Introduction
Hypercalcemia is a rare finding in children but is most common in neonates.1 Unlike adults, in whom the vast majority of cases are due to hyperparathyroidism and malignancy, the etiology of childhood hypercalcemia is diverse and commonly presents with nonspecific signs and symptoms.1, 2 Despite the often subtle and nonspecific presentation, left untreated it can lead to serious end-organ injury. As a result, prompt and thorough workup of children with hypercalcemia is extremely important.
Calcium homeostasis is maintained through the interaction of parathyroid hormone and vitamin D. Vitamin D accomplishes its primary function by maintaining normal calcium and phosphorus balance through careful regulation of active metabolites. Vitamin D is supplied to the body either as a supplement or through endogenous photolysis of precursors in the skin to the 2 primary forms of inactive vitamin D: vitamin D3 (cholecalciferol) or vitamin D2 (ergocalciferol). These inactive forms are first metabolized in the liver by 25-hydroxylase (CYP2R1) to 25-hydroxy-vitamin D3. This enables the critical second hydroxylation by 1α-hydroxylase (CYP27B1) in the kidney to generate the biologically active vitamin D metabolite, 1,25-dihydroxyvitamin D3. When calcium is sufficient, 24-hydroxylase (CYP24A1) leads to reduced 1,25-dihydroxyvitamin D3 levels by converting it to 1,24,25(OH)3D3, which is eventually excreted as calcitroic acid.3, 4, 5
Nephrocalcinosis is a rare etiology of kidney disease in infants but, when present, raises an important differential diagnosis. We present a case of infantile nephrocalcinosis due to a novel pathogenic mutation in CYP24A1.
Case Presentation
Clinical History and Initial Laboratory Data
An 11-month-old American Indian boy presented to the local emergency department after a visit to his primary care physician revealed hypercalcemia, 13.9 mg/dl, and an elevated creatinine, 0.9 mg/dl. He had a past medical history significant for persistent poor weight gain since birth, as well as gross motor delay. The patient was born at 41 weeks’ estimated gestational age by cesarean delivery due to fetal decelerations. His mother had a slow and prolonged rupture of membranes for 1 week before delivery. His diet included Similac Advance formula (Abbott Laboratories, Chicago, IL) ad libitum and he did not receive any medications or vitamin supplements. The mother was unsure about the exact quantity of formula intake. Routine laboratory data obtained on admission confirmed the hypercalcemia, 14.2 mg/dl; hypermagnesemia, 2.9 mg/dl; and an elevated serum creatinine, 0.87 mg/dl. Urinalysis was negative for hemoglobin and protein. A review of systems revealed that he had gross motor delays, with an inability to crawl, and difficulty feeding, with frequent gagging. Physical examination demonstrated normal vital signs for the patient’s age with a temperature of 36.6 °C, blood pressure of 96/65 mm Hg, heart rate of 137 beats per minute, and a respiratory rate of 32 breaths per minute. Physical examination was remarkable only for hypotonia, with no evidence of tetany or neuromuscular compromise. He was started on i.v. fluids (normal saline at 65 ml/h) and admitted directly to the pediatric intensive care unit for further workup.
A workup was initiated to further investigate the etiology of the patient’s hypercalcemia. Parathyroid hormone levels as an outpatient, on admission, and during hospitalization were 9, 16, and 7 pg/ml, respectively, making primary hyperparathyroidism an unlikely etiology of the hypercalcemia (parathyroid hormone reference range 15–55 pg/ml). A urine calcium level was elevated at 166 mg per 24 hours (21 mg/kg per 24 hours). Vitamin D 25-OH was 34 ng/ml (reference rage 30–100 ng/ml) and vitamin D 1,25 was 35.2 pg/ml (normal range 19.9–79.3 pg/ml). An echocardiogram was performed and was unremarkable, demonstrating an intact atrial septum and a patent aortic arch with no coarctation. A skeletal survey was within normal limits. Renal ultrasound demonstrated bilateral increase in echogenicity of the renal cortex with mildly increased echogenicity within the renal pyramids. Mild dilation of the renal pelvis also was seen, bilaterally. Although aggressive hydration initially improved his calcium to 11.5 mg/dl, the hypercalcemia recurred on hospital day 2 (12.2 mg/dl) and the patient received i.v. pamidronate, 8 mg (1 mg/kg) run over 3 hours. A renal biopsy was performed on hospital day 3 to determine the etiology of the elevated serum creatinine.
Additional Investigations
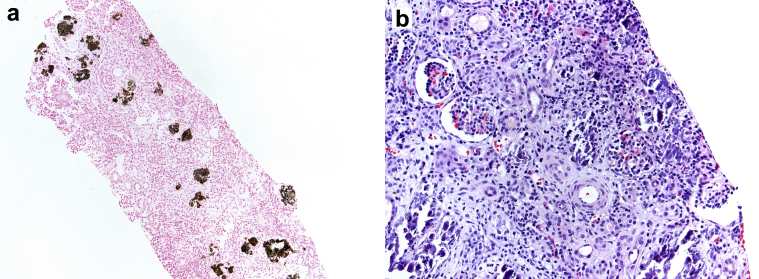
The kidney biopsy specimen contained 15 glomeruli for light microscopic examination. All glomeruli were normal without proliferative changes or sclerosis. Extensive calcium phosphate deposition, which stained positive with von Kossa stain, was present diffusely throughout the tubulointerstitium. There was severe tubular atrophy and dropout along with interstitial fibrosis encompassing approximately 50% of the interstitial compartment of the cortex. Minimal inflammation was present within the intact tubulointerstitium. Arteries were unremarkable (Figure 1). Immunofluorescence was negative for immune reactants throughout the glomeruli and tubulointerstitium. Electron microscopic evaluation of a glomerulus showed unremarkable mesangial matrix and basement membranes with no evidence of electron-dense deposits.
Figure 1.
Kidney biopsy specimen. (a) Low-power photomicrograph with tubulointerstitial crystalline deposits highlighted by von Kossa stain (original magnification ×100). (b) Interstitial edema, inflammation, and numerous basophilic tubulointerstitial calcium phosphate crystals (hematoxylin and eosin; original magnification ×200).
Kidney Biopsy Diagnosis
The diagnosis of nephrocalcinosis was made, with numerous calcium phosphate crystals throughout the tubulointerstitium, as well as severe interstitial fibrosis and tubular atrophy.
Clinical Follow-up
Because of the renal biopsy findings of diffuse nephrocalcinosis in an 11-month-old with developmental delay, poor feeding, and poor weight gain, a wide differential was entertained. Whole-exome sequencing (Invitae Genetics, San Francisco, CA) was performed to further evaluate for a genetic etiology of the short stature, nephrocalcinosis, and failure to thrive. A homozygous pathogenic variant, c.1396C>T (p.Arg466*), was identified in the CYP24A1 gene. Further analysis revealed that both parents are carriers of this mutation. This variant was present in the gnomaAD database (rs988715134) with an allele frequency of 0.000007954. It fulfilled American College of Medical Genetics and Genomics criteria to be classified as a pathogenic variant.
The patient has been seen for follow-up every 6 months, with mildly elevated calcium levels ranging from 10.4 to 10.5 mg/dl. His estimated glomerular filtration rate was 69.7 ml/min per 1.73 m2 at his 12-month follow-up and 74 ml/min per 1.73 m2 at his 24-month follow-up while continuing on sodium citrate–citric acid at 5 ml twice daily and rifampin. A follow-up renal ultrasound performed 1 year after admission demonstrated normal echogenicity and no evidence of nephrocalcinosis.
Discussion
Nephrocalcinosis refers to the histologic finding of calcium phosphate precipitates within the renal tubules, interstitium, and tubular basement membranes. This histopathology is typically seen in association with hypercalcemia, but is not specific for a single etiology. Although it can affect children of all ages, it most commonly presents in the first years of life, and the underlying cause is commonly genetic in nature.6 Nephrogenesis, specifically renal tubular maturation, is not complete until 34 to 36 weeks’ gestation, making the kidneys of premature infants at particularly increased risk of nephrocalcinosis. The increased risk is thought to be the consequence of low urine velocity in the setting of long loops of Henle resulting in conditions that are favorable for crystal formation.7
Timely diagnosis of nephrocalcinosis is important to halt progression of renal injury. A thorough history should be obtained, including birth history (specifically prematurity), diet, fluid intake, medications, vitamin supplementation, developmental history, and other known diseases or conditions.6, 8 A detailed family history also is warranted, and may include the use of a pedigree for initial evaluation of possible heritable etiologies. Physical examination findings are typically nonspecific, but the presence of signs that are syndromic in nature may provide helpful information to narrow the differential diagnosis. Aside from renal biopsy, high-resolution renal ultrasound is useful for the identification of nephrocalcinosis8; however, it is important to know the pitfalls of this technique in neonates and preterm infants. In this population, Tamm-Horsfall protein deposited in renal calyces can mimic the appearance of nephrocalcinosis but will disappear within 1 to 2 weeks on reevaluation. In addition, the presence of physiologically increased echogenicity of renal cortices within neonates also can complicate the identification of nephrocalcinosis by ultrasound in this age group.8
The diagnosis of nephrocalcinosis is not specific, and should initiate a thorough investigation to determine the etiology of a patient’s underlying kidney disease so that the appropriate treatment can be initiated. Possible etiologies include nongenetic conditions, such as primary hyperparathyroidism, as well as medications such as loop diuretics and vitamin D toxicity.7, 8 Genetic etiologies of nephrocalcinosis are particularly common in the pediatric setting. These include Dent disease (mutation in CLCN5), Lowe syndrome (Dent 2, OCRL mutation), William-Beuren syndrome, and Bartter syndrome, CASR mutations, as well as idiopathic infantile hypercalcemia due to mutations in CYP24A1, involved in vitamin D metabolism (Table 1).6, 8, 9 Laboratory tests commonly used for identification of the pathogenesis of nephrocalcinosis include blood and urine electrolytes, vitamin D levels, hormone levels (such as parathyroid hormone), and genetic testing.8
Table 1.
Genetic etiologies of pediatric nephrocalcinosis
| Disease | Gene | Gene product | Inheritance pattern | Phenotype |
|---|---|---|---|---|
| Bartter syndrome type 1 | SLC12A1 | Bumetadine-sodium-potassium-chloride cotransporter | Autosomal recessive | Polyhydramnios, prematurity, failure to thrive, constipation, dehydration, osteopenia, intellectual disability, muscle weakness, cramping, fatigue, hypercalciuria, nephrocalcinosis |
| Bartter syndrome type 2 | KCNJ1 | Renal outer-medullary potassium channel | Autosomal recessive | Polyhydramnios, prematurity, failure to thrive, constipation, dehydration, osteopenia, intellectual disability, muscle weakness, cramping, fatigue, hypercalciuria, nephrocalcinosis |
| Bartter syndrome type 3 | CLCNKB | Voltage-gated chloride channel | Autosomal recessive | Typically less severe than types 1/2, wide variation in phenotype hypokalemia, metabolic alkalosis, hypercalciuria, nephrocalcinosis |
| Bartter syndrome type 4 | BSND | Beta subunit for chloride channels CLCNKA and CLCNKB | Autosomal recessive | Usually antenatal presentation, failure to thrive, hypotonia, hyporeflexia, intellectual disability, motor delay, polyhydramnios, prematurity, sensorineural hearing loss, nephrocalcinosis |
| Bartter syndrome type 5 | CASR | Calcium-sensing receptor | Autosomal dominant | Mild, usually asymptomatic hypocalcemia, nephrocalcinosis |
| Dent disease type 1 | CLCN5 | Chloride voltage-gated channel 5 | X-linked recessive | Fanconi syndrome, rickets, nephrocalcinosis, nephrolithiasis |
| Dent disease type 2/Lowe syndrome | OCRL | Inositol polyphosphate-5-phosphatase | X-linked recessive | Oculocerebrorenal syndrome including congenital cataracts, intellectual disability, nephrolithiasis, Fanconi syndrome |
| Familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC) |
CLDN16 CLDN19 |
Claudin 16 Claudin 19 |
Autosomal recessive | Hypomagnesemia, hypocalcemia, nephrocalcinosis, urolithiasis |
| Williams-Beuren syndrome | Continuous deletion syndrome, hemizygous deletion of 1.5–1.8 Mb on chromosome 7q11.23 Includes ELN, LIMK1, RFC2 |
Elastin lim domain kinase 1 replication factor C, subunit 2 |
Mostly sporadic Autosomal dominant |
Multisystemic disorder, intellectual disability, distinctive facial features (including broad forehead, periorbital fullness, strabismus, stellate iris, flat nasal bridge, malar flattening, long smooth philtrum, pointed chin), short stature, hypertension, supravalvular aortic stenosis, arterial stenosis, friendly social personality, endocrine abnormalities (hypercalcemia, diabetes mellitus, hypothyroidism), nephrolithiasis, constipation, rectal prolapse, spasticity, hypotonia, joint laxity, contractures |
| Idiopathic infantile hypercalcemia | CYP24A1 | 25-hydroxymitamin D 24-hydrolase | Autosomal recessive | Increased sensitivity to vitamin D leading to hypercalcemia and nephrocalcinosis |
We present here the case of an 11-month-old American Indian boy with gross motor delay and poor weight gain who was found to have severe hypercalcemia along with renal insufficiency. The patient was formula-fed but was not taking excessive amounts of vitamin D and was not premature at birth. Whole-exome sequencing revealed a homozygous pathogenic variant within the CYP24A1 gene, c.1396C>T (p.Arg466*), resulting in a premature stop signal during translation leading to an absent or truncated protein product. Among the CYP24A1 mutations leading to nephrocalcinosis that have been described previously, our case demonstrates a novel pathogenic mutation.S1–S7 CYP24A1 encodes 1,25(OH)2D-24-hydroxylase, an enzyme vital in vitamin D metabolism. Specifically, it functions to hydroxylate 1,25-dihydroxyvitamin D3 (activated form) as well as 25-hydroxyvitamin D3 at the C24 position, leading to inactivation of 1,25-dihydroxyvitamin D3, enabling calcium homeostasis.S1,S8 Classic associated findings with CYP24A1 mutations include high vitamin D 25-(OH)D3 and 1,25-(OH)2D3, low 24,25-(OH)2D3, and normal to high serum calcium.S1,S8 However, our case, as well as previous reports, show that these generalizations are inconsistent, and 1,25-(OH)2D3 can be in the normal range despite the enzyme that breaks it down being abnormal.S2,S3 Thus, CYP24A1 mutations cannot be excluded as the etiology of nephrocalcinosis based on the vitamin D levels. The explanation for this is unknown, although it might suggest some residual activity of the mutant CYP24A1 protein in these cases.
Long-term management of patients with nephrocalcinosis has largely been directed at minimizing large peaks in urine solute concentration through adequate hydration distributed over the whole day.6 Conventional management also includes limiting sunlight exposure and dietary vitamin D and calcium.S7,S9 The addition of citrate preparations is aimed at reducing precipitation of calcium in the urine and can reduce progression of nephrocalcinosis.6 Therapies including azoles (notably ketoconazole and fluconazole) directed at reducing the active 1,25 dihydroxy vitamin D3 through inhibition of 25-hydroxylase and 1-alpha-hydroxylase have been used in recent years to treat patients with vitamin D–mediated hypercalcemia, including those with CYP24A1 mutations.S10,S11 However, it is important to note that when treating with azoles, patients should be monitored for possible hepatotoxicity.S10 Rifampin has recently been reported as an effective treatment of nephrocalcinosis due to mutations in CYP24A1through induction of the p450 enzyme, CYP3A4. The utility of this upregulation is the creation of an alternative pathway whereby 25-hydroxy vitamin D3 and active 1,25 dihydroxy vitamin D3 are metabolized to inactive forms, 4-beta,25 dihydroxy vitamin D3 and 1,23,25 trihydroxy vitamin D3.S9 Reports have shown this therapy to improve clinical symptoms, normalize serum and urinary calcium concentrations, and normalize serum intact parathyroid hormone level.S9
Conclusion
Nephrocalcinosis is a rare finding on renal biopsy in infants that raises a broad differential diagnosis of genetic as well as nongenetic etiologies. The identification of nephrocalcinosis as the driver of kidney failure is only a first step in an important clinical evaluation. Diagnostic measures should continue until a definite underlying pathogenic mechanism can be identified, as this will determine treatment and prevent further renal injury. This evaluation should include a careful history and physical in combination with appropriate laboratory and imaging techniques. The case presented here demonstrates the utility of genetic testing in identifying the etiology in a patient with a novel pathogenic CYP24A1 mutation (Table 2).
Table 2.
Key teaching points
| 1. Timely diagnosis of the underlying etiology of nephrocalcinosis is important to halt progression of renal injury. |
| 2. Genetic etiologies of nephrocalcinosis are particularly common in the pediatric setting. |
| 3. CYP24A1 mutations are an autosomal recessive etiology of idiopathic infantile hypercalcemia and nephrocalcinosis. |
Disclosure
All the authors declared no competing interests.
Footnotes
Supplementary References.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
References
- 1.McNeilly J.D., Boal R., Shaikh M.G. Frequency and aetiology of hypercalcaemia. Arch Dis Child. 2016;101:344–347. doi: 10.1136/archdischild-2015-309029. [DOI] [PubMed] [Google Scholar]
- 2.Cullas Ilarslan N.E., Siklar Z., Berberoglu M. Childhood sustained hypercalcemia:a diagnostic challenge. J Clin Res Pediatr Endocrinol. 2017;9:315–322. doi: 10.4274/jcrpe.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tebben P.J., Singh R.J., Kumar R. Vitamin D-mediated hypercalcemia:mechanisms, diagnosis, and treatment. Endocr Rev. 2016;37:521–547. doi: 10.1210/er.2016-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stokes V.J., Nielsen M.F., Hannan F.M. Hypercalcemic disorders in children. J Bone Miner Res. 2017;32:2157–2170. doi: 10.1002/jbmr.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones G., Prosser D.E., Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. 2014;55:13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habbig S., Beck B.B., Hoppe B. Nephrocalcinosis and urolithiasis in children. Kidney Int. 2011;80:1278–1291. doi: 10.1038/ki.2011.336. [DOI] [PubMed] [Google Scholar]
- 7.Schell-Feith E.A., Kist-van Holthe J.E., van der Heijden A.J. Nephrocalcinosis in preterm neonates. Pediatr Nephrol. 2010;25:221–230. doi: 10.1007/s00467-008-0908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoppe B., Kemper M.J. Diagnostic examination of the child with urolithiasis or nephrocalcinosis. Pediatr Nephrol. 2010;25:403–413. doi: 10.1007/s00467-008-1073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Berkel Y., Ludwig M., van Wijk J.A.E. Proteinuria in Dent disease: a review of the literature. Pediatr Nephrol. 2017;32:1851–1859. doi: 10.1007/s00467-016-3499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.