Abstract
Introduction
This study examined the relationship among age, measures of social deprivation, and incidence and outcome of acute kidney injury (AKI).
Methods
The Welsh National electronic AKI reporting system was used to identify all cases of AKI in patients 18 years or older between March 2015 and January 2017. Socioeconomic classification of patients was derived from the Welsh Index of Multiple Deprivation (WIMD). Patients were grouped according to their WIMD score, and Multivariate Cox proportional hazard modeling was used to adjust the data for age. The ranked data were categorized into percentiles and correlated with incidence, and measures of AKI severity and outcome.
Results
Analysis included 57,654 patients. For the whole cohort, the highest 90-day survival was associated with the most socially deprived cohorts. There was a significant negative relationship between age-adjusted incidence of AKI and the WIMD score. In patients 60 years or older, there was an inverse correlation between WIMD score and survival that was not evident in those younger than 60. AKI severity at presentation was worse in patients from areas of social deprivation. Social deprivation was associated with a significantly higher proportion of preexisting chronic kidney disease (CKD) in patients with AKI older than 60, but not in those younger than 60.
Conclusion
Overall mortality following AKI was higher in least-deprived areas, reflecting an older patient cohort. In contrast, social deprivation was associated with higher age-adjusted AKI incidence and age-adjusted mortality following AKI. The excess mortality observed in more deprived areas was associated with more severe AKI and a higher proportion of preexisting CKD.
Keywords: AKI, CKD, epidemiology, mortality
AKI, characterized by a sudden decline in renal function, is associated with increased patient morbidity and mortality.1, 2 Most publications of large series characterizing AKI rely on making and recording an accurate diagnosis of AKI through hospital coding or retrospective review of hospital records.3, 4, 5, 6 Based on a presumption that early identification may help raise standards of care and improve patient outcomes, an automated real-time electronic (e)-alert system for AKI based on the Kidney Disease: Improving Global Outcomes change in creatinine diagnostic criteria has been established and implemented nationally across all areas of the National Health Service in England and Wales. Across Wales, an AKI alert is generated when the all Wales Laboratory Information Management System (Intersystems TrakCare Lab, Cambridge, MA) automatically compares measured serum creatinine values on an individual patient against previous results on the system database. Using the electronic AKI alert, we have developed a centralized data collection system to provide a comprehensive characterization of the incidence of AKI identified by an electronic alert, and its outcome in Wales.7, 8, 9
In renal medicine, the relationship between socioeconomic deprivation, severity of CKD, and poor clinical outcomes is well documented,10, 11, 12, 13, 14 as are the effects of social deprivation in renal transplantation.15, 16, 17 Much less is known regarding the relationship between social deprivation and AKI. We have previously demonstrated that the incidence of AKI is related to measures of social deprivation, with a higher incidence in the most disadvantaged areas.7 In contrast, a higher mortality was seen in AKI in the most affluent areas.18 We postulate that this discrepancy relates to a longer life expectancy in areas of affluence, which influences the nature of patients presenting with AKI. In the current study, we have further examined the relationship among age, measures of social deprivation, and incidence and outcome of AKI.
Methods
Setting
Data were collected from all Health boards in the National Health Service in Wales, representing a population of 3.06 million people. The Medical Record Number, a unique reference number allocated to patients registered in the National Laboratory Information Management System, was used as the patient identifier. The study has been approved under the conditions of “Service Evaluation Project Registration.”
The Electronic AKI Reporting System
The AKI alert is generated by comparing a current creatinine value with historic creatinine measurements for the same patient in real time. It defines AKI according to Kidney Disease: Improving Global Outcomes increase in creatinine parameters.7 Patients with no previous recorded creatinine values will not generate an alert. For patients with preexisting CKD, the algorithm will only generate alerts for acute or chronic AKI (i.e., only if a significant acute rise is detected).
Data Collection
Data were collected on AKI alerts generated on all individuals older than 18 in any location across Wales between March 2015 and January 2017. To avoid spurious results resultant from fluctuations in creatinine related to renal replacement therapies, dialysis patients, patients with a known renal transplant, and AKI alerts generated on the renal tertiary center base ward were excluded from the analysis.7 Patients were counted only once in the analysis (i.e., any alert for the same patient other than the first was excluded).
In addition to measurements of renal function, data were collected on patient age, gender, stage of AKI, preexisting CKD (estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate equation19 and preexisting CKD was defined as an estimated glomerular filtration rate <60 ml/min per 1.73 m2 derived from the baseline serum creatinine), and the clinical location at which the alert was generated. All stages of AKI were included in the analysis; however, severity of AKI at presentation was determined by the percentage of patients presenting with AKI stages 2 and 3.
Incidence rates were calculated using mid-2013 Office for National Statistics population estimates and patient-level post code analyses.20 Data on patient mortality at 90 days following each episode were collected from the Welsh Demographic Service.21 Socioeconomic classification of patients was derived from the WIMD score.22 The overall WIMD is a weighted area level aggregation of 8 domains of deprivation that can be recognized and measured separately (income, employment, education, health, geographical access to services, housing, and physical environment). Patients were grouped according to the WIMD score by their postcode and corresponding Lower-layer Super Output Area of residence, with results presented as ranked data. The ranked data were categorized into percentiles, with percentile 1 being the most socioeconomically deprived and percentile 100 being the least deprived.
Statistical analysis was carried out using SPSS software, version 20 (IBM SPSS, Chicago, IL). Pearson’s coefficient was calculated to determine correlation between measures of social deprivation of AKI readouts. Multivariate Cox proportional hazard modeling was used to analyze patient survival. To exclude the influence of age on survival a Cox proportional hazards regression model was used to generate an age β correction factor of 0.034 per year. This enabled the adjustment of each WIMD percentile population to age 60. P values <0.05 were considered statistically significant.
Results
A total of 57,654 patients triggered an electronic AKI alert between March 2015 and January 2017. Overall 90-day mortality for the whole cohort was 25.8%.
Survival, WIMD, and Age Distribution of AKI
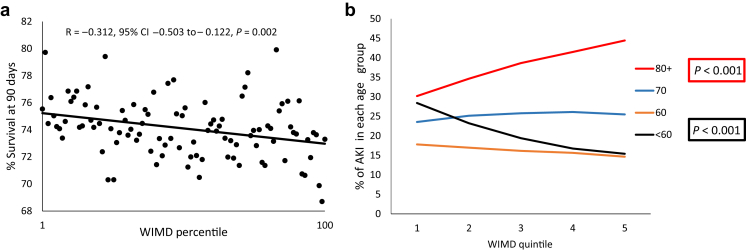
The relationship between socioeconomic measures and 90-day survival following AKI is shown in Figure 1. There was a strong positive correlation between ranking by WIMD score and 90-day survival (Figure 1a), with a higher proportion of patients with AKI surviving at 90 days in the most socially deprived areas (R = 0.312; 95% confidence interval [CI]: −0.503 to −0.122; P = 0.002). In line with the hypothesis that reduced survival associated with AKI in affluent areas is a reflection of longer life expectancy in the most affluent areas, the percentage of patients with AKI who were 80 years or older increased significantly with increasing affluence (Figure 1b). This was mirrored by a significant fall in the percentage of patients presenting with AKI who were younger than 60 years.
Figure 1.
The relationship between 90-day survival (a), and age distribution (b) of patients with acute kidney injury (AKI) and social deprivation. For 90-day survival, social deprivation was measured by Welsh Index of Multiple Deprivation (WIMD) percentile, where percentile 1 is the most deprived and percentile 100 is the least deprived. For age distribution, the data represent percentage of patients in each category of social deprivation with AKI in each of the age groups <60 years, 60–69 years, 70–79 years, and ≥80 years with the social deprivation, expressed as WIMD quintiles with quintile 1 representing the most deprived and quintile 5 the least deprived. CI, confidence interval.
Cox regression proportional hazard modeling analysis was used to assess corrected patient mortality. For the whole cohort, higher hazard of death was associated with older age (hazard ratio: 1.037; 95% CI: 1.036–1.38; P < 0.001) and a higher WIMD ranking (i.e., less social deprivation) (hazard ratio: 1.001; 95% CI: 1.001–1.002; P < 0.001). Adjusted for age, however, the hazard ratio of death was lower in patients with a higher WIMD ranking (adjusted hazard ratio: 0.999; 95% CI: 0.998–0.999; P < 0.001).
Incidence of AKI by WIMD Population Age
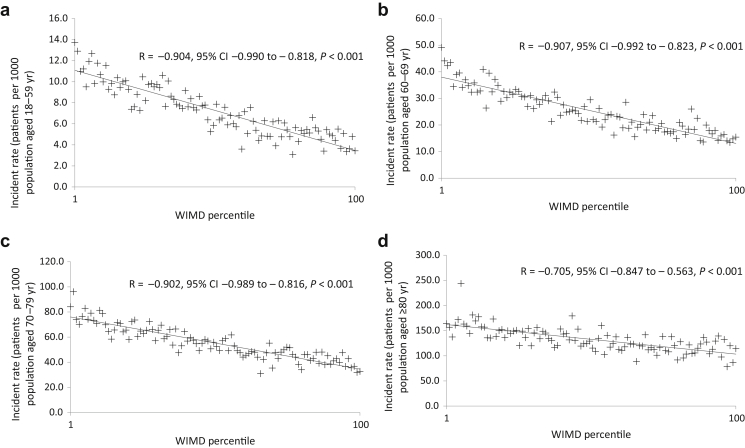
The incidence of AKI according to patient age using the population of each WIMD geographic area within that age group as the denominator is shown in Figure 2. The data are subdivided into 4 age groups: younger than 60 years (Figure 2a), 60 to 69 years (Figure 2b), 70 to 79 years (Figure 2c), and 80 years or older (Figure 2d). In all age groups, there was a significant negative relationship by linear regression, between age-adjusted incidence of AKI and the WIMD percentile, with the highest incidence in the most socially deprived areas.
Figure 2.
The relationship among age, incidence of acute kidney injury (AKI), and social deprivation as measured by Welsh Index of Multiple Deprivation (WIMD) percentile. The data are expressed as the incidence of AKI according to patient age using the population of each WIMD geographic area within that age group as the denominator. (a) AKI incidence rate in patients aged <60 years. (b) AKI incidence rate in patients aged 60–69 years. (c) AKI incidence rate in patients aged 70–79 years. (d) AKI incidence rate in patients aged ≥80 years. Percentile 1 is the most deprived and percentile 100 is the least deprived. CI, confidence interval.
Social Deprivation Impact on AKI-Associated Mortality Is Age Dependent
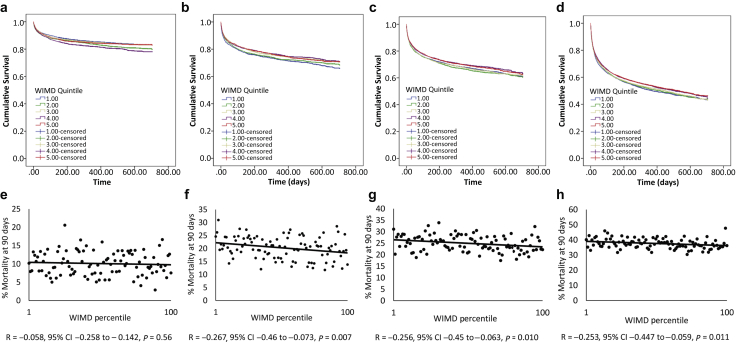
The influence of patient age on the relationship between social deprivation and 90-day mortality is shown in Figure 3. Kaplan-Meier survival curves for each age group are presented as survival by WIMD quintile, with quintile 1 representing the most socially deprived and quintile 5 the most affluent geographical areas (Figure 3a-d). Mortality is also presented as percentage mortality at 90 days for each age group by WIMD percentile (Figure 3e–h).
Figure 3.
Age-dependent acute kidney injury (AKI)-associated mortality. Kaplan-Meier survival curves for AKI patients aged <60 years (a), aged 60–69 years (b), aged 70–79 years (c), and aged ≥80 years (d), stratified by Welsh Index of Multiple Deprivation (WIMD) quintile. Quintile 1 represents the most deprived and quartile 5 the most affluent ranked geographical areas. Data were censored at 2 years. Correlation between 90-day mortality and social deprivation as measured by WIMD percentile for patients with AKI aged <60 years (e), aged 60–69 years (f), aged 70–79 years (g), and aged ≥80 years (h). Percentile 1 is the most deprived and percentile 100 is the least deprived. CI, confidence interval.
As would be expected, overall mean survival was longest in the cohort aged <60 years (600.1 days, 95% CI: 596.5–603.6) and fell progressively in each of the age groups 60 to 69 years (531.9 days, 95% CI: 526.1–537.8), 70 to 79 years (492.0 days, 95% CI: 487.1–497.0), and 80 years and older (387.7 days, 95% CI: 383.263–392.1, P < 0.0001). In patients younger than 60 years, there was no significant relationship between mortality and social deprivation expressed either as Kaplan-Meier survival curves censored at 2 years or percentage mortality at 90 days (Figure 3a and e). In contrast, in each of the patient groups aged 60 to 69 (Figure 3b and f), 70 to 79 (Figure 3c and g), and 80 years and older (Figure 3d and h), there was a significant negative impact of social deprivation on survival, with the highest mortality in each of the age groups seen in the most socially deprived areas. In each of these age groups, mean survival (censored at 2 years) was lowest in the first WIMD quintile and highest in the fifth WIMD quintile (age 60–69, mean survival quintile 1, 518.3 days 95% CI: 505.1–530.6; quintile 5, 539.8 days 95% CI: 525.1–537.8, χ2 P = 0.024: age 70–79, mean survival quintile 1, 488.7 days 95% CI: 477.6–499.8; quintile 5, 501.1 days 95% CI: 489.3–512.9, χ2 P = 0.04: age older than 80 years, mean survival quintile 1, 377.9 days 95% CI: 367.4–388.5, quintile 5, 397.5 days 95% CI: 387.7–407.4, χ2 P = 0.016). This suggests that patients living in socially deprived areas need to survive to 60 years or older for the impact of prolonged deprivation to translate into increased AKI mortality.
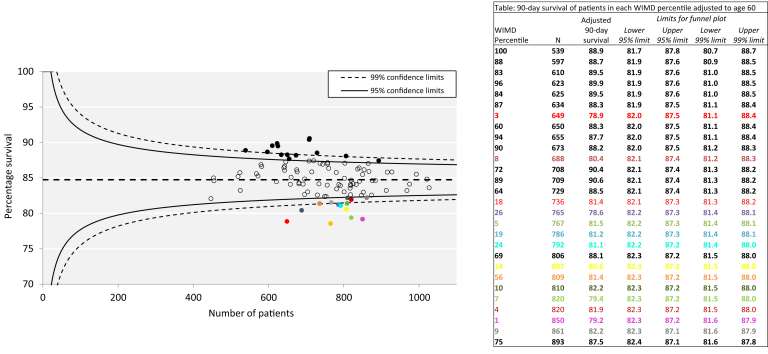
As social deprivation directly influences the incidence of AKI, and patient age influences survival, analysis of the influence of the index of deprivation and AKI-associated survival was also presented by a funnel plot, with patient survival reported as survival adjusted to age 60 years (Figure 4). This gives an estimate of what the survival would be if all patients in each WIMD percentile had been aged 60 at the time of the AKI episode. The plot identified 14 WIMD percentiles below the 95% confidence limits for age-adjusted survival, representing areas with excess mortality. Of these 14 percentiles, 12 represented WIMD percentiles in the lowest quarter (i.e., representing the most socially deprived). In contrast, 14 WIMD percentiles fell above 95% confidence limits for survival, representing better survival. Of these outlying WIMD percentiles with the best age-adjusted survival, 10 represented percentiles in the top quarter (i.e., the least socially deprived quarter). This is therefore consistent with social deprivation being an independent predictor of poor outcome.
Figure 4.
Funnel plot for age-adjusted acute kidney injury (AKI) survival. Ninety-day survival of patients in each Welsh Index of Multiple Deprivation (WIMD) percentile is adjusted to mortality at age 60. The data in the insert provide the detail for data points that lie either below the 95% confidence limits (colored text and bullets), or above the 95% confidence limits (bold text and bullets).
Severity of AKI
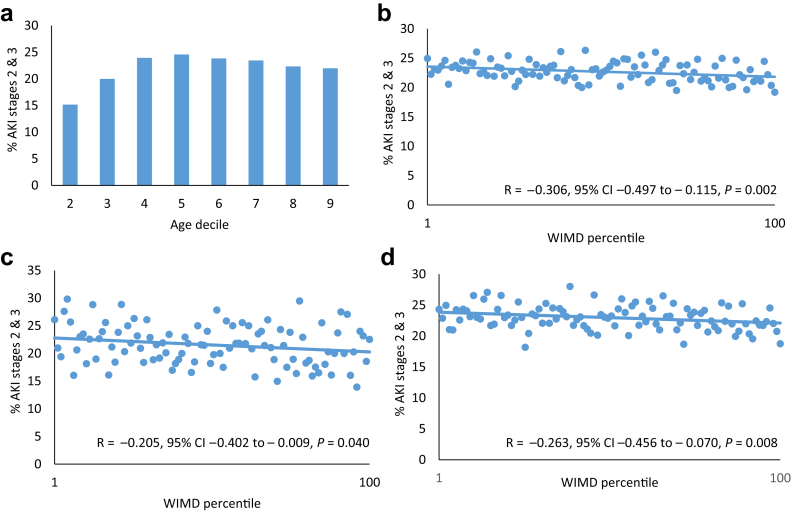
Next, we sought to examine the contribution of severity of AKI to the excess mortality in elderly patients from socially deprived areas. Severity of AKI at presentation was determined by the percentage of patients presenting with AKI stages 2 and 3. The effect of age on AKI severity is shown in Figure 5a. The percentage of patients presenting with AKI stages 2 and 3 increased in the age deciles 20 to 50 years, but thereafter, age did not alter the severity of AKI at presentation. For the whole patient group (Figure 5b), AKI severity at presentation was worse in patients from areas of social deprivation. This relationship was the same for both patients younger than 60 years (Figure 5c) and those older than 60 years (Figure 5d).
Figure 5.
Acute kidney injury (AKI) severity. (a) Distribution of AKI stage by age. Relationship between AKI severity and social deprivation measured by Welsh Index of Multiple Deprivation (WIMD) percentile (b) for the whole cohort, (c) for patients younger than 60 years (d) and patients older than 60 years (d). Severity of AKI was assessed by the percentage presenting with AKI stages 2 and 3. Percentile 1 is the most deprived and percentile 100 is the least deprived. CI, confidence interval.
Preexisting CKD as a Marker of Patient-Associated Comorbidities
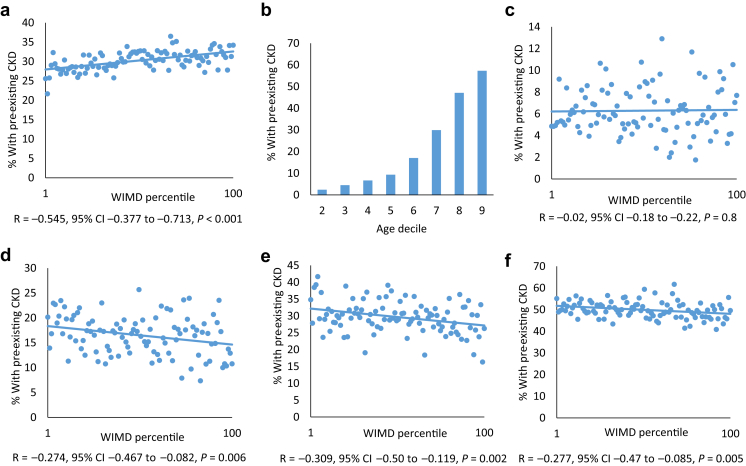
Overall the percentage of preexisting CKD was higher in areas of affluence (Figure 6a). This is consistent with the prevalence of preexisting CKD increasing with age in patients with AKI (Figure 6b). Although there was no relationship between social deprivation and preexisting CKD in patients with AKI younger than 60 years (Figure 6c), in patients aged 60 to 69 (Figure 6d), 69 to 70 (Figure 6e), and older than 80 years (Figure 6f), social deprivation was associated with a significantly higher proportion of preexisting CKD in the patients with incident AKI.
Figure 6.
Preexisting chronic kidney disease (CKD) as a marker of patient-associated comorbidities: (a) Relationship between social deprivation and the percentage of patients with preexisting CKD for the whole cohort. (b) Distribution of preexisting CKD by age group. Relationship between social deprivation and preexisting CKD in patients with acute kidney injury younger than 60 years (c), in patients aged 60–69 (d), 69–70 (e), and older than 80 years (f). For Welsh Index of Multiple Deprivation (WIMD) percentiles, percentile 1 is the most deprived and percentile 100 is the least deprived. CI, confidence interval.
Discussion
Socioeconomic deprivation has been shown to be an important determinant of poor health and life expectancy.11, 23, 24 In renal medicine, increased prevalence of renal failure, higher incidence of dialysis-associated mortality, reduced access to specialist care, reduced access to renal transplantation, and poorer outcomes following renal transplantation have all been reported for patients from more socioeconomically deprived areas.10, 11, 12, 13, 14, 25 In this article we sought to examine the influence of age and social deprivation on outcome following AKI.
The principal findings of this study are that age is a predictor of outcome and also a predictor of the impact of social deprivation on outcome following an episode of AKI. We report a high mortality associated with AKI in affluent areas that is not due to the severity of AKI in the elderly but associated with a higher percentage of older patients who have more preexisting CKD. Previous data defining the epidemiology of AKI in Wales demonstrated increased higher hazards of death associated with older age.7 The current data reflect this in that the AKI-associated mortality in the affluent areas is a reflection of increased life expectancy and an aging population.26, 27
When comparing patients of a similar age, social deprivation was associated with a higher incidence of age-adjusted incidence of AKI, although in the socially deprived areas this represents smaller absolute numbers of elderly patients. It also is of note that, in patients who survive beyond age 60, social deprivation adversely affects mortality. Similarly, analysis of age-standardized mortality demonstrates that areas of social deprivation are overrepresented in the WIMD percentiles, with age-adjusted patient survival below the 95% confidence limits (i.e., the highest mortality). We postulate that the adverse influence of social deprivation in those older than 60 years is related to a greater accumulation of comorbidities. That there is more comorbidity at any particular age in deprived areas is supported by the increased proportion of CKD within each of the age groups. This is consistent with the hypothesis that socioeconomic deprivation is associated with “premature aging,” which results in a difference between the “healthy life expectancy” within Wales being 18.7 years for men and 18.2 years for women in the least affluent areas. The severity of the AKI episode in the socially deprived population also contributes to the worse outcome associated with social deprivation as a higher proportion of AKI alerting at stage 2 and stage 3 at the time of presentation is associated with social deprivation. These data therefore suggest that both the burden of comorbidity and severity of AKI at presentation contribute to the impact of social deprivation on AKI-associated mortality.
Previous studies describing the impact of social deprivation on health outcomes have suggested that patient-related health beliefs and behavior, and access to care contribute to adverse outcome. Presentation of patients from the most deprived areas, with more advanced AKI, is consistent with delayed presentation/access to medical services. Using UK-based data where health care provision is free for all at the point of delivery does, however, provide an opportunity to exclude the confounding effect of systematic access to treatment and medication. In this regard, it is perhaps reassuring that in a health care service that is free at the point of access, in our cohort for the surviving patients, deprivation appears to have no impact on renal outcomes (data not shown), although this represents a competing risk resultant from a higher death rate from the more deprived areas. It is likely that delayed access and presentation in this setting relates at least in part to patient-related behavioral factors rather than inequity of access to hospital services. Changing attitudes to encourage early presentation, however, is only one aspect of improving outcome associated with social deprivation. Encouraging and facilitating early presentation does not address the confounding effect of the increased burden of comorbidities associated with presentation of AKI and social deprivation, which also may be related to patient behaviors. For example, a higher incidence of smoking, obesity, poor diet, inactivity, and alcohol consumption associated with social deprivation,28, 29, 30, 31, 32, 33 are aetiologically linked to the 4 diseases that account for 64% of early deaths in this cohort: cancer, heart disease, stroke, and diabetes. Unhealthy patient-related behaviors are therefore likely to contribute to hypertension, obesity, and type 2 diabetes and, therefore, the increased incidence of CKD associated with AKI and social deprivation. A challenge is, therefore, to educate and support patients to make positive lifestyle changes to reduce the burden of comorbidities and improve life expectancy in socially deprived areas. In addition to access and patient behavior, biological factors, such as genetics and race, also have been found to contribute to adverse health outcomes by way of social deprivation. It is of note that Wales, however, is not ethnically diverse, with 4.1% of the population coming from minority ethnic groups, and ethnicity therefore is unlikely to influence our findings.
Although this study uses a large national data set to define the relationship between measures of social deprivation and AKI, its findings need to be qualified by its limitations. As the e-alert system is information technology driven it lacks “intelligence” and therefore there is no clinical context applied. Using an information technology–based approach precludes inclusion of clinical information, such as patient comorbidity and linkage to primary care data sets, and lacks the detail of the cause of AKI, the need for renal replacement therapy, and does not shed light on the cause of death. The study is also limited in that any patient presenting with AKI but without a measurement of renal function in the previous 365 days will not be included. Similarly, the reliance on a definition of AKI based on serial changes in serum creatinine does not take into account urine output–based AKI diagnosis, which also may lead to failure to include all cases of AKI.
In conclusion, we demonstrate that social deprivation is associated with a poor outcome following AKI. The influence of social deprivation is, however, age dependent. Social deprivation–related excess mortality was also associated with more severe disease, late presentation, and more associated comorbidity as determined by preexisting CKD. Influencing the complex and long-term inequalities that contribute to the increased incidence and mortality in AKI requires population-level social and economic interventions.
Disclosure
All the authors declared no competing interests.
Acknowledgments
JH and DP collected and analyzed the data, produced the figures, and wrote the report. JDW, KD, and JG interpreted the data and wrote the report. AOP set up the program of work, designed the study, interpreted the data, and wrote the report. The work was carried out under the auspices of the Welsh AKI Steering Group which is sponsored by the Welsh Renal Clinical Network and Welsh Government.
References
- 1.Lafrance J.P., Miller D.R. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010;21:345–352. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lameire N., Van Biesen W., Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 3.Bucaloiu I.D., Kirchner H.L., Norfolk E.R. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81:477–485. doi: 10.1038/ki.2011.405. [DOI] [PubMed] [Google Scholar]
- 4.Hsu C.Y., Chertow G.M., McCulloch C.E. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu C.Y., McCulloch C.E., Fan D. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liangos O., Wald R., O'Bell J.W. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 7.Holmes J., Rainer T., Geen J. Acute kidney injury in the era of the AKI E-alert. Clin J Am Soc Nephrol. 2016;11:2123–2131. doi: 10.2215/CJN.05170516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes J., Allen N., Roberts G. Acute kidney injury electronic alerts in primary care - findings from a large population cohort. QJM. 2017;110:577–582. doi: 10.1093/qjmed/hcx080. [DOI] [PubMed] [Google Scholar]
- 9.Holmes J., Roberts G., May K. The incidence of pediatric acute kidney injury is increased when identified by a change in a creatinine-based electronic alert. Kidney Int. 2017;92:432–439. doi: 10.1016/j.kint.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Caskey F.J., Roderick P., Steenkamp R. Social deprivation and survival on renal replacement therapy in England and Wales. Kidney Int. 2006;70:2134–2140. doi: 10.1038/sj.ki.5001999. [DOI] [PubMed] [Google Scholar]
- 11.Drey N., Roderick P., Mullee M., Rogerson M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis. 2003;42:677–684. doi: 10.1016/s0272-6386(03)00916-8. [DOI] [PubMed] [Google Scholar]
- 12.Merkin S.S., Coresh J., Diez Roux A.V. Area socioeconomic status and progressive CKD: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2005;46:203–213. doi: 10.1053/j.ajkd.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 13.Vart P., Gansevoort R.T., Crews D.C. Mediators of the association between low socioeconomic status and chronic kidney disease in the United States. Am J Epidemiol. 2015;181:385–396. doi: 10.1093/aje/kwu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vart P., Gansevoort R.T., Joosten M.M. Socioeconomic disparities in chronic kidney disease: a systematic review and meta-analysis. Am J Prev Med. 2015;48:580–592. doi: 10.1016/j.amepre.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Stephens M.R., Evans M., Ilham M.A. The influence of socioeconomic deprivation on outcomes following renal transplantation in the United Kingdom. Am J Transplant. 2010;10:1605–1612. doi: 10.1111/j.1600-6143.2010.03041.x. [DOI] [PubMed] [Google Scholar]
- 16.Udayaraj U., Ben-Shlomo Y., Roderick P. Social deprivation, ethnicity, and uptake of living kidney donor transplantation in the United Kingdom. Transplantation. 2012;93:610–616. doi: 10.1097/TP.0b013e318245593f. [DOI] [PubMed] [Google Scholar]
- 17.Udayaraj U., Ben-Shlomo Y., Roderick P. Social deprivation, ethnicity, and access to the deceased donor kidney transplant waiting list in England and Wales. Transplantation. 2010;90:279–285. doi: 10.1097/TP.0b013e3181e346e3. [DOI] [PubMed] [Google Scholar]
- 18.Phillips D., Holmes J., Davies R. The influence of socioeconomic status on presentation and outcome of acute kidney injury. QJM. 2018;111:849–857. doi: 10.1093/qjmed/hcy180. [DOI] [PubMed] [Google Scholar]
- 19.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh Government Office for National Statistics National level population estimates by year, age and UK country. 2016. https://statswales.gov.wales/Catalogue/Population-and-Migration/Population/Estimates/nationallevelpopulationestimates-by-year-age-ukcountry Available at: Accessed October 1, 2018.
- 21.NHS Wales Informnatics Service Welsh Demographic Services. 2016. http://www.wales.nhs.uk/nwis/page/52552 Available at: Accessed October 1, 2018.
- 22.Welsh Index of Multiple Deprivation. 2014. http://gov.wales/statistics-and-research/welsh-index-multiple-deprivation/?lang=en Available at: Accessed October 1, 2018.
- 23.Diez Roux A.V., Merkin S.S., Arnett D. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 24.Singh G.K., Miller B.A., Hankey B.F., Edwards B.K. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer. 2004;101:1051–1057. doi: 10.1002/cncr.20467. [DOI] [PubMed] [Google Scholar]
- 25.Vart P., Reijneveld S.A., Bultmann U., Gansevoort R.T. Added value of screening for CKD among the elderly or persons with low socioeconomic status. Clin J Am Soc Nephrol. 2015;10:562–570. doi: 10.2215/CJN.09030914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw M., Gordon D., Dorling D. Increasing mortality differentials by residential area level of poverty: Britain 1981–1997. Soc Sci Med. 2000;51:151–153. doi: 10.1016/s0277-9536(99)00434-7. [DOI] [PubMed] [Google Scholar]
- 27.Waitzman N.J., Smith K.R. Phantom of the area: poverty-area residence and mortality in the United States. Am J Public Health. 1998;88:973–976. doi: 10.2105/ajph.88.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diez-Roux A.V., Nieto F.J., Caulfield L. Neighbourhood differences in diet: the Atherosclerosis Risk in Communities (ARIC) Study. J Epidemiol Community Health. 1999;53:55–63. doi: 10.1136/jech.53.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diez-Roux A.V., Nieto F.J., Muntaner C. Neighborhood environments and coronary heart disease: a multilevel analysis. Am J Epidemiol. 1997;146:48–63. doi: 10.1093/oxfordjournals.aje.a009191. [DOI] [PubMed] [Google Scholar]
- 30.Duncan C., Jones K., Moon G. Smoking and deprivation: are there neighbourhood effects? Soc Sci Med. 1999;48:497–505. doi: 10.1016/s0277-9536(98)00360-8. [DOI] [PubMed] [Google Scholar]
- 31.Asderakis A., Khalid U., Madden S., Dayan C. The influence of socioeconomic deprivation on outcomes in pancreas transplantation in England: registry data analysis. Am J Transplant. 2018;18:1380–1387. doi: 10.1111/ajt.14633. [DOI] [PubMed] [Google Scholar]
- 32.Krieger N., Chen J.T., Waterman P.D. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156:471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 33.Krieger N., Williams D.R., Moss N.E. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]