Abstract
Objective:
To evaluate follow-up and timing of sleep-disordered breathing diagnosis and treatment in urban children referred from primary care.
Study Design:
Retrospective longitudinal cohort analysis
Setting:
Tertiary health system
Subjects and Methods:
Pediatric outpatients with sleep-disordered breathing, referred from primary care for subspecialty appointment or polysomnography in 2014, followed for 2-years. Timing of polysomnography/subspecialty appointments, loss to follow-up, and sleep-disordered breathing severity were main outcomes. Chi-square and t-test identified differences in children referred for polysomnography, surgery, and loss to follow-up. Logistic regression identified predictors of loss to follow-up. Days to polysomnography/surgery were evaluated using Kaplan-Meier estimator, with Cox regression comparing estimates by polysomnography receipt and disease severity.
Results:
Of 216 children,188(87%) had public insurance. Half [109(50%)] were lost to follow-up after primary care referral. More children were lost to follow-up when referred for polysomnography [50(76%)] compared to subspecialty evaluation [35(32%);(p<0.001)]. Loss to follow-up was associated with Black race (OR=2.44,95% CI 1.23–4.86; p=0.011) and polysomnography referral (OR=6.70,95% CI 0.18–0.81; p=0.012). For children who obtained PSG an asymmetric distribution of obstructive sleep apnea severity was not observed (p=0.152). Median time to polysomnography and surgery was 75 and 226 days respectively. Obstructive sleep apnea severity did not influence time to surgery (p=0.410).
Conclusion:
In this urban population, half of children referred for sleep-disordered breathing evaluation are lost to follow-up from primary care. Obstructive sleep apnea severity did not predict follow-up or timeliness of treatment. These findings suggest social determinants may pose barriers to care in addition to the clinical burden of sleep-disordered breathing.
Keywords: sleep-disordered breathing, obstructive sleep apnea, polysomnography, adenotonsillectomy, treatment, access to care, health disparities, socioeconomic status, children, pediatrics
Introduction
The National Academy of Medicine defines a healthcare disparity as a “difference in treatment or access not justified by the differences in health status or preferences of the groups”. 1 The elimination of health disparities is a public health priority and key to achieving healthcare equity. Disparities in pediatric surgical care have been noted in the management of appendicitis, retinoblastoma, and congenital heart surgery, with ethnic minorities and children in low income settings being more likely to have greater time intervals from referral to treatment as well as poorer outcomes. 2–6 Socioeconomic status strongly influences children’s health status with children in low income settings frequently having poor to fair health status.7–8 In addition, children from low-income families tend to experience greater barriers to obtaining care for their health problems.1,9–12
Socioeconomic disparities have been described in pediatric otolaryngologic conditions such as otitis media, pharyngitis, and sinusitis with low socioeconomic status children often experiencing longer time with disease prior to treatment and more clinical complications of disease.12−16 Furthermore, children with low socioeconomic status may have less access to otolaryngology surgical services including tonsillectomy or cochlear implantation due to insurance restrictions. 13–14,17–18 Specifically, disparities in pediatric sleep medicine have been noted. Wang et al. showed that children with Medicaid in California were far less likely to obtain an appointment for evaluation or surgery for treatment of obstructive sleep apnea (OSA) compared to children with private insurance. 19 Although children with low socioeconomic status have an increased risk of sleep-disordered breathing (SDB) and OSA, these children are less or only equally as likely to have adenotonsillectomy for treatment. 13, 19–20 Due to social determinants, they are also at increased risk of co-morbidities such as obesity, poor academic performance, and behavior issues including ADHD, which are also consequences of untreated SDB.19–24 Proposed explanations for these disparities have been attributed to factors such as household crowding, increased prevalence of obesity, or higher exposure to secondhand smoke for children with low socioeconomic status.14
In a previous study, we described that children with public insurance experience longer time intervals from initial subspecialty evaluation to polysomnography (PSG) or surgery, as compared to children with private insurance.25 While there was no association between insurance status and loss to follow-up, loss to follow-up was prevalent in nearly half of children referred for PSG.25 However, this study was unable to determine specific patient- or family-level reasons for the large proportion of loss to follow-up, and the children were identified after they had reached subspecialty care. In the present study, we aim to expand this body of knowledge by evaluating loss to follow-up and time to treatment in children referred for SDB evaluation from primary care in a low-income urban population.
Methods
We performed a retrospective longitudinal cohort analysis of pediatric outpatients seen in Johns Hopkins Hospital affiliated pediatric primary care clinics. This study was approved by the Johns Hopkins Institutional Review Board. Using electronic health record referral data, we pulled data for all referrals made in 2014 from pediatric primary care in the Johns Hopkins Health System to the following departments: Sleep Medicine, Otolaryngology, Pediatric Otolaryngology, Pediatric Sleep Medicine and Pediatric Pulmonology. We included children (≤18 years) referred for evaluation by PSG or subspecialty appointment (Otolaryngology or Pulmonology) for diagnoses related to SDB (adenotonsillar hypertrophy, noisy breathing, snoring, and OSA). We excluded patients over age 18 and those referred for evaluation of diagnoses unrelated to SDB. We collected demographic variables including patient age, gender, race/ethnicity, and insurance type. Public insurance was used as a proxy for low socioeconomic status.26–29 Clinical information abstracted included PSG results (Respiratory Disturbance Index, Oxygen saturation nadir, peak end tidal CO2, Apnea-Hypopnea Index [AHI]) and comorbidities. Severity of OSA was determined based on results of PSG, with an AHI of 1–4.9 indicating mild OSA, AHI of 5–9.9 indicating moderate OSA and an AHI > 10 indicating severe OSA.30 Records were also analyzed to assess timeline of care: date of the initial primary care visit, date of subspecialty visit, date of PSG, and date of surgery were each recorded. Time intervals in days from initial primary care visit to subspecialty visit, PSG, and surgery were calculated. Patients were considered lost to follow-up if they were referred from primary care to PSG or subspecialty evaluation and did not have any encounters documented for 2 years after referral date.
Statistical Analysis
Main outcome measures were timing of PSG and subspecialty appointments as well as loss to follow-up. Chi-square analysis was conducted to identify differences in receipt of PSG or surgery, insurance type and loss to follow up between racial/ethnic groups and referral types. Mean age was compared using t test. Univariate logistic regression analyses were conducted to identify variables predictive of loss to follow-up. Linear regression analysis was done to identify differences between days to PSG and surgery. Kaplan-Meier (K-M) survival analysis and cox regression tests were used to compare time-to-treatment distributions and attrition rates by PSG receipt, and OSA severity. Kolmogorov-Smirnov test was used to assess equality of OSA severity distribution. All analysis was performed using Stata statistical software (version 14; Stata Corp; College Station, TX). P < .05 was considered significant for all analyses.
Results
216 children were referred from primary care for SDB evaluation. Table 1 lists patient demographics and clinical characteristics. The majority of children referred were Black [171 (79%)] with a smaller number of White [5 (2%)], Hispanic [28 (13%)], Bi/Multiracial [6 (3%)] and children with unidentified race [6 (3%)]. Children lost to follow-up were older compared to children who did follow-up (median age 8.01 years vs. 6.81 years; p=0.049). There were no significant differences in race/ethnicity between the children who were lost to follow-up and the children who did follow-up (p=0.056). The majority of children, [188 (87%)] had public insurance. Half of the children were lost to follow-up after primary care referral [109 (50%)]. There were fewer children lost to follow-up when referred for subspecialty evaluation vs referral to PSG [16 (32%) vs 50 (76%); (p < 0.001)]. For children who obtained PSG an asymmetric distribution of OSA severity was not observed (p=0.152), where relatively the same proportion of children had mild, moderate, severe, or absence of sleep apnea [Figure 1].
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristics | Total (N=216) | Lost to Follow-Up (n=109) | Not Lost to Follow-Up (n=107) | p-value |
|---|---|---|---|---|
| Age | ||||
| Mean (SD) | 7.7 years (SD=4.16) | 8.25 years (SD=4.46) | 7.14 years (SD = 3.78) | 0.049 |
| Median | 7.41 years | 8.01 years | 6.81 years | |
| Range | 0.33 – 17.72 years | 0.33– 17 years | 0.51– 17.72 years | |
| Sex | ||||
| Male | 116 (54) | 60 (55) | 56 (52) | 0.690 |
| Female | 100 (46) | 49 (45) | 51 (48) | |
| Race/Ethnicity | ||||
| White | 5 (2) | 2 (2) | 3 (3) | 0.056 |
| Black | 171 (79) | 94 (86) | 77 (72) | |
| Hispanic/Latino | 28 (13) | 7 (6) | 21 (19) | |
| Bi/Multiracial | 6 (3) | 3 (3) | 3 (3) | |
| Unidentified | 6 (3) | 3 (3) | 3 (3) | |
| Insurance | ||||
| Private | 27 (12.5) | 12 (11) | 15 (14) | 0.388 |
| Public | 188 (87) | 97 (89) | 91 (85) | |
| Unknown | 1 (0.5) | 0 | 1 (0.9) | |
| Comorbidities | ||||
| Asthma | 82 (38) | 41 (38) | 41 (38) | 0.915 |
| Obesity ¥ | 81 (38) | 46 (42) | 35 (33) | 0.149 |
| ADHD | 26 (12) | 14 (13) | 12 (11) | 0.713 |
| Downs | 4 (2) | 1 (0.9) | 3 (3) | 0.294 |
| Autism | 2 (1) | 1 (0.9) | 1 (0.9) | 0.990 |
| Craniofacial | 1 (0.5) | 1 (0.9) | 0 | 0.235 |
| Referral type* | ||||
| Referred to Otolaryngology/Pulmonology | 108 (50) | 35 (32) | 73 (68) | p<0.001 |
| Referred to Polysomnography | 66 (31) | 50 (45) | 16 (15) | p<0.001 |
| Referred for both | 42 (19) | 24 (22) | 18 (17) | 0.334 |
Obesity was defined as body mass index greater than or equal to the 95th percentile for-age percentile. 42
Figure 1. OSA Severity for Children Who Obtained Polysomnography (n=107) *.
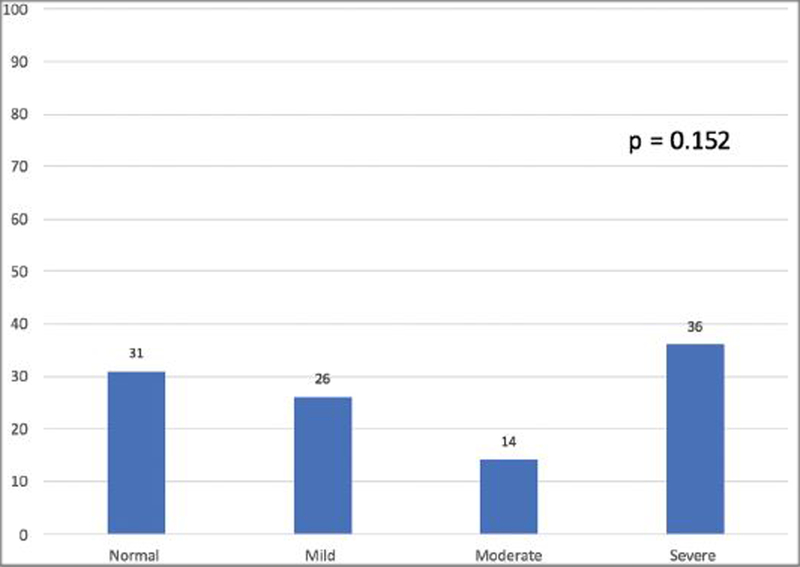
*Includes children referred for polysomnography by subspecialist (n=28). **Chi-square analysis was used for referral type comparison.
Univariate analysis for predictors of loss to follow-up are shown in Table 2. Black children were more likely to be lost to follow-up (OR = 2.44, 95% CI = 1.23–4.86, p = 0.011) as well as children referred to PSG (OR=4.82, 95% CI= 2.51–9.25, p<0.001). Children referred for subspecialty evaluation were less likely to be lost to follow-up (OR = 0.22, 95% CI = 0.12–0.39, p<0.001). Univariate analysis comparing loss to follow-up by subspecialty referral revealed a decreased likelihood of being lost to follow-up if referred to Otolaryngology vs Pulmonology (OR = 0.12, 95% CI = 0.12–0.42; p<0.001). For the children who underwent PSG, OSA severity was not a predictor for likelihood to follow-up (p=0.231).
Table 2.
Univariate analysis for Predictors of Loss to Follow-up
| Characteristic | OR (95% CI) | p-value |
|---|---|---|
| Age | 1.07 (1.00–1.14) | 0.050 |
| Race | ||
| Black | 2.44 (1.23–4.86) | 0.011 |
| Gender | 1.12 (0.65–1.90) | 0.690 |
| Referred to Otolaryngology/Pulmonology | 0.22 (0.12–0.39) | <0.001 |
| Referred to Polysomnography | 4.82 (2.51–9.25) | <0.001 |
| Referred to Both | 1.40(0.71–2.75) | 0.336 |
Overall 46 (21%) children underwent surgical intervention with adenotonsillectomy (3 White, 30 Black, 11 Hispanic, 1 bi/multiracial child and 1 child with unidentified race) independent of race/ethnicity (p=0.608). A larger proportion of children with severe OSA underwent surgical intervention (5 no OSA, 12 mild, 8 moderate, 27 severe OSA; p=0.046).
Time to treatment data is shown in table 3. There was a median interval of 75 days from initial primary care visit to PSG (mean=114.92, range =7–711) and a median interval of 226 days from primary care visit to surgery (mean = 288.43, range = 21–749). For children who obtained PSG and underwent surgery there was a median interval of 129 days from PSG to surgery (mean = 157.80, range = 31–455. Children with OSA experienced longer median time intervals from initial primary care visit and surgery compared to children without OSA (224 days vs. 244, p < 0.001). We dichotomized age into 2 groups: ≤ 6 years old and > 6 years old. This age was chosen as it is near the mean age of the overall cohort (7.7 ± 4.16 years) and represents the beginning of the school age range. Linear regression analysis adjusting for ethnicity and gender revealed no significant difference in days to PSG by age (93.4 vs 130.6 days; 95%CI = −19.2–99.9; p=0.182). Cox regression adjusting for age, gender and race/ethnicity comparing Kaplan-Meier curves of days from initial primary care visit to surgery by receipt of PSG and OSA severity revealed no significant difference in time to treatment (p=0.148, p=0.483) [Figures 2 and 3].
Table 3.
Time intervals to diagnosis and treatment
| Days PCP¥ to PSG† (n=79) mean ± SD (range; median) | p-value | Days PCP to Surgery (n=46) mean ± SD (range; median) | p-value | Days PSG to Surgery* (n=25) mean ± SD (range; median) | p-value | |
|---|---|---|---|---|---|---|
| Overall | 114.92 ± 132.33 (7–711;75) | 288.43 ± 190.08 (21–749;226) | 157.8 ± 123.24 (31–455;129) | |||
| Black | 122.87 ± 145.04 (7–711;73) | 0.329 | 307.2 ± 177.81 (101–749;286) | 0.365 | 153.39 ± 120.44 (39–418;129) | 0.781 |
| Other | 85.94 ± 63.49 (16–278;76) | 253.25± 212.71 (21–680;182) | 169.14 ± 139.45 (31–455;124) | |||
| Obese | 113.13± 88.29 (7–398;94) | 0.853 | 295.61 ± 236.11 (61–749;264.5) | 0.840 | 140.88 ± 121.44 (45–418;119.5) | 0.648 |
| Non-obese | 115.71 ± 148.24 (9–711;72) | 283.82 ± 158.31 (21–676;170) | 165.76 ± 126.95 (31–455;138) | |||
| OSA | 102.6± 102.36 (7–593;74.5) | 0.328 | 292.83 ± 158.31 (21–676;224) | <0.001 | 157.8 ± 123.24 (31–455;129) | |
| No OSA | 136.17 ± 172.37 (11–711;74) | 252.4 ± 196.44 (55–561;244) | n/a** |
PCP: primary care physician.
PSG: Polysomnography
Includes the 25 children who underwent PSG and surgery.
all kids who had PSG and surgery had OSA. The categories represented are not mutually exclusive.
Figure 2. Cox Regression of Kaplan Meier Estimates of time to treatment by Polysomnography receipt adjusted by Age, Gender, and Race.
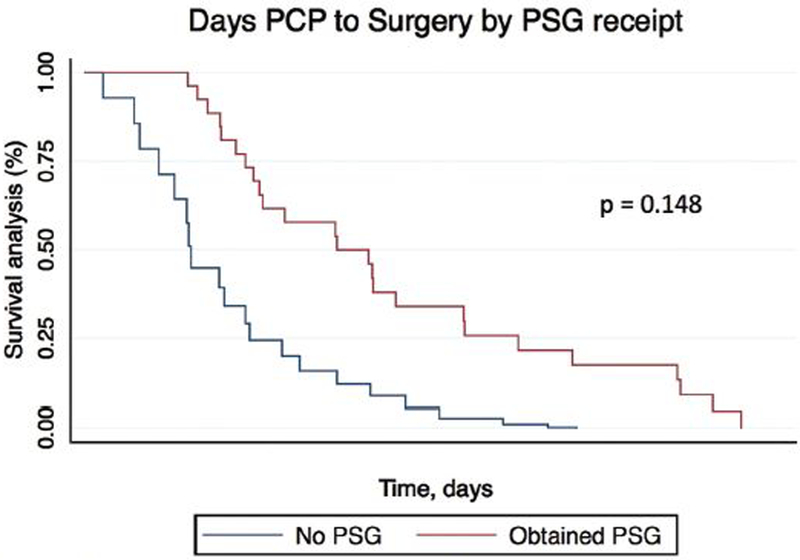
PSG: Polysomnography
25 Children obtained PSG and Surgery.
21 Children underwent surgery alone without PSG
Figure 3.
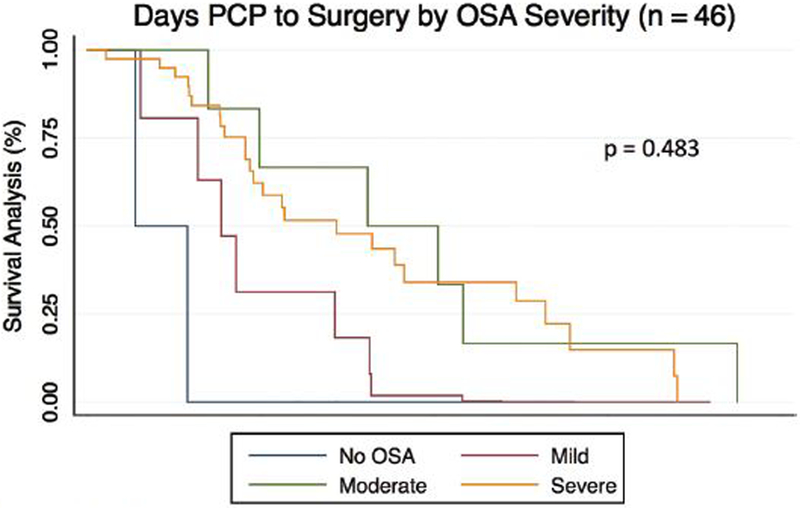
Cox Regression of Kaplan Meier Estimates of time to treatment by OSA severity adjusted by Race, Age, Gender
Discussion
To our knowledge this is the first study to assess success of obtaining follow-up and timing to treatment for low-income children with SDB referred from primary care, and evaluate the impact of clinical parameters on these outcome measures. In addition, no study has followed continuum of care for low income children with SDB from point of primary care referral to surgical treatment. A noteworthy finding was that half of the children referred for PSG and/or subspecialty SDB care were lost to follow-up. The high rate of loss to follow-up may illustrate a high prevalence of undiagnosed or untreated OSA in this low-income population. Untreated OSA can impact growth and development, contribute to behavior problems, cardiopulmonary disease and poor school performance. 31–32 Children with low socioeconomic status are already at increased risk for these co-morbidities, and untreated SDB or OSA may exaggerate these adverse effects and further impact quality of life. Therefore, understanding social determinants that impact receipt of care and finding ways to facilitate timely diagnosis and treatment are imperative to improve quality of care for children with low socioeconomic status.
Racial distribution of children in this population was limited, however most children were Black with a smaller group being Hispanic and White. When comparing racial groups, Black race was a significant predictor of loss to follow-up and a smaller percentage of Black children initially referred underwent surgery. These findings of less healthcare resource use by Black children are similar to findings in a previous study by Kum-Nji et al that found that Black children in rural Mississippi used fewer health care resources compared to White children. 33 The racial/ethnic differences in loss to follow-up may also be due to other social and cultural factors contributing to barriers in access to care and difficulty navigating the health care system for Black children and families in this setting. Furthermore, Black and Hispanic race, as well as low socioeconomic status, is associated with higher emergency department use and clinical sequelae post-tonsillectomy which may highlight patient/family-provider communication issues.25 Due to the nature of the administrative data used for this analysis, we are unable to determine the role patient/family preferences played in the decision-making to pursue scheduling appointments and treatment. A subsequent qualitative study is underway to understand how patient/family preference and other social/cultural factors influence treatment decisions for families of children with SDB.
All children in this primarily low-income population experienced prolonged time intervals from point of primary care referral to PSG and surgery. This finding is similar to findings in previous publications which showed longer time intervals to surgery for SDB, epilepsy surgery and cleft lip repair for children with public insurance. 26,34–35 While there are no established acceptable wait times for pediatric elective surgery in the United States, the Canadian Wait Time Alliance has established benchmarks of surgery within 3 months for children with OSA and surgery within 3 weeks for children with severe OSA.36 Due to the differences in healthcare systems between the United States and Canada these benchmarks may not be applicable but may serve as a starting point to establishing wait time targets in the United States. The long intervals between initial primary care visit, PSG and surgery in this population may be due to poor access to subspecialty care, possibly due to limited provider scheduling of patients with public insurance, patient/family-provider incompatibility, cultural perceptions of sleep, and/or functional limitations. Findings show an opportunity for health system quality improvement, as there is currently no system at this institution to remind patients to schedule referral appointments. Also, the children diagnosed with OSA underwent PSG in order to obtain the diagnosis. The process of scheduling and obtaining PSG likely accounts for the longer time interval to surgery. Of note we only have data on PSG and Surgery date for the patients that followed-up.
Previous studies have shown that children with low socioeconomic status have longer wait times to outpatient subspecialty care and may have less access to otolaryngology surgical services in particular. 12–18,37 Children with low socioeconomic status often have public insurance and reimbursement concerns may affect physicians’ willingness and ability to deliver timely care. 33 Therefore, future efforts to eliminate socioeconomic disparities in SDB healthcare may also focus on improving or equalizing insurance policy and reimbursement.
There was a higher likelihood of being lost to follow-up for children referred to PSG alone compared to other referral dispositions. The American Academy of Otolaryngology Head and Neck (AAOHNS) Surgery guidelines recommends obtaining PSG prior to tonsillectomy only for a select group of children.31 However, there is wide variation in PSG referral patterns amongst primary care providers. Possible reasons for this finding may be due to differences in guidelines recommended by AAOHNS and the American Academy of Pediatrics with the latter recommending PSG be performed in any child with snoring and symptoms/signs of OSA. 38 The high rate of loss to follow-up after PSG referral may be due to social or cultural factors that limit likelihood to obtain PSG such as burden of time, cost and/or missed school or work. Children referred to Otolaryngology were also less likely to be lost to follow-up compared to referral to Pulmonology. This may be due to parents considering a referral to a surgeon as a more proactive approach. This pattern will again be examined in a qualitative study examining barriers and facilitators in obtaining pediatric SDB care. Direct referral to sleep subspecialty evaluation without first referring to PSG from primary care may increase the likelihood of obtaining treatment for children with social or cultural barriers to care. Primary care providers and sleep subspecialist may consider a focused shared-decision making model on choosing PSG or going directly to surgery. Social factors may be a barrier to obtaining PSG in urban populations so providers may need to make this a specific focus of shared decision-making in urban populations.
One of our most important findings was that the success of obtaining follow-up and the timeliness of treatment were not influenced by OSA severity, in contrast to our expectations that children with more severe disease would be more likely to follow-up for quicker treatment. This finding may indicate that social determinants such as caregiver educational level, social support system and economic stability may play a large role in loss to follow-up. A 2011 survey of physicians by the Robert Wood Johnson foundation showed that while most physicians are aware of the link between social conditions and poor health outcomes, they are unable to intervene to improve health care. 39 While this is a difficult problem to overcome, considering how social factors may influence patients’/families’ ability to adhere to treatment recommendations could be the first step in improving quality of care.
There were several limitations. This study utilized a retrospective analysis using electronic health records which may have coding errors or data entry inaccuracies, and is subject to incorrect data abstraction. In addition, by only using administrative data we were unable to identify other systemic and cultural factors for prolonged time to care or loss to follow-up. Furthermore, this analysis did not assess specific practice patterns or decision-making algorithms of providers. Therefore, we are unable to explain why children with normal PSGs underwent adenotonsillectomy. Severity of comorbid symptoms or presence of chronic adenotonsillitis may be some reasons. It is also possible that some referrals were missed. We are also unable to determine how disease severity or perception of disease severity by parents/caregivers affected timeliness of care or loss to follow-up. It is a single-institution study and may not be generalizable on a national level. The low number of privately insured children did not allow adequate power for statistical comparison by insurance type. The low number of children that both obtained PSG and underwent surgical intervention did not allow adequate power for a statistically significant comparison of time to surgery by PSG receipt. The use of insurance type as a proxy for socioeconomic status may not be the most reliable measure. The Johns Hopkins Children’s Center offers primary health care services to the community in East Baltimore. The East Baltimore community has a population that is 70% Black, with 34% of the population below poverty level41–42. This is reflected in the demographics of the study cohort; therefore, these findings may apply to other large urban areas but may not be generalizable on a national level.40–41 Because the majority of the children were Black race, findings related to race would be better investigated in a more diverse population. Further qualitative studies may be needed to identify patient/family specific reasons for loss to follow-up or other systemic and cultural factors that may be barriers limiting access to care and healthcare utilization. A subsequent study is under way using a broader more diverse data to make more reliable comparisons by race and insurance.
Despite these limitations, this study is the first to follow the continuum of care for children with SDB from point of primary care referral. As more emphasis is placed on eliminating health disparities, examining referral patterns and health care utilization in low-income settings can aid in designing interventions to reduce care variation for SDB. Findings from this study will serve as a foundation for further research dedicated to equitable and efficient care for children with SDB.
Conclusions
In this urban health system, half of all children referred for evaluation or management of SDB do not complete the recommended referrals. All children experienced long time intervals to PSG and surgery. Disease severity did not influence likelihood to follow-up, implying that clinical disease is not a predictor of receipt of treatment. These findings suggest the need for further evaluation of social and cultural barriers to diagnosis of pediatric SDB. Assessing referral patterns and access within the healthcare system for these at-risk children and developing tailored communication approaches based on socioeconomic status and race/ethnicity may aid in reducing care variation for this prevalent condition.
Acknowledgments
Financial support: Dr. Harris is support by grant 512808 from the American Academy of Otolaryngology – Head & Neck Surgery Resident Research Award sponsored by Cook Medical and grant 5T32DC000027–27 from the National Institute on Deafness and Other Communication Disorders (NIDCD) for research Training in Otolaryngology. Dr. Boss is supported by grant number K08HS022932 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Dr. Boss is also supported by the American Society of Pediatric Otolaryngology Career Development Award.
Footnotes
Presented at the Academy of Otolaryngology – Head & Neck Surgery 2017 Meetings, Chicago, IL, USA, September 12th 2017.
The authors have no financial relationships, or conflicts of interest to disclose.
References:
- 1.Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care Washington, DC: National Academy Press; 2002 [PMC free article] [PubMed] [Google Scholar]
- 2.Kokoska ER, Bird TM, Robbins JM, Smith SD, Corsi JM, Campbell BT. Racial disparities in the management of pediatric appendicitis. J Surg Res 2007;137(1): 83–88. [DOI] [PubMed] [Google Scholar]
- 3.Jablonski KA, Guagliardo MF. Pediatric appendicitis rupture rate: a national indicator of disparities in healthcare access. Popul. Health Metr 2005; 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bratu I, Martens PJ, Leslie WD, Dik N, Chateau D, Katz A. Pediatric appendicitis rupture rate: disparities despite universal health care. J. Pediatr. Surg 2008; 43:1964–1969. [DOI] [PubMed] [Google Scholar]
- 5.Truong B, Green AL, Friedrich P, Ribeiro KB, Rodriguez-Galindo C. Ethnic, Racial, and Socioeconomic Disparities in Retinoblastoma. JAMA Pediatr 2015;169(12):1096–104. [DOI] [PubMed] [Google Scholar]
- 6.Chan T, Lion KC, Mangione-Smith R. Racial disparities in failure-to-rescue among children undergoing congenital heart surgery. J Pediatr 2015;166(4):812–8. e1–4. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health Care Management. Reducing Health Disparities Among Children: Strategies and Programs for Health Plan 2007. http://www.nihcm.org/pdf/HealthDisparitiesFinal.pdf Accessed August 15th, 2017.
- 8.Newacheck P, Jameson WJ, Halfon N. Health status and income: The impact of poverty on child health. Journal of School Health 1994;64(6):229–33. [DOI] [PubMed] [Google Scholar]
- 9.Alegria M, Vallas M, Pumariega A. Racial and Ethnic Disparities in Pediatric Mental Health. Child Adolesc Psychiatr Clin N Am 2010;19(4):759–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandell DS, Listerud J, Levy SE, Pinto-Martin JA. Race differences in the age at diagnosis among medicaid-eligible children with autism. J Am Acad Child Adolesc Psychiatry 2002; 41(12):1447–53. [DOI] [PubMed] [Google Scholar]
- 11.Weech-Maldonado R, Morales LS, Spritzer K, Elliott M, Hays RD. Racial and ethnic differences in parents’ assessments of pediatric care in Medicaid managed care. Health Serv Res 2001; 36(3): 575–594. [PMC free article] [PubMed] [Google Scholar]
- 12.Patel S, Schroeder JW. Disparities in children with otitis media the effect of insurance status. Otolaryngol Head Neck Surg 2011;144(1):73–7. [DOI] [PubMed] [Google Scholar]
- 13.Boss EF, Marsteller JA, Simon AE. Outpatient Tonsillectomy in Children: Demographic and Geographic Variation in the United States, 2006. J Pediatr 2012;160(5):814–9. [DOI] [PubMed] [Google Scholar]
- 14.Smith DF, Boss EF. Racial/ethnic and socioeconomic disparities in the prevalence and treatment of otitis media in children in the United States. Laryngoscope 2010;120(11):2306–12. [DOI] [PubMed] [Google Scholar]
- 15.Smith DF, Ishman SL, Tunkel DE, Boss EF. Chronic rhinosinusitis in children: race and socioeconomic status. Otolaryngol Head Neck Surg 2013;149(4):639–44. [DOI] [PubMed] [Google Scholar]
- 16.Stern RE, Yueh B, Lewis C, Norton S, Sie KC. Recent epidemiology of pediatric cochlear implantation in the United States: disparity among children of different ethnicity and socioeconomic status. Laryngoscope 2005;115(1):125–131. [DOI] [PubMed] [Google Scholar]
- 17.Bradham T, Jones J. Cochlear implant candidacy in the United States: prevalence in children 12 months to 6 years of age. Int J Pediatr Otorhinolaryngol 2008;72(7):1023–1028. [DOI] [PubMed] [Google Scholar]
- 18.Wang EC, Choe MC, Meara JG, Koempel JA. Inequality of access to surgical specialty health care: why children with government-funded insurance have less access than those with private insurance in Southern California. Pediatrics 2004;114(5): e584–90. [DOI] [PubMed] [Google Scholar]
- 19.Spilsbury JC, Storfer-Isser A, Kirchner HL, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr 2006;149 (3):342–347. [DOI] [PubMed] [Google Scholar]
- 20.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: Association with race and prematurity. J Pediatr 2003;142(4):383–9. [DOI] [PubMed] [Google Scholar]
- 21.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med 2007;176(4):401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 2004; 291(23): 2847–2850. [DOI] [PubMed] [Google Scholar]
- 23.Lee NE, De AK, Simon PA. School-based physical fitness testing identifies large disparities in childhood overweight in Los Angeles. J Am Diet Assoc 2006;106(1): 118–121. [DOI] [PubMed] [Google Scholar]
- 24.Stevens GD, Seid M, Mistry R, Halfon N. Disparities in primary care for vulnerable children: the influence of multiple risk factors. Health Serv Res 2006; 41(2): 507–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhattacharyya N, Shapiro NL. Associations between Socioeconomic status and race with complications after tonsillectomy in Children. Otolaryngol Head Neck Surg 2014;151(6):1055–60. [DOI] [PubMed] [Google Scholar]
- 26.Boss EF, Benke JR, Tunkel DE, Ishman SL, Bridges JFP, Kim JM. Public Insurance and Timing of Polysomnography and Surgical Care for Children with Sleep-Disordered Breathing. JAMA Otolaryngol Head Neck Surg 2015;141(2):106–11 [DOI] [PubMed] [Google Scholar]
- 27.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The Relation between Health Insurance Coverage and Clinical Outcomes among Women with Breast Cancer. N Engl J Med 1993; 329:326–331. [DOI] [PubMed] [Google Scholar]
- 28.Harnick DJ, Cohen JL, Schechter CB, Fuster V, Smith DA. Effects of practice setting on quality of lipid-lowering management in patients with coronary artery disease. Am J Cardiol 1998; 81:1416–1420. [DOI] [PubMed] [Google Scholar]
- 29.Shen JJ, Wan TT, Perlin JB. An exploration of the complex relationship of socioecologic factors in the treatment and outcomes of acute myocardial infarction in disadvantaged populations. Health Serv Res 2001; 36:711–732. [PMC free article] [PubMed] [Google Scholar]
- 30.Foraker RE, Rose KR, Whitsel EA, Chirayath MS, Wood JL, Rosamond WD. Neighborhood socioeconomic status, Medicaid coverage and medical management of myocardial infarction: Atherosclerosis risk in communities (ARIC) community surveillance. BMC Public Health 2010; 10:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roland PS, Rosenfeld RM, Brooks LJ, et al. American Academy of Otolaryngology–Head and Neck Surgery Foundation. Clinical practice guideline: polysomnography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg 2011;145 (1) (suppl): S1-S15. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell RB, Kelly J. Child behavior after adenotonsillectomy for obstructive sleep apnea syndrome. Laryngoscope, 2005;115(11):2051–2055. [DOI] [PubMed] [Google Scholar]
- 33.Kum-Nji P, Mangrem CL, Wells PJ, Klesges LM, Herrod HG. Black/white differential use of health services by young children in a rural Mississippi community. South. Med. J 2006; 99:957–962. [DOI] [PubMed] [Google Scholar]
- 34.Hauptman JS, Dadour A, Oh T, et al. Time to pediatric epilepsy surgery is longer and developmental outcomes lower for government compared with private insurance. Neurosurgery 2013. ;73(1):152–7. [DOI] [PubMed] [Google Scholar]
- 35.Abbott MM, Kokorowski PJ, Meara JG. Timeliness of surgical care in children with special health care needs: delayed palate repair for publicly insured and minority children with cleft palate. J Pediatr Surg 2011; 46 (7): 1319–24. [DOI] [PubMed] [Google Scholar]
- 36.Wright JG, Li K, Seguin C, et al. Development of pediatric wait time access targets. Can J Surg 2011;54(2):107–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bisgaier J, Rhodes KV. Auditing Access to Specialty Care for Children with Public Insurance. N Engl J Med 2011; 364:2324–33. [DOI] [PubMed] [Google Scholar]
- 38.Marcus CL, Brooks LJ, Draper KA, Gozal D, et al. American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130(3):576–84. [DOI] [PubMed] [Google Scholar]
- 39.Health care’s blind side: the overlooked connection between social needs and good health Princeton (NJ): Robert Wood Johnson Foundation;2011. http://www.rwjf.org/content/dam/farm/reports/surveys_and_polls/2011/rwjf71795. Accessed August 15th, 2017. [Google Scholar]
- 40.East Baltimore neighborhood in Baltimore, Maryland (MD), 21218, 21202, 21205, 21213 detailed profile. East Baltimore neighborhood in Baltimore, Maryland (MD), 21218, 21202, 21205, 21213 subdivision profile - real estate, apartments, condos, homes, community, population, jobs, income, streets. http://www.city-data.com/neighborhood/East-Baltimore-Baltimore-MD.html. Accessed November 2, 2017. [Google Scholar]
- 41.The Johns Hopkins Hospital & Johns Hopkins Bayview Medical Center Community Health Needs Assessment & Implementation Strategy Johns Hopkins Medicine;2016. http://web.jhu.edu/administration/gca/CHNA%20and%20CBR%20Docs/CHNA%20IS%20FY16.pdf. Accessed November 2, 2017. [Google Scholar]
- 42.Centers for Disease Control and Prevention. Defining Over- weight and Obesity 2010. http://www.cdc.gov/obesity/defining.html. Accessed November 1, 2017.


