Abstract
Surgical treatment of patients with massive rotator cuff tears is unpredictable because of a low healing rate and high incidence of clinical failure. Arthroscopic superior capsular reconstruction has emerged as a promising technique in treating younger, active patients with massive irreparable rotator cuff tears. Superior capsular insufficiency has been theorized to be a factor in the higher failure rate for repairs of massive tears, and there have been proposals of superior capsular repair in addition to rotator cuff repair to facilitate better healing of massive rotator cuff tears. This article presents our technique of functional rotator cuff augmentation, which is concomitant superior capsular reconstruction with arthroscopic rotator cuff repair, to treat massive, atrophic rotator cuff tears. This technique is used in patients with massive rotator cuff tears and superior capsular insufficiency.
Treatment options for young, active patients with massive, retracted rotator cuff tears have historically been considered unpredictable and fraught with high failure rates because of tendon inelasticity and the poor tissue quality typically present in these retracted tears.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 In 2013, Mihata et al.15 described arthroscopic superior capsular reconstruction (SCR) as a successful procedure in treating massive, irreparable rotator cuff tears. They reported excellent clinical results using a fascia lata autograft to arthroscopically reconstruct the superior capsule, with significant improvements in pain, function, and range of motion in forward flexion and abduction.15 As this procedure has evolved, techniques of arthroscopic SCR with acellular dermal allograft have been developed as an alternative to their original technique.4, 5 When performing arthroscopic SCR in patients with massive rotator cuff tears, we initially would attempt to mobilize the residual rotator cuff tissue through release techniques. Prior to SCR, residual rotator cuff tissue was often unable to be advanced to the native rotator cuff footprint, leading us to assume irreparability of the tear. We then noted the phenomenon that, after SCR, rotator cuff tears that were unable to be reduced to the native footprint on initial arthroscopic evaluation and attempted repair were able to be advanced to the rotator cuff footprint under significantly less tension. This led us to evaluate and create the concept of the superior capsular distance.19 It was our hypothesis that restoration of glenohumeral mechanics with SCR would effectively re-center the glenohumeral articulation and decrease the distance of excursion of the residual rotator cuff tissue, allowing reapproximation of the rotator cuff tissue to its native footprint. The decreased superior capsular distance resulting from SCR allows us to repair rotator cuff tears overlying the reconstruction with significantly less tension. We hypothesized that this technique would allow the SCR graft to serve the following purposes in treating those patients with massive rotator cuff tears with superior capsular insufficiency:
-
1
Superior capsular stability that is lost in chronic massive tears is restored.
-
2
The superior capsular distance is decreased and the humeral head is re-centered, resulting in less tension on the repair.
-
3
Maintenance of the decreased superior capsular distance during early healing protects the repair, with the graft serving as a “biologic internal brace.”
-
4
Soft-tissue augmentation from the graft provides a biologic scaffold to the repair in these tears that uniformly have poor tissue quality.
As previously stated, it has been hypothesized that the high failure rate of rotator cuff repairs of massive tears may be due to persistent superior capsular insufficiency. Our technique restores superior capsular stability while providing biologic augmentation, leading us to refer to this as a “functional biologic augmentation” owing to its biomechanical function as a stabilizer and the biologic function in augmentation of poor tissue quality.
Technique
The indications for this technique are patients presenting with massive rotator cuff tears that are unable to be easily advanced to the native rotator cuff footprint despite release techniques such as rotator interval slides. Typically, these patients have superior capsular insufficiency with no ability to repair the native superior capsule (Fig 1). The described procedure is typically performed in patients with massive rotator cuff tears, retraction, superior capsular insufficiency, and poor tissue quality. We have also used this technique in patients with retraction and advanced muscular atrophy (Fig 2). Preoperative radiographic examination is used to predict whether the technique may be used (Fig 2). When a suggestion of superior capsular insufficiency is present on plain radiographs or magnetic resonance imaging with evidence of superior humeral head migration or if advanced retraction and atrophy are present, we always have the equipment available to perform this technique.
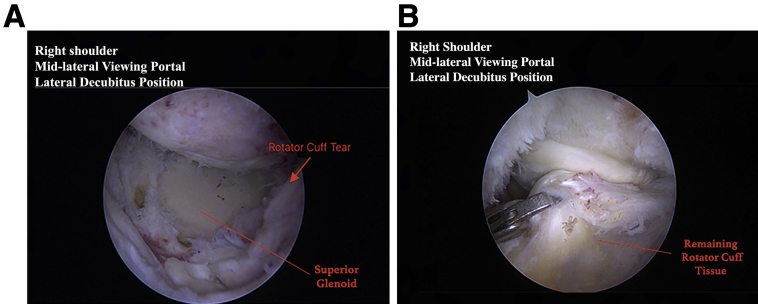
Fig 1.
Arthroscopic images taken from the midlateral viewing portal in a right shoulder with the patient in the lateral decubitus position. The initial arthroscopic findings of a typical patient undergoing this procedure are shown. (A) Massive rotator cuff tear. (B) A large tear of the supraspinatus and infraspinatus is present that, when mobilized, is not able to be approximated to the native rotator cuff footprint.
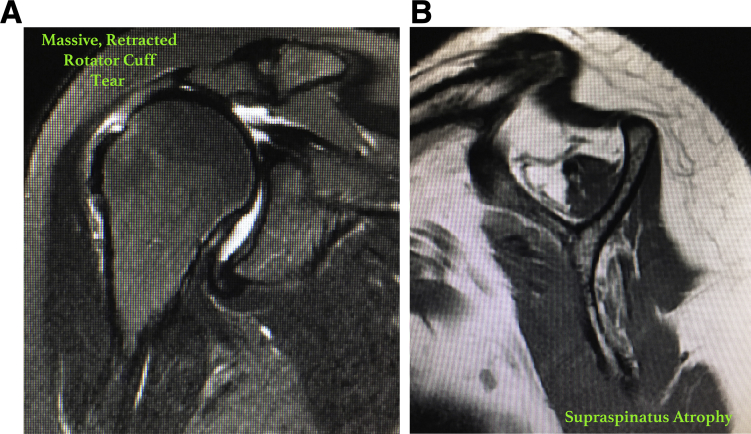
Fig 2.
Typical findings on magnetic resonance imaging in a patient undergoing the functional biologic augmentation procedure: coronal (A) and sagittal (B) views of a patient presenting with significant right shoulder muscular atrophy in the supraspinatus tendon and a massive, retracted rotator cuff tear.
All of our cases are performed with patients in the lateral decubitus position with general anesthesia and a single-shot interscalene block or single-shot block with an indwelling pain catheter. The shoulder is first examined to assess range of motion and stability. The patient is placed in the lateral decubitus position with the arm positioned in 45° of abduction, 10° of forward flexion, and neutral rotation. A posterior glenohumeral viewing portal is created along with an anterior outflow and/or working portal, and diagnostic arthroscopy is performed. Any intra-articular pathology is assessed and addressed appropriately. The arthroscope is then reconfigured into the subacromial space, and a midlateral portal is created. Specific attention is given to the rotator cuff as it is assessed for degree of retraction, mobility, tissue quality, and atrophy. Reparability of the rotator cuff is determined initially with release techniques and attempts in multiple vectors to reapproximate the torn rotator cuff to the native footprint. When it is determined that the remaining tissue is of poor quality, that the eventual repair would be under increased tension, and that the native superior capsular layer is not present and able to be repaired, the decision is made to use this technique.
The biceps tendon, if present, has been treated with tenotomy to facilitate exposure of the superior glenoid in all of our functional augmentation procedures. The superior labrum is debrided to expose and create a bleeding bed of bone on the superior glenoid to facilitate graft healing to the glenoid. We perform this technique with 4 portals: midlateral portal, anterior portal, posterior portal, and juxta-acromial working portal (Fig 3). These are the same portals we use to perform a standard rotator cuff repair. The juxta-acromial portal is able to be used for placement of glenoid fixation and humeral fixation, as well as graft introduction into the joint. The midlateral portal is typically used as our viewing portal. The juxta-acromial portal is placed to facilitate appropriate anchor placement in the superior glenoid rim, as well as anchor placement to the proximal humerus, for a standard arthroscopic transosseous-equivalent double-row fixation technique of the graft to the humerus.15 This portal is similar to a standard rotator cuff repair portal used for anchor placement and is placed with the assistance of visualization with a spinal needle. A flexible large-diameter cannula (Passport; Arthrex, Naples, FL) is placed in the juxta-acromial portal for graft advancement. For glenoid and humeral fixation, large twist-in threaded cannulas (8.25 mm × 7 cm; Arthrex) are placed in the posterior portal and a standard or large threaded cannula is placed in the anterior portal to be used as working portals (Fig 3).
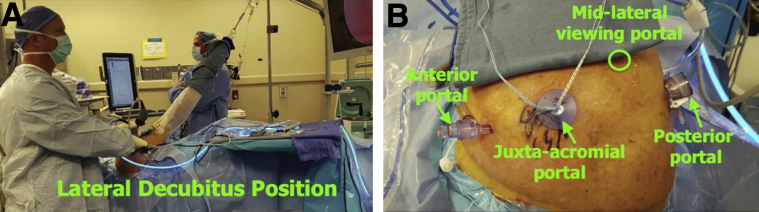
Fig 3.
Typical preparation for our procedure. (A) The patient is in the lateral decubitus position. (B) The procedure is performed with 4 standard portals. In our technique, the midlateral portal is used for visualization; the posterior and anterior portals are used as working portals; and the juxta-acromial portal is used for graft advancement, as well as graft fixation to the glenoid and graft and rotator cuff fixation to the humerus.
The undersurface of the acromion and coracoacromial ligament is assessed for evidence of impingement. Any evidence of abrasion on the undersurface of the acromion is addressed with an arch-sparing and/or gentle subacromial smoothing procedure. The acromioclavicular joint is assessed, and if required (acromioclavicular joint tenderness), a distal clavicle excision is performed.
We use the same surgical technique for the glenoid fixation step described in our previously published reports on short-term clinical outcomes of arthroscopic SCR with our modification of glenoid fixation of the graft using a push-in type of anchor.19 After abrasion of the superior glenoid rim and the footprint of the greater tuberosity of the humerus, 2.9-mm drill holes are placed in the superior glenoid rim in preparation for graft fixation (Fig 4).
Fig 4.
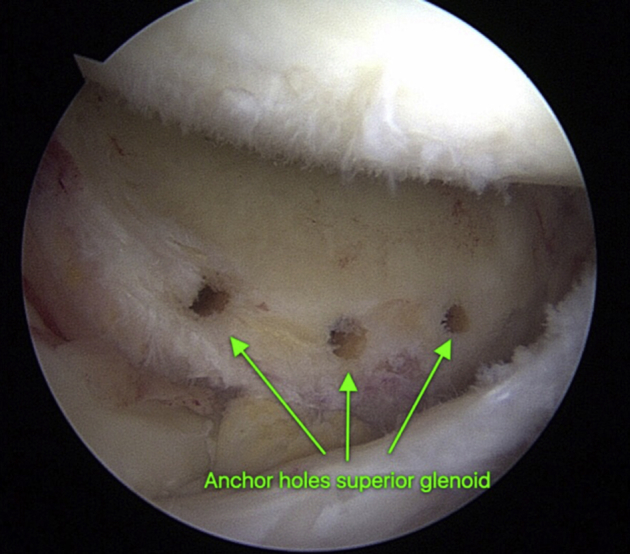
View of the right shoulder from the midlateral viewing portal in a patient in the lateral decubitus position, showing the anchor holes placed in the superior glenoid for glenoid fixation of the acellular dermal allograft.
The 2 medial-row anchors (BioComposite, 4.75 mm × 19.1 mm; Arthrex) on the humerus are placed with associated suture tapes. Graft dimensions are obtained medially on the superior glenoid rim, laterally on the humeral footprint between remnant rotator cuff tissues, and anteriorly and posteriorly between the superior glenoid and the humerus.
A 3.0-mm-thick (2.75-3.25 mm) acellular dermal allograft (LifeNet Health, Virginia Beach, VA) is prepared using the dimensions obtained. Three suture tapes to be used for glenoid fixation are placed in the medial aspect of the graft. We mark the middle suture tape with a surgical marker at the graft–suture tape interface, as well as at the free ends, to facilitate identification during graft advancement and fixation (Fig 5). Two holes of sufficient diameter are placed in the lateral (humeral) aspect of the graft to allow passage of the medial-row suture tapes that are in the rotator cuff anchors.
Fig 5.
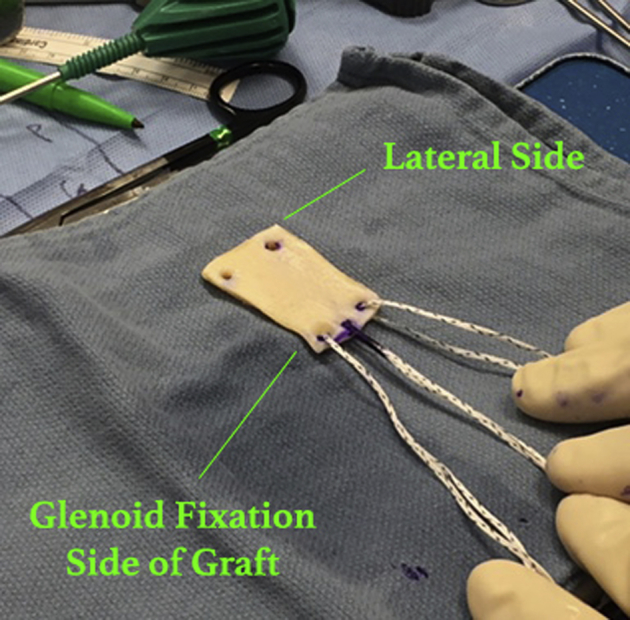
Outside of the shoulder, preparation of the acellular dermal allograft is shown. The prepared acellular dermal allograft features 3 suture tapes on the medial (glenoid) aspect and 2 holes laterally to facilitate passage of the suture tapes from the medial-row anchors into the humerus.
Glenoid fixation in our technique uses a push-in anchor technique (BioComposite, 2.9 mm × 12.5 mm; Arthrex) that allows us to use the anchor suture tape–graft interface to push the graft in through the juxta-acromial portal and securely fix the graft to the glenoid adjacent to the articular margin immediately medial to the superior articular surface of the glenoid. We favor this fixation technique over the double-pulley technique because of ease of graft advancement using the anchor to advance the graft, as well as the fixation provided by the anchor being placed in the typically strong subchondral bone of the glenoid immediately adjacent to the superior glenoid rim. This technique is knotless, providing strong, smooth fixation of the medial aspect of the graft. Typically, we advance the graft through the juxta-acromial portal with the suture tape and anchor of the posterior-superior glenoid fixation site, followed by fixation of the graft to the middle anchor of the superior glenoid and, last, the anterosuperior glenoid anchor. In all cases that we have performed, we have used 3 push-in anchors on the glenoid. Figure 6 shows the SCR graft fixed to the glenoid. Medial-row sutures are passed through the graft. The residual native rotator cuff tissue is visualized adjacent to the graft, as shown at the bottom of the arthroscopic photograph.
Fig 6.
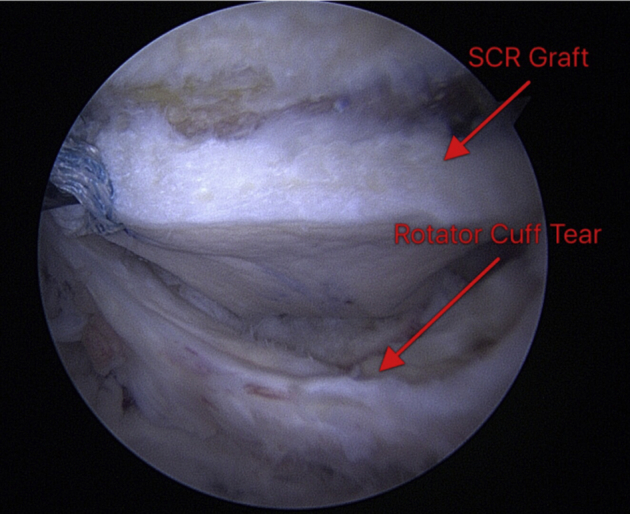
Arthroscopic view from the midlateral portal of the rotator cuff and dermal allograft. A right shoulder is shown, with the patient in the lateral decubitus position. The graft has been fixed to the glenoid, and the remnants of the rotator cuff can be seen below this graft. By use of the midlateral portal from this configuration, the greater tuberosity of the humerus is visualized at the superior aspect of the photograph. (SCR, superior capsular reconstruction.)
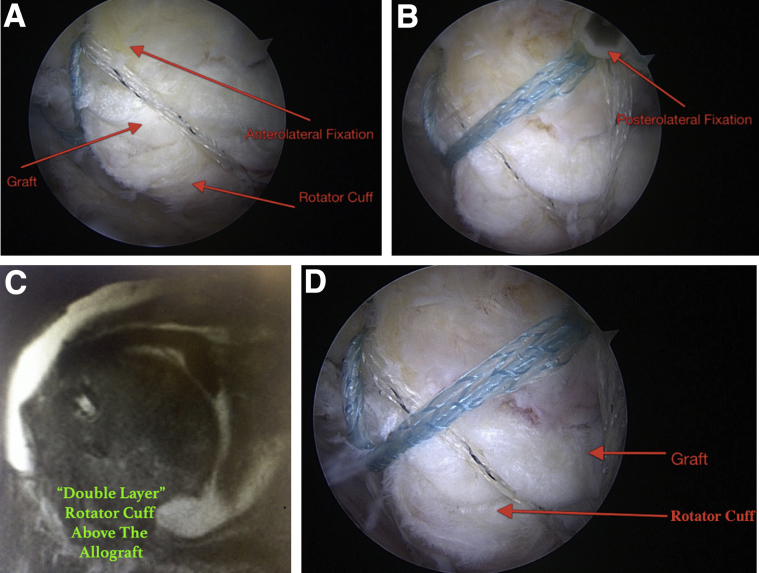
In our earlier cases, when we noted that rotator cuff tissue would be able to be advanced after SCR to the native footprint more easily, presumably because of the decreased superior capsular distance provided by the graft, we would perform repair of the rotator cuff over the top of the SCR graft with separate fixation. Because we are now better at identifying the ability to decrease the superior capsular distance by reconstruction of the superior capsule with acellular dermal allograft, we use the medial-row tapes to fix the graft and the native remaining rotator cuff tissue to the greater tuberosity of the humerus. After the graft is placed and fixed to the glenoid, the humeral suture tapes from the medial anchor row are passed through the rotator cuff anteriorly and posteriorly, as shown in Figure 7. Lateral fixation is provided by placement of these sutures with 2 additional lateral anchors placed into the humerus lateral to the native footprint, securely fixing the rotator cuff and graft to the humerus. The final construct provides a double-row transosseous-equivalent repair of the lateral aspect of the SCR graft and native rotator cuff tissue to the humerus. When necessary, additional suture-tape links are placed in the anterior and posterior corners of the residual native rotator cuff tissue and are incorporated into the lateral-row anchors during repair. In this technique, the rotator cuff tissue is repaired over the acellular dermal allograft (Fig 8).
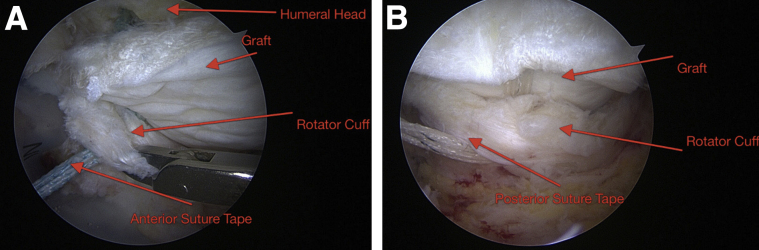
Fig 7.
Two arthroscopic views of a right shoulder from the midlateral viewing portal, showing the anterior and posterior suture tapes being passed through the rotator cuff tissue. (A) The humeral head and graft are visualized. (B) The medial-row tapes are traversing the acellular dermal allograft and the rotator cuff.
Fig 8.
Both the anterolateral fixation (A) and posterolateral fixation (B) of the rotator cuff and acellular dermal allograft onto the humerus are shown. These images were taken from the midlateral viewing portal in a right shoulder with the patient in the lateral decubitus position. (C) A magnetic resonance image of the same patient shows the typical “double layer” of this repair construct, with the rotator cuff overlying the dermal allograft, 6 months after our repair technique was used. (D) The double layer with the graft positioned beneath the rotator cuff, fixed to the humeral head, viewed from the midlateral portal, is shown in the same patient. The theoretical advantage of this technique is less initial tension on the repair owing to the decreased superior capsular distance provided by the superior capsular reconstruction.
Postoperatively, we treat all of these patients with a similar rehabilitation protocol to that used for our rotator cuff repair patients. This includes sling immobilization for comfort with passive range of motion for 6 weeks. Active-assisted motion is commenced at 6 weeks, with progression to active motion by 8 weeks postoperatively. Pearls and pitfalls of our technique are shown in Table 1, and advantages and disadvantages are listed in Table 2.
Table 1.
Pearls and Pitfalls of Technique
| Pearls |
| The anchor shaft should be used to advance the graft through the flexible cannula. |
| Careful suture management is required on graft introduction. |
| Visualization for the glenoid holes is best achieved through the midlateral portal. |
| When the posterior portal is used for visualization, the glenoid drill holes may be better visualized with a 70° arthroscope. |
| In cases in which there may be a higher risk of nonhealing, we inject the acellular dermal allograft with PRP during graft preparation. The vascular channels that are present in the graft can serve as a substrate for growth factors and platelets provided in the injection to remain in the environment for an extended period. |
| Pitfalls |
| In cases with poor suture management, removal of the graft for reintroduction can be challenging. |
| Regarding the indications for any placement of superior capsular reconstruction alone or with the described augmentation technique, either an intact subscapularis attachment or a reparable tear of the subscapularis tendon needs to be present. Restoration of the force couples is not possible without a functioning subscapularis tendon. |
| Care needs to be taken not to cause iatrogenic injury to the face of the glenoid or to damage the suprascapular nerve during insertion of glenoid anchors. |
PRP, platelet-rich plasma.
Table 2.
Advantages and Disadvantages
| Advantages |
| Glenoid fixation of the SCR graft is theoretically improved owing to the technique using suture tapes with 3 points of fixation in the strong subchondral bone of the glenoid. |
| Theoretic improvement in the ease of graft introduction occurs in comparison with the double-pulley technique because the graft can be pushed into the fixation point with the anchor and shaft of the anchor inserter. |
| Restoring superior capsular stability through superior capsular reconstruction theoretically decreases rotator cuff repair failures due to superior capsular insufficiency that is typically present in these massive rotator cuff tears. |
| The decreased superior capsular distance and re-centering of the humeral head decrease tension on the repair and theoretically improve healing through early protection of the repair. |
| Maintenance of the decreased SCD during early healing protects the repair, with a theoretical “biologic internal brace” of the rotator cuff repair. |
| Soft-tissue augmentation provides a biologic scaffold and increases the strength of the repair construct. |
| Disadvantages |
| The need for direct suture management is increased. |
| The expense of the repair, because of the need for additional anchors and tapes in the glenoid, as well as the cost of acellular dermal allograft, must be considered. |
SCD, superior capsular distance; SCR, superior capsular reconstruction.
Discussion
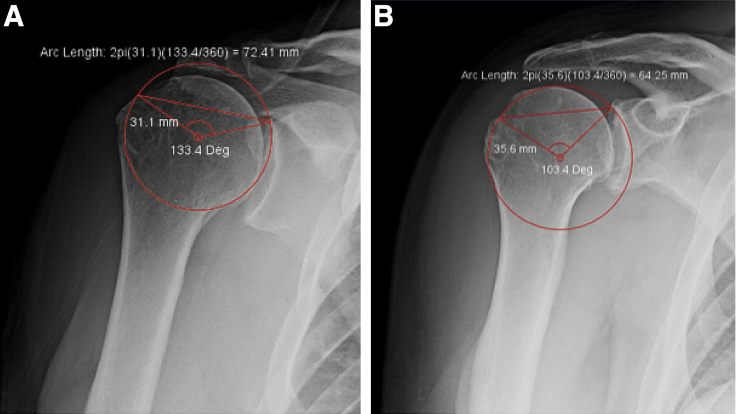
We call our technique a “functional biologic augmentation” of the rotator cuff because it theoretically provides mechanical advantages, as described earlier, by restoring superior capsular stability, decreasing the superior capsular distance, and providing biologic augmentation. Adams et al.20 recently discussed the importance of achieving superior capsular repair when treating patients with massive rotator cuff tears surgically to achieve more predictable results. When superior capsular tissue is present, we certainly try to incorporate this tissue into the repair and do not proceed with reconstruction. When repair of the native superior capsular tissue is not possible, we use this technique to restore superior stability to theoretically decrease stress on the repair. Recently, Cabarcas et al.21 reported a similar technique in Arthroscopy Techniques that they termed “over-the-top repair” of native rotator cuff tissue over an SCR graft in accordance with our view. We believe that decreasing the superior capsular distance through placement of the SCR graft decreases tension on the repair by decreasing the length that the rotator cuff has to be stretched to reach the native attachment site. This, along with restoration of superior capsular mobility, results in a theoretically enhanced healing environment for these massive repairs. Figure 9 illustrates the concept of the superior capsular distance measured preoperatively as well as postoperatively in a patient who underwent this procedure.
Fig 9.
Our method for measuring the superior capsular distance (SCD) is shown in a right shoulder. The SCD is defined as the arc length (in millimeters) between the medial aspect of the greater tuberosity and the superior aspect of the glenoid (2∏r × ∡°/360°). (A) Preoperative radiograph showing an SCD of 72.41 mm. (B) Radiographic image taken after superior capsular reconstruction and arthroscopic rotator cuff repair, indicating a 64.25-mm SCD. (Deg, degrees.)
This technique of concomitant SCR with rotator cuff repair has risks inherent to standard SCR and rotator cuff repair techniques. For our glenoid fixation, because we use 3 points of anchor fixation, there is theoretically an increased potential risk of glenoid bone stock injury. If this is the case, a primary limitation of this technique would be poor fixation or failure of fixation in patients who have pre-existing or iatrogenic poor superior glenoid bone stock.
In summary, this technique has been a reproducible method for us to achieve successful surgical management of patients with massive rotator cuff tears and radiographic evidence of advanced atrophy and superior capsular insufficiency who are not candidates for treatment with reverse total shoulder arthroplasty because of age and activity-level concerns. Video 1 shows our procedure in a step-wise process. We are currently updating our clinical series to report our patient results with more than 2 years of follow-up to ascertain the true value of this procedure long-term. Given the expense of this procedure, we need to continue to monitor its long-term efficacy to determine its value in this patient population.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: W.T.P. receives consulting fees from Arthrex and is a physician owner of Midwest Orthopedic Specialty Hospital and The Surgery Center at Associated Medical and Surgical Specialists. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Arthroscopic rotator cuff repair with concomitant superior capsular reconstruction. Our procedure is shown in a step-wise process, clearly demonstrating our technique, through the mid-lateral viewing portal, in a right shoulder, with the patient in the lateral decubitus position.
References
- 1.Le B.T., Wu X.L., Lam P.H., Murrell G.A. Factors predicting rotator cuff tears: An analysis of 1000 consecutive shoulders. Am J Sports Med. 2014;42:1134–1142. doi: 10.1177/0363546514525336. [DOI] [PubMed] [Google Scholar]
- 2.Mihata T., McGarry M., Pirolo J., Kinoshita M., Lee T. Superior capsular reconstruction to restore superior stability in irreparable rotator cuff tears: A biomechanical cadaveric study. Am J Sports Med. 2012;40:2248–2255. doi: 10.1177/0363546512456195. [DOI] [PubMed] [Google Scholar]
- 3.Adams C.R., Denard P.J., Brady P.C., Hartzler R.U., Burkhart S.S. The arthroscopic superior capsular reconstruction. Am J Orthop (Belle Mead NJ) 2016;45:320–324. [PubMed] [Google Scholar]
- 4.Hirohara A.M., Adams C.R. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4:e637–e641. doi: 10.1016/j.eats.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerber C., Rahm S.A., Cantanzaro S., Farshad M., Moor B. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: Long-term follow-up of ten years. J Bone Joint Surg Am. 2013;95:1920–1926. doi: 10.2106/JBJS.M.00122. [DOI] [PubMed] [Google Scholar]
- 6.El-Azab H.M., Rott O., Irlenbusch U. Long term follow-up after latissimus dorsi transfer for irreparable posterosuperior rotator cuff tears. J Bone Joint Surg Am. 2015;97:462–469. doi: 10.2106/JBJS.M.00235. [DOI] [PubMed] [Google Scholar]
- 7.Hanseler J.F., Nagels J., Van der Zwall P., Nelissen R.G. Teres major tendon transfer for patients with massive irreparable posterosuperior rotator cuff tears: Short term clinical results. J Bone Joint Surg Am. 2013;95:523–529. doi: 10.1302/0301-620X.95B4.30390. [DOI] [PubMed] [Google Scholar]
- 8.Kanatli U., Ozer M., Ataoglu M.B. Arthroscopic assisted latissimus dorsi tendon transfer for massive, irreparable rotator cuff tears: Technique and short-term follow-up of patients with pseudoparalysis. Arthroscopy. 2017;33:929–937. doi: 10.1016/j.arthro.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Barber F.A., Burns J.P., Deutsch A., Labbe M.R., Litchfield R.B. A prospective randomized evaluation of acellular dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28:8–15. doi: 10.1016/j.arthro.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 10.Jones C.R., Snyder S.J. Massive irreparable rotator cuff tears: A solution that bridges the gap. Sports Med Arthrosc. 2015;23:130–138. doi: 10.1097/JSA.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 11.Bond J.L., Dopirak R.M., Higgins J., Burns J., Snyder S.J. Arthroscopic replacement of massive, irreparable rotator cuff tears using a graft jacket allograft: Technique and preliminary results. Arthroscopy. 2008;24:403–409.e1. doi: 10.1016/j.arthro.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A.K., Hug K., Boggess B., Gavigan M., Toth A.P. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: Clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41:872–879. doi: 10.1177/0363546512475204. [DOI] [PubMed] [Google Scholar]
- 13.Boileau P., Baque F., Valerio L., Ahrens P., Chuinard C., Trojani C. Isolated arthroscopic biceps tenotomy of tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89:747–757. doi: 10.2106/JBJS.E.01097. [DOI] [PubMed] [Google Scholar]
- 14.Park M.C., Elattreche N.S., Ahmad C.S., Tibone J.E. “Transosseous-equivalent” rotator cuff repair technique. Arthroscopy. 2006;22:1360.e1–1360.e5. doi: 10.1016/j.arthro.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Mihata T., Lee T.Q., Watanabe C. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29:459–470. doi: 10.1016/j.arthro.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Hirahara A.M., Anderson W.J., Panero A.J. Superior capsular reconstruction: Clinical outcomes after minimum 2-year follow-up. Am J Orthop (Belle Mead NJ) 2017;46:266–278. [PubMed] [Google Scholar]
- 17.Denard P.J., Brady P.C., Adams C.R., Tokish J.M., Burkhart S.S. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy. 2018;34:93–99. doi: 10.1016/j.arthro.2017.08.265. [DOI] [PubMed] [Google Scholar]
- 18.Ruiwen L., Lam P.H., Shepherd H., Murrell G.A. Tape versus suture—A biomechanical and clinical analysis in arthroscopic rotator cuff repair of large tears. Orthop J Sports Med. 2016;4(suppl) doi: 10.1177/2325967117701212. 2325967116S00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pennington W.T., Bartz B.A., Pauli J.M., Walker C., Schmidt W. Arthroscopic superior capsular reconstruction with acellular dermal allograft for the treatment of massive irreparable rotator cuff tears: Short-term clinical outcomes and the radiographic parameter of superior capsular distance. Arthroscopy. 2018;34:1764–1773. doi: 10.1016/j.arthro.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Adams C.R., DeMartino A., Rego G., Denard P.J., Burkhart S.S. The rotator cuff and the superior capsule: Why we need both. Arthroscopy. 2016;32:2628–2637. doi: 10.1016/j.arthro.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Cabarcas B.C., Garcia G.H., Gowd A.K., Liu J.N., Romeo A.A. Arthroscopic superior capsular reconstruction and over-the-top rotator cuff repair incorporation for treatment of massive rotator cuff tears. Arthrosc Tech. 2018;7:e829–e837. doi: 10.1016/j.eats.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Arthroscopic rotator cuff repair with concomitant superior capsular reconstruction. Our procedure is shown in a step-wise process, clearly demonstrating our technique, through the mid-lateral viewing portal, in a right shoulder, with the patient in the lateral decubitus position.