Key Points
Question
What is the cost-effectiveness of evolocumab in patients with very high-risk atherosclerotic cardiovascular disease at its new annual list price of $5850?
Findings
This economic analysis determined that incremental cost-effectiveness ratios varied by baseline cardiovascular event rate, ranging from $56 655 per quality-adjusted life-year gained at an event rate of 6.4 events per 100 patient-years to $7667 per quality-adjusted life-year gained at an event rate of 12.3 events per 100 patient-years.
Meaning
The addition of evolocumab to standard background therapy at its current list price meets accepted cost-effectiveness thresholds across a range of risk profiles in the population with very high-risk atherosclerotic cardiovascular disease.
This economic analysis uses an updated cost-effectiveness analysis of evolocumab added to standard therapy in patients with very high-risk atherosclerotic cardiovascular disease, reflecting new guidelines and a new evolocumab list price.
Abstract
Importance
In October 2018, evolocumab was made available at a reduced annual list price of $5850 in the United States. This 60% reduction was aimed at improving patient access by lowering patient copays. Shortly thereafter, the 2018 American College of Cardiology/American Heart Association cholesterol management guideline was released. An updated cost-effectiveness analysis of evolocumab in the United States may be therefore of interest to payers and prescribers.
Objective
To present an updated cost-effectiveness analysis of evolocumab added to standard background therapy compared with standard background therapy alone in patients with very high-risk atherosclerotic cardiovascular disease, reflecting the 2018 ACC/AHA guideline definition and using the new evolocumab list price.
Design, Setting, and Participants
This study used the Markov model originally used in a previous study by Fonarow et al in 2017. A US societal perspective was considered, and a range of baseline cardiovascular event rates were modeled to reflect varying risk profiles in clinical practice within patients with very high-risk atherosclerotic cardiovascular disease.
Exposures
Addition of evolocumab to standard background therapy, including maximally tolerated statin therapy (ie, the maximum intensity of statin therapy a patient can safely receive), with or without ezetimibe.
Main Outcomes and Measures
Major cardiovascular events (myocardial infarction, ischemic stroke, and cardiovascular death), costs, quality-adjusted life-years, and incremental cost-effectiveness ratios.
Results
Evolocumab was associated with both increased costs and improved outcomes when added to standard background therapy. Incremental costs ranged from $22 228 to $3411, depending on the varying level of risk within the defined population. Incremental quality-adjusted life years ranged from 0.39 to 0.44. Incremental cost-effectiveness ratios ranged from $56 655 to $7667 per quality-adjusted life-year gained. For a range of baseline cardiovascular event rates in patients with very high-risk atherosclerotic cardiovascular disease, incremental cost-effectiveness ratios were below the generally accepted willingness-to-pay thresholds. Moreover, the ratios were below the threshold of $50 000 per quality-adjusted life-years gained for any baseline rate of 6.9 or more events per 100 patient-years.
Conclusions and Relevance
At its current list price, the addition of evolocumab to standard background therapy meets accepted cost-effectiveness thresholds across a range of baseline cardiovascular event rates in patients with very high-risk atherosclerotic cardiovascular disease as defined by the 2018 ACC/AHA guideline.
Introduction
Based on the results of the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) clinical trial,1 Fonarow et al2 evaluated the cost-effectiveness of evolocumab when added to standard background therapy (maximally tolerated statin therapy, or the highest intensity of statin that a patient can safely receive, with or without ezetimibe) in patients with atherosclerotic cardiovascular disease (ASCVD). Previous cost-effectiveness analyses of proprotein convertase/subtilisin type 9 (PCSK9) inhibitors in the United States raised debate on value and access implications.3 In October 2018, evolocumab was made available at a reduced annual list price of $5850 in the United States. This 60% reduction was aimed at improving patient access by lowering patient copays, especially for Medicare beneficiaries. Shortly thereafter, the 2018 American College of Cardiology/American Heart Association Multisociety Clinical Guideline on the Management of Blood Cholesterol (2018 ACC/AHA guideline) recommended PCSK9 inhibitors for, among other patient populations, patients with very high-risk (VHR) ASCVD whose low-density lipoprotein cholesterol levels remain at 70 mg/dL or more (≥1.8 mmol/L; to convert to millimoles per liter, multiply by 0.0259) despite a heart-healthy lifestyle and treatment with standard background therapy.4 Based on the Fonarow et al2 economic model, we present an updated cost-effectiveness analysis of evolocumab added to standard background therapy, compared with standard background therapy alone, in patients with VHR ASCVD, per the 2018 ACC/AHA guideline, using the new evolocumab list price.
Methods
We used the previously published Fonarow et al2 Markov model, which considered a US societal perspective and assumed a lifetime horizon to capture the progression of ASCVD. The model was used to assess subsequent major cardiovascular (CV) events as a function of age, sex, low-density lipoprotein cholesterol level, and CV event history. Model outcomes included major CV events (myocardial infarction, ischemic stroke, and CV death), costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs). Institutional review board approval was not required because this was a model-based analysis, and no human participants were involved.
The previously published inputs for ASCVD costs, utilities, and evolocumab intervention effects from the original model were used in this updated analysis.2 Accordingly, total costs and total QALYs determined by the model for standard background therapy remain unchanged from the original article2 when baseline CV event rates are aligned. This updated analysis, however, incorporated new data for 2 key model parameters: (1) an evolocumab annual list price of $5850, reflecting the 60% reduction, and (2) a baseline CV event rate in a clinical practice population in the United States, reflecting the definition of patients with VHR ASCVD in the 2018 ACC/AHA guideline.4
Because the 2018 ACC/AHA guideline has been recently published,4 there is an understandable lack of real-world evidence for patients with VHR ASCVD. We therefore modeled a range of plausible baseline CV event rates to represent patients with varying risk profiles within the VHR population. In the FOURIER clinical trial,1 the baseline CV event rate for patients at VHR was approximately 2-fold that for patients who were not at VHR. The lower bound of the baseline CV event rate range in this analysis was anchored by a rate of 6.4 events per 100 patient-years, using the original population in Fonarow et al,2 referred to as patients in US clinical practice. These event rates integrated total CV events, including recurrent events. The upper bound was calculated at 12.3 events per 100 patient-years by multiplying the rate of patients in US clinical practice by the rate ratio of patients at VHR to those not at VHR observed in the FOURIER clinical trial. Finally, a scenario analysis considering a baseline CV event rate of 4.4 events per 100 patient-years, representing FOURIER clinical trial patients at VHR, was considered. Excel 2016 (Microsoft) was used for all statistical analyses.
Results
Evolocumab was associated with both increased costs and improved outcomes when added to standard background therapy compared with standard background therapy alone, including maximally tolerated statin therapy with or without ezetimibe (Table). Incremental costs varied across the spectrum of CV risk modeled within the population with VHR ASCVD, ranging from $22 228 for a baseline CV event rate of 6.4 events per 100 patient-years to $3411 for a baseline CV event rate of 12.3 events per 100 patient-years. Incremental QALYs gained ranged from 0.39 to 0.44, respectively. The ICERs therefore ranged from $56 655 to $7667 per QALY gained, respectively. Of note, the ICER of $56 665 reflects the original patients in US clinical practice per Fonarow et al2 after only the reduction in the price of evolocumab to $5850 is considered, before modeling any increase in baseline CV event rate to characterize the spectrum of increased risk across the patients at VHR.
Table. Cost-effectiveness Results at an Evolocumab List Price of $5850 in Patients With Very High-risk Atherosclerotic Cardiovascular Diseasea.
| Base Case and Scenario Analysesb | Cost, $ | Quality-Adjusted Life-Years, y | Incremental Cost- effectiveness Ratio, $ |
||
|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | ||
| Base cases involving treatment with or without ezetimibe | |||||
| Patients at VHR in US clinical practice, event rate of 6.4 events per 100 patient-years | |||||
| Maximally tolerated statin therapy | 234 877 | NA | 7.23 | NA | 56 655 |
| Maximally tolerated statin therapy plus evolocumab | 257 105 | 22 228 | 7.62 | 0.39 | |
| Patients at VHR in US clinical practice, event rate of 12.3 events per 100 patient-years | |||||
| Maximally tolerated statin therapy | 258 519 | NA | 5.48 | NA | 7667 |
| Maximally tolerated statin therapy plus evolocumab | 261 931 | 3411 | 5.93 | 0.44 | |
| Scenarios involving treatment with ezetimibe | |||||
| Patients at VHR in US clinical practice, event rate of 6.4 events per 100 patient-years | |||||
| Maximally tolerated statin therapy | 262 677 | NA | 7.23 | NA | 59 331 |
| Maximally tolerated statin therapy plus evolocumab | 285 955 | 23 278 | 7.62 | 0.39 | |
| Patients at VHR in US clinical practice, event rate of 12.3 events per 100 patient-years | |||||
| Maximally tolerated statin therapy | 280 221 | NA | 5.48 | NA | 10 584 |
| Maximally tolerated statin therapy plus evolocumab | 284 931 | 4709 | 5.93 | 0.44 | |
| Scenario involving treatment with or without ezetimibe | |||||
| Patients at VHR in FOURIER trial, event rate of 4.4 events per 100 patient-years | |||||
| Maximally tolerated statin therapy | 227 451 | NA | 8.11 | NA | 91 610 |
| Maximally tolerated statin therapy plus evolocumab | 257 748 | 30 297 | 8.44 | 0.33 | |
Abbreviations: FOURIER, Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk; NA, not applicable; PCSK9, proprotein convertase/subtilisin type 9; VHR, very high risk.
Very high-risk atherosclerotic cardiovascular disease was defined as per the 2018 American College of Cardiology/American Heart Association guideline4: a history of multiple major atherosclerotic cardiovascular disease events (recent acute coronary syndrome, a history of myocardial infarction or ischemic stroke, or symptomatic peripheral artery disease) or 1 major atherosclerotic cardiovascular disease event and multiple high-risk conditions (≥65 years of age, heterozygous familial hypercholesterolemia, history of congestive heart failure, prior coronary artery bypass graft or percutaneous coronary intervention, diabetes mellitus, hypertension, chronic kidney disease, current smoking, or persistently elevated low-density lipoprotein cholesterol ≥100 mg/dL [to convert to millimoles per liter, multiply by 0.0259] despite maximally tolerated statin therapy and ezetimibe).
Cardiovascular events include myocardial infarction, ischemic stroke, and cardiovascular death.
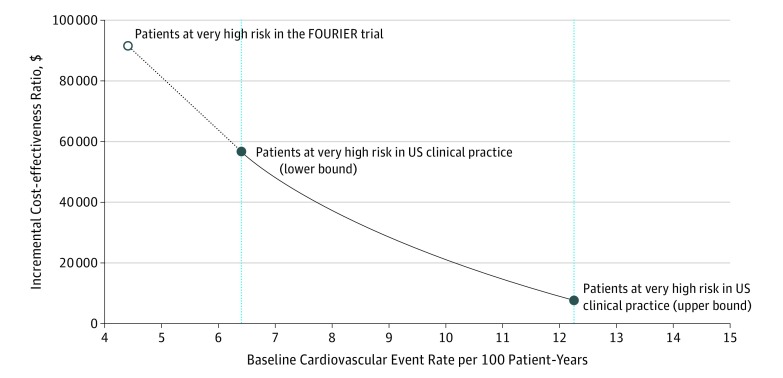
For the range of baseline CV event rates in patients with VHR ASCVD in clinical practice, all ICERs were within what the 2018 ACC/AHA guideline considers a high or intermediate value (<$50 000 to <$150 000 per QALY gained).4 Moreover, most ICERs were below $50 000 per QALY gained (the high value), corresponding to baseline CV event rate of 6.9 or more events per 100 patient-years (Figure).
Figure. Incremental Cost-effectiveness Ratio as a Function of Baseline Cardiovascular Event Rate.
Cardiovascular events include myocardial infarction, ischemic stroke, and cardiovascular death. An annual evolocumab list price of $5850 was used. FOURIER indicates the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk trial.
If standard background therapy included maximally tolerated statin therapy with ezetimibe, ICERs would range from $59 331 to $10 584, depending on the varying level of risk within the population at VHR. In the conservative scenario analysis in which a baseline CV event rate of 4.4 events per 100 patient-years was considered, based on the FOURIER clinical trial observed rates for patients at VHR, the ICER was $91 610 (Table).
Discussion
We present an updated cost-effectiveness analysis of evolocumab added to standard background therapy, compared with standard background therapy alone, in patients with VHR ASCVD per the 2018 ACC/AHA guideline, using the new evolocumab list price. As noted in the 2018 ACC/AHA guideline, at any given price, the value of PCSK9 inhibitors will be improved by selecting patients at higher risk for the occurrence of CV events.4 At its new annual list price of $5850, the addition of evolocumab to standard background therapy in patients with VHR ASCVD yields ICERs that meet currently accepted cost-effectiveness thresholds and are consistent with providing high-value care.
This updated cost-effectiveness analysis may be useful for payers, clinicians, and those informing the appropriate use of lipid-lowering therapies in the United States, particularly in light of the current access and reimbursement barriers. The 2018 ACC/AHA guideline questioned the value of PCSK9 inhibitors, likely because mid-2018 prices were considered. Likewise, previously published cost-effectiveness analyses of evolocumab based on the FOURIER clinical trial estimated ICERs that were above the generally accepted willingness-to-pay thresholds; however, these analyses were not specifically conducted in patients with VHR ASCVD and did not reflect the current evolocumab list price.5,6 For instance, the base case ICER in Fonarow et al2 is reduced by 79% to an ICER of $56 665 as a result of the decrease in annual list price of 60%. The reduction in list price improved the cost-effectiveness for patients with ASCVD before characterizing patients at VHR, for whom baseline CV event rates would be higher. Despite the improvement in value at the new current list price, a comprehensive disease management approach including a heart-healthy lifestyle across the life course and adherence to standard background therapy, as per current guidelines, should be applied.
A strength of this analysis is that it offers ICERs across a range of baseline CV event rates as a proxy for patients with varying risk profiles within the VHR population. Despite the understandably limited real-world evidence in this very recently defined population, the range of baseline CV event rates considered in this study is plausible and supported by the available literature. For instance, a recent study in Medicare patients at VHR with a history of recent myocardial infarction or ischemic stroke reported a composite 1-year rate of acute coronary syndrome, ischemic stroke, or death of 38.2 events per 100 patient-years.7 In that study, the 1-year rate of nonfatal myocardial infarction or ischemic stroke was approximately 9 events per 100 patient-years, even before capturing CV death. On the other hand, a recent evaluation of a PCSK9 inhibitor in a subgroup of patients at VHR in the United States applied a baseline CV event rate of 7.2 events per 100 patient-years.8 These results highlight the importance of future analyses that more precisely quantify the baseline CV event rates estimated from patients with VHR ASCVD in the real world to further understand the cost-effectiveness of evolocumab.
Limitations
There are potential limitations to this study, and the findings should be interpreted within the context of the data inputs, event rates, and modeling assumptions used. The event rate range for patients with VHR ASCVD was estimated using available data from the FOURIER clinical trial and patients in US clinical practice. The results are based on the assumption that clinical benefits of treatment extend beyond the period with direct follow-up. If the levels of persistence with and adherence to evolocumab therapy differ from those modeled, costs, and clinical effectiveness may differ.
Conclusions
At its current list price, the addition of evolocumab to standard background therapy meets accepted cost-effectiveness thresholds across a range of baseline CV event rates in patients with VHR ASCVD in the United States, with very high risk defined by the 2018 ACC/AHA guideline. This updated cost-effectiveness analysis may be informative for payers, health systems, and clinicians regarding the appropriate use and value of lipid-lowering therapies in the United States.
References
- 1.Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2(10):1069-1078. doi: 10.1001/jamacardio.2017.2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mark DB, Richman I, Hlatky MA. Proprotein convertase subtilisin/kexin type 9 inhibitor therapy-breakthrough in low-density lipoprotein cholesterol lowering, breakdown in value. JAMA Cardiol. 2017;2(10):1066-1068. doi: 10.1001/jamacardio.2017.2911 [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines [published online November 8, 2018]. J Am Coll Cardiol. 2018. doi: 10.1016/j.jacc.2018.11.003 [DOI] [Google Scholar]
- 5.Arrieta A, Hong JC, Khera R, Virani SS, Krumholz HM, Nasir K. Updated cost-effectiveness assessments of PCSK9 inhibitors from the perspectives of the health system and private payers: insights derived from the FOURIER trial. JAMA Cardiol. 2017;2(12):1369-1374. doi: 10.1001/jamacardio.2017.3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazi DS, Penko J, Coxson PG, et al. Updated cost-effectiveness analysis of PCSK9 inhibitors based on the results of the FOURIER trial. JAMA. 2017;318(8):748-750. doi: 10.1001/jama.2017.9924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li S, Peng Y, Wang X, et al. Cardiovascular events and death after myocardial infarction or ischemic stroke in an older Medicare population. Clin Cardiol. 2019;42(3):391-399. doi: 10.1002/clc.23160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazi DS, Penko J, Coxson PG, Guzman D, Wei PC, Bibbins-Domingo K. Cost-effectiveness of alirocumab: a just-in-time analysis based on the ODYSSEY outcomes trial. Ann Intern Med. 2019;170(4):221-229. doi: 10.7326/M18-1776 [DOI] [PubMed] [Google Scholar]