Introduction
Glomerular crescents are not generally regarded as a distinct pathology in renal amyloidosis. Yet up to 13.3% of cases of renal amyloidosis that have been reported show at least focal crescent formation.1 Thus, crescents may be a frequently overlooked finding in cases of renal amyloidosis.
Amyloidosis is a relatively uncommon disease (the incidence is about 5−10 cases per million patient-years), resulting from abnormal deposition of autologous protein fibrils that aggregate to form a cross-β sheet quaternary structures. The kidney is 1 of the most affected organs. The etiology of renal amyloidosis is heterogeneous. The most common types of amyloid to involve the kidney are light chain amyloidosis (AL-amyloidosis), serum amyloid A (AA amyloidosis), and leukocyte cell–derived chemotaxin 2 (ALECT2 amyloidosis). Serum amyloid A amyloidosis makes up approximately 40% of renal amyloidosis cases. The primary etiologies for AA amyloidosis include rheumatoid arthritis and familial Mediterranean fever. A rare etiology for AA amyloidosis is solid tumors (etiology in <10% of AA amyloidosis cases).2, 3
Here we present 2 cases of renal AA amyloidosis in patients with underlying solid organ malignancies (renal cell carcinoma and cervical squamous cell carcinoma), both of which manifested as a crescentic glomerulonephritis.
Case Presentation
Case 1
The patient was a 60-year old Caucasian woman who presented with 8 weeks of lower extremity edema and mild dyspnea on exertion. She was found to have a creatinine of 8 mg/dl, nephrotic syndrome (urine protein to creatinine ratio, 20.6 g/g), and microhematuria. A renal ultrasound revealed a 10-cm left upper pole renal mass. Subsequent serologic workup including autoimmune serologies (antinuclear antibody, anti-dsDNA), infectious workup, anti-neutrophil cytoplasmic antibody (ANCA) studies, and serum and urine protein electrophoresis were all negative. The patient underwent biopsy of the right kidney to interrogate the cause of her acute kidney injury and nephrotic syndrome and subsequently underwent a left radical nephrectomy for her renal mass.
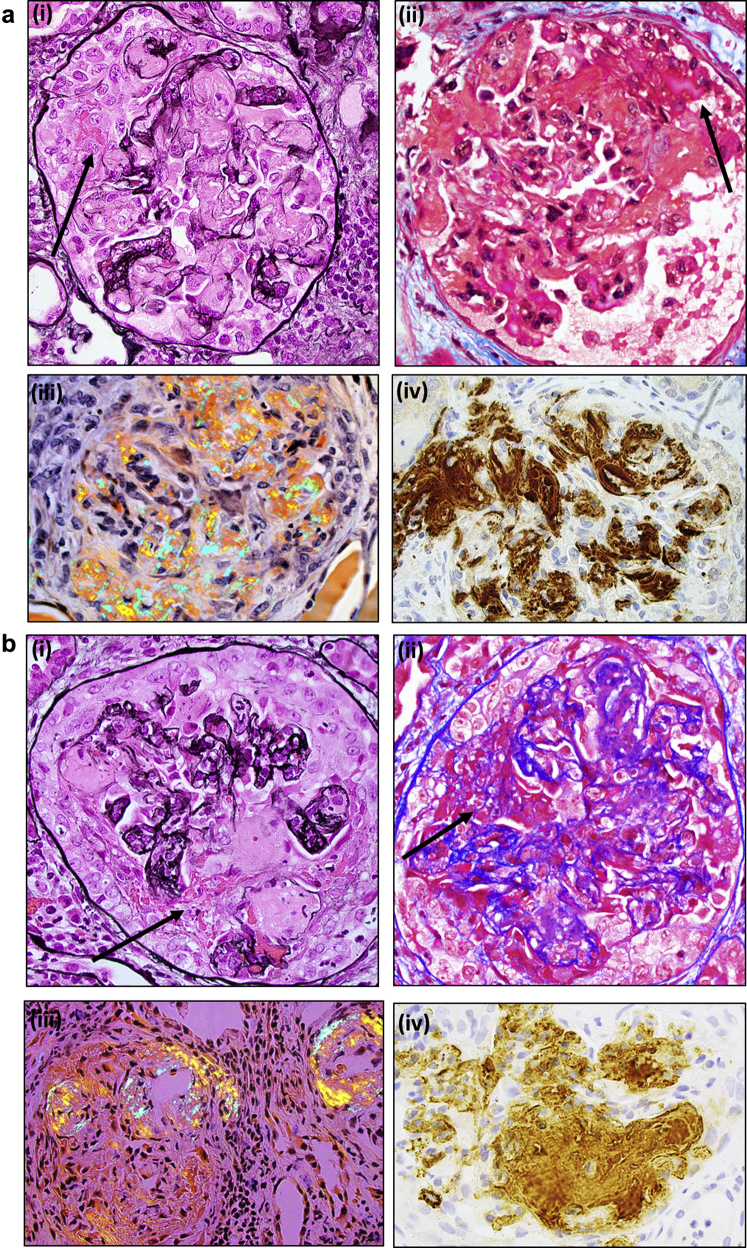
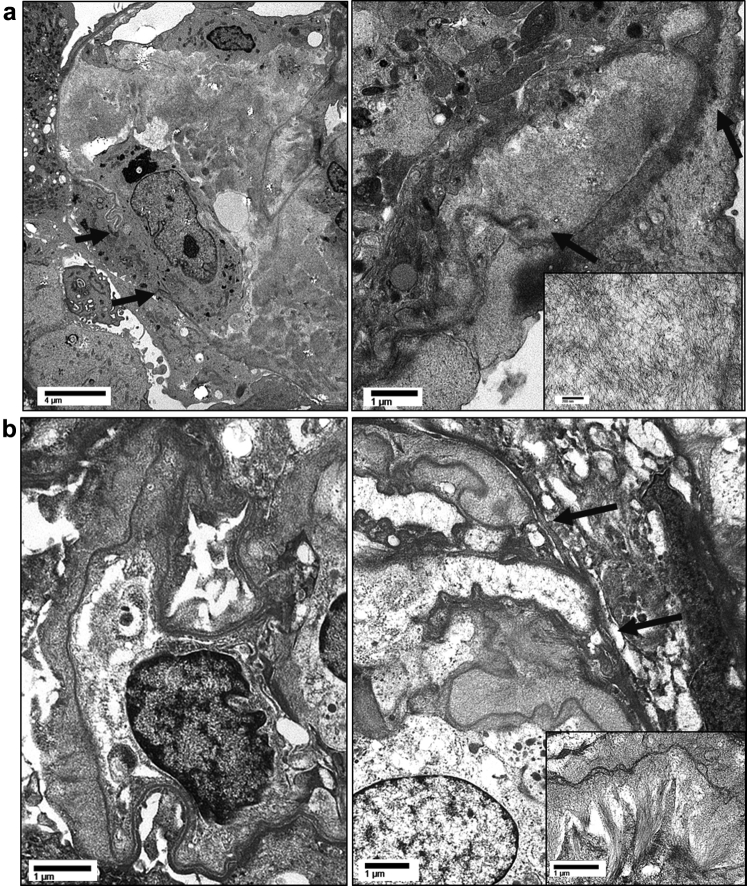
Light microscopy evaluation of the right-sided renal biopsy specimen (48 glomeruli, 1 globally sclerotic) revealed the majority (60%−70%) of glomeruli to be involved, with active cellular crescents represented by disruption of glomerular basement membranes with exudation of fibrin and cells into the urinary space (Figure 1). Several crescents were associated with segmental necrotizing lesions. A minority of glomeruli (<5%) showed fibrocellular-to-fibrous crescents. All glomeruli exhibited segmental to global capillary loop and nodular mesangial infiltration by amyloid (confirmed by Congo red stain, which showed apple-green birefringence under polarized light). The amyloid deposits were associated with basement membrane dissolution and crescent formation. Amyloid deposits were also seen in scattered arterioles as well as interstitial spaces (predominantly in the medulla). There was no arteritis. Capillary loops uninvolved with crescents or amyloid deposits showed mild segmental endocapillary hypercellularity composed of mononuclear leukocytes and occasionally neutrophils. There was marked tubular injury and mild interstitial inflammation as well as edema. There was early mild tubulointerstitial scarring. The immunofluorescence findings were significant only for fibrinogen staining of crescentic lesions. There was no significant glomerular staining with any Ig or complement factor. The electron microscope studies (Figure 2) showed extensive amyloid fibril infiltration of glomerular mesangial regions and basement membranes. Several foci of glomerular basement membrane disruption in areas of amyloid infiltration were seen. No immune complex−type deposits were identified. The nonneoplastic renal parenchyma from the left-sided nephrectomy specimen showed similar findings.
Figure 1.
Light micrographs of glomeruli from both cases ([a] case 1; [b] case 2) showing amyloid infiltration of glomerular capillary loops and mesangial regions associated with glomerular basement membrane disruption, fibrinoid necrosis (arrows point to fibrin), and crescent formation. Amyloid deposits stained with Congo red show apple-green birefringence under polarized light and are positive for serum amyloid A by immunohistochemistry. (i) Jones silver stain, (ii) trichrome stain, (iii) Congo red stain, polarized (iv) serum amyloid A immunostain. Original magnification ×400.
Figure 2.
Representative electron micrographs from both cases ([a] case 1; [b] case 2), demonstrating heavy amyloid fibril infiltration of glomerular basement membranes and mesangial regions. Foci of glomerular basement membrane disruption are present (arrows).
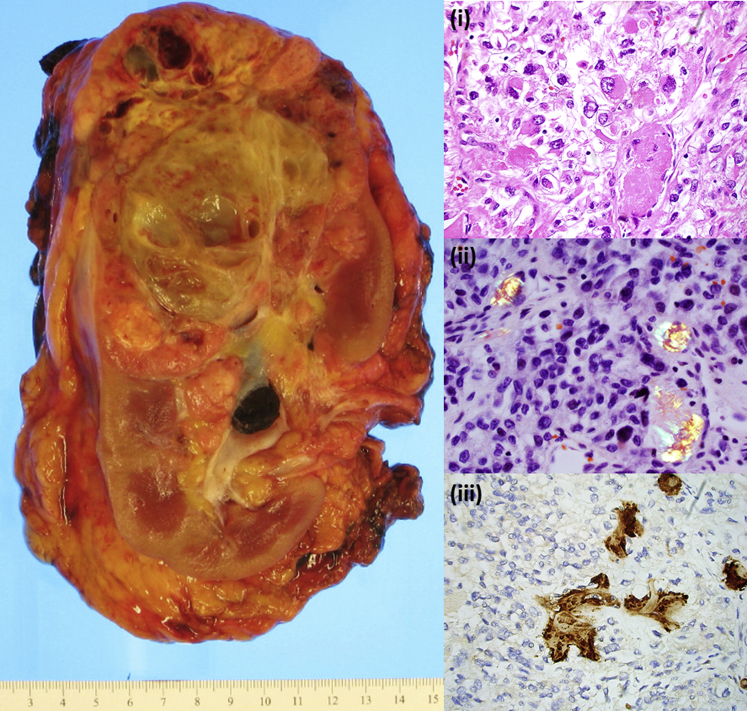
The left nephrectomy specimen contained a 13.0-cm tumor with clear-cell morphology, high-grade nuclear features (World Health Organization/International Society of Urological Pathology grade 4 of 4), and expressed CA-IX and CD10 without expression of CK7, CD117, or TFE-3, consistent with high-grade clear-cell renal cell carcinoma (Figure 3). The tumor also showed ∼50% sarcomatoid morphology as well as a prominent rhabdoid component. Scattered amyloid deposition was observed throughout the tumor (confirmed by Congo red stain), which was confirmed to be composed of serum amyloid A protein on immunohistochemistry. Scattered tumor cells displays cytoplasmic amyloid A protein staining.
Figure 3.
Nephrectomy specimen and light micrographs from concurrent clear-cell renal cell carcinoma tumor (case 1) exhibiting intratumoral AA amyloid deposition. Amyloid deposits stained with Congo red show apple-green birefringence under polarized light and are positive for serum amyloid A by immunohistochemistry. (i) Hematoxylin and eosin stain, (ii) Congo red stain, polarized (iii) serum amyloid A immunostain. Original magnification ×400.
Diagnosis
A diagnosis was made of clear-cell renal cell carcinoma, World Health Organization/International Society of Urological Pathology grade 4 of 4 with intratumoral amyloid A deposition and coexisting severe active crescentic glomerulonephritis with AA amyloidosis.
Clinical Follow-up
Clinical follow-up consisting of methylprednisolone 500 mg i.v. for 3 days was started the day after the biopsy, and dialysis was started 2 days after the biopsy. Complications of ischemic colitis, urinary tract infection, and slow return of bowel function resulted in a total hospital stay of about 1 month. The patient has now been on dialysis for more than 6 months, without recovery of kidney function, and is in the process of obtaining long-term dialysis access.
Case 2
Clinical Information
A 58-year-old Hispanic woman with poorly differentiated adenocarcinoma of the cervix stage IIB undergoing X-ray therapy was admitted to the hospital after acute kidney injury (serum creatinine at 2.4 mg/dl from baseline of 1.2 mg/dl found on routine laboratory test results prior to planned initiation of chemotherapy). Urinalysis was positive for protein (3+) leukocyte esterase (1+) blood (3+, >50 white blood cells, >50 red blood cells) with urine culture returning Escherichia coli. Over the course of admission, however, her serum creatinine continued to rise up to 3.15 mg/dl, and urine sediment remained active despite antibiotics. Renal ultrasound showed mild echogenicity without other abnormality. A 24-hour urine protein demonstrated nephrotic-range proteinuria (7.7 g). Serologic evaluation was negative for hepatitis, HIV, antinuclear antibody, ANCA, serum protein electrophoresis, and dsDNA. She had normal C3/C4 levels and a normal light chain ratio. A kidney biopsy was performed.
Renal Biopsy Findings
By light microscopy, nearly all glomeruli (27 of 28) exhibited cellular crescents represented by disruption of basement membranes with extravasation of fibrin and cells segmentally to globally filling the urinary space (Figure 1). The remaining glomerulus was globally sclerotic. Many glomeruli and rare arterioles showed extensive mesangial and capillary loop infiltration by amyloid (confirmed by Congo red stain). The amyloid deposits were intimately associated with capillary loop disruption and crescent formation. Outside of the crescentic lesions, glomerular capillary loops and mesangial regions were otherwise unremarkable. There was diffuse tubular injury and extensive tubulointerstitial inflammation as well as mild early tubular atrophy involving 5%−10% of the cortex. There was no evidence of arteritis. Immunohistochemistry for amyloid A was strongly positive in glomerular amyloid deposits. The immunofluorescence studies showed weak nonspecific smudgy staining of the amyloid deposits with all antibody and complement factors. There was no evidence for light chain restriction in glomerular deposits or tubular casts.
Electron microscope studies (Figure 2) showed extensive and nearly confluent infiltration of glomerular basement membranes by amyloid fibrils (average, 9.3 nm; range, 6.8−11.4 nm) with frequent spicule-like projections perpendicular to basement membranes. Marked nodular mesangial amyloid fibril infiltration was also seen. Areas of glomerular basement membrane disruption associated with crescent formation show heavy amyloid infiltration. There were no immune complex−type deposits in any locations. There were no tubuloreticular inclusions.
Diagnosis
A diagnosis was made of severe active crescentic glomerulonephritis with AA amyloidosis.
Clinical Follow-up
After discharge, the patient’s creatinine continued to rise, peaking at 5.16 mg/dl, accompanied by nausea, vomiting, and dehydration. She was readmitted to the hospital with improvement in serum creatinine to 2.3 mdg/dl. She continued to receive X-ray therapy, but because of functional status was not believed to be a chemotherapy candidate with progression of disease to include metastasis to lymph and lungs. Creatinine had remained stable for approximately 1 month before steadily rising to a value of ∼7 mg/dl 4 months after her initial diagnosis, when dialysis was initiated. She continued to decline functionally on dialysis with significant malnutrition, recurrent nausea and vomiting, recurrent fevers thought to be secondary to her malignancy, and was transitioned to comfort care 7 months after her diagnosed rapidly progressive glomerulonephritis.
Discussion
Historical characterizations of renal injury in amyloidosis4, 5 show <0.4% involvement by crescents, and, as such, glomerular crescents are not generally regarded as a distinct pathology in renal amyloidosis. However, the coexistence of crescentic glomerulonephritis and renal amyloidosis has now been reported a handful of times in the literature.1, 4, 6, 7, 8, 9 In the largest case series to date, Nagata et al. suggest that when sufficient glomeruli are examined, crescents may complicate up to 13% of renal amyloidosis cases, often with >50% crescents (6 of 14 reported cases).1 They also observed that localization of amyloid deposition was closely related to crescent formation, and that the incidence did not correlate with the overall extent of amyloid deposition or the presence of nephrotic syndrome.
The 2 cases presented here highlight that amyloidosis should be considered in the differential list for any crescentic glomerulonephritis, especially in the setting of a negative serologic workup (Table 1). Most reported cases of reported crescentic glomerulonephritis and concurrent amyloidosis have been seen with type AA amyloidosis due to serum amyloid A protein, most often in the clinical setting of rheumatoid arthritis. In a retrospective case series, Verine et al. observed at least 1 glomerulus involved with a necrotizing lesion or cellular crescent in 12 of 68 cases (∼18%) of renal involvement with AA amyloidosis.9 Again, rheumatoid arthritis was the underlying etiology in the majority of cases. Other etiologies included sigmoiditis, malignancy, malaria, Sezary syndrome, and familial Mediterranean fever. Within these case series, rare AL-type amyloidosis and 1 Waldenstrom macroglobulinemia−type amyloidosis cases have also been reported to show crescent formation.
Table 1.
Teaching points
| Amyloid deposition can cause glomerular crescents, which have been observed in up to 13% of renal amyloidosis cases |
| Amyloid should be on the differential diagnosis for any crescentic glomerulonephritis, especially with negative serologic workup |
| Congo red staining can be used to differentiate capillary loop fibrin from amyloid deposits |
| Solid tumors are a rare association with amyloid A amyloidosis (AA amyloidosis) |
The pathogenesis of amyloid-induced crescent formation is uncertain. Some authors argue that amyloid infiltration results in both mesangial cell dysfunction and increased glomerular basement membrane fragility. This hypothesis is supported by co-localization of amyloid deposition in areas of glomerular basement membrane rupture. Other authors argue that the AA amyloid itself may induce an inflammatory response resulting in a crescentic glomerulonephritis, which may show other features of a proliferative glomerulonephritis (e.g, mesangial and endocapillary hypercellularity).9,S1
In both cases presented here, the electron microscope studies do indeed show extensive amyloid fibril infiltration of glomerular basement membranes in areas of glomerular basement membrane disruption and crescent formation. In addition, case 1 shows increased endocapillary hypercellularity in glomerular loops.
Malignancy is reported to be the underlying etiology in ∼7% of systemic AA amyloidosis cases.S2,S3 Renal cell carcinoma is the most common solid organ malignancy to cause AA amyloidosis; however, AA amyloidosis complicates only 2%−3% of renal cell carcinomas.S4 Although no cases of systemic AA amyloidosis due to cervical carcinoma have yet been reported, cervical carcinomas are reportedly associated with increased serum levels of serum amyloid A.S5 In addition, some cervical carcinomas exhibit localized amyloid deposition.S6 Thus, in both cases reported here, malignancy provides a plausible underlying etiology for the systemic AA amyloidosis. Furthermore, the finding of intratumoral amyloidosis in renal cell carcinoma in case 1 is suggestive that the production of serum amyloid A protein by the tumor itself may be the underlying etiology for the glomerular and extrarenal amyloidosis.
In the setting of renal cell carcinoma−associated systemic amyloidosis, resection of the primary tumor has been associated with at least partial resolution of the amyloidosis.S7,S8 However, in patients with renal amyloidosis, progression of kidney disease to dialysis is common. A cohort of 86 patients in Italy with AA amyloid had a mean rate of decline of glomerular filtration rate of 2.3 ml/min per month, a median time to dialysis of 25 months, and an overall rate of progression to dialysis of 47%, with no cases of renal recovery reported.S9 Other cohorts reported had mean times to dialysis of 64.7 months and 69.3 months.S10,S11 For all types of amyloidosis, rates of renal recovery are very low, reported at 3% in a cohort of AL patients in France and 0.8% in a large Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) cohort.S12,S13 No reports of recovery of kidney function for patients with dialysis-dependent AA amyloidosis were found in a PubMed literature search.
Finally, 2 additional differential diagnoses for the crescentic process could be considered: collapsing glomerulopathy and an ANCA-negative pauci-immune crescentic glomerulonephritis. Collapsing glomerulopathy can occur in the setting of amyloidosis.S14 The pseudo-crescents of collapsing glomerulopathy can sometimes be difficult to distinguish from true crescents. True crescents are identified by the presence of glomerular basement membrane disruption, fibrinoid necrosis, and in some cases endocapillary inflammatory cells. The amyloid-associated crescents seen in the 2 cases reported here showed glomerular basement membrane disruption, fibrinoid necrosis, and increased endocapillary inflammatory cells. In addition, no areas of capillary loop collapse were seen.
Up to 10% to 30% of cases of pauci-immune glomerulonephritis show negative ANCA serologies.S15 In the 2 cases presented here, it is difficult to entirely exclude this possibility; however, no other systemic evidence for vasculitis or other histologic features suggestive of an ANCA-like process (e.g., necrotizing vasculitis or granulomatous inflammation) were present in either case. Thus the amyloid deposition provides the most convincing underlying etiology for the crescentic processes.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Funding for this manuscript comes from J.E.Z. professorial educational enrichment fund (UCLA Department of Pathology).
Footnotes
Supplementary References.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
References
- 1.Nagata M., Shimokama T., Harada A. Glomerular crescents in renal amyloidosis: an epiphenomenon or distinct pathology. Pathol Int. 2001;51:179–186. doi: 10.1046/j.1440-1827.2001.01188.x. [DOI] [PubMed] [Google Scholar]
- 2.de Asúa D.R., Costa R., Galván J.M. Systemic AA amyloidosis: epidemiology, diagnosis, and management. Clin Epidemiol. 2014;6:369–377. doi: 10.2147/CLEP.S39981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Hutten H., Mihatsch M., Lobeck H. Prevalence and origin of amyloid in kidney biopsies. Am J Surg Pathol. 2009;33:1198–1205. doi: 10.1097/PAS.0b013e3181abdfa7. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe T., Toyoshima H., Nagafuchi Y. Renal amyloidosis with crescents. Hum Pathol. 1984;15:684–686. doi: 10.1016/s0046-8177(84)80295-6. [DOI] [PubMed] [Google Scholar]
- 5.Kyle R.A., Bayrd E.D. Amyloidosis: review of 236 cases. Medicine (Baltimore) 1975;54:271–299. doi: 10.1097/00005792-197507000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Panner B.J. Rapidly progressive glomerulonephritis and possible amyloidosis. Arch Pathol Lab Med. 1980;104:603–609. [PubMed] [Google Scholar]
- 7.Harada A., Tomita Y., Yamamoto H. Renal amyloidosis associated with crescentic glomerulonephritis. Am J Nephrol. 1984;4:52–55. doi: 10.1159/000166774. [DOI] [PubMed] [Google Scholar]
- 8.Vernier I., Pourrat J.P., Mignon-Conte M.A. Rapidly progressive glomerulonephritis associated with amyloidosis: efficacy of plasma exchange. J Clin Apher. 1987;3:226–229. doi: 10.1002/jca.2920030407. [DOI] [PubMed] [Google Scholar]
- 9.Verine J., Mourad N., Desseaux K. Clinical and histological characteristics of renal AA amyloidosis: a retrospective study of 68 cases with a special interest to amyloid-associated inflammatory response. Hum Pathol. 2007;38:1798–1809. doi: 10.1016/j.humpath.2007.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.