Abstract
Introduction
Magnesium (Mg) may protect against arterial calcification. We tested the hypotheses that a higher serum Mg concentration is associated with less arterial calcification and stiffness in patients on hemodialysis (HD) and that these associations are modified by diabetes mellitus.
Methods
We performed cross-sectional analyses of 367 incident HD patients from the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease (PACE) cohort. Measures of arterial calcification and stiffness included coronary arterial calcification (CAC) and thoracic aortic calcification (TAC) scores, ankle brachial index (ABI; high ABI: >1.4 or incompressible vessels), pulse wave velocity (PWV), and pulse pressure.
Results
Mean Mg was 1.8 ± 0.2 mEq/l and 58% had diabetes. Among nondiabetic individuals, per 0.1 mEq/l higher Mg, non-zero CAC score was lower (% difference: −15.4%; 95% confidence interval [CI]: −28% to −0.55%; P = 0.03), the odds of having TAC score >0 and the odds of having high ABI were lower (odds ratio [OR]: 0.66; 95% CI 0.47–0.93; P = 0.02, and 0.23; 95% CI: 0.06–0.83, P = 0.03, respectively) while adjusting for demographics, comorbidities, markers of mineral metabolism, and dialysis clearance. Among diabetic individuals, per 0.1 mEq/l higher Mg, the odds of having TAC score >0 was higher (OR: 1.57; 95% CI: 1.09–2.26; P = 0.02). Mg was not associated with CAC or high ABI among diabetic individuals. Mg was not associated with PWV or pulse pressure regardless of diabetes status.
Conclusion
Diabetes modified the associations of serum Mg with arterial calcification and stiffness in incident HD patients. Higher Mg was associated with less arterial calcification and less peripheral arterial stiffness among nondiabetic individuals, but Mg was only associated with TAC among diabetic individuals with higher Mg being associated with higher likelihood of having TAC score >0.
Keywords: arterial calcification, arterial stiffness, diabetes mellitus, magnesium, mineral metabolism
Arterial calcification is common in patients with chronic kidney disease (CKD).1 Calcification can lead to arterial stiffness, systolic hypertension, left ventricular hypertrophy, and ultimately contribute to cardiovascular death.1, 2, 3 The extent of arterial calcification independently predicts cardiovascular morbidity and mortality.1, 2 In patients with CKD, the disturbances in the metabolism of divalent ions such as calcium, phosphate, and magnesium (Mg) play an important role in the development of arterial calcification.4
Whereas calcium and phosphate retention are known to promote arterial calcification,5 in the past decade, several studies have suggested that Mg may protect against arterial calcification.6, 7, 8, 9, 10 In rodents, dietary Mg supplementation prevented vascular and cardiac calcification.6, 7, 8 In 2 small studies of dialysis patients, oral Mg supplementation decreased carotid intima-medial thickness.9, 10 Both in vitro and in vivo studies also showed that Mg attenuated phosphate-induced arterial calcification.11, 12 Although the precise mechanism by which Mg may protect against arterial calcification is unknown, Mg inhibits calcium pyrophosphate dehydrate crystal formation,13 increases the expression of calcification inhibitors, and regulates the activity of transient receptor potential cation channel subfamily M member 7 in vascular smooth muscle cells.14
In this study, we tested the hypothesis that a higher serum Mg concentration is associated with less arterial calcification and stiffness among incident hemodialysis (HD) patients from the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease (PACE) study. Because diabetes mellitus is a risk factor for both hypomagnesemia and arterial calcification,15, 16 we also examined whether diabetes modifies the relationship of serum Mg with arterial calcification and stiffness.
Materials and Methods
Study Population
PACE is a prospective study designed to determine cardiovascular and dialysis-related risk factors associated with cardiac dysfunction and incidence of sudden cardiac death in patients on HD. Incident HD patients receiving regular outpatient HD thrice weekly for less than 6 months were recruited from 25 free-standing outpatient HD units and 2 hospital-based outpatient units in Baltimore, Maryland, and its surrounding area from 2008 to 2012. The details of eligibility criteria and recruitment were described previously.17 The PACE study was approved by the Johns Hopkins School of Medicine and MedStar Institutional Review Boards. A total of 568 participants were consented and enrolled at baseline. For this study, 367 participants were included for analyses after excluding those with missing serum Mg (n = 201). The flow chart of study population is in Supplementary Figure S1.
Measurement of Serum Magnesium
Total serum Mg concentration was measured in the blood collected on a non-HD day after approximately 8 hours of fasting at the baseline visit. All participants received HD on a dialysate Mg concentration of 1.0 mEq/l. For the comparisons of participant characteristics, serum Mg levels were categorized into tertiles of the entire study population: <1.7 mEq/l (n = 112), 1.7–1.8 mEq/l (n = 135), and >1.8 mEq/l (n = 120). We considered using the normal range of Mg levels (1.5–2.0 mEq/l)18 as the reference group, but this approach results in small groups for comparison (n = 34 for Mg <1.5 mEq/l and n = 44 for Mg >2.0 mEq/l). For the main analyses of serum Mg with arterial calcification and stiffness, serum Mg was examined as a continuous variable.
Measurement of Arterial Calcification and Stiffness
Outcome variables were coronary arterial calcification (CAC) score, thoracic aortic calcification (TAC) score, ankle brachial index (ABI), pulse wave velocity (PWV), and pulse pressure. Arterial calcification was measured in the coronary arteries and thoracic aorta using computed tomography (Aquilon One; Toshiba, Tokyo, Japan) at the baseline visit.19 To minimize the effect of cardiac motion, a single prospective electrocardiographic-triggered acquisition was taken and images were acquired in mid-diastole within 1 R to R interval of a single heartbeat. CAC score was quantified using Agatston score.17 TAC score was calculated as the sum of calcium scores from ascending and descending thoracic aorta. Of the 367 participants, 287 participants had computed tomography examination and available CAC scores. TAC scores were only available in 200 participants because the computed tomography image window was too small to include the whole thoracic aorta in 87 participants. Median time for computed tomography measurement was 3.6 months (interquartile range 2.7–5.4) after the initiation of chronic HD.
Arterial stiffness was defined as high ABI, PWV, or pulse pressure. ABI is the ratio of ankle to brachial systolic blood pressure, and the lower of the bilateral ABI measurements was used for analyses.20 Because development of arterial stiffness is a relatively slow process,21 we included ABI measurements that were obtained either at baseline (n = 280) or year 1 (n = 32). Participants with ABI ≤0.9 (n = 51) were excluded from the analyses because an ABI ≤0.9 indicates the presence of peripheral arterial disease.22 High ABI was defined as an ABI >1.4 or having incompressible vessels, and normal ABI as >0.9 and ≤1.4.23 PWV measurements were taken supine in the carotid and femoral arteries using Sphygmocor PVx system (AtCor Medical, West Ryde, Australia), and measured either at baseline (n = 278) or year 1 (n = 23). Pulse pressure was defined as seated systolic minus diastolic blood pressure on a non-HD day. Blood pressure was taken 3 times and the readings were averaged. There were 261 participants available for the analyses of ABI, 301 for PWV, and 364 for pulse pressure.
Measurement of Covariates
Confounders were selected a priori and included self-reported demographic factors (age, sex, and race), education level, smoking history, medical history, serum markers of mineral metabolism, and parameters of HD. Comorbidities such as diabetes and hypertension were adjudicated by a committee of physicians. Serum calcium, phosphate, intact parathyroid hormone, albumin, and hemoglobin levels were averaged using 3 months of laboratory values collected before a dialysis session. Fetuin-A level was measured using an enzyme-linked immunosorbent assay with a coefficient of variation of 18% (Epitope Diagnostics, San Diego, CA) in blood collected on a non-HD day. HD adequacy was assessed by 3-month average single-pool Kt/V before the study visit. Medication use was recorded during the study visit. Vitamin D therapy included both nutritional vitamin D supplement and activated vitamin D therapy.
Statistical Analyses
Baseline participant characteristics were examined by diabetes status and serum Mg tertiles. For continuous variables, 2-sample t-test or Mann-Whitney U test was used to examine characteristics by diabetes status, and analysis of variance or Kruskal-Wallis test was used for serum Mg tertiles. For categorical variables, χ2 tests were used.
CAC and TAC scores were examined as categorical variables after being dichotomized at 0 (score >0 vs. =0). Non-zero CAC and TAC scores24 were examined as continuous variables after log-transformation to meet the normality assumption. As sensitivity analyses, we examined the combination of zero and non-zero CAC and TAC scores as continuous variables after the log-transformation of the scores plus 1 (i.e., log[score+1]). For arterial stiffness, high ABI was compared with normal ABI. PWV and pulse pressure were examined as continuous variables. PWV was log-transformed to meet the normality assumption.
Multiple linear regression models were used to examine continuous outcome variables. Multiple logistic regression models were used for binary outcome variables. Models were adjusted for age, sex, race, diabetes status, smoking history, body mass index, serum calcium, phosphate, intact parathyroid hormone, fetuin-A, albumin, hemoglobin, low-density lipoprotein, and single-pool Kt/V. The percentage changes in non-zero CAC, TAC scores, and PWV were calculated by transforming the β coefficients (% change = 100 × [eβ − 1]). Effect modification of the association between serum Mg and arterial calcification/stiffness by diabetes status was evaluated by stratified analyses and using first-order interaction terms. A 2-sided P value <0.05 was considered statistically significant for all analyses. All analyses were conducted using STATA 14.1 (StataCorp, College Station, TX).
Results
Participant Characteristics
Mean age of the participants was 55 years; 40% were women; 72% were black; 211 (58%) participants had diabetes and all had hypertension (Table 1). These baseline characteristics were similar compared with the PACE participants without Mg levels (Supplementary Table S1). Compared with nondiabetic individuals, people with diabetes were older and less likely to have a history of smoking (Table 1). Diabetic individuals also had higher body mass index and lower single-pool Kt/V than nondiabetic individuals.
Table 1.
Demographic and clinical characteristics of participants by diabetes status and serum Mg tertiles
| Characteristics | Total (n = 367) | Nondiabetic individuals (n = 156) |
Diabetic individuals (n = 211) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total nondiabetic individuals | Mg <1.7 mEq/l (n = 36) | Mg 1.7–1.8 mEq/l (n = 61) | Mg >1.8 mEq/l (n = 59) | Total individuals with diabetes | Mg <1.7 mEq/l (n = 76) | Mg 1.7–1.8 mEq/l (n = 74) | Mg >1.8 mEq/l (n = 61) | ||
| Age, yr | 55 ± 13 | 53 ± 15 | 53 ± 17 | 53 ± 16 | 54 ± 14 | 57 ± 11a | 57 ± 12 | 57 ± 11 | 56 ± 12 |
| Women, n (%) | 145 (40) | 65 (42) | 12 (33) | 25 (41) | 28 (47) | 80 (38) | 32 (42) | 26 (35) | 22 (36) |
| Black, n (%) | 266 (72) | 110 (71) | 26 (72) | 42 (69) | 42 (71) | 156 (74) | 52 (68) | 58 (78) | 46 (75) |
| Body mass index | 30 ± 8 | 27 ± 8 | 27 ± 7 | 29 ± 9 | 26 ± 5 | 31 ± 8a | 32 ± 8 | 30 ± 8 | 31 ± 8 |
| History of smoking, n (%) | 223 (61) | 108 (69) | 22 (61) | 43 (70) | 43 (73) | 115 (55)a | 40 (53) | 37 (50) | 38 (62) |
| Education, <grade 11, n (%) | 130 (36) | 61 (39) | 14 (39) | 30 (50) | 17 (29) | 69 (33) | 22 (29) | 27 (36) | 20 (33) |
| Serum calcium, mg/dl | 8.6 ± 0.6 | 8.8 ± 0.7 | 8.6 ± 0.8 | 8.7 ± 0.6 | 8.9 ± 0.7b | 8.6 ± 0.6a | 8.5 ± 0.5 | 8.6 ± 0.6 | 8.6 ± 0.6 |
| Serum phosphate, mg/dl | 5.2 ± 1.1 | 5.2 ± 1.2 | 4.8 ± 1.1 | 5.1 ± 1.2 | 5.5 ± 1.2b | 5.1 ± 1.1 | 5.0 ± 1.0 | 5.2 ± 1.2 | 5.3 ± 0.9 |
| Serum magnesium, mEq/l | 1.8 ± 0.2 | 1.8 ± 0.3 | 1.4 ± 0.2 | 1.8 ± 0.1 | 2.0 ± 0.2b | 1.8 ± 0.2 | 1.5 ± 0.1 | 1.8 ± 0.1 | 2.0 ± 0.1b |
| Intact parathyroid hormone, pg/ml | 383 (253–570) | 371 (243–580) | 319 (196–456) | 400 (257–638) | 375 (252–580) | 391 (266–564) | 403 (290–570) | 391 (247–590) | 390 (245–510) |
| Fetuin-A, g/l | 0.51 ± 0.18 | 0.52 ± 0.18 | 0.47 ± 0.18 | 0.52 ± 0.17 | 0.56 ± 0.19 | 0.50 ± 0.17 | 0.47 ± 0.13 | 0.51 ± 0.18 | 0.54 ± 0.19 |
| Serum albumin, g/dl | 3.7 ± 0.5 | 3.7 ± 0.5 | 3.6 ± 0.6 | 3.7 ± 0.5 | 3.8 ± 0.4 | 3.6 ± 0.4a | 3.5 ± 0.4 | 3.6 ± 0.4 | 3.6 ± 0.5 |
| Hemoglobin, g/dl | 10.8 ± 1.2 | 10.8 ± 1.3 | 10.5 ± 1.6 | 10.6 ± 1.4 | 11.1 ± 1.1 | 10.8 ± 1.1 | 10.6 ± 1.1 | 10.8 ± 1.2 | 11.1 ± 1.0b |
| LDL, mg/dl | 82 (61–108) | 89 (63–115) | 89 (59–106) | 94 (66–106) | 81 (59–124) | 80 (60–106) | 72 (54–101) | 82 (62–110) | 79 (59–103) |
| Calcium-based phosphate binder, n (%) | 135 (37) | 55 (35) | 8 (22) | 26 (43) | 21 (36) | 80 (38) | 27 (35) | 28 (38) | 25 (41) |
| Vitamin D therapy,cn (%) | 283 (77) | 112 (72) | 23 (64) | 45 (74) | 44 (75) | 171 (81)a | 63 (83) | 56 (76) | 52 (85) |
| RAAS blockage, n (%) | 141 (42) | 59 (41) | 15 (45) | 21 (38) | 23 (43) | 82 (43) | 26 (38) | 29 (45) | 27 (47) |
| Single-pool Kt/V | 1.8 ± 0.3 | 1.9 ± 0.3 | 1.9 ± 0.4 | 1.8 ± 0.3 | 1.9 ± 0.3 | 1.8 ± 0.3a | 1.7 ± 0.3 | 1.8 ± 0.3 | 1.8 ± 0.3 |
LDL, low-density lipoprotein; RAAS, renin-angiotensin-aldosterone system.
Note: If normally distributed, values for continuous variables with normal distribution are provided as mean ± SD. Otherwise, they are provided as median (interquartile range). Categorical variables are presented as absolute number with percentage.
P < 0.05 compared with nondiabetic individuals.
P for trend <0.05 across serum Mg tertiles for individuals with and without diabetes.
Vitamin D therapy includes both nutritional vitamin D supplement and activated vitamin D therapy.
Overall serum Mg concentration (mean ± SD) was 1.8 ± 0.2 mEq/l and ranged from 1.1 to 2.4 mEq/l (Table 1). Serum Mg was similar between nondiabetic individuals and people with diabetes. Among nondiabetic individuals, higher serum Mg was associated with higher serum calcium and phosphate levels. Among people with diabetes, higher serum Mg was associated with higher hemoglobin level.
Association Between Mg and Arterial Calcification
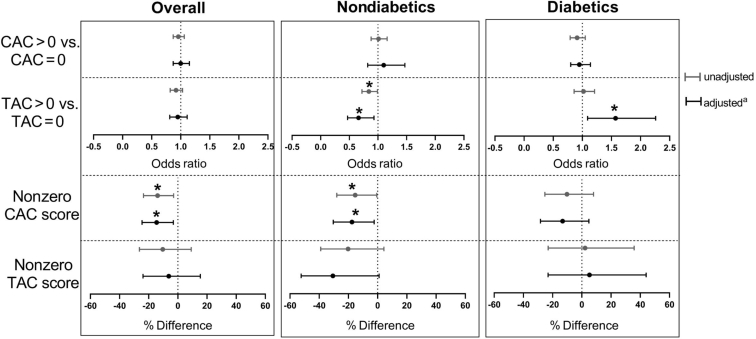
A total of 182 (63%) participants had CAC score >0 and 100 (50%) had TAC score >0. Among all participants, serum Mg level was associated only with non-zero CAC scores (Figure 1). Per 0.1 mEq/l higher Mg, non-zero CAC score was 14.6% lower (adjusted % difference: −14.6%; 95% CI: −24.6% to −3.1%; P = 0.02). Among nondiabetic individuals, higher serum Mg was associated with lower non-zero CAC score and lower odds of having a TAC score >0. Per 0.1 mEq/l higher Mg, non-zero CAC score was 15.4% lower (adjusted % difference: −15.4%; 95% CI: −28% to −0.55%; P = 0.03) and the odds of having a TAC score >0 versus having a score of 0 was 34% lower (adjusted OR: 0.66; 95% CI: 0.47–0.93; P = 0.02). Among people with diabetes, serum Mg was only associated with TAC score >0 in the adjusted model. The odds of having a TAC score >0 versus having a score of 0 was 57% greater (adjusted OR: 1.57; 95% CI: 1.09–2.26; P = 0.02) per 0.1 mEq/l higher Mg. The interaction term for serum Mg and diabetes status was only significant for TAC when the TAC score was dichotomized at 0 (P = 0.003, Supplementary Table S2). Sensitivity analyses examining the combination of zero and non-zero CAC and TAC scores as continuous variables yielded similar results (Supplementary Figure S2).
Figure 1.
Association between serum magnesium (Mg) and arterial calcification (per 0.1 mEq/l higher serum Mg). CAC, coronary arterial calcification; TAC, thoracic aortic calcification. aAdjusted for age, sex, race, history of diabetes mellitus, smoking, body mass index, serum calcium, phosphate, intact parathyroid hormone, fetuin-A, albumin, hemoglobin, low-density lipoprotein, and single-pool Kt/V. In the stratified models, diabetes status was not included as a covariate. *P < 0.05.
Association Between Serum Mg and Arterial Stiffness
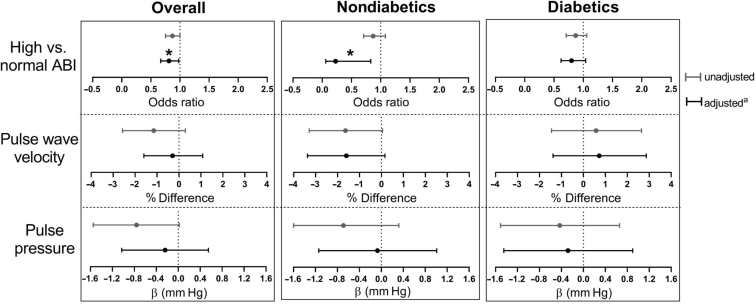
Thirty-nine (15%) participants had a high ABI. Median PWV was 10.4 (interquartile range 8.0–12.7) meter per second. Mean pulse pressure was 63 ± 18 mm Hg. Among all participants, serum Mg was only associated with high ABI in the adjusted model (Figure 2). Per 0.1 mEq/l higher serum Mg, the odds of having high versus normal ABI was 19% lower (adjusted OR: 0.81; 95% CI: 0.67–0.98; P = 0.03). Similarly, among nondiabetic individuals, serum Mg was only associated with high ABI in the adjusted model. Per 0.1 mEq/l higher serum Mg, the odds of having high versus normal ABI was 77% lower (adjusted OR: 0.23; 95% CI: 0.06–0.83; P = 0.03). Among people with diabetes, serum Mg was not associated with any measure of arterial stiffness. None of the interaction terms for serum Mg and diabetes status were statistically significant (Supplementary Table S2).
Figure 2.
Association between serum Mg and arterial stiffness (per 0.1 mEq/l higher serum Mg). aAdjusted for age, sex, race, history of diabetes mellitus, smoking, body mass index, serum calcium, phosphate, intact parathyroid hormone, fetuin-A, albumin, hemoglobin, low-density lipoprotein, and single-pooled Kt/V. In the stratified models, diabetes status was not included as a covariate. *P < 0.05.
Conclusion
In this study, we found that higher serum Mg was associated with lower arterial calcification and stiffness in incident HD patients, but only among those without diabetes. Diabetes mellitus qualitatively modified the associations of serum Mg concentration with arterial calcification and stiffness, especially the association between serum Mg and TAC. Among nondiabetic individuals, higher serum Mg was associated with lower CAC scores, lower likelihood of having TAC score >0, and lower likelihood of having high ABI. However, among people with diabetes, higher serum Mg was associated with higher likelihood of having TAC score >0 and not associated with other measures of arterial calcification or stiffness. Prior studies have demonstrated that a higher Mg level is associated with less arterial calcification,25, 26, 27, 28, 29 but we found no studies that have reported whether diabetes modifies this relationship.
Why diabetes modifies the association between serum Mg and aortic calcification is unclear but may be due to the differences in the pathways that CKD and diabetes induce arterial calcification. Both CKD and diabetes are proinflammatory states with elevated cytokines that can induce osteogenic differentiation of vascular smooth muscle cells30 and can lead to both medial and intimal calcification.31 Patients with CKD have disordered mineral metabolism that contributes to the development of arterial calcification,4, 32 whereas patients with diabetes often develop arterial calcification due to poor glycemic control and insulin resistance. Patients with diabetes generally do not have disordered metabolism of calcium or phosphate before developing advanced CKD.33 In our study, serum Mg was not associated with CAC or arterial stiffness among people with diabetes. The protective effect of Mg on arterial calcification may be independent of the metabolic abnormalities solely associated with diabetes; or the factors inducing arterial calcification in diabetes may mask any effect of Mg on preventing calcification. Why TAC but not CAC was higher with increasing serum Mg in people with diabetes is not clear and will require further study.
Diabetic nephropathy is one of the most common causes of end stage renal disease.34 If confirmed in larger studies, the findings of this study may have important implications on Mg supplementation in preventing arterial calcification. In animal studies, dietary supplementation of Mg prevented the development of arterial and cardiac calcification,6, 7, 8 and this effect was independent of the action of Mg as an intestinal phosphate binder.8 In patients on HD, observational studies showed an inverse relationship between serum Mg and arterial calcification.25, 26, 27, 28, 29 Two small clinical trials (n = 47 and n = 54) demonstrated that oral Mg supplementation decreased carotid intima-media thickness in patients on HD.9, 10 Using a serum marker of calcification propensity, a recent single-center, randomized controlled trial35 showed that increasing dialysate Mg from 1.0 to 2.0 mEq/l for 4 weeks increased the potency of serum Mg to inhibit calcification.36 These studies have led to the idea of supplementing Mg in dialysis patients,6, 7, 8, 9, 10, 37 even before the results of an on-going clinical trial.38 The results of our study suggest that the diabetes status of patients should be considered in clinical trials.
Over the past decades, the commonly used concentration of dialysate Mg decreased from approximately 1.5 mEq/l in the 1970s to 1.0 mEq/l currently.39 This change was due, in part, to the concern that hypermagnesemia could suppress parathyroid hormone and interfere with the process of bone mineralization, contributing to adynamic bone disease.40, 41 In a Japanese cohort of patients on HD,42 higher serum Mg was associated with lower intact parathyroid hormone. In this study, serum Mg was not associated with intact parathyroid hormone in participants with or without diabetes. (Table 1). Compared with the Japanese cohort, serum Mg level in our cohort was lower (mean 1.76 vs. 2.1 mEq/l) and intact parathyroid hormone level was higher (median 383 vs.126 pg/ml). Future studies are needed to determine the potential benefits and risks of Mg supplementation on not only arterial calcification, but also on parathyroid hormone levels, bone strength, and bone architecture in patients with CKD.
Our study has limitations. First, because it is a cross-sectional study, temporal relationship of serum Mg with arterial calcification and stiffness could not be studied, thus limiting the inference of a potential causal relationship. Second, because Mg is mainly intracellular, serum Mg does not accurately reflect total body Mg; however, serum Mg is the only Mg measurement available in clinical setting. Third, serum Mg was collected on different non-HD days during the study visit. We do not have the day of the week on which blood was collected with respect to participants’ dialysis schedules. All participants received dialysis thrice weekly, thus there was a 2-day break between the last session of the week and the first session of the next one. Serum Mg drawn in the beginning of the week might be higher than that done later during the week. Fourth, the interaction terms for serum Mg and diabetes were not significant except for the comparison between TAC >0 and TAC = 0. This could be because we need a bigger sample to test the interactions. However, we performed stratified analyses and found that the associations of serum Mg with arterial calcification and stiffness were qualitatively different between participants with and without diabetes. Last, residual renal function may influence the association of serum Mg with arterial calcification and stiffness. Only a third of participants had available data on residual renal function. Due to the limitation in sample size, we did not include residual renal function in our analyses.
There are several strengths in our study. We demonstrated that diabetes modified the relationship of serum Mg with arterial calcification and stiffness in incident HD patients. This finding not only provides insight into the development of arterial calcification in CKD and diabetes, it also has an important clinical implication, as Mg supplementation is being considered as a therapy for arterial calcification in CKD. We attempted a thorough evaluation of arterial calcification by quantifying CAC and TAC and by assessing arterial stiffness using ABI, PWV, and pulse pressure with standardized protocols.17 Prior studies examined the relationship between Mg and arterial calcification using only 1 measure of calcification.26, 27, 28, 43 As some arteries are more prone to develop medial calcification than others,44 it is important to study calcification in different arterial beds.
In this study, we evaluated the association of serum Mg concentration with arterial calcification and stiffness by diabetes status in incident HD patients, and found that diabetes modified these relationships. Among nondiabetic individuals, higher serum Mg was associated with less arterial calcification and less peripheral arterial stiffness; however, among people with diabetes, Mg was not associated with arterial calcification or stiffness except that higher serum Mg was associated with higher likelihood of have a TAC score >0. If confirmed in larger studies, the findings may have a significant clinical implication on the use of Mg supplements in HD patients. Diabetes status of participants should be considered in designing clinical trials that examine the effect of Mg on arterial calcification.
Disclosure
DAB is a consultant for Relyspa/Vifor/Fresenius, Amgen, Sanofi/Genzyme, and Tricida and has an equity interest in Amgen and Tricida. All the other authors declared no competing interests.
Acknowledgments
We thank participants, nephrologists, and staff of the DaVita and MedStar dialysis units in the Baltimore region who contributed to the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease (PACE) study. The PACE study was funded by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; R01 DK072367 and DK090070) and National Kidney Foundation of Maryland. WC is supported by the American Society of Nephrology Carl W. Gottschalk Research Grant and NIDDK (K23 DK114476). DAB is supported by NIDDK (R01 DK075462). RSP is supported by the Canada Research Chair in chronic kidney disease epidemiology. Funders had no role in any aspect of the publication.
Footnotes
Table S1. Demographic and clinical characteristics of PACE participants with and without Mg level.
Table S2. P values for interaction between serum Mg and diabetes status.
Figure S1. Flow chart of the study population.
Figure S2. Association between serum Mg and arterial calcification scores (combining zero and non-zero scores, as continuous variables) (per 0.1 mEq/l higher serum Mg).
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Demographic and clinical characteristics of PACE participants with and without Mg level.
P values for interaction between serum Mg and diabetes status.
Flow chart of the study population.
Association between serum Mg and arterial calcification scores (combining zero and non-zero scores, as continuous variables) (per 0.1 mEq/l higher serum Mg).
References
- 1.Gorriz J.L., Molina P., Cerveron M.J. Vascular calcification in patients with nondialysis CKD over 3 years. Clin J Am Soc Nephrol. 2015;10:654–666. doi: 10.2215/CJN.07450714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.London G.M., Guerin A.P., Marchais S.J. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 3.London G.M. Mechanisms of arterial calcifications and consequences for cardiovascular function. Kidney Int Suppl. 2013;3:442–445. doi: 10.1038/kisup.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shroff R.C., McNair R., Skepper J.N. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol. 2010;21:103–112. doi: 10.1681/ASN.2009060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giachelli C.M. Vascular calcification mechanisms. J Am Soc Nephrol. 2004;15:2959–2964. doi: 10.1097/01.ASN.0000145894.57533.C4. [DOI] [PubMed] [Google Scholar]
- 6.Gorgels T.G., Waarsing J.H., de Wolf A. Dietary magnesium, not calcium, prevents vascular calcification in a mouse model for pseudoxanthoma elasticum. J Mol Med. 2010;88:467–475. doi: 10.1007/s00109-010-0596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Broek F.A., Beynen A.C. The influence of dietary phosphorus and magnesium concentrations on the calcium content of heart and kidneys of DBA/2 and NMRI mice. Lab Anim. 1998;32:483–491. doi: 10.1258/002367798780599758. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Tocados J.M., Peralta-Ramirez A., Rodriguez-Ortiz M.E. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int. 2017;92:1084–1099. doi: 10.1016/j.kint.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Turgut F., Kanbay M., Metin M.R. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int Urol Nephrol. 2008;40:1075–1082. doi: 10.1007/s11255-008-9410-3. [DOI] [PubMed] [Google Scholar]
- 10.Mortazavi M., Moeinzadeh F., Saadatnia M. Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: a double-blind, randomized, placebo-controlled trial. Eur Neurol. 2013;69:309–316. doi: 10.1159/000346427. [DOI] [PubMed] [Google Scholar]
- 11.Louvet L., Buchel J., Steppan S. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol Dial Transplant. 2013;28:869–878. doi: 10.1093/ndt/gfs520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi Y., Fujii N., Shoji T. Committee of Renal Data Registry of the Japanese Society for Dialysis T. Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: a cohort study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0116273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ter Braake A.D., Tinnemans P.T., Shanahan C.M. Magnesium prevents vascular calcification in vitro by inhibition of hydroxyapatite crystal formation. Sci Rep. 2018;8:2069. doi: 10.1038/s41598-018-20241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montezano A.C., Zimmerman D., Yusuf H. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension. 2010;56:453–462. doi: 10.1161/HYPERTENSIONAHA.110.152058. [DOI] [PubMed] [Google Scholar]
- 15.Katz R., Budoff M.J., O'Brien K.D. The metabolic syndrome and diabetes mellitus as predictors of thoracic aortic calcification as detected by non-contrast computed tomography in the Multi-Ethnic Study of Atherosclerosis. Diabet Med. 2016;33:912–919. doi: 10.1111/dme.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pham P.C., Pham P.M., Pham S.V. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2007;2:366–373. doi: 10.2215/CJN.02960906. [DOI] [PubMed] [Google Scholar]
- 17.Parekh R.S., Meoni L.A., Jaar B.G. Rationale and design for the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease (PACE) study. BMC Nephrol. 2015;16:63. doi: 10.1186/s12882-015-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bringhurst F.R., Demay M.B., Krane S.M., Kronenberg H.M. Bone and mineral metabolism in health and disease. In: Longo D.L., Fauci A.S., Kasper A.S., editors. Harrison's Principles of Internal Medicine. 17 ed. The McGraw-Hill Companies; New York, NY: 2018. p. 2372. [Google Scholar]
- 19.Jaar B.G., Zhang L., Chembrovich S.V. Incidental findings on cardiac computed tomography in incident hemodialysis patients: the predictors of arrhythmic and cardiovascular events in end-stage renal disease (PACE) study. BMC Nephrol. 2014;15:68. doi: 10.1186/1471-2369-15-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aboyans V., Criqui M.H., Abraham P. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 21.Avramovski P., Janakievska P., Sotiroski K., Sikole A. Accelerated progression of arterial stiffness in dialysis patients compared with the general population. Korean J Intern Med. 2013;28:464–474. doi: 10.3904/kjim.2013.28.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohler E.R., 3rd Peripheral arterial disease: identification and implications. Arch Intern Med. 2003;163:2306–2314. doi: 10.1001/archinte.163.19.2306. [DOI] [PubMed] [Google Scholar]
- 23.Hendriks E.J., Westerink J., de Jong P.A. Association of high ankle brachial index with incident cardiovascular disease and mortality in a high-risk population. Arterioscler Thromb Vasc Biol. 2016;36:412–417. doi: 10.1161/ATVBAHA.115.306657. [DOI] [PubMed] [Google Scholar]
- 24.Block G.A., Wheeler D.C., Persky M.S. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23:1407–1415. doi: 10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikee R. Cardiovascular disease, mortality, and magnesium in chronic kidney disease: growing interest in magnesium-related interventions. Renal Replacement Therapy. 2018;4:1. [Google Scholar]
- 26.Tzanakis I., Pras A., Kounali D. Mitral annular calcifications in haemodialysis patients: a possible protective role of magnesium. Nephrol Dial Transplant. 1997;12:2036–2037. [PubMed] [Google Scholar]
- 27.Tzanakis I., Virvidakis K., Tsomi A. Intra- and extracellular magnesium levels and atheromatosis in haemodialysis patients. Magnes Res. 2004;17:102–108. [PubMed] [Google Scholar]
- 28.Ishimura E., Okuno S., Kitatani K. Significant association between the presence of peripheral vascular calcification and lower serum magnesium in hemodialysis patients. Clin Nephrol. 2007;68:222–227. doi: 10.5414/cnp68222. [DOI] [PubMed] [Google Scholar]
- 29.Liu F., Zhang X., Qi H. Correlation of serum magnesium with cardiovascular risk factors in maintenance hemodialysis patients—a cross-sectional study. Magnes Res. 2013;26:100–108. doi: 10.1684/mrh.2013.0344. [DOI] [PubMed] [Google Scholar]
- 30.Tintut Y., Patel J., Parhami F., Demer L.L. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102:2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 31.Hassan N.A., D'Orsi E.T., D'Orsi C.J., O'Neill W.C. The risk for medial arterial calcification in CKD. Clin J Am Soc Nephrol. 2012;7:275–279. doi: 10.2215/CJN.06490711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shroff R., Long D.A., Shanahan C. Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol. 2013;24:179–189. doi: 10.1681/ASN.2011121191. [DOI] [PubMed] [Google Scholar]
- 33.Mehrotra R., Budoff M., Hokanson J.E. Progression of coronary artery calcification in diabetics with and without chronic kidney disease. Kidney Int. 2005;68:1258–1266. doi: 10.1111/j.1523-1755.2005.00522.x. [DOI] [PubMed] [Google Scholar]
- 34.Krolewski M., Eggers P.W., Warram J.H. Magnitude of end-stage renal disease in IDDM: a 35 year follow-up study. Kidney Int. 1996;50:2041–2046. doi: 10.1038/ki.1996.527. [DOI] [PubMed] [Google Scholar]
- 35.Bressendorff I., Hansen D., Schou M. The effect of increasing dialysate magnesium on serum calcification propensity in subjects with end stage kidney disease: a randomized, controlled clinical trial. Clin J Am Soc Nephrol. 2018;13:1373–1380. doi: 10.2215/CJN.13921217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasch A. Novel assessments of systemic calcification propensity. Curr Opin Nephrol Hypertens. 2016;25:278–284. doi: 10.1097/MNH.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 37.Apetrii M., Covic A., Massy Z.A. Magnesium supplementation: a consideration in dialysis patients. Semin Dial. 2018;31:11–14. doi: 10.1111/sdi.12653. [DOI] [PubMed] [Google Scholar]
- 38.Bressendorff I., Hansen D., Schou M. The effect of magnesium supplementation on vascular calcification in chronic kidney disease-a randomised clinical trial (MAGiCAL-CKD): essential study design and rationale. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alhosaini M., Leehey D.J. Magnesium and dialysis: the neglected cation. Am J Kidney Dis. 2015;66:523–531. doi: 10.1053/j.ajkd.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 40.Gonella M., Ballanti P., Della Rocca C. Improved bone morphology by normalizing serum magnesium in chronically hemodialyzed patients. Miner Electrolyte Metab. 1988;14:240–245. [PubMed] [Google Scholar]
- 41.Floege J. Magnesium concentration in dialysate: is higher better? Clin J Am Soc Nephrol. 2018;13:1309–1310. doi: 10.2215/CJN.08380718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakaguchi Y., Hamano T., Wada A. magnesium and risk of hip fracture among patients undergoing hemodialysis. J Am Soc Nephrol. 2018;29:991–999. doi: 10.1681/ASN.2017080849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamashiro M., Iseki K., Sunagawa O. Significant association between the progression of coronary artery calcification and dyslipidemia in patients on chronic hemodialysis. Am J Kidney Dis. 2001;38:64–69. doi: 10.1053/ajkd.2001.25195. [DOI] [PubMed] [Google Scholar]
- 44.Schlieper G. Vascular calcification in chronic kidney disease: not all arteries are created equal. Kidney Int. 2014;85:501–503. doi: 10.1038/ki.2013.423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic and clinical characteristics of PACE participants with and without Mg level.
P values for interaction between serum Mg and diabetes status.
Flow chart of the study population.
Association between serum Mg and arterial calcification scores (combining zero and non-zero scores, as continuous variables) (per 0.1 mEq/l higher serum Mg).