Graphical abstract
To the Editor:
Automated peritoneal dialysis (APD) is an important treatment option for patients that preserves renal function, improves survival, and improves quality of life compared with hemodialysis.S1 Remote patient monitoring (RPM) offers 2-way communication between the patient and the clinical team, and provides an opportunity for high-quality, timely services based on data transmitted from the patient’s home. Remote patient monitoring ensures improved patient confidence in performing APD at home and provides support and encouragement.1 High patient satisfaction has been reported with RPM and treatment.S2
Enhanced communication between the patient and the clinical team may decrease APD complications, improve clinical outcomes, and identify mechanical complications earlier, leading to better fluid balance control and cardiovascular health.2,S3-S5 RPM may have a direct impact on the continued use of PD therapy by reducing hospital stays and drop-out rates. Furthermore, RPM is an excellent platform that provides dialysis to patients living in rural or remote areas.S6
At the end of 2014, an APD cycler coupled with RPM capability (Home Choice Claria cycler with Sharesource, Baxter Healthcare, Deerfield, IL) was launched. Claria with Sharesource allows health care providers to retrieve data that are transmitted from the device through a modem to a cloud-based data repository review data, to program the unit, to edit configurations of the device, and to analyze treatment results. On-demand access to patient data allows the clinical team to remotely adjust APD prescriptions and to help patients comply with their prescriptions. This technology may increase physician confidence in offering home dialysis to patients.
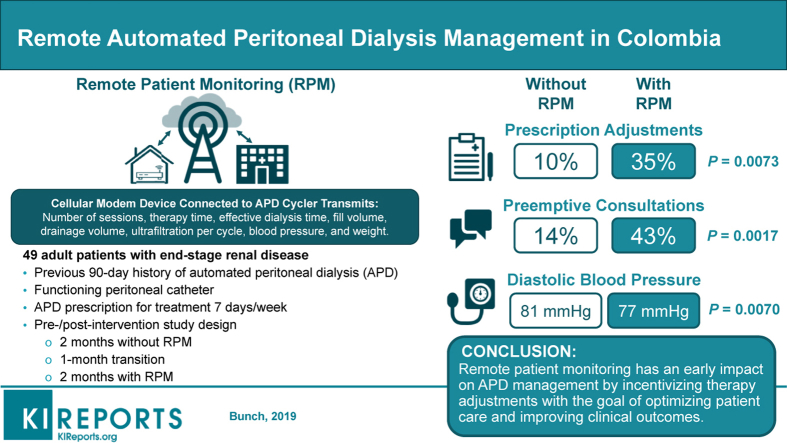
Our goal was to explore the clinical effects of a new APD with RPM program for 2 months (APD+RPM) compared with the same APD program before the introduction of RPM (pre-RPM). We hypothesize that RPM will have an early impact on improving and optimizing patient care.
Results
A total of 49 patients were admitted to the study. Their mean age was 59.3 years; 29% had only an elementary school education; and 37% lived at a low socioeconomic level (Table 1). Most patients lived in an urban setting. No patients were lost to follow-up.
Table 1.
Demographic characteristics at baseline (N = 49)
| Characteristics | Patients, n (%) |
|---|---|
| Age, mean (SD), yr | 59.3 (17.31) |
| Sex, n (%) | |
| Male | 27 (55) |
| Female | 22 (45) |
| Place of residence, n (%) | |
| Urban | 48 (98) |
| Rural | 1 (2) |
| Educational level, n (%) | |
| Elementary school | 14 (29) |
| High school diploma | 26 (53) |
| Technical diploma | 4 (8) |
| University degree | 5 (10) |
| Socioeconomic level, n (%) | |
| 1 (lowest) | 2 (4) |
| 2 | 16 (33) |
| 3 | 22 (45) |
| 4 | 8 (16) |
| 5 | 1 (2) |
| 6 (highest) | 0 |
| Requires a caregiver, n (%) | 25 (51) |
| Time on PD, median (IQR), mo | 32.1 (35.70) |
| Cause of chronic kidney disease, n (%) | |
| Hypertension | 21 (43) |
| Diabetes | 13 (27) |
| Nephrotic syndrome | 9 (18) |
| Other | 6 (12) |
| History of congestive heart failure, n (%) | 8 (16) |
IQR, interquartile range.
The APD prescription characteristics were similar with RPM or without (Table 2). The proportion of patients prescribed with exchanges during the day (wet day) was similar between the pre-RPM and APD/+RPM phases by either a last APD infusion dwell or an additional exchange in the afternoon. The basal frequency of clinic visits was 1 per month; patients in both RPM phases complied with all visits.
Table 2.
Prescription and adherence characteristics (N = 49)
| Characteristics | Pre-RPM (n = 49) | APD+RPM (n = 49) |
|---|---|---|
| Prescription | ||
| Total dialyzed volume, mean (SD), ml | 9787.8 (2594.95) | 9682.7 (2391.35) |
| Time programmed for each session, mean (SD), h | 9.7 (1.24) | 9.6 (1.26) |
| Number of cycles per session, mean (SD) | 4.2 (1.23) | 4.1 (1.05) |
| Volume of infusion per cycle, mean (SD), ml | 1975.5 (277.38) | 2000 (267.70) |
| Glucose per session, mean (SD), kcal | 248.5 (66.35) | 249.0 (68.03) |
| Last APD infusion dwell, n (%) | 29 (59) | 30 (61) |
| Afternoon dwell, n (%) | 17 (35) | 20 (41) |
APD, automated peritoneal dialysis; RPM, remote patient monitoring.
During the APD+RPM phase, 2534 (85%) of the 2989 prescribed PD sessions were adhered to and completed. Just 147 (5%) of prescribed sessions were interrupted, and 308 (10%) were missed treatments. The mean (SD) lost dwelling time was 78 (158) minutes per patient-month, and lost treatment time per patient was 175 (366) minutes. Although patients during the pre-RPM phase attended monthly consultations and brought a notebook in which daily ultrafiltration was recorded, reliable information about adherence and completion of prescribed sessions was not available.
We found significant differences between the 2 RPM phases in the proportion of patients with a change in prescription (P = 0.0073), the number of prescription changes (P = 0.006), the proportion of patients requiring additional medical consultations (P = 0.0017), the number of additional medical consultations (P = 0.003), the proportion of patients with signs of edema (P = 0.000), and changes in mean diastolic blood pressure (BP) (P = 0.007) (Table 3). Among patients who had a change in prescription during the APD+RPM phase, 8 patients (47%) experienced an increase in infusion volume and time spent on treatment, 4 (23%) an additional exchange at noon, 3 (18%) an increase in infusion volume, and 2 (12%) an increase in the time spent on treatment. When we performed a multivariate analysis of variance, we found a significant change in diastolic pressure during the post-phase (P = 0.035). There were 6 unscheduled and 1 preemptive APD consultations during the pre-RPM phase, and 12 unscheduled and 9 preemptive consultations in the APD+RPM phase.
Table 3.
Comparison of clinical outcomes before and after remote monitoring (N = 49)
| Characteristics | Pre-RPM | APD+RPM | P value |
|---|---|---|---|
| Number of treatments completed per patient/mo, mean (SD) | N/A | 25.9 (4.22) | — |
| Patients with a change in prescription, n (%) | 5 (10) 95% CI: 3.4–22.2 | 17 (35) 95% CI: 21.7–49.6 | 0.0073a |
| Number of prescription changes, n (%) | 0.0060a | ||
| 0 changes | 44 (90) | 32 (65) | |
| 1 change | 5 (10) | 14 (29) | |
| 2 changes | 0 | 3 (6) | |
| Patients with an episode of peritonitis, n (%) | 2 (4) 95% CI: 0.4–14.0 | 0 95% CI: 0–7.3 | 0.1573a |
| Number of peritonitis episodes, n (%) | 0.1600b | ||
| 0 episodes | 47 (96) | 49 (100) | |
| 1 episode | 2 (4) | 0 | |
| Patients with technique failure, n (%) | 0 | 0 | — |
| Patients with hospitalization, n (%) | 5 (10) 95% CI: 3.4–22.2 | 4 (8) 95% CI: 2.3–19.6 | 0.7389a |
| Number of hospitalizations, n (%) | 0.7400 | ||
| 0 hospitalizations | 44 (90) | 45 (92) | |
| 1 hospitalization | 5 (10) | 4 (8) | |
| Patients requiring preemptive outpatient medical consultations, n (%) | 7 (14) 95% CI: 5.9–27.2 | 21 (43) 95% CI: 28.8–57.8 | 0.0017a |
| Number of preemptive outpatient medical consultations, n (%) | 0.0030b | ||
| 0 appointments | 42 (86) | 28 (57) | |
| 1 appointment | 7 (14) | 17 (35) | |
| 2 appointments | 0 | 4 (8) | |
| Ultrafiltration, mean (SD) ml/d | 1107.9 (461.30) | 1111.3 (386.11) | 0.0750b |
| Ultrafiltration ≥750 ml/d, n (%) | 40 (82) 95% CI: 70.8–92.5 | 41 (84) 95% CI: 73.3–94.0 | 0.7896a |
| Weight, mean (SD), kg | 66.0 (13.88) | 66.0 (12.23) | 0.3100b |
| Patients without signs of edema, n (%) | 39 (79) 95% CI: 68.3–90.9 | 42 (86) 95% CI: 75.9–95.5 | 0.0000a |
| Systolic blood pressure, mean (SD), mm Hg | 134.3 (22.73) | 128.9 (17.18) | 0.3100b |
| Diastolic blood pressure, mean (SD), mm Hg | 81.2 (15.90) | 76.7 (11.22) | 0.0070b |
| Patients requiring antihypertensive medicines, n (%) | 34 (69) 95% CI: 54.6–81.7 | 41 (84) 95% CI: 70.3–92.7 | 0.0952a |
| Number of antihypertensive medicines, mean (SD) | 1.3 (1.11) | 1.8 (1.23) | 0.0000b |
APD, automated peritoneal dialysis; CI, confidence interval; RPM, remote patient monitoring.
McNemar test.
Wilcoxon-signed rank test.
There was no significant difference in technique failures, episodes of peritonitis, ultrafiltration, or body weight between the RPM phases (Table 3). There were 2 adverse events: 1 patient experienced hypotension in the pre-RPM phase, and 1 patient experienced dehydration in the APD+RPM phase. Neither adverse event was considered related to RPM. There were no serious adverse events.
Discussion
Nearly 30% of patients undergoing PD do not comply with the prescribed therapy.3 Nonadherent patients require more follow-up visits to guarantee adequacy, to minimize hospital stays, and to increase the time-to-hemodialysis transition. Without RPM, accurate and timely adherence data cannot be captured because patients need to physically report to the clinic. Use of RPM eliminates this problem by reliably monitoring patient adherence. We found complete patient adherence for 85% of treatment sessions, which is higher than previously reported3, 4 and may reflect the success of the enhanced patient educational program in the RTS network. It was not possible to report any predictive trends for long-term patient-adherence with RPM after the conclusion of the short follow-up period of 2 months of this study. However, another form of remote monitoring in telenephrology (televideoconferencing) improved long-distance patient adherence and provided comparable clinical outcomes over a 2-year follow-up period.S7
We observed a significant increase of 25% (P = 0.0073) in real-time therapy adjustments (new prescriptions) with RPM, which illustrates another advantage of RPM. Patients do not need to carry the APD unit to their next appointment,5 which avoids unnecessary visits to the renal care center by patients and caregivers.
We observed a significant increase of 29% (P = 0.0017) in the number of times that patient visits were preempted during the APD+RPM phase compared with the pre-RPM phase. This difference could be explained because the clinic staff was already aware of the patient’s condition—specifically weight, ultrafiltration, BP, and adherence. Use of RPM allows health care providers to continuously monitor patients, and, if deviations from prescribed therapy are noted, to suggest that the patient visit the renal clinic for evaluation and potential changes in medications, update APD prescriptions, or participate in adherence education activities. This increase in the number of early and preemptive interventions may increase workload for the health care team.
We observed a significant decrease in mean diastolic BP of approximately 5 mm Hg during the APD+RPM phase, which may be due to a higher awareness of BP, adjustments in the APD prescription, or adjustments in antihypertensive medication. Despite the increasing number of hypertensive medications prescribed with RPM, patients were still adherent and did not drop out of the study, because of the better personalized treatment plan. A trend toward an increase in the proportion of patients without edema may serve as an indicator for improved BP, as reflected by the number of patients who achieved the target ultrafiltration at 84% (n = 41). However, given that there was no significant difference in the ultrafiltration volume, the degree of edema may not be precise, and the improvement in BP control could be due to a more judicious use of antihypertensive medication.
The early increase in interventions is an indicator of better and active monitoring of patients’ health and needs. Other short-term studies have shown that remote monitoring allows for early interventions that improve personalization of APD prescriptions.6
We must highlight that the pre- and post-design has a potential for bias, from the observer and the observed. The adherence rate could be influenced by our investigation’s study design, which may bias patient behavior toward the improvement of adherence. Another limitation of this study is the absence of outcomes from the patients’ perspectives, such as improvement in the quality of life, burden of disease, and health care satisfaction. Although we did not assess the effect of RPM on hospitalization events or emergency department visits, we hypothesize that long-term use of RPM will have a positive impact on the cost-effectiveness of the treatment. For example, remote biometric monitoring of just BP and weight may be associated with lower inpatient and outpatient costs among subgroups of patients receiving PD.7
Remote patient monitoring used in urban areas may improve patient convenience by decreasing time spent in traffic and reducing wait time at a clinic. Use of RPM as a prescription tool can be included in models of telemedicine that are affordable and useful for patients in remote areas, especially in developing countries. Regardless of a patient’s location, RPM/telemedicine programs can empower patients and help address the inherent “therapy gaps” of home therapies, which include lack of adherence and limited availability of medical supervision.S8,S9
Remote patient monitoring may improve home dialysis patient care, reduce health care costs, and improve patient quality of life. Results from a small randomized controlled trial have demonstrated a significant reduction in hospitalizations, hospital days, and emergency department visits among hemodialysis patients using RPM compared with patients using traditional care.8 More studies are needed to analyze the most important parameters to monitor that will lead to an effective use of telemedicine for dialysis patients and providers.9 Remote patient monitoring may be a valuable tool to improve the quality of PD care and clinical outcomes, enabling effective connectivity between patients and clinicians and paving the way toward improved individualized care.
Despite the exploratory nature of this study, we identify early findings for generating hypotheses. Future studies investigating the long-term impact of RPM on patient adherence, quality of life, clinical outcomes (such as hospitalization events or technique failure) overall survival, health care provider workload and job satisfaction, and health care costs are critical in determining the role of RPM in patients undergoing PD.
Disclosure
AB and RMS are full-time employees of Renal Therapy Services-Latin America, Bogotá, Colombia. JIV, DOC, LC, APM, MCR, and CPR are full-time employees of Renal Therapy Services-Colombia, Bogotá, Colombia. MED is retired and was a full-time employee of Renal Therapy Services-Colombia when the study was conducted. Renal Therapy Services has received grant/research support from Baxter. ASR is a full-time employee of Baxter Healthcare Corporation, Chicago, Illinois, USA. RMS has declared no competing interests.
Acknowledgments
This study was supported by Renal Therapy Services-Colombia, an independent entity owned by Baxter International, Inc. Funding to support the preparation of this article was provided by Baxter Healthcare Corporation, Deerfield, Illinois, USA.
The authors thank all the PD nursing teams who participated in the study. We also thank Lamara D. Shrode, PhD, CMPP, who, on behalf of Baxter Healthcare Corporation, provided editorial support and assisted in implementing author revisions throughout the editorial process.
Author Contributions
AB, JIV, DOC, LC, APM, MED, MCR, PR, RS, AR, and MS have all made substantial contributions to project conception and design, the acquisition of data, and the analysis and interpretation of data. AB, JIV, DOC, LC, APM, MED, MCR, PR, RS, ASR, and MS have been involved in the drafting of the manuscript and revising it critically for important intellectual content. AB, JIV, DOC, LC, APM, MED, MCR, PR, RS, AR, and MS have given final approval of the version to be published. All authors verify that they have met all the journal’s requirements for authorship. All authors agree to be accountable for all aspects of the work, ensuring the accuracy and integrity of the publication. All authors approved the final manuscript draft submitted for publication. The authors received no financial compensation for the development of this manuscript.
Availability of Data and Material
The full protocol and database for the study can be acquired from the principal investigator (alfonso_bunch@baxter.com).
Footnotes
Supplementary Methods.
Supplementary References.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
References
- 1.Nayak K.S., Ronco C., Karopadi A.N. Telemedicine and remote monitoring: supporting the patient on peritoneal dialysis. Perit Dial Int. 2016;36:362–366. doi: 10.3747/pdi.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makhija D., Alscher M.D., Becker S. Remote monitoring of automated peritoneal dialysis patients: assessing clinical and economic value. Telemed J E Health. 2018;24:315–323. doi: 10.1089/tmj.2017.0046. [DOI] [PubMed] [Google Scholar]
- 3.Bernardini J., Nagy M., Piraino B. Pattern of noncompliance with dialysis exchanges in peritoneal dialysis patients. Am J Kidney Dis. 2000;35:1104–1110. doi: 10.1016/s0272-6386(00)70047-3. [DOI] [PubMed] [Google Scholar]
- 4.Griva K., Lai A.Y., Lim H.A. Non-adherence in patients on peritoneal dialysis: a systematic review. PLoS One. 2014;9:e89001. doi: 10.1371/journal.pone.0089001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nayak A., Antony S., Nayak K.S. Remote monitoring of peritoneal dialysis in special locations. Contrib Nephrol. 2012;178:79–82. doi: 10.1159/000337816. [DOI] [PubMed] [Google Scholar]
- 6.Milan Manani S., Crepaldi C., Giuliani A. Remote monitoring of automated peritoneal dialysis improves personalization of dialytic prescription and patient's independence. Blood Purif. 2018;46:111–117. doi: 10.1159/000487703. [DOI] [PubMed] [Google Scholar]
- 7.Lew SQ, Sikka N, Thompson C, et al. Impact of remote biometric monitoring on cost and hospitalization outcomes in peritoneal dialysis [e-pub ahead of print]. J Telemed Telecare. 1357633X18784417. 10.1177/1357633X1878441. [DOI] [PubMed]
- 8.Berman S.J., Wada C., Minatodani D. Home-based preventative care in high-risk dialysis patients: a pilot study. Telemed J E Health. 2011;17:283–287. doi: 10.1089/tmj.2010.0169. [DOI] [PubMed] [Google Scholar]
- 9.Wallace E.L., Rosner M.H., Alscher M.D. Remote patient management for home dialysis patients. Kidney Int Rep. 2017;2:1009–1017. doi: 10.1016/j.ekir.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.