This randomized withdrawal study compares the efficacy of esketamine nasal spray plus an oral antidepressant with an oral antidepressant plus placebo nasal spray in delaying relapse of depressive symptoms in patients with treatment-resistant depression who were in stable remission after treatment with esketamine nasal spray plus an oral antidepressant.
Key Points
Question
What are the long-term effects of esketamine nasal spray in patients with treatment-resistant depression?
Findings
Of the 297 adults with treatment-resistant depression who were randomized in the maintenance phase of this clinical trial, those who continued treatment with intermittently administered esketamine nasal spray plus an oral antidepressant had a significantly delayed time to relapse vs those treated with oral antidepressant plus placebo nasal spray after 16 weeks of initial treatment with esketamine and an antidepressant.
Meaning
Continued treatment with esketamine nasal spray plus an antidepressant can sustain antidepressant effects among patients with treatment-resistant depression to a greater extent than an oral antidepressant alone.
Abstract
Importance
Controlled studies have shown short-term efficacy of esketamine for treatment-resistant depression (TRD), but long-term effects remain to be established.
Objective
To assess the efficacy of esketamine nasal spray plus an oral antidepressant compared with an oral antidepressant plus placebo nasal spray in delaying relapse of depressive symptoms in patients with TRD in stable remission after an induction and optimization course of esketamine nasal spray plus an oral antidepressant.
Design, Setting, and Participants
In this phase 3, multicenter, double-blind, randomized withdrawal study conducted from October 6, 2015, to February 15, 2018, at outpatient referral centers, 705 adults with prospectively confirmed TRD were enrolled; 455 entered the optimization phase and were treated with esketamine nasal spray (56 or 84 mg) plus an oral antidepressant. After 16 weeks of esketamine treatment, 297 who achieved stable remission or stable response entered the randomized withdrawal phase.
Interventions
Patients who achieved stable remission and those who achieved stable response (without remission) were randomized 1:1 to continue esketamine nasal spray or discontinue esketamine treatment and switch to placebo nasal spray, with oral antidepressant treatment continued in each group.
Main Outcomes and Measures
Time to relapse was examined in patients who achieved stable remission, as assessed using a weighted combination log-rank test.
Results
Among the 297 adults (mean age [SD], 46.3 [11.13] years; 197 [66.3%] female) who entered the randomized maintenance phase, 176 achieved stable remission; 24 (26.7%) in the esketamine and antidepressant group and 39 (45.3%) in the antidepressant and placebo group experienced relapse (log-rank P = .003, number needed to treat [NNT], 6). Among the 121 who achieved stable response, 16 (25.8%) in the esketamine and antidepressant group and 34 (57.6%) in the antidepressant and placebo group experienced relapse (log-rank P < .001, NNT, 4). Esketamine and antidepressant treatment decreased the risk of relapse by 51% (hazard ratio [HR], 0.49; 95% CI, 0.29-0.84) among patients who achieved stable remission and 70% (HR, 0.30; 95% CI, 0.16-0.55) among those who achieved stable response compared with antidepressant and placebo treatment. The most common adverse events reported for esketamine-treated patients after randomization were transient dysgeusia, vertigo, dissociation, somnolence, and dizziness (incidence, 20.4%-27.0%), each reported in fewer patients (<7%) treated with an antidepressant and placebo.
Conclusions and Relevance
For patients with TRD who experienced remission or response after esketamine treatment, continuation of esketamine nasal spray in addition to oral antidepressant treatment resulted in clinically meaningful superiority in delaying relapse compared with antidepressant plus placebo.
Trial Registration
ClinicalTrials.gov identifier: NCT02493868
Introduction
Depression is the leading cause of disability worldwide and is associated with a 10-year reduction in life expectancy.1,2 Achieving and maintaining remission, the goals of treatment for this recurrent disease, improves functioning, reduces suicide risk, and leads to greater clinical stability.3 Patients who have not responded to at least 2 different antidepressants in the current depressive episode are considered to have treatment-resistant depression (TRD).4 Patients with treatment-resistant major depressive disorder (MDD) experience relapse at a higher rate than do those with treatment-responsive MDD. Even when patients with TRD respond to treatment, the overall relapse rate while continuing treatment with the same antidepressant is high after 2 (65%; within 3.1 months) and 3 failed trials (71.1%; within 3.3 months).3 There is a substantial unmet need for effective treatments that can sustain antidepressant benefits for the population with TRD.
Several short-term studies5,6,7,8,9,10,11,12 with racemic ketamine and a stereoisomer, esketamine, have demonstrated efficacy for TRD. In contrast to available data about short-term antidepressant effects of esketamine and ketamine,13,14 little is known about maintaining antidepressant effects in the long term. We report the findings of, to our knowledge, the first controlled maintenance study of esketamine that evaluated whether continued use of intermittently administered esketamine nasal spray plus an oral antidepressant can sustain antidepressant effects among patients with TRD to a greater extent than an oral antidepressant alone.
Methods
Study Population
Patients were enrolled directly or were transferred into this study after achieving treatment response (≥50% reduction from baseline in Montgomery-Åsberg Depression Rating Scale [MADRS] total score) to esketamine nasal spray in 1 of 2 short-term double-blind, active-controlled studies (1 fixed dose and 1 flexible dose), with all patients meeting identical entrance criteria (reported elsewhere12,15. Participants were outpatients who were in treatment or referred to a variety of academic and nonacademic clinic settings across the United States, Canada, and Europe. Enrolled patients were approached by their treating physician or responded to institutional review board– or independent ethics committee–approved patient recruitment materials. In addition, a web-based prescreening tool was developed to assist sites in identifying appropriate study candidates.
The trial protocol can be found in Supplement 1. Institutional review boards and independent ethics committees (eAppendix 1 in Supplement 2) approved the study protocol and amendments. The study was conducted in accordance with ethical principles of the Declaration of Helsinki,16 Good Clinical Practices, and applicable regulatory requirements. All patients provided written informed consent before entering the study.
Eligible patients (aged 18-64 years) had recurrent or single-episode (≥2 years) MDD (DSM-5),17 a total score of 34 or higher on the Clinician-Rated Inventory of Depressive Symptomatology,18 and a total MADRS score of 28 or higher, indicating moderate to severe depression. At screening, all patients were nonresponders to at least 1, but no more than 5, antidepressants in the current depressive episode, with nonresponse to a different oral antidepressant confirmed by 4 weeks or more of observed treatment during the prospective screening phase.12 Key exclusion criteria were history of psychotic disorder, suicidal behavior within the prior year, current or recent homicidal or suicidal ideation or intent, diagnosis of MDD with psychotic features, and moderate or severe substance or alcohol use disorder within 6 months. A history (lifetime) of ketamine use disorder was exclusionary. (A full list of the inclusion and exclusion criteria is presented in eAppendix 2 in Supplement 2.) Urine drug screening (eg, barbiturates, methadone, opiates, cocaine, cannabinoids, phencyclidine, and amphetamine or methamphetamine) was conducted intermittently before dosing throughout the study.
Study Design
This double-blind, randomized clinical trial (A Study of Intranasal Esketamine Plus an Oral Antidepressant for Relapse Prevention in Adult Participants With Treatment-Resistant Depression [SUSTAIN-1]) used a randomized withdrawal design and was conducted from October 6, 2015, to February 15, 2018. Ninety-nine sites randomized patients.
The study consisted of up to 5 phases: (1) a 4-week screening and prospective observation phase (direct-entry patients only); (2) a 4-week open-label induction phase (direct-entry patients only); (3) a 12-week optimization phase (open-label, direct-entry patients or double-blind, transfer-entry patients); (4) a maintenance phase (double-blind, randomized withdrawal, event driven, variable duration); and (5) a 2-week posttreatment follow-up phase. The study continued until the requisite number of relapses occurred, specified by a preplanned interim analysis (described below).
Direct-Entry Patients
During the 4-week screening and observation phase, nonresponse to the ongoing oral antidepressant treatment was assessed prospectively in eligible patients. Those with nonresponse at the end of this phase discontinued use of the prior antidepressant(s), with the option of a 3-week or less taper period. In the induction phase, patients received esketamine nasal spray (56 or 84 mg, flexibly dosed) twice weekly plus a new oral antidepressant (duloxetine, escitalopram, sertraline, or extended-release venlafaxine) administered daily.
Transfer-Entry and Direct-Entry Patients
Transfer-entry and direct-entry patients who achieved treatment response at the end of the induction phase (ie, ≥50% reduction in MADRS score from baseline) entered a 12-week optimization phase during which study drug dosages at the end of the induction phase remained fixed but the frequency of intranasal dosing was reduced to once weekly for 4 weeks then individualized to weekly or every 2 weeks based on the severity of depressive symptoms. To preserve the blinding, transfer-entry patients continued treatment assignment (esketamine or placebo) from the induction phase.
Maintenance Phase
At week 16 of the optimization phase, esketamine-treated direct-entry (open-label treatment) and transfer-entry patients (double-blind treatment) who had achieved stable remission (primary analysis set; defined as MADRS score ≤12 for ≥3 of the last 4 weeks, with 1 excursion [MADRS score >12] or 1 missing MADRS assessment permitted at week 13 or 14 only) and patients with stable response (secondary analysis set; defined as ≥50% reduction in MADRS score from baseline in the last 2 weeks of the optimization phase but without achieving remission) continued into the maintenance phase. Because patients with treatment resistance who achieve remission reportedly have lower relapse rates compared with those who respond but do not experience remission,3 the prespecified primary analysis was conducted using the analysis of patients who achieved stable remission. However, those who met the less conservative criteria for stable response (but not stable remission) were also evaluated because a reduction in MADRS score from baseline of 50% or more for 2 weeks in this patient population is considered as clinically meaningful. Patients who achieved stable remission and those who achieved stable response (without remission) were separately randomized 1:1 according to a computer-generated schedule to continue esketamine treatment or discontinue esketamine treatment and switch to placebo nasal spray, each in addition to oral antidepressant treatment. The dosage of antidepressant throughout the maintenance phase remained unchanged from the induction phase. Randomization was balanced using randomly permuted blocks and stratified by country.
Transfer-entry patients who were assigned to the antidepressant and placebo group in the short-term studies and achieved stable remission or stable response continued the same treatment in the maintenance phase and were included in safety, but not efficacy, analyses of this study. Treatment administration frequency during the maintenance phase was based on an algorithm using the MADRS score and was reevaluated every 4 weeks, with nasal spray treatment self-administered either once weekly or every 2 weeks.
Patients who met the criteria for experiencing relapse could proceed into a long-term safety study of esketamine nasal spray.19 Otherwise, patients continued to a 2-week posttreatment follow-up phase after their participation in the maintenance phase ended.
Intranasal Study Drug and Administration
Esketamine and placebo were provided in nasal spray devices, each containing 200 μL of solution per device (ie, 2 sprays). Each device contained 32.28 mg of esketamine hydrochloride (28 mg of esketamine base) or placebo. The placebo solution contained a bittering agent (denatonium benzoate) to simulate the taste of esketamine solution and maintain the blinding.
Efficacy Assessments
Independent, blinded, remote raters performed MADRS assessments throughout the study (weeks 1, 2, and 4 of the screening and observation phase and weekly during the induction, optimization, maintenance, and follow-up phases).
Safety Assessments
Adverse events (AEs) and other safety assessments, including clinical laboratory tests, physical examination, electrocardiography, Columbia Suicide Severity Rating Scale20 (C-SSRS) (with 0 indicating no suicidal ideation or behavior; 1-5, suicidal ideation; and 6-10, suicidal behavior; item descriptions in eAppendix 3 in Supplement 2) were monitored throughout the study. Vital signs, the Clinician-Administered Dissociative States Scale21 (CADSS), and the Brief Psychiatric Rating Scale22 (4-item positive symptom subscale) were assessed at baseline and all treatment administration visits (before dosing and at 40 minutes, 1 hour [vital signs only], and 1.5 hours after dosing).
The 20-item Physician Withdrawal Checklist23 was administered to assess for potential withdrawal symptoms after cessation of intranasal study medication. Cognitive testing was performed before dosing to assess for a potential effect on cognition; these data will be reported in a separate article.
Statistical Analysis
Sample Size Determination
On the basis of assumptions (accrual period and rate, maximum study duration, and dropout rate), 211 patients who achieved stable remission needed to be randomized (1:1 ratio) to obtain 84 relapses, providing 90% power to detect a hazard ratio (HR) of 0.49 at a 2-sided α of .05 for a fixed-sample design to detect superiority of esketamine and antidepressant over antidepressant and placebo in delaying relapse. A 2-stage group-sequential design was implemented for the analysis set of patients who achieved stable remission, and an independent data-monitoring committee performed a prespecified interim analysis after 31 relapses to assess early efficacy.
The interim analysis on patients who achieved stable remission did not show superiority of esketamine and antidepressant over antidepressant and placebo (at a 2-sided significance level of .0097, log-rank test); therefore, the study continued, and the number of relapses in patients who achieved stable remission was reestimated to 59 relapses in total with an adjusted significance level of .046 (2-sided) for the final efficacy analysis (based on the Wang-Tsiatis boundary α-spending function24), ensuring a conditional power of 90% or higher after the interim analysis.
Efficacy End Points and Analyses
Cumulative distribution of time to relapse during the maintenance phase among patients who achieved stable remission (primary efficacy end point) and those who achieved stable response without remission (secondary end point) was estimated by the Kaplan-Meier method. Relapse was defined as a MADRS total score of 22 or higher for 2 consecutive assessments separated by 5 to 15 days or hospitalization for worsening depression, suicide attempt, suicide prevention or completed suicide, or another clinically relevant event suggestive of relapse (assessed by a relapse adjudication committee).
The between-group difference in time to relapse was analyzed using a log-rank test (weighted combination [interim and final analyses] for patients who achieved stable remission because of conducting an interim analysis). The estimated HRs and 95% CIs were based on weighted estimates for patients who achieved stable remission and on a Cox proportional hazards regression model with treatment as a factor for patients who achieved stable response. A similar post hoc analysis was performed combining the analysis set of patients who achieved stable remission and the analysis set of patients who achieved stable response.
Results
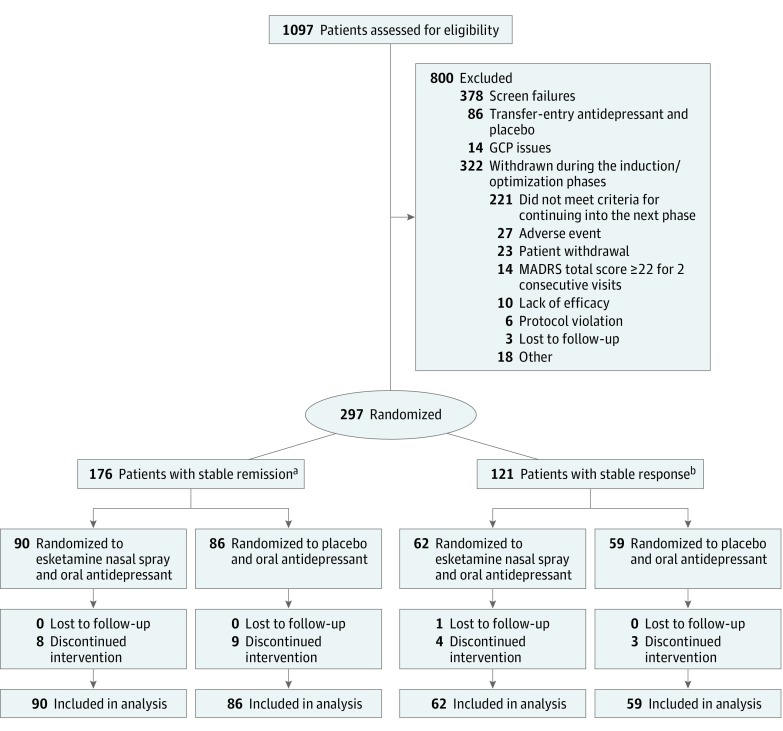
A total of 297 adults (mean age [SD], 46.3 [11.13] years; 197 [66.3%] female) were randomized in the maintenance phase of the study. A CONSORT diagram is presented in Figure 1. The median number of patients per site was 2 (range, 1-25). The treatment groups were comparable based on demographic and baseline clinical characteristics (Table 1). Median exposure to intranasal esketamine during the maintenance phase was 17.7 weeks among patients who achieved stable remission and 19.4 weeks among patients who achieved stable response. Median exposure to placebo during the maintenance phase was 10.2 weeks among patients who achieved stable remission and 10.1 weeks among those who achieved stable response.
Figure 1. CONSORT Diagram.
This study used data for those patients who had been undergoing treatment with esketamine nasal spray plus an oral antidepressant for 16 weeks and who, after meeting criteria for either stable remission (primary analysis) or stable response (secondary analysis), were randomized (separately) to continue treatment with esketamine nasal spray plus an oral antidepressant or to discontinue treatment with esketamine and switch to placebo nasal spray and continue use of the oral antidepressant. Stable remission was defined as a Montgomery-Åsberg Depression Rating Scale (MADRS) total score of 12 or lower for 3 or more of the last 4 weeks of the optimization phase, with up to 1 excursion (MADRS total score >12) or 1 missing MADRS assessment permitted at week 13 or 14 only. Stable response was defined as 50% or greater reduction in MADRS total score from baseline in the last 2 weeks of the optimization phase, but without achieving stable remission criteria. Patients who were lost to follow-up or discontinued treatment after randomization were included in the analysis.
aOne patient with stable response was incorrectly randomized in the group with stable remission.
bOne patient who did not meet stable remission or stable response criteria at the end of the optimization phase was incorrectly randomized in the group with stable response.
Table 1. Demographic and Baseline Characteristicsa.
| Characteristic | Stable Remission at Baseline | Stable Response at Baseline | ||
|---|---|---|---|---|
| Esketamine Nasal Spray and Oral Antidepressant (n = 90) | Oral Antidepressant and Placebo Nasal Spray (n = 86) | Esketamine Nasal Spray and Oral Antidepressant (n = 62) | Oral Antidepressant and Placebo Nasal Spray (n = 59) | |
| Age, mean (SD) [range], y | 45.4 (12.12) [19-64] | 46.2 (11.16) [19-64] | 47.2 (11.00) [23-63] | 46.7 (9.76) [24-64] |
| Sex | ||||
| Male | 32 (35.6) | 27 (31.4) | 24 (38.7) | 17 (28.8) |
| Female | 58 (64.4) | 59 (68.6) | 38 (61.3) | 42 (71.2) |
| Race | ||||
| American Indian or Alaskan Native | 0 | 1 (1.2) | 0 | 0 |
| Asian | 0 | 0 | 0 | 1 (1.7) |
| Black | 4 (4.4) | 6 (7.0) | 2 (3.2) | 1 (1.7) |
| White | 80 (88.9) | 76 (88.4) | 57 (91.9) | 55 (93.2) |
| Other | 2 (2.2) | 1 (1.2) | 3 (4.8) | 1 (1.7) |
| Multiple | 1 (1.1) | 0 | 0 | 1 (1.7) |
| Not reported | 3 (3.3) | 2 (2.3) | 0 | 0 |
| Region | ||||
| Europe | 52 (57.8) | 50 (58.1) | 34 (54.8) | 35 (59.3) |
| North America | 22 (24.4) | 20 (23.3) | 18 (29.0) | 16 (27.1) |
| Brazil and Mexico | 16 (17.8) | 16 (18.6) | 10 (16.1) | 8 (13.6) |
| Age diagnosed with MDD, mean (SD) [range], y | 32.5 (11.42) [5-55] | 33.4 (11.41) [10-60] | 36.2 (13.25) [15-61] | 34.0 (10.54) [14-60] |
| Duration of current episode, mean (SD) [range], wk | 112.2 (171.30) [12-1040] | 110.5 (147.41) [9-884] | 121.6 (193.85) [13-1080] | 141.8 (254.43) [9-1248] |
| No. of previous antidepressants before screening | ||||
| ≤2 | 71 (78.9) | 62 (73.8) | 41 (66.1) | 34 (57.6) |
| >2 | 19 (21.1) | 22 (26.2) | 21 (33.9) | 25 (42.4) |
| History of suicidal ideation in previous 6 mo | 18 (20.0) | 14 (16.3) | 20 (32.3) | 14 (23.7) |
| Class of oral antidepressant | ||||
| SNRI | 62 (68.9) | 58 (67.4) | 35 (56.5) | 36 (61.0) |
| SSRI | 28 (31.1) | 28 (32.6) | 27 (43.5) | 23 (39.0) |
| Baseline MADRS total score, mean (SD) | ||||
| All patients | 37.4 (5.20) | 37.6 (4.66) | 40.1 (5.56) | 38.9 (4.92) |
| Direct-entry patientsb | 37.8 (5.28) | 37.8 (4.26) | 40.5 (4.88) | 38.5 (4.65) |
| Transfer-entry patientsc | 36.8 (5.10) | 37.3 (5.38) | 39.6 (6.22) | 39.9 (5.49) |
| Baseline PHQ-9 score, mean (SD) | 19.2 (4.16) | 19.8 (3.43) | 20.5 (4.12) | 20.4 (4.15) |
| Dose of esketamine before randomizationd | ||||
| 56 mg | 40 (44.4) | 33 (38.4) | 20 (32.3) | 19 (32.2) |
| Direct-entry patients | 14 (15.6) | 9 (10.5) | 7 (11.3) | 6 (10.2) |
| Transfer-entry 3001 patientse | 5 (5.6) | 4 (4.7) | 5 (8.1) | 3 (5.1) |
| Transfer-entry 3002 patientsf | 21 (23.3) | 20 (23.3) | 8 (12.9) | 10 (16.9) |
| 84 mg | 50 (55.6) | 53 (61.6) | 42 (67.7) | 40 (67.8) |
| Direct-entry patients | 12 (13.3) | 11 (12.8) | 8 (12.9) | 2 (3.4) |
| Transfer-entry 3001 patientse | 5 (5.6) | 6 (7.0) | 11 (17.7) | 7 (11.9) |
| Transfer-entry 3002 patientsf | 33 (36.7) | 36 (41.9) | 23 (37.1) | 31 (52.5) |
| Dosing frequency at baseline | ||||
| Weekly | 37 (41.1) | 41 (47.7) | 51 (83.6) | 43 (72.9) |
| Every other week | 53 (58.9) | 45 (52.3) | 10 (16.4) | 16 (27.1) |
| Missing | 0 | 0 | 1 | 0 |
Abbreviations: MADRS, Montgomery-Åsberg Depression Rating scale; MDD, major depressive disorder; PHQ-9, Patient Health Questionnaire 9; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Data are presented as number (percentage) of patients unless otherwise indicated.
Patients who achieved stable remission: 54 for esketamine nasal spray and oral antidepressant and 56 for oral antidepressant and placebo nasal spray; patients who achieved stable response: 31 for esketamine nasal spray and oral antidepressant and 41 for oral antidepressant and placebo nasal spray.
Patients who achieved stable remission: 36 for esketamine nasal spray and oral antidepressant and 30 for oral antidepressant and placebo nasal spray; patients who achieved stable response: 31 for esketamine nasal spray and oral antidepressant and 18 for oral antidepressant and placebo nasal spray.
During the optimization phase and before randomization.
The 3001 indicates transferred from Janssen-sponsored fixed-dose esketamine study TRD3001.15
The 3002 indicates transferred from Janssen-sponsored flexible-dose esketamine study TRD3002.12
Of the 90 patients who achieved stable remission in the esketamine and antidepressant group, 40 (44.4%) received 56 mg of esketamine on day 1 of the maintenance phase and 50 (55.6%) received 84 mg, with 62 (68.9%) receiving treatment every 2 weeks for most of the maintenance phase. A greater proportion of the 62 patients who achieved stable response in the esketamine and antidepressant group received the higher esketamine dose in the maintenance phase (56 mg: n = 20 [32.3%]; 84 mg: n = 42 [67.7%]), with 34 (54.8%) receiving treatment once weekly most of the time.
Efficacy Results
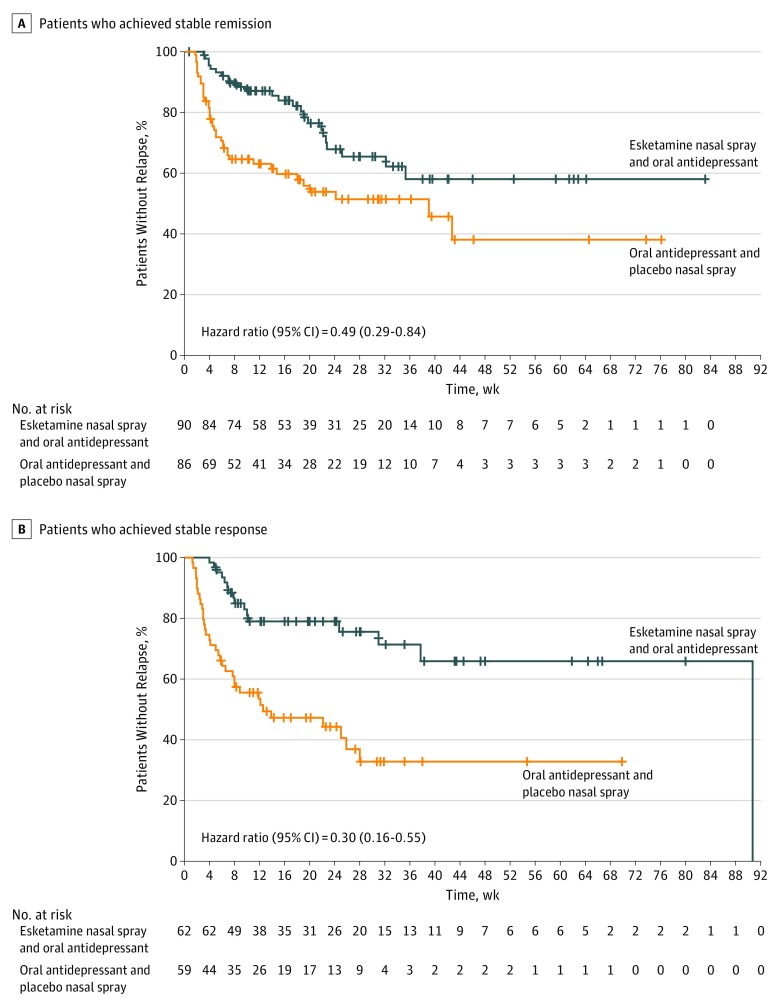
Overall, among patients who achieved stable remission, 24 patients (26.7%) in the esketamine and antidepressant group and 39 patients (45.3%) in the antidepressant and placebo group experienced a relapse event during the maintenance phase; among the patients who achieved stable response (but not remission), 16 patients (25.8%) in the esketamine and antidepressant group and 34 patients (57.6%) in the antidepressant and placebo group experienced relapse (Table 2). Continued treatment with esketamine and antidepressant significantly delayed relapse compared with treatment with antidepressant and placebo (patients who achieved stable remission: HR, 0.49; 95% CI, 0.29-0.84; P = .003, number needed to treat [NNT], 6; patients who achieved stable response: HR, 0.30; 95% CI, 0.16-0.55: P < .001, NNT, 4). According to HR estimates, treatment with esketamine and antidepressant decreased relapse risk by 51% among patients who achieved stable remission and by 70% among patients who achieved stable response compared with antidepressant and placebo (Figure 2). In addition, in a post hoc analysis, esketamine and antidepressant delayed relapse compared with antidepressant and placebo among patients who achieved stable remission and patients who achieved stable response combined (HR, 0.38; 95% CI, 0.26-0.57; P < .001). In a post hoc sensitivity analysis for the primary end point, using a MADRS score cutoff of 10 for remission, the between-group difference remained statistically significant (2-sided P = .005) (eTable 1 in Supplement 2). Time to relapse for patients who achieved stable remission was also assessed by study entry (direct vs transfer). The HRs were 0.49 (95% CI, 0.27-0.90) for direct-entry patients and 0.45 (95% CI, 0.17-1.18) for transfer-entry patients.
Table 2. Time to Relapse and Number of Patients Who Remained Relapse Free in the Maintenance Phasea.
| Group | Esketamine Nasal Spray and Oral Antidepressant | Oral Antidepressant and Placebo Nasal Spray |
|---|---|---|
| Patients Who Achieved Stable Remission | ||
| No. assessed | 90 | 86 |
| No. (%) censored | 66 (73.3) | 47 (54.7) |
| No. (%) of relapses | 24 (26.7) | 39 (45.3) |
| 25th Percentile (95% CI) | 153.0 (105.0-225.0) | 33.3 (22.0-48.0) |
| Median (95% CI) | NE | 273.0 (97.0 to NE) |
| 75th Percentile (95% CI) | NE | NE |
| HR (95% CI)b | 0.49 (0.29-0.84) | |
| 2-Sided P valuec | .003 | |
| Patients Who Achieved Stable Response | ||
| No. assessed | 62 | 59 |
| No. (%) censored | 46 (74.2) | 25 (42.4) |
| No. (%) of relapses | 16 (25.8) | 34 (57.6) |
| 25th Percentile (95% CI) | 217.0 (56.0-635.0) | 24.0 (17.0-46.0) |
| Median (95% CI) | 635.0 (264.0-635.0) | 88.0 (46.0-196.0) |
| 75th Percentile (95% CI) | 635.0 (NE) | NE |
| HR (95% CI)d | 0.30 (0.16-0.55) | |
| 2-Sided P valuee | <.001 | |
Abbreviations: HR, hazard ratio; NE, not estimable.
Censoring was done for patients who remained relapse free at the end of the study, defined by achieving the target number of relapse events, or who withdrew early without relapse in the maintenance phase. Most censored patients (ie, when participation was ended) were considered as administrative based on the study having reached its end point (ie, based on the target number of relapse events having been achieved and the study stopping). Only 13 (8 patients who achieved stable remission and 5 patients who achieved stable response) in the esketamine nasal spray and oral antidepressant group and 12 (9 patients who achieved stable remission and 3 patients who achieved stable response) in the oral antidepressant and placebo group were censored because they discontinued the maintenance phase before having a relapse and before the end of the study.25 Data are based on Kaplan-Meier product-limit estimates.
HR and CI are weighted estimates based on Wassmer25 and calculated using gsDesign and mvtnorm packages in R.
Two-sided P value is based on the final test statistic, which is a weighted combination of the log-rank test statistics calculated on the interim and final analysis sets.
Regression analysis of survival data based on Cox proportional hazards regression model with treatment as a factor.
Log-rank test.
Figure 2. Kaplan-Meier Estimates of Time to Relapse.
One patient who achieved stable response was incorrectly randomized as a patient who achieved stable remission at the end of the optimization phase. One patient did not meet stable remission or stable response criteria and was incorrectly randomized as a patient with stable response. The most common cause of censoring participants was based on being relapse free at study end (see Table 2 legend). Vertical lines indicate censored observations.
Given the low median number of patients per site (2; range, 1-25), to further evaluate the effect of site on the estimation of treatment effect (ie, HR), a post hoc sensitivity analysis was performed using a Cox proportional hazards regression model by excluding one site at a time. On the basis of this analysis, the HR was estimated to range from 0.42 to 0.57, which is consistent with the overall HR of 0.47 (unweighted).
Nineteen of the 39 relapses in patients who achieved stable remission and who were switched to placebo nasal spray occurred in the first month after discontinuation of esketamine treatment (6 by week 2 and the remainder by week 4), with 11 of these 19 early relapses occurring in patients who had required weekly treatment administration in the last 4 weeks of the optimization phase before randomization (eFigure 1 in Supplement 2).
After completing induction and optimization treatment (16 weeks total), in patients who achieved stable remission and those who achieved stable response, there was separation in MADRS total scores between patients randomized to continue vs discontinue esketamine treatment, each in the presence of antidepressant therapy, with MADRS total scores being lower over time for esketamine-treated patients. This separation was maintained in both patients who achieved stable remission and those who achieved stable response. Mean MADRS total score over time using last observation carried forward data during the induction, optimization, and maintenance phases is presented in eFigure 2 in Supplement 2.
Safety Results
The 5 most common AEs reported in the esketamine and antidepressant group during the maintenance phase were dysgeusia, vertigo, dissociation, somnolence, and dizziness (Table 3). Most AEs were mild to moderate, observed after dosing, and generally resolved in the same day. No cases of respiratory depression or interstitial cystitis were observed.
Table 3. Most Frequently Reported Adverse Events in the Maintenance Phase in Patients Who Achieved Stable Remission and Those Who Achieved Stable Responsea.
| Adverse Event | No. (%) of Patients | |
|---|---|---|
| Esketamine Nasal Spray and Oral Antidepressant (n = 152) | Oral Antidepressant and Placebo Nasal Spray (n = 145) | |
| Dysgeusia | 41 (27.0) | 10 (6.9) |
| Vertigo | 38 (25.0) | 8 (5.5) |
| Dissociation | 35 (23.0) | 0 |
| Somnolence | 32 (21.1) | 3 (2.1) |
| Dizziness | 31 (20.4) | 7 (4.8) |
| Headache | 27 (17.8) | 14 (9.7) |
| Nausea | 25 (16.4) | 1 (0.7) |
| Vision blurred | 24 (15.8) | 1 (0.7) |
| Hypoesthesia oral | 20 (13.2) | 0 |
| Anxiety | 12 (7.9) | 5 (3.4) |
| Nasal discomfort | 11 (7.2) | 4 (2.8) |
| Paresthesia | 11 (7.2) | 0 |
| Viral upper respiratory tract infection | 11 (7.2) | 12 (8.3) |
| Blood pressure increased | 10 (6.6) | 5 (3.4) |
| Dizziness postural | 10 (6.6) | 3 (2.1) |
| Sedation | 10 (6.6) | 1 (0.7) |
| Vomiting | 10 (6.6) | 1 (0.7) |
| Confusional state | 9 (5.9) | 0 |
| Diplopia | 9 (5.9) | 0 |
| Hypoesthesia | 9 (5.9) | 0 |
| Paresthesia oral | 8 (5.3) | 1 (0.7) |
| Throat irritation | 8 (5.3) | 1 (0.7) |
Adverse events are listed in decreasing order based on incidence within the esketamine plus antidepressant group and in alphabetical order for events with the same incidence. The incidence was 5% or greater in either treatment group.
No deaths were reported during the study. Serious AEs considered by the investigator as related to study drug were reported for 6 patients in the esketamine and antidepressant group (autonomic nervous system imbalance, disorientation, hypothermia, lacunar stroke [ie, ischemic lesion, day 1, 6 hours after dosing], sedation, simple partial seizures [day 5, 45 minutes after dosing; no seizure history], and suicidal ideation) during the induction phase. No serious AEs considered as related to esketamine were reported during the optimization or maintenance phases.
Seven patients experienced 1 or more AEs during the maintenance phase, leading to discontinuation of the intranasal study drug; 4 (2.6%) of 152 were in the esketamine and antidepressant group (worsening depression, 3 patients; anxiety and confusional state [transient], 1 patient) and 3 (2.1%) of 145 were in the antidepressant and placebo group (worsening depression for each).
Transient blood pressure increases were observed with esketamine on treatment days; the maximum value was reached at 40 minutes after the start of administration in most cases and typically returned to the predose range by 1.5 hours after administration (eFigure 3 in Supplement 2). Few patients experienced treatment-emergent transient hypertension, defined as a systolic blood pressure of 180 mm Hg or higher and/or a diastolic blood pressure of 110 mm Hg or higher (ie, systolic hypertension: 1 [0.7%] esketamine-treated patient and 0 antidepressant- and placebo-treated patients; diastolic hypertension: 2 [1.3%] esketamine-treated patients and 0 antidepressant-and placebo-treated patients) during the maintenance phase. No clinically significant change in electrocardiographic findings was observed during the study.
Most direct-entry patients (362 [85.4%]) had baseline C-SSRS scores of 0, indicating no suicidal ideation or behavior. Of those patients treated with esketamine and oral antidepressant who reported no suicidal ideation or behavior at baseline, 42 (11.6%) had a higher postbaseline score (maximum C-SSRS score of 1 [n = 35], 2 [n = 3], 3 [n = 2], 5 [n = 1], and 8 [n = 1]) during the open-label induction phase; 22 (5.7%) (direct- and transfer-entry patients) had a higher postbaseline score (maximum C-SSRS score of 1 [n = 17], 2 [n = 3], and 3 [n = 2]) in the optimization phase; and 3 (2.4%) had a higher postbaseline score (maximum C-SSRS score of 1 [n = 2] and 4 [n = 1]) compared with 6 patients (4.5%) receiving antidepressant and placebo (maximum C-SSRS score of 1 [n = 6]) in the maintenance phase. On the basis of the C-SSRS, there were no reports of suicidal behavior in the optimization or maintenance phases. None of the patients who experienced relapse had a significant elevation in C-SSRS score (ie, the most severe postbaseline score was 2 for patients who experienced relapse in the esketamine and antidepressant group and 3 for patients who experienced relapse in the antidepressant and placebo group).
Present-state dissociative symptoms, as measured by CADSS (eFigure 4 in Supplement 2), began shortly after the start of esketamine treatment, peaked at 40 minutes, and generally resolved by 1.5 hours. The magnitude of symptoms attenuated with repeated administrations over time in the induction phase, with a relatively low magnitude reported in the optimization and maintenance phase. No symptoms or AEs of psychosis were observed.
Of note, 1 patient received 1 dose of ketamine (10 mg intravenously) during the study for the treatment of an AE of nephrolithiasis, but no AEs were reported by any participant related to use or abuse of ketamine. No evidence of a distinct withdrawal syndrome was observed during the 2 weeks after cessation of esketamine nasal spray as assessed by the 20-item Physician Withdrawal Checklist.
Discussion
In this first study, to our knowledge, of esketamine nasal spray for relapse prevention in patients with TRD, continued treatment with esketamine and an antidepressant demonstrated clinically meaningful and statistically significant superiority compared with antidepressant and placebo in delaying relapse in patients who had achieved stable remission or stable response after 16 weeks of treatment with esketamine and an antidepressant. No major difference in efficacy was seen by direct- or transferred-entry status.
One concern often cited in interpretation of randomized withdrawal studies is that the increased rate of depression observed after switching to placebo is a pharmacologic consequence of antidepressant withdrawal.26 A high relapse rate early in the withdrawal period could indicate a possible withdrawal or rebound effect. In this study, although there were a high number of relapses in the first month in those switched to placebo nasal spray, it is unlikely that a pharmacologic withdrawal effect contributed given that the decrease in esketamine plasma concentrations is rapid for the initial 2 to 4 hours and more gradual thereafter (mean terminal half-life, 7-12 hours), with steady state never reached with intermittent dosing. Moreover, this high rate of early relapse is similar to that observed after cessation of electroconvulsive therapy.27 There are no known rebound effects after electroconvulsive therapy discontinuation. The high rates of early relapse after esketamine discontinuation and those observed by Rush et al3 for patients in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study at level 3 or 4 (ie, who had failed 2 and 3 prior antidepressant treatments, respectively) more likely reflect a greater vulnerability to relapse among patients with TRD during maintenance treatment with an antidepressant alone.
In recognition of interindividual variability, the MADRS-based treatment algorithm individualized dosing frequency to the lowest frequency that maintained remission or response. Dosing frequency was reduced to once every 2 weeks if the patient had achieved remission (ie, MADRS score ≤12), whereas those unable to achieve or maintain remission were assigned to a weekly dosing frequency. Of note, more than half of the patients who experienced relapse during the first month after discontinuation of esketamine treatment required weekly dosing to sustain remission, reflecting the higher vulnerability in this subpopulation. Taken together, the evidence suggests that relapses seen in the first weeks after discontinuing esketamine treatment are likely attributable to more vulnerable patients and not a withdrawal or rebound phenomenon.
No new or unexpected safety concern was observed in this long-term study of esketamine nasal spray administered weekly or every 2 weeks. Results were consistent with previous findings from completed short-term (4-week) phase 2 and 3 studies.10,12,15
Limitations
Study limitations include the fact that esketamine has known transient dissociative and sedative effects that are difficult to blind; these symptoms could have biased the staff who observed treatment administration. To ensure unbiased efficacy evaluation, independent, remote, blinded MADRS raters assessed treatment response throughout this study. In addition, a post hoc analysis assessed participants randomized to discontinue intranasal esketamine treatment who subsequently experienced relapse in the first 4 weeks of the maintenance phase (n = 19). A sensitivity analysis, performed by censoring the patients who experienced relapse and showed a clear change in CADSS score before and after randomization (n = 3), resulted in an HR of 0.50 (95% CI, 0.30-0.84) with a 2-sided P = .008 (consistent with the primary analysis), based on an unweighted Cox proportional hazards regression model and log rank test.
Conclusions
This study demonstrated that, after 16 weeks of initial treatment, continued treatment with esketamine plus antidepressant leads to significant, clinically meaningful superiority compared with an antidepressant plus placebo for relapse prevention among patients with TRD and provides further safety data supporting a positive benefit-risk ratio of long-term treatment.
Trial Protocol
eAppendix 1. List of Institutional Review Boards and Independent Ethics Committees
eAppendix 2. Patient Inclusion and Exclusion Criteria
eAppendix 3. Columbia Suicide Severity Rating Scale (C-SSRS) Item Descriptions
eTable 1. Time-to-Relapse and Number (%) of Stable Remitters That Remained Relapse Free (Based on Stable Remission Defined as MADRS Total Score ≤10) in the Maintenance Phase
eFigure 1. Kaplan-Meier Estimates of Time to Relapse for Stable Remitters by Dosing Frequency in the Maintenance Phase
eFigure 2. Mean (±SE) in MADRS Total Score Over Time LOCF During the Induction, Optimization, and Maintenance Phases
eFigure 3. Mean (±SE) Blood Pressure Over Time in the Maintenance Phase for Stable Remitters and Stable Responders
eFigure 4. Mean (±SE) CADSS Total Score Over Time During the Induction, Optimization, and Maintenance Phases for Stable Remitters and Stable Responders
Data Sharing Statement
References
- 1.World Health Organization, Media Centre, Depression Fact Sheet, Updated 22 March 2018. https://www.who.int/en/news-room/fact-sheets/detail/depression. Accessed December 26, 2018.
- 2.Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341. doi: 10.1001/jamapsychiatry.2014.2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rush AJ, Trivedi MH, Wisniewski SR, et al. . Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. doi: 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- 4.Agency for Healthcare Research and Quality Definition of treatment-resistant depression in the Medicare population. https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/ta/drafts-for-review/trd-draft.pdf. Accessed October 23, 2018. [PubMed]
- 5.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB; APA Council of Research Task Force on Novel Biomarkers and Treatments . Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966. doi: 10.1176/appi.ajp.2015.15040465 [DOI] [PubMed] [Google Scholar]
- 6.Zarate CA Jr, Singh JB, Carlson PJ, et al. . A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864. doi: 10.1001/archpsyc.63.8.856 [DOI] [PubMed] [Google Scholar]
- 7.Murrough JW, Iosifescu DV, Chang LC, et al. . Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142. doi: 10.1176/appi.ajp.2013.13030392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh JB, Fedgchin M, Daly E, et al. . Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80(6):424-431. doi: 10.1016/j.biopsych.2015.10.018 [DOI] [PubMed] [Google Scholar]
- 9.Singh JB, Fedgchin M, Daly EJ, et al. . A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173(8):816-826. doi: 10.1176/appi.ajp.2016.16010037 [DOI] [PubMed] [Google Scholar]
- 10.Daly EJ, Singh JB, Fedgchin M, et al. . Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: results of a double-blind, doubly-randomized, placebo-controlled study. JAMA Psychiatry. 2018;75(2):139-148. doi: 10.1001/jamapsychiatry.2017.3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry. 2008;192(1):52-58. doi: 10.1192/bjp.bp.106.032532 [DOI] [PubMed] [Google Scholar]
- 12.Popova V, Daly E, Trivedi M, et al. Randomized, double-blind study of flexibly-dosed intranasal esketamine plus oral antidepressant vs active control in treatment-resistant depression. Paper presented at: 2018 American Society for Clinical Pathology Annual Meeting; May 30, 2018; Miami, FL. https://pmg.joynadmin.org/documents/1005/5afde1ec68ed3f2e245822b9.pdf. Accessed May 7, 2019.
- 13.Blier P, Zigman D, Blier J. On the safety and benefits of repeated intravenous injections of ketamine for depression. Biol Psychiatry. 2012;72(4):e11-e12. doi: 10.1016/j.biopsych.2012.02.039 [DOI] [PubMed] [Google Scholar]
- 14.Barenboim I, Lafer B. Maintenance use of ketamine for treatment-resistant depression: an open-label pilot study. Braz J Psychiatry. 2018;40(1):110. doi: 10.1590/1516-4446-2017-2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fedgchin M, Trivedi M, Daly EJ, et al. Randomized, double-blind study of fixed-dose intranasal esketamine plus oral antidepressant vs. active control in treatment-resistant depression (abstract 18). Presented at the 9th Biennial Conference of the International Society for Affective Disorders (ISAD) and the Houston Mood Disorders Conference; September 21, 2018; Houston, Texas. [Google Scholar]
- 16.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 18.Trivedi MH, Rush AJ, Ibrahim HM, et al. . The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82. doi: 10.1017/S0033291703001107 [DOI] [PubMed] [Google Scholar]
- 19.ClinicalTrials.gov A Long-term Safety Study of Intranasal Esketamine in Treatment-resistant Depression (SUSTAIN-3). NCT02782104. https://clinicaltrials.gov/ct2/show/NCT02782104. Accessed April 2, 2019.
- 20.Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164(7):1035-1043. doi: 10.1176/ajp.2007.164.7.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bremner JD, Krystal JH, Putnam FW, et al. . Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress. 1998;11(1):125-136. doi: 10.1023/A:1024465317902 [DOI] [PubMed] [Google Scholar]
- 22.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799-812. doi: 10.2466/pr0.1962.10.3.799 [DOI] [Google Scholar]
- 23.Rickels K, Garcia-Espana F, Mandos LA, Case GW. Physician Withdrawal Checklist (PWC-20). J Clin Psychopharmacol. 2008;28(4):447-451. doi: 10.1097/JCP.0b013e31817efbac [DOI] [PubMed] [Google Scholar]
- 24.Wang SK, Tsiatis AA. Approximately optimal one-parameter boundaries for group sequential trials. Biometrics. 1987;43(1):193-199. doi: 10.2307/2531959 [DOI] [PubMed] [Google Scholar]
- 25.Wassmer G. Planning and analyzing adaptive group sequential survival trials. Biom J. 2006;48(4):714-729. doi: 10.1002/bimj.200510190 [DOI] [PubMed] [Google Scholar]
- 26.Borges S, Chen YF, Laughren TP, et al. . Review of maintenance trials for major depressive disorder: a 25-year perspective from the US Food and Drug Administration. J Clin Psychiatry. 2014;75(3):205-214. doi: 10.4088/JCP.13r08722 [DOI] [PubMed] [Google Scholar]
- 27.Sackeim HA, Haskett RF, Mulsant BH, et al. . Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA. 2001;285(10):1299-1307. doi: 10.1001/jama.285.10.1299 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. List of Institutional Review Boards and Independent Ethics Committees
eAppendix 2. Patient Inclusion and Exclusion Criteria
eAppendix 3. Columbia Suicide Severity Rating Scale (C-SSRS) Item Descriptions
eTable 1. Time-to-Relapse and Number (%) of Stable Remitters That Remained Relapse Free (Based on Stable Remission Defined as MADRS Total Score ≤10) in the Maintenance Phase
eFigure 1. Kaplan-Meier Estimates of Time to Relapse for Stable Remitters by Dosing Frequency in the Maintenance Phase
eFigure 2. Mean (±SE) in MADRS Total Score Over Time LOCF During the Induction, Optimization, and Maintenance Phases
eFigure 3. Mean (±SE) Blood Pressure Over Time in the Maintenance Phase for Stable Remitters and Stable Responders
eFigure 4. Mean (±SE) CADSS Total Score Over Time During the Induction, Optimization, and Maintenance Phases for Stable Remitters and Stable Responders
Data Sharing Statement