NT-pro-BNP, N-terminal pro-brain natriuretic peptide.
Fiber intake density defined as daily fiber intake in grams per day adjusted per 1000 kcal energy intake.
Out of the model including age, gender, high-sensitivity C-reactive protein, fetuin-A, interleukin-6, and hemoglobin.
Discussion
In this study, we observed an important relationship between higher fiber intake and higher fiber intake density with a lower risk of MACE in a prospective dialysis cohort being followed for 4 years. Every 1 g higher daily fiber intake was independently associated with an 11% reduction in the risk of MACE. Every 1 g/d per 1000 kcal higher daily fiber intake density was independently associated with a 13% reduction in the adjusted risk of MACE. The magnitude of risk reduction associated with every gram increase of fiber intake and every g/d per 1000 kcal increase of fiber intake density appeared larger than that observed in the general population.24 Notably, the significance of higher fiber intake in predicting a lower risk of MACE outweighed other traditional and kidney disease–related cardiovascular risk factors or markers such as hypoalbuminemia; arterial stiffening; low fetuin-A; high hs-CRP, cardiac troponin T, and NT-pro-BNP; and left ventricular mass index. This observation is novel and extends the National Health and Nutrition Examination Survey data, showing that a higher dietary fiber intake was associated with a lower risk of all-cause mortality in those with CKD.12 There was some suggestion that higher dietary fiber intake may also retard kidney function decline, reduce proinflammatory factors and indoxyl sulfate and thus lower cardiovascular risk in advanced CKD.25
The general health benefits of dietary fiber are well recognized and have been quite extensively studied in the general population. Several general population cohorts showed that higher fiber intake was associated with a lower risk of cardiovascular disease and myocardial infarction.4, 26 In a systematic review of 12 prospective general population cohort studies, every 7 g/d higher fiber intake was associated with a 9% lower risk of coronary heart disease and cardiovascular disease.24 Our current observations add to the existing evidence, suggesting that increasing dietary fiber intake may have a similar cardiovascular protective benefit in the dialysis population and warrant further exploration.
Several biologically plausible mechanisms may explain the association between higher fiber intake and higher fiber intake density with a lower risk of MACE in dialysis patients. First, high fiber intake favorably modifies the gut microbiota populations, decreases gut dysbiosis, improves the integrity of intestinal barrier, and increases colonization of acetate-producing bacteria or saccharolytic forms of bacteria in the gut, leading to the release of short-chain fatty acids and acetate, into the circulation and reduces the generation of colon-derived uremic toxins including indoxyl sulfate and p-cresol sulfate from the metabolism of tryptophan and tyrosine, respectively.27, 28 Dietary fiber in the form of resistant starch has been shown to increase the ratio of Bacteroides to Firmicutes and reduce the generation of uremic solutes indoxyl sulfate and p-cresol sulfate in adenine-induced CKD rats.29 In keeping with these experimental data, vegetarians were noted to have at least 50% less production of indoxyl sulfate and p-cresol sulfate than in healthy individuals consuming an unrestricted diet.30 In a recent small randomized trial of hemodialysis subjects, resistant starch supplementation was associated with reduced plasma levels of colon-derived solutes indoxyl sulfate and possibly p-cresol sulfate compared with control subjects, without the need to intensify dialysis treatments.31 P-cresol sulfate and indoxyl sulfate have both been related to overall mortality and cardiovascular disease in the CKD and dialysis population.32, 33, 34
In this study, dialysis patients having higher fiber intake and higher fiber intake density were notably associated with less cardiac hypertrophy, lower hs-CRP and cardiac troponin T levels, and a trend toward lower NT-pro-BNP, suggesting a possible link between higher fiber intake with less myocardial injury and inflammation. A recent study from hypertensive mice showed that higher fiber diet and acetate supplementation significantly reduced systolic and diastolic blood pressures, cardiac fibrosis, and cardiac hypertrophy. Transcriptome analysis showed that the protective effects of high fiber and acetate were accompanied by downregulation of cardiac and renal Egr1, which is a master cardiovascular regulator involved in cardiac hypertrophy, fibrosis, and inflammation.35 Although this experimental data may not be directly translatable to the dialysis population, it adds important mechanistic insights on a possible link between higher fiber intake, modulation of gut microbiota, and regulation of cardiac hypertrophy and fibrosis and lends important support for the novel observations from this study. Reshaping the gut microbiota using dietary fiber may emerge as a novel, potentially therapeutic strategy for cardiovascular disease in CKD and dialysis patients and warrants further investigation.
The other biologically plausible mechanism that explains lower MACE risk with higher fiber intake and higher fiber intake density in dialysis patients may relate to the lower inflammatory response associated with higher fiber intake, as supported by the current finding. Several studies in the general population have reported a similar, inverse relationship between higher fiber intake and lower levels of inflammatory markers such as CRP.36, 37 In the National Health and Nutrition Examination Survey data, higher fiber intake was associated with lower inflammatory response and more so in those with CKD. Every 10 g/d increase in total fiber intake was associated with an 11% and 38% decrease in the odds of having an elevated CRP in patients without and with kidney disease, respectively.12 Having an elevated CRP was well recognized to be associated with an increased risk of mortality and cardiovascular death in the dialysis population.38 Contrary to data from the general population suggesting that increasing fiber intake may lower the risk of cardiovascular disease by improving cholesterol levels,24 this seems not to be the case in our dialysis patients, as we did not find any significant relations between fiber intake quantities and serum lipid profile.
The recommended daily dietary fiber intake of the general population is 25 g for adult women and 30 g for men by the American Dietetic Association,39 and 30 g by the Australia and New Zealand National Medical Research Council40 and British Nutrition Foundation.41 However, no recommendation has been specifically drawn for the CKD and dialysis population. In this study, the average daily fiber intake of our dialysis patients was only 5 g, which was alarmingly low. This finding may, however, not be surprising, as our patients are very often educated to restrict their intake of fruits, vegetables, and whole grains because of potassium and phosphate concerns. Further study is needed to define the recommended fiber intake in the CKD and dialysis population and whether the type of fiber may differ in terms of their effects on cardiovascular outcomes in this population.
Our study has several important strengths. First, it was prospectively designed with comprehensive clinical, biochemical, echocardiographic, and dialysis data collection; the outcomes were purposely defined a priori; and the study had a rather long follow-up duration with no loss to follow-up. Second, we quantified dietary fiber intake using a locally validated 7-day dietary questionnaire. Nevertheless, several limitations are worth mentioning. First, we were not able to make causal implications based on some of the cross-sectional associations observed and there could be reverse causality. That is, patients who had worse health condition and cardiac status may have poorer appetite and as a consequence, had lower overall intake and also less willing to eat a more diverse diet or a diet with higher fiber content. Second, our questionnaire quantified the total fiber intake without differentiating between soluble and insoluble fiber and without identifying the food source of fiber, that is, whether it is constituted by fruits, vegetables, resistant starch, cereals, or others. This may be important information to know as the effects of different types of fiber may not be the same. Third, dietary recall had the limitation of reporting bias and may under- or overestimate dietary intake and have measurement errors. Fourth, the use of self-report and 1-time assessment of diet could lead to misclassification bias. Because dietary intake and all the other parameters were assessed only once at baseline, we were not able to take into account changes over time. It is possible that patients’ diet at baseline may change during follow-up. Finally, there could be residual confounding not accounted for in the multivariable Cox regression analysis.
In conclusion, higher fiber intake and higher fiber intake density were independently associated with a lower risk of MACE in the dialysis population. Our novel findings call on the need for a large randomized trial to further explore this inexpensive but important lifestyle modification strategy for cardiovascular protection in the dialysis population.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was partly funded by the Health Services Research Fund (931009) of which Angela Yee-Moon Wang is the Principal Investigator, and the Bristol-Myers Squibb Mead Johnson Unrestricted Nutrition Grant of which Jean Woo is the Principal Investigator.
Footnotes
Table S1. Univariate Cox regression analysis in relation to first MACE.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Univariate Cox regression analysis in relation to first MACE.
References
- 1.Trowell H. Ischemic heart disease and dietary fiber. Am J Clin Nutr. 1972;25:926–932. doi: 10.1093/ajcn/25.9.926. [DOI] [PubMed] [Google Scholar]
- 2.Hu F.B., Willett W.C. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569–2578. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- 3.Pietinen P., Rimm E.B., Korhonen P. Intake of dietary fiber and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Circulation. 1996;94:2720–2727. doi: 10.1161/01.cir.94.11.2720. [DOI] [PubMed] [Google Scholar]
- 4.Liu S., Buring J.E., Sesso H.D. A prospective study of dietary fiber intake and risk of cardiovascular disease among women. J Am Coll Cardiol. 2002;39:49–56. doi: 10.1016/s0735-1097(01)01695-3. [DOI] [PubMed] [Google Scholar]
- 5.Rimm E.B., Ascherio A., Giovannucci E. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA. 1996;275:447–451. doi: 10.1001/jama.1996.03530300031036. [DOI] [PubMed] [Google Scholar]
- 6.Wolk A., Manson J.E., Stampfer M.J. Long-term intake of dietary fiber and decreased risk of coronary heart disease among women. JAMA. 1999;281:1998–2004. doi: 10.1001/jama.281.21.1998. [DOI] [PubMed] [Google Scholar]
- 7.Li S., Flint A., Pai J.K. Dietary fiber intake and mortality among survivors of myocardial infarction: prospective cohort study. BMJ. 2014;348:g2659. doi: 10.1136/bmj.g2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson J.W., Baird P., Davis R.H., Jr. Health benefits of dietary fiber. Nutr Rev. 2009;67:188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 9.Satija A., Hu F.B. Cardiovascular benefits of dietary fiber. Curr Atheroscler Rep. 2012;14:505–514. doi: 10.1007/s11883-012-0275-7. [DOI] [PubMed] [Google Scholar]
- 10.James S.L., Muir J.G., Curtis S.L., Gibson P.R. Dietary fibre: a roughage guide. Intern Med J. 2003;33:291–296. doi: 10.1046/j.1445-5994.2003.00404.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang A.Y., Sea M.M., Ng K. Nutrient intake during peritoneal dialysis at the Prince of Wales Hospital in Hong Kong. Am J Kidney Dis. 2007;49:682–692. doi: 10.1053/j.ajkd.2007.02.257. [DOI] [PubMed] [Google Scholar]
- 12.Krishnamurthy V.M., Wei G., Baird B.C. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012;81:300–306. doi: 10.1038/ki.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo J., Leung S.S.F., Ho S.C. A food frequency questionnaire for use in the Chinese population in Hong Kong: description and examination of validity. Nutr Res. 1997;17:1633–1641. [Google Scholar]
- 14.Hsu-Hage B.H., Wahlqvist M.L. A food frequency questionnaire for use in Chinese populations and its validation. Asia Pac J Clin Nutr. 1992;1:211–223. [PubMed] [Google Scholar]
- 15.Holland B., Welch A.A., Unwin I.D. 5th ed. The Royal Society of Chemistry and Ministry of Agriculture, Fisheries and Food; London, UK: 1992. McCance and Widdowson’s The Composition of Foods. [Google Scholar]
- 16.Tsang Y.S., Fung S.W. Zhongshan University Press; Guangzhou, China: 1991. Diet and Health Guide and Composition of Food in Southern China. Chinese. [Google Scholar]
- 17.Institute of Health, Chinese Medical Science Institute . Peoples Health Press; Beijing, China: 1992. Food Composition Tables. [Google Scholar]
- 18.Schiller N.B., Shah P.M., Crawford M Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 19.Otterstad J.E., Froeland G., St John Sutton M., Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J. 1997;18:507–513. doi: 10.1093/oxfordjournals.eurheartj.a015273. [DOI] [PubMed] [Google Scholar]
- 20.Nolph K.D., Moore H.L., Twardowski Z.J. Cross-sectional assessment of weekly urea and creatinine clearances in patients on continuous ambulatory peritoneal dialysis. ASAIO J. 1992;38:M139–M142. doi: 10.1097/00002480-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 21.van Olden R.W., Krediet R.T., Struijk D.G., Arisz L. Measurement of residual renal function in patients treated with continuous ambulatory peritoneal dialysis. J Am Soc Nephrol. 1996;7:745–750. doi: 10.1681/ASN.V75745. [DOI] [PubMed] [Google Scholar]
- 22.Myerburg R.J., Castellanos A. Cardiac arrest and sudden death. In: Braunwald E., editor. Heart Disease: A Textbook of Cardiovascular Medicine. WB Saunders; Philadelphia, PA: 1997. pp. 742–779. [Google Scholar]
- 23.Engelstein E.D., Zipes D.P. Sudden cardiac death. In: Alexander R.W., Schlant R.C., Fuster V., editors. The Heart, Arteries and Veins. McGraw-Hill; New York, NY: 1998. pp. 1081–1111. [Google Scholar]
- 24.Threapleton D.E., Greenwood D.C., Evans C.E. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2013;347:f6879. doi: 10.1136/bmj.f6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu L., Huang Y.F., Wang M.Q. Dietary fiber intake is associated with chronic kidney disease (CKD) progression and cardiovascular risk, but not protein nutritional status, in adults with CKD. Asia Pac J Clin Nutr. 2017;26:598–605. doi: 10.6133/apjcn.072016.08. [DOI] [PubMed] [Google Scholar]
- 26.Mozaffarian D., Kumanyika S.K., Lemaitre R.N. Cereal, fruit, and vegetable fiber intake and the risk of cardiovascular disease in elderly individuals. JAMA. 2003;289:1659–1666. doi: 10.1001/jama.289.13.1659. [DOI] [PubMed] [Google Scholar]
- 27.Poesen R., Meijers B., Evenepoel P. The colon: an overlooked site for therapeutics in dialysis patients. Semin Dial. 2013;26:323–332. doi: 10.1111/sdi.12082. [DOI] [PubMed] [Google Scholar]
- 28.Ramezani A., Massy Z.A., Meijers B. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis. 2016;67:483–498. doi: 10.1053/j.ajkd.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieffer D.A., Piccolo B.D., Vaziri N.D. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am J Physiol Renal Physiol. 2016;310:F857–F871. doi: 10.1152/ajprenal.00513.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel K.P., Luo F.J., Plummer N.S. The production of p-cresol sulfate and indoxyl sulfate in vegetarians versus omnivores. Clin J Am Soc Nephrol. 2012;7:982–988. doi: 10.2215/CJN.12491211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirich T.L., Plummer N.S., Gardner C.D. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol. 2014;9:1603–1610. doi: 10.2215/CJN.00490114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barreto F.C., Barreto D.V., Liabeuf S. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bammens B., Evenepoel P., Keuleers H. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006;69:1081–1087. doi: 10.1038/sj.ki.5000115. [DOI] [PubMed] [Google Scholar]
- 34.Niwa T. Indoxyl sulfate is a nephro-vascular toxin. J Ren Nutr. 2010;20:S2–S6. doi: 10.1053/j.jrn.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Marques F.Z., Nelson E., Chu P.Y. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135:964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 36.Fujii H., Iwase M., Ohkuma T. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ajani U.A., Ford E.S., Mokdad A.H. Dietary fiber and C-reactive protein: findings from national health and nutrition examination survey data. J Nutr. 2004;134:1181–1185. doi: 10.1093/jn/134.5.1181. [DOI] [PubMed] [Google Scholar]
- 38.Wang A.Y., Woo J., Lam C.W. Is a single time point C-reactive protein predictive of outcome in peritoneal dialysis patients? J Am Soc Nephrol. 2003;14:1871–1879. doi: 10.1097/01.asn.0000070071.57901.b3. [DOI] [PubMed] [Google Scholar]
- 39.Marlett J.A., McBurney M.I., Slavin J.L., American Dietetic Association Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2002;102:993–1000. doi: 10.1016/s0002-8223(02)90228-2. [DOI] [PubMed] [Google Scholar]
- 40.National Health and Medical Research Council, Australian Government Department of Health and Aging. New Zealand Ministry of Health . Australia; Canberra: 2006. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; pp. 1–304. [Google Scholar]
- 41.British Nutrition Foundation . British Nutritional Foundation; London, UK: 2016. Healthy Diet Recommendations. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Univariate Cox regression analysis in relation to first MACE.