Abstract
Introduction
Cardiovascular (CV) morbidity and mortality are excessively high among hemodialysis (HD) patients. Anemia is a common complication of chronic kidney disease (CKD) and a known risk factor for CV events. To understand the impact of the recent regulatory and guideline changes in anemia management, we examined regional CV event rates in high-risk and erythropoiesis-stimulating agent (ESA)−hyporesponsive HD patients.
Methods
A prospective cohort study including 16,560 HD patients, 8660 CV high-risk, and 884 hyporesponsive to ESAs, from the Dialysis Outcomes and Practice Patterns Study (DOPPS) phase 4 (2009−2011) and phase 5 (2012−2015) was conducted to quantify all-cause mortality, major adverse cardiovascular events (MACE), and MACE plus heart failure and thromboembolic events (MACE+).
Results
The MACE+ rates (per 100 patient-years) were highest in North America (NA) (19.4; 95% CI = 18.2−20.7), followed by Europe (EU) (17.4; 95% CI = 16.6−18.1) and lowest in Japan (7.5; 95% CI = 6.9−8.1). When restricted to the high CV risk population, rates increased by 36% in NA, 45% in EU, and 72% in Japan. Mortality accounted for >74% of MACE+ events. MACE+ rates in ESA-hyporesponsive patients and high CV risk patients were similar in NA and EU cohorts. There were minimal differences in outcomes between the DOPPS phases 4 and 5.
Conclusion
Cardiovascular event rates are high in the HD population, vary by geographic region, and are substantially higher in high CV risk patients and ESA-hyporesponsive patients; however, the rates appear not to be affected by anemia guideline changes. The findings from this study will be essential to contextualize the design of future CV anemia-related outcome studies and clinical trials.
Keywords: cardiovascular events, cohort study, DOPPS, ESA hyporesponsivness, hemodialysis
CV morbidity and mortality are excessively high among HD patients. The pathogenesis of CV events is likely multifactorial, and anemia and related treatments (ESAs, i.v. iron, and blood transfusions) may contribute. Patients with CKD should be considered at increased risk for cardiovascular disease (CVD), as CKD-specific risk factors (i.e., vascular calcification) seem to play a role in CVD.1
Anemia is a common complication of CKD and is estimated to affect between 24% and 85% of those with CKD and >85% of those with end-stage renal disease (ESRD).2, 3, 4 Regulatory changes, such as the 2011 Food and Drug Administration (FDA) revision to the ESA label,5 the 2011 implementation of the new Centers for Medicare and Medicaid Services prospective (bundled) payment system in the United States (US), and publication of revised anemia guidelines6 have led to fundamental changes in anemia management. These, in turn, may have affected CV event rates. In the US, important clinical trends observed since implementation of the new bundled payment system include a decrease in ESA dose and associated lower hemoglobin (Hgb) levels, an increase in i.v. iron use and higher ferritin levels, and an increase in transfusion rates.7, 8, 9 The changes since this implementation stabilized by mid to late 2012.10
Given the global impact of other policies (e.g., publication of the Kidney Disease: Improving Global Outcomes [KDIGO] guidelines6 and European Best Practice Anemia Guidelines), it is anticipated that changes in anemia management might have also occurred in Europe and other regions of the world. It is currently unknown what impact (if any) these changes may have had on clinical outcomes and CV event rates. In addition, understanding the current CV event rates will also provide more precise estimates of CV endpoints essential to inform the design of clinical trials and CV outcome studies. DOPPS was designed to address some limitations of existing international registries, which often rely on voluntary reporting and collect little information about practice patterns and individual patient characteristics. The DOPPS uses a common protocol and standardized questionnaires to capture detailed longitudinal information on generally representative facilities and patients in participating countries. Key advantages of this design include the following: (i) highly-detailed patient-level data, enabling comprehensive description; (ii) facility practice data; and (iii) generalizability of findings to national HD populations.
Using 2 recent DOPPS cohorts, Phase 4 (2009−2011) and Phase 5 (2012−2015), the objectives of this study were to better understand the short-term impact of recent anemia guidelines changes on CV event rates, and to examine CV event rates in HD patients with and without high CV risk factors and in ESA-hyporesponsive patients.
Methods
DOPPS Cohort
DOPPS Phase 4 (2009−2011) and Phase 5 (2012−2015) cohorts consists of an HD population from 10 countries across 3 regions: NA (i.e., Canada and US); EU (i.e., Belgium, France, Germany, Italy, Spain, Sweden, and the United Kingdom [UK]); and Japan. The study population comprised all patients on HD enrolled in the DOPPS and dialyzing 3 to 5 times per week. Subjects with a history of malignancy within the prior 10 years (as collected in DOPPS questionnaires) or receiving treatment for cancer were excluded.
A subset of high CV risk patients was also examined. High-risk patients were defined as HD patients that met criteria for at least 2 of 6 predetermined risk factors for CV events: (i) age >65 years, (ii) history of diabetes, (iii) history of myocardial infarction (MI), (iv) history of stroke or transient ischemic attack (TIA), (v) history of congestive heart failure (CHF), or (vi) history of peripheral vascular disease (PVD).
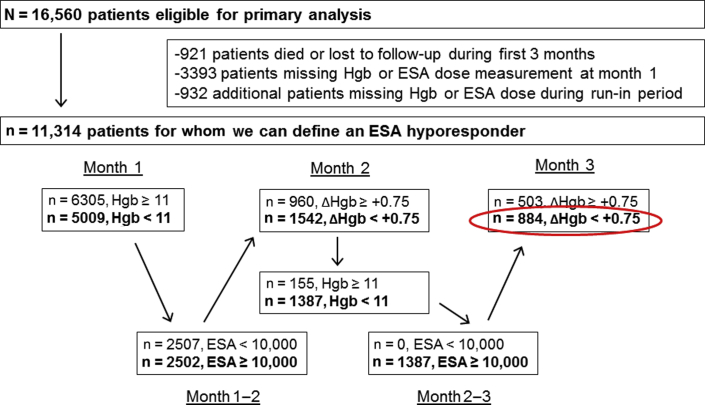
ESA-hyporesponsive patients are another subset of HD patients of interest with potential high mortality, as recently suggested by 2 different groups.11, 12 In the DOPPS data, Hgb is collected monthly, along with the ESA prescription at the end of each month. ESA-hyporesponsive groups were determined by cross-tabulating baseline Hgb value with the subsequent ESA dose and the following month’s Hgb concentrations to identify the proportion of patients in each ESA-by-Hgb stratum. To be included in this subgroup, all patients required 3 months of Hgb data and at least 2 months of ESA dose information (denominator). The hyporesponder group was then defined as patients who were “hyporesponsive” for the first 2 months of follow-up, defined as Hgb <11g/dl and ESA dose ≥10,000 units/wk (≥5000/wk in Japan), with a subsequent increase in Hgb <0.75 g/dl. Figure 1 details the number of patients meeting each criterion. Patients with at least 3 months of data but who did not meet the hyporesponsive criteria were classified as “nonhyporesponsive.” There was a heterogenous group consisting of normo-responsive patients as well as patients who did not meet all specific criteria to be hyporesponsive. Patients without sufficient data during these 3 months were not classified as ESA hyporesponsive or nonhyporesponsive and were excluded from the analysis.
Figure 1.
Defining erythropoiesis-stimulating agents (ESA) hyporesponsivness.
CV Outcomes
Evaluation of mortality and CV event rates included time to the first instance of the following events: (i) all-cause mortality, (ii) MACE, and (iii) MACE plus HF and thromboembolic events (nonvascular) (MACE+). Composite outcomes, such as MACE, were created by recording the first instance of any of the component events (all-cause mortality, fatal/nonfatal myocardial infarction, or fatal/nonfatal stroke). Specific definitions of each event are shown in Table 1; the codes are defined using standardized DOPPS questionnaires and not through International Classification of Diseases (ICD) 9/10 or otherwise externally identifiable.
Table 1.
Outcome definitions
| Clinical outcome | Cause of death | Hospitalizations |
|
|---|---|---|---|
| Diagnosis | Procedure | ||
| (2) Fatal and nonfatal MI | Acute myocardial infarction | Acute myocardial infarction | Coronary angioplasty |
| Atherosclerotic heart disease | Cardiac arrest/sudden death | Coronary artery bypass graft | |
| (3) Fatal and nonfatal stroke | Stroke, hemorrhagic | TIA | |
| Stroke, ischemic | Stroke (CVA) | ||
| (4) Hospitalization for HF | Congestive heart failure | Congestive heart failure | |
| (5) Thromboembolic events, nonvascular access | Pulmonary embolus | Deep vein thrombosis | Arterial bypass surgery |
CVA, cardiovascular accident; MACE, major adverse cardiovascular events, defined as all-cause mortality or outcomes (2) and (3) above; MACE+, MACE plus heart failure and thromboembolic events (additionally includes outcomes [4] or [5]); TIA, transient ischemic attack.
Rate Calculation
For each patient, we counted the first recorded event during the designated follow-up period. Follow-up began at study entry when analyzing all patients or stratifying by CV risk. When stratifying by ESA hyporesponsivness, the follow-up began after the 3-month run-in period as defined in Figure 1. If the patient survived to the end of follow-up without experiencing any of the events, the patient was censored, and no event was recorded. We divided the number of patients with an event by the sum of the total patient-years in the sample to calculate the event rates for each of the outcomes. Event rates in each region were calculated among all HD patients, by CV risk status, and by ESA hyporesponder status. Facilities, and their patients, without data on cause-specific hospitalizations were excluded from analyses. The cumulative event-free survival probability for MACE+ was illustrated by region using Kaplan−Meier plots. All analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC).
Results
Patient Characteristics
Characteristics for all 16,560 HD patients are shown in Table 2. Differences based on CV risk status were largely due to the definition: high-risk patients were much older and had a higher prevalence of comorbidities. They also had lower albumin levels and were more likely to use a catheter for HD. Hgb levels and ESA dose in the high-risk population was similar to that in the overall cohort. A subset of 11,314 HD patients had sufficient longitudinal data to define ESA hyporesponsivness. The hyporesponsive patients had higher doses of ESA and lower Hgb levels (by definition) and were more likely to be Asian (i.e., from Japan-DOPPS), potentially due to the lower ESA hyporesponsivness definition in the Japanese population. These patients also had lower ferritin levels, longer dialysis vintage, and less HD catheter use, all characteristic of Japanese HD patients.
Table 2.
Study population characteristics by CV risk and ESA hyporesponsive status
| Patient characteristic | All | High CV risk |
ESA hyporesponsive |
||
|---|---|---|---|---|---|
| No | Yes | No | Yes | ||
| N patients | 16,560 | 7900 | 8660 | 10,430 | 884 |
| N patients by region | |||||
| North America | 3893 | 1507 | 2386 | 2816 | 164 |
| Europe | 8746 | 4185 | 4561 | 4997 | 257 |
| Japan | 3921 | 2208 | 1713 | 2617 | 463 |
| Demographics | |||||
| Age (yr) | 63.8 ± 14.7 | 57.0 ± 15.0 | 70.1 ± 11.1 | 63.6 ± 14.6 | 65.1 ± 12.9 |
| Gender (% male) | 60.4 | 58.4 | 62.2 | 60.3 | 56.6 |
| Race (%) | |||||
| White | 65.4 | 60.2 | 70.1 | 63.4 | 40.7 |
| Black | 5.8 | 6.7 | 5.0 | 6.3 | 2.9 |
| Asian | 24.3 | 28.7 | 20.3 | 25.6 | 52.6 |
| Other | 4.5 | 4.4 | 4.6 | 4.7 | 3.7 |
| Vintage (yr) | 4.6 ± 6.1 | 5.5 ± 7.2 | 3.7 ± 4.8 | 4.7 ± 6.2 | 5.5 ± 6.9 |
| Smoking status (%) | |||||
| Active smoker | 11.5 | 13.8 | 9.5 | 11.5 | 12.0 |
| Former smoker | 24.3 | 18.9 | 29.3 | 24.3 | 20.8 |
| Never smoker | 36.4 | 39.0 | 34.1 | 37.3 | 35.5 |
| Unknown/missing | 27.7 | 28.4 | 27.2 | 26.9 | 31.7 |
| Body mass index (kg/m2) | 25.7 ± 6.1 | 24.9 ± 5.9 | 26.4 ± 6.2 | 25.7 ± 6.1 | 24.4 ± 6.4 |
| Comorbid conditions (%) | |||||
| CAD (incl. prior MI) | 35.5 | 14.2 | 54.8 | 35.7 | 35.5 |
| Other CVD | 28.8 | 16.7 | 39.7 | 28.4 | 31.4 |
| Cerebrovascular disease | 15.6 | 3.0 | 27.1 | 15.1 | 16.3 |
| CHF | 22.6 | 4.4 | 39.1 | 22.7 | 24.7 |
| Diabetes | 43.0 | 16.7 | 66.8 | 42.7 | 46.4 |
| Hypertension | 86.9 | 81.5 | 91.7 | 87.0 | 86.0 |
| Peripheral vascular disease | 27.0 | 3.2 | 48.7 | 26.5 | 27.6 |
| Other treatment variables | |||||
| ESA use (%) | 85.4 | 84.4 | 86.4 | 83.5 | 100 |
| ESA dose (1000 units/wk) | 6.0 (2.5,12.0) | 5.2 (2.3,10.0) | 6.6 (2.5,12.0) | 5.4 (2.3,10.0) | 10.0 (6.3,15.0) |
| Systolic BP (mm Hg) | 141.9 ± 22.5 | 141.3 ± 21.6 | 142.4 ± 23.2 | 142.4 ± 22.4 | 144.6 ± 23.1 |
| Diastolic BP (mm Hg) | 73.7 ± 13.0 | 77.1 ± 13.1 | 70.7 ± 12.2 | 74.1 ± 13.0 | 74.3 ± 12.7 |
| Hemodiafiltration (%) | 12.9 | 13.8 | 12.1 | 10.7 | 9.5 |
| Catheter use (%) | 25.8 | 21.2 | 30.0 | 23.9 | 19.4 |
| Laboratory values | |||||
| Hemoglobin (g/dl) | 11.0 ± 1.4 | 11.0 ± 1.4 | 11.0 ± 1.4 | 11.1 ± 1.4 | 9.9 ± 1.2 |
| Albumin (g/dl) | 3.68 ± 0.54 | 3.76 ± 0.52 | 3.61 ± 0.54 | 3.72 ± 0.52 | 3.60 ± 0.54 |
| TSAT (%) | 26.2 ± 12.4 | 26.8 ± 12.7 | 25.7 ± 12.1 | 26.3 ± 12.1 | 24.7 ± 12.0 |
| Ferritin (ng/ml) | 312 (126,579) | 278 (108,541) | 336 (146,613) | 311 (120,581) | 229 (72,523) |
| Total cholesterol (mg/dl) | 158.2 ± 41.6 | 162.3 ± 41.7 | 154.3 ± 41.2 | 159.4 ± 41.1 | 153.0 ± 42.4 |
BP, blood pressure; CAD, coronary artery disease; CHF, congestive heart failure; CVD, cardiovascular disease; ESA, erythropoiesis-stimulating agent; incl., including; MI, myocardial infarction; TSAT, transferrin saturation.
Data are mean ± SD, median (interquartile range), or percentage shown.
High CV risk patients are those meeting criteria for at least 2 of 6 factors: (i) age >65 years, (ii) history of MI, (iii) history of stroke or TIA, (iv) history of CHF, (v) history of diabetes, and (vi) history of peripheral vascular disease.
Hyporesponder definition detailed in Methods. Note that because of dose differences across countries, a different definition of “hyporesponder” was used in Japan (>5000 units/wk instead of >10,000 units/wk). Note the number of patients in the Hyporesponder + Nonhyporesponder groups do not sum to the total because of missing data, deaths, and losses to follow-up during the 3-month run-in period.
Cardiovascular Event Rates
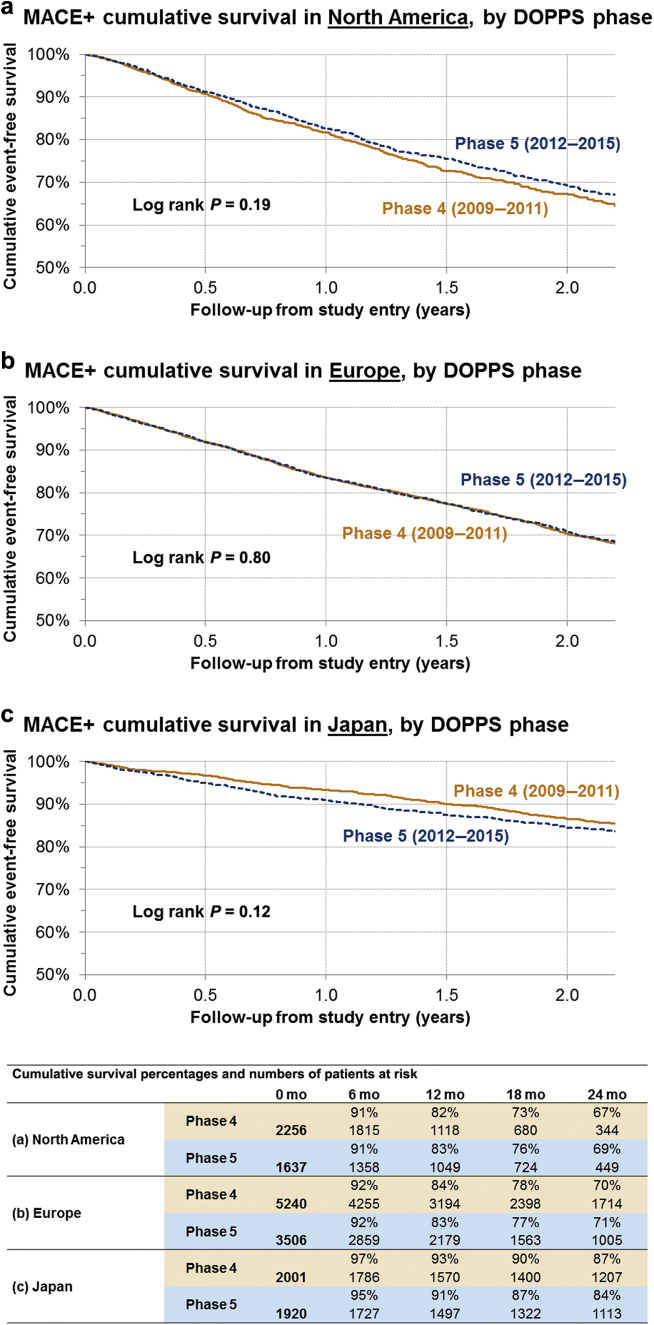
All-cause mortality, MACE, and MACE+ rates by DOPPS phase for each region are shown in Table 3 and Figure 2. There were no significant differences in the CV event rates by DOPPS phase in each of the regions. The point estimate for MACE+ rates for phase 5 compared to earlier phase 4 was slightly higher in NA, similar in EU, and slightly lower in Japan; the CI overlapped substantially (Table 3, Figure 2). Thus, for the remaining analysis, the CV event rates were assessed in the phase 4 and 5 combined cohort.
Table 3.
Event rates by DOPPS phase and region
| N patients | All-cause mortality |
MACE |
MACE+ |
||||
|---|---|---|---|---|---|---|---|
| N events | Rate (95% CI) | N events | Rate (95% CI) | N events | Rate (95% CI) | ||
| North America | |||||||
| Phase 4 (2009−2011) | 2256 | 388 | 14.3 (13.0–15.8) | 466 | 17.7 (16.2–19.4) | 520 | 20.2 (18.6–22.1) |
| Phase 5 (2012−2015) | 1637 | 317 | 13.5 (12.1–15.1) | 377 | 16.6 (15.0–18.3) | 412 | 18.4 (16.8–20.3) |
| Europe | |||||||
| Phase 4 (2009−2011) | 5240 | 1032 | 13.2 (12.4–14.1) | 1198 | 15.8 (14.9–16.7) | 1300 | 17.5 (16.5–18.4) |
| Phase 5 (2012−2015) | 3506 | 644 | 13.1 (12.1–14.2) | 740 | 15.4 (14.3–16.5) | 813 | 17.2 (16.1–18.4) |
| Japan | |||||||
| Phase 4 (2009−2011) | 2001 | 204 | 4.8 (4.2–5.6) | 256 | 6.2 (5.5–7.0) | 283 | 6.9 (6.1–7.7) |
| Phase 5 (2012−2015) | 1920 | 208 | 6.0 (5.3–6.9) | 255 | 7.5 (6.7–8.5) | 273 | 8.1 (7.2–9.2) |
CI, confidence interval; DOPPS, Dialysis Outcomes and Practice Patterns Study; MACE, major adverse cardiovascular events; MACE+, MACE plus heart failure and thromboembolic events; non-VA, nonvascular access.
MACE includes all-cause mortality plus hospitalization due to myocardial infarction or stroke. MACE+ includes MACE plus heart failure and thromboembolic events (non-VA). Rates expressed per 100 patient-years.
Figure 2.
Kaplan−Meier cumulative event-free survival in MACE+ patients, by Dialysis Outcomes and Practice Patterns Study (DOPPS) phase in (a) North America, (b) Europe, and (c) Japan. MACE+, major adverse cardiovascular events (MACE) plus heart failure and thromboembolic events.
All-cause mortality, MACE, and MACE+ rates across both DOPPS phases are shown by region and CV risk in Table 4. All-cause mortality rates (per 100 patient-years) were much higher in NA (14.0; 95% CI = 13.0−15.0) and EU (13.2; 95% CI = 12.6−13.8) than in Japan (5.4; 95% CI = 4.9−5.9). MACE and MACE+ rates were incrementally higher in each region, with regional differences persisting. When restricted to the high-risk population, event rates increased by about 36% in North America, 45% in Europe, and 72% in Japan. Event rates were similarly elevated among ESA-hyporesponsive patients in NA and EU. In Japan, event rates were lower among the hyporesponsive group as compared to the high CV risk group, but remained elevated compared to the ESA nonhyporesponsive group. Regional differences also persisted within all subgroups.
Table 4.
Event rates by region, and by CV risk and ESA hyporesponsive status
| N patients | All-cause mortality |
MACE |
MACE+ |
||||
|---|---|---|---|---|---|---|---|
| N events | Rate (95% CI) | N events | Rate (95% CI) | N events | Rate (95% CI) | ||
| North America | |||||||
| All patients | 3893 | 705 | 14.0 (13.0–15.0) | 843 | 17.2 (16.1–18.4) | 932 | 19.4 (18.2–20.7) |
| Low CV risk | 1507 | 139 | 6.9 (5.8–8.1) | 170 | 8.5 (7.3–9.9) | 189 | 9.6 (8.3–11.0) |
| High CV risk | 2386 | 566 | 18.7 (17.2–20.3) | 673 | 23.1 (21.4–24.9) | 743 | 26.3 (24.4–28.2) |
| Nonhyporesponder | 2816 | 492 | 12.7 (11.7–13.9) | 601 | 16.0 (14.8–17.3) | 664 | 18.0 (16.7–19.5) |
| Hyporesponder | 164 | 42 | 19.1 (14.1–25.8) | 47 | 22.0 (16.6–29.3) | 53 | 25.9 (19.8–33.9) |
| Europe | |||||||
| All patients | 8746 | 1676 | 13.2 (12.6–13.8) | 1938 | 15.6 (14.9–16.3) | 2113 | 17.4 (16.6–18.1) |
| Low CV risk | 4185 | 402 | 6.6 (6.0–7.3) | 483 | 8.0 (7.3–8.8) | 542 | 9.1 (8.4–9.9) |
| High CV risk | 4561 | 1274 | 19.3 (18.3–20.4) | 1455 | 22.8 (21.7–24.0) | 1571 | 25.3 (24.1–26.6) |
| Nonhyporesponder | 4997 | 927 | 11.6 (10.9–12.4) | 1080 | 13.8 (13.0–14.7) | 1182 | 15.4 (14.6–16.3) |
| Hyporesponder | 257 | 74 | 18.7 (14.9–23.5) | 86 | 22.3 (18.0–27.5) | 95 | 25.4 (20.8–31.1) |
| Japan | |||||||
| All patients | 3921 | 412 | 5.4 (4.9–5.9) | 511 | 6.8 (6.2–7.4) | 556 | 7.5 (6.9–8.1) |
| Low CV risk | 2208 | 126 | 2.7 (2.3–3.3) | 162 | 3.6 (3.0–4.1) | 178 | 3.9 (3.4–4.5) |
| High CV risk | 1713 | 286 | 9.4 (8.4–10.5) | 349 | 11.7 (10.6–13.0) | 378 | 12.9 (11.7–14.3) |
| Nonhyporesponder | 2617 | 216 | 4.0 (3.5–4.6) | 281 | 5.3 (4.8–6.0) | 313 | 6.0 (5.4–6.7) |
| Hyporesponder | 463 | 66 | 7.4 (5.8–9.4) | 79 | 8.9 (7.1–11.1) | 83 | 9.4 (7.6–11.7) |
CI, confidence interval; CV, cardiovascular; ESA, erythropoiesis-stimulating agent; MACE, major adverse cardiovascular events; MACE+, MACE plus heart failure and thromboembolic events.
MACE includes all-cause mortality plus hospitalization due to MI or stroke; MACE+ includes MACE plus HF and thromboembolic events (non-VA); rates expressed per 100 patient-years.
High CV risk patients are those meeting criteria for at least 2 of 6 factors: (1) age >65 years, (2) history of myocardial infarction, (3) history of stroke or transient ischemic attack, (4) History of congestive heart failure, (5) History of diabetes, (6) History of peripheral vascular disease.
Hyporesponder definition detailed in Methods (note: because of dose differences across countries, a different definition of hyporesponder was used in Japan (>5000 units/wk instead of >10,000 units/wk). Note that the number of patients in the Hyporesponder + Nonhyporesponder groups do not sum to the total because of missing data, deaths, and losses to follow-up during the 3-month run-in period. DOPPS phase 4 and 5 data combined.
The Kaplan−Meier plots in Figure 3 illustrate the proportion of HD patients remaining event (MACE+) free up to 2 years after study entry. After 1 year, 92% of patients in Japan remained event free, compared to 83% in EU and 82% in NA. After 2 years, 86% of patients in Japan remained event free, compared to 70% in EU and 68% in NA.
Figure 3.
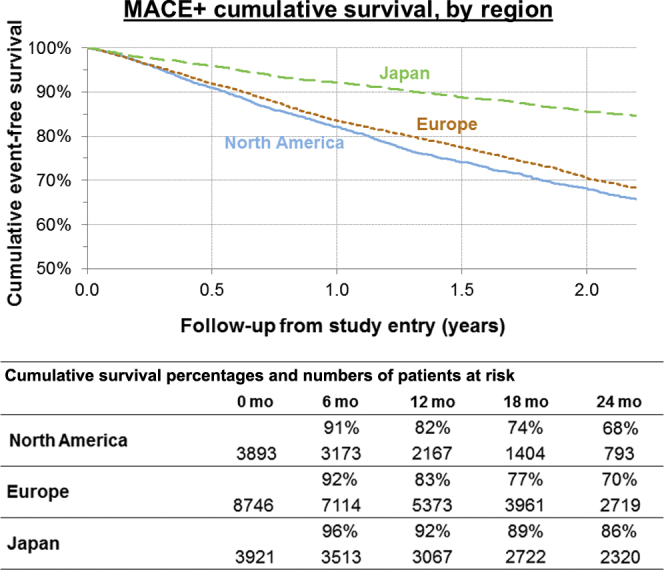
Kaplan−Meier cumulative event-free survival in MACE+ patients, by region, Dialysis Outcomes and Practice Patterns Study (DOPPS) phases 4 and 5 combined. MACE+, major adverse cardiovascular events (MACE) plus heart failure and thromboembolic events.
Discussion
In the current report, we leveraged the most recent data available from the international DOPPS program to estimate CV event rates in this unique sample that is generally representative of the dialysis populations within each of the participating countries. We have shown that CV event rates differ by geographical region and CV risk group, but not by DOPPS phase 4 (2009−2011) compared to phase 5 (2012−2015).
Overall CV event rates in the most recent study period did not differ substantially from prior years. Thus, despite lower ESA doses used in the US in DOPPS Cohort 5, CV events were less pronounced than expected. It is conceivable that the follow-up period after the anemia guideline changes may not have been long enough to reflect any differences.
All-cause mortality explained a large proportion of the MACE variable. This is consistent with a recent study showing no regional differences in overall mortality in HD subjects.13 For all subsequent analysis, data for DOPPS phases 4 and 5 were combined because of a lack of temporal changes.
Overall, CV event and mortality rates among dialysis patients were high, in keeping with reports by renal registries within many of the participating countries.14, 15, 16 Crude rates of the composite outcome of death or CV event varied widely across regions, being 2- to 3-fold higher in NA (19.4 per 100 patient-years) than in Japan (7.5 per 100 patient-years). The very low rates of CV events in Japan align with prior DOPPS findings17 and reports from the Japanese Society of Dialysis and Transplantation. The reasons for these regional differences is not completely clear at this time. Prior DOPPS analyses indicate that many beneficial dialysis practices (e.g., longer treatment time, adherence to dialysis schedule, mineral and bone disorder control) are more common in Japan than in other countries.18 It is conceivable that a combination of clinical practices, behavioral factors, and background event rates play a role in the very low CV events in Japan.
Independent of study region, CV event rates were higher in ESA-hyporesponsive subjects compared with the overall study sample. Previous result from the CHOIR trial showed that high ESA dose and not achieving the Hgb target was associated with increased mortality and CV events.19 These findings are consistent with a recent study showing that ESA dose (>8000 UI/wk) was significantly associated with increased mortality.18 In addition, we showed that CV event rates were significantly higher in the high CV risk sample without noticeable differences in ESA use, ESA dose, or Hgb levels compared to the low CV risk sample. This highlights the importance of both disease state and anemia management on CV event rates in the HD population.
The frequency of cardiovascular disease in ESRD in the US has increased from 67% in individuals 45 to 64 years old to 81.1% in those 75+ years old.14 However, the proportion of HD patients of the DOPPS population at high CV risk (combination of at least 2 risk factors) across all countries was lower than expected (mean, 53%; range by country, 44%−61%).
This report has unique strengths related to the international nature, consistent protocol across sites, and rigorous design with respect to site selection. Specifically, the DOPPS study design achieves national samples that are generally representative of the HD population within each participating country20; therefore, our reported patient characteristics and event rates could be applicable to the majority of HD patients in DOPPS countries.
The current study has a few limitations. First, sampling bias could be a concern, although it is reduced because of the rigorous sampling strategy in DOPPS. In addition, because mortality comprises a large proportion of MACE, the impact of nonfatal CV events on event rates was minimal. The greatest limitation was that cause-specific event rates in the US DOPPS sample may not be generalizable to all US HD patients, because outcome data from facilities with electronic data collection (approximately two-thirds of facilities in phases 4 and 5) were limited to all-cause mortality; these patients were thus excluded from all analyses due to missing information on the outcomes of interest. Overall, all rates are unadjusted and do not account for differences in patient characteristics.
In conclusion, this large global study of DOPPS HD cohorts quantified CV event rates. High CV event rates were demonstrated in the HD population, varying by geographic region, were even higher among patients with pre-existing CV risk factors, and in ESA-hyporesponsive patients based on a relatively new definition. To our surprise, event rates did not change appreciably over the study period in any DOPPS region.
The quantification of CV outcomes is essential to understand potential clinical trial populations and to place designs and results into the context of real-world findings. Enrichment strategies must be balanced with recruitment strategies in clinical trials, as the frequency of high CV risk patients is much smaller than previously anticipated; and recent regulatory and reimbursement changes, as well as clinical guidelines, have contributed to decreased ESA and Hgb levels in recent years, especially in the US. Further studies looking at the less selective ESRD population are needed to clarify whether the mortality and MACE rates are higher in ESA hyporesponsive population since the regulatory change on ESAs were introduced. Finally, current phase 3 studies of newer anemia investigational products, hypoxia prolyl-hydroxylase inhibitors such as daprodustat, may provide us an answer as to whether the ESA hyporesponsivness and higher mortality or MACE events are the result of higher ESA dose or other contributing factors.
Disclosure
HAS-F, BC, LK, DJ, ARC are employees and hold stocks of GlaxoSmithKline. AK, BAB, and BMR are employees of the nonprofit research organization Arbor Research Collaborative for Health, that has designed and carries out the Dialysis Outcomes and Practice Patterns Study (DOPPS) Program.
Acknowledgments
The DOPPS Program is supported by Amgen, Kyowa Hakko Kirin, and Baxter Healthcare. Additional support for specific projects and countries is provided by Amgen, AstraZeneca, European Renal Association-European Dialysis & Transplant Association (ERA-EDTA), German Society of Nephrology (DGfN), Hexal AG, Janssen, Japanese Society for Peritoneal Dialysis (JSPD) Keryx, Proteon, Relypsa, Roche, Società Italiana di Nefrologia (SIN), Spanish Society of Nephrology, Vifor Fresenius Medical Care Renal Pharma. Public funding and support is provided for specific DOPPS projects, ancillary studies, or affiliated research projects by Agence Nationale de la Recherche in France, Canadian Institutes of Health Research (CIHR) and Ontario Renal Network in Canada, National Health & Medical Research Council (NHMRC) in Australia, National Institute for Health Research (NIHR) via the Comprehensive Clinical Research Network (CCRN) in the United Kingdom, National Institutes of Health (NIH) and Patient-Centered Outcomes Research Institute (PCORI) in the United States, Thailand Research Foundation (TRF), Chulalongkorn University Matching Fund, King Chulalongkorn Memorial Hospital Matching Fund, and the National Research Council of Thailand (NRCT) in Thailand. All support is provided without restrictions on publications. This study was financially supported by GlaxoSmithKline.
References
- 1.Vassalotti J.A., Centor R., Turner B.J. Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. 2016;129:153–162. doi: 10.1016/j.amjmed.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L., Williet N., Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr. 2015;102:1585–1594. doi: 10.3945/ajcn.114.103366. [DOI] [PubMed] [Google Scholar]
- 3.Kutuby F., Wang S., Desai C. Anemia of chronic kidney disease. Disease-a-Month. 2015;61:421–424. doi: 10.1016/j.disamonth.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Bolignano D., D’Arrigo G., Pisano A. Pentoxifylline for anemia in chronic kidney disease: a systematic review and meta-analysis. PLoS One. 2015;10:1–16. doi: 10.1371/journal.pone.0134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration FDA Drug Safety Communication: Modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm Available at:
- 6.KDIGO (Kidney Disease Improving Global Outcomes) Anemia Work Group: KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int. 2012;2:279. [Google Scholar]
- 7.Fuller D.S., Pisoni R.L., Bieber B.A. The DOPPS Practice Monitor for US dialysis care: trends through December 2011. Am J Kidney Dis. 2013;61:342–346. doi: 10.1053/j.ajkd.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Fuller D.S., Pisoni R.L., Bieber B.A. The DOPPS Practice Monitor for US dialysis care: update on trends in anemia management 2 years into the bundle. Am J Kidney Dis. 2013;62:1213–1216. doi: 10.1053/j.ajkd.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Karaboyas A., Zee J., Morgenstern H. Understanding the recent increase in ferritin levels in United States dialysis patients: potential impact of changes in intravenous iron and erythropoiesis-stimulating agent dosing. J Am Soc Nephrol. 2015;10:1814–1821. doi: 10.2215/CJN.02600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dialysis Outcomes and Practice Patterns Study Practice Monitor. www.dopps.org/DPM/ Available at:
- 11.Luo J., Jensen D.E., Maroni Spectrum and burden of erythropoiesis-stimulating agent hyporesponsiveness among contemporary hemodialysis patients. Am J Kidney Dis. 2016;68:763–771. doi: 10.1053/j.ajkd.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Stirnadel-Farrant H.A., Luo J., Kler L. Anemia and mortality in patients with nondialysis-dependent chronic kidney disease. BMC Nephrology. 2018;19:135–146. doi: 10.1186/s12882-018-0925-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pérez-García R., Varas J., Cives A. Increased mortality in haemodialysis patients administered high doses of erythropoiesis-stimulating agents: a propensity score-matched analysis. Nephrol Dial Transplant. 2018;33:690–699. doi: 10.1093/ndt/gfx269. [DOI] [PubMed] [Google Scholar]
- 14.United States Renal Data System, 2017 annual data report: an overview of the epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2017. [Google Scholar]
- 15.Kato S., Abe T., Lindholm B., Maruyama S. Neutrophil/lymphocyte ratio: a promising prognostic marker in patients with chronic kidney disease. Inflam Cell Signal. 2015;2:1–5. [Google Scholar]
- 16.ERA-EDTA Registry: ERA-EDTA Registry Annual Report 2012. Academic Medical Center, Department of Medical Informatics; Amsterdam, Netherlands: 2014. [Google Scholar]
- 17.Goodkin D.A., Bragg-Gresham J.L., Koenig K.G. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc of Nephrol. 2003;14:3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 18.Robinson B.M., Akizawa T., Jager K.J. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and hemodialysis practices. Lancet. 2016;388:294–306. doi: 10.1016/S0140-6736(16)30448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szczech L.A., Barnhart H.X., Inrig J.K. Secondary analysis of the CHOIR trial epoetin-α dose and achieved hemoglobin outcomes. Kidney Int. 2008;74:791–798. doi: 10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pisoni R.L., Gillespie B.W., Dickinson D.M. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44:7–15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]