Key Points
Question
What is the association of patient survival and intensity of treatment with total Medicare costs that are associated with radical cystectomy vs trimodal therapy for older adults with localized muscle-invasive bladder cancer?
Findings
This Surveillance, Epidemiology, and End Results–Medicare cohort study identified 2963 patients aged 66 to 85 years with a diagnosis of clinical stage T2 to T4a bladder cancer. The inverse probability of treatment-weighted propensity score models revealed that outpatient, radiology, and pathology/laboratory services, along with medication expenses, significantly contributed to $136 935 higher costs associated with trimodal therapy compared with radical cystectomy within 1 year after bladder cancer diagnosis.
Meaning
The excess spending associated with trimodal therapy largely attributed to medication and radiology expenses along with the less favorable survival outcomes compared with radical cystectomy should be discussed during clinical decision making for patients with bladder cancer.
Abstract
Importance
Earlier studies on the cost of muscle-invasive bladder cancer treatments lack granularity and are limited to 180 days.
Objective
To compare the 1-year costs associated with trimodal therapy vs radical cystectomy, accounting for survival and intensity effects on total costs.
Design, Setting, and Participants
This population-based cohort study used the US Surveillance, Epidemiology, and End Results–Medicare database and included 2963 patients aged 66 to 85 years who had received a diagnosis of clinical stage T2 to T4a muscle-invasive bladder cancer from January 1, 2002, through December 31, 2011. The data analysis was performed from March 5, 2018, through December 4, 2018.
Main Outcomes and Measures
Total Medicare costs within 1 year of diagnosis following radical cystectomy vs trimodal therapy were compared using inverse probability of treatment–weighted propensity score models that included a 2-part estimator to account for intrinsic selection bias.
Results
Of 2963 participants, 1030 (34.8%) were women, 2591 (87.4%) were white, 129 (4.4%) were African American, and 98 (3.3%) were Hispanic. Median costs were significantly higher for trimodal therapy than radical cystectomy in 90 days ($83 754 vs $68 692; median difference, $11 805; 95% CI, $7745-$15 864), 180 days ($187 162 vs $109 078; median difference, $62 370; 95% CI, $55 581-$69 160), and 365 days ($289 142 vs $148 757; median difference, $109 027; 95% CI, $98 692-$119 363), respectively. Outpatient care, radiology, medication expenses, and pathology/laboratory costs contributed largely to the higher costs associated with trimodal therapy. On inverse probability of treatment–weighted adjusted analyses, patients undergoing trimodal therapy had $136 935 (95% CI, $122 131-$152 115) higher mean costs compared with radical cystectomy 1 year after diagnosis.
Conclusions and Relevance
Compared with radical cystectomy, trimodal therapy was associated with higher costs among patients with muscle-invasive bladder cancer. The differences in costs were largely attributed to medication and radiology expenses associated with trimodal therapy. Extrapolating cost figures resulted in a nationwide excess spending of $468 million for trimodal therapy compared with radical cystectomy for patients who received a diagnosis of bladder cancer in 2017.
This SEER–Medicare cohort study compares the costs associated with radical cystectomy vs trimodal therapy for older adults with localized muscle-invasive bladder cancer.
Introduction
Although radical cystectomy with extended pelvic lymphadenectomy is the guideline-recommended treatment for patients with muscle-invasive bladder cancer, trimodal therapy may be a suitable treatment option.1,2,3,4,5,6,7 Given the lack of randomized trials comparing these 2 treatments, we have relied largely on population-based studies reporting varying survival outcomes between the 2 treatments.7,8,9,10 Despite these conflicting findings, trimodal therapy has been increasingly used in treating patients with muscle-invasive bladder cancer.
Clinical decision making increasingly incorporates the costs of treatment. The costs associated with bladder cancer treatment, monitoring, and the management of treatment adverse effects are substantial.11,12,13 Although prior studies have evaluated the costs of radical cystectomy, to our knowledge, the costs associated with trimodal therapy have only been preliminarily assessed up to 180 days with a lack of granularity.8,13,14 Estimating the marginal association of trimodal therapy vs radical cystectomy with costs should incorporate survival (ie, increased costs due to differences in survival between 2 treatments) and intensity (ie, cost accumulation over time when patients are alive) effects.15 The purpose of this study was to compare the total 1-year costs of trimodal therapy vs radical cystectomy and assess the independent contributions of survival and intensity to total costs.
Methods
Data Source
We used the Surveillance, Epidemiology, and End Results (SEER)–linked Medicare database. The SEER database is sponsored by the National Cancer Institute and aggregates data from 18 cancer registries. The ascertainment of cancer cases in SEER meets the standards of the North American Association of Central Cancer Registries (98% complete).16 Medicare provides information on health care use. The study was exempt from review by the institutional review board at the University of Texas Medical Branch and informed consent was waived as there was no increased harm to patients because the data were retrospective.
Study Cohort
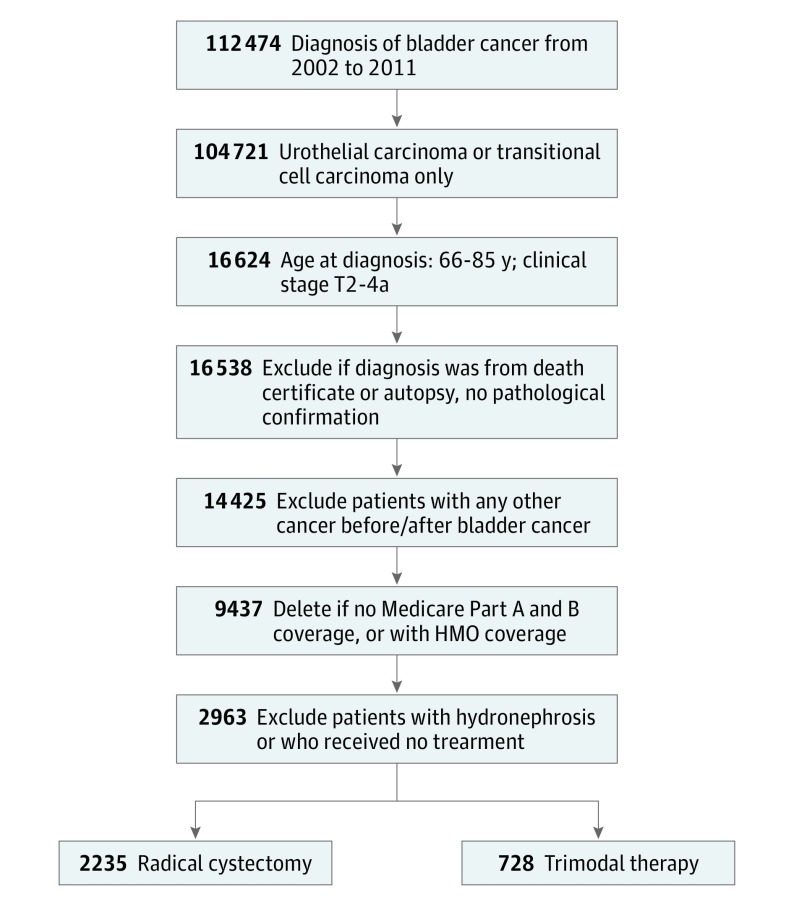
This cohort study included older adults (age 66-85 years) with a diagnosis of stage T2 to T4a bladder cancer (transitional cell or urothelial cell carcinoma) from January 1, 2002, through December 31, 2011 (Figure 1). Medicare claims data were reviewed through December 31, 2012. We excluded patients older than 85 years, those who received a diagnosis of node-positive and/or metastatic disease, those with hydronephrosis, those without continuous Medicare fee-for-service coverage, and those without available Medicare Part A and Part B claims data.
Figure 1. Patient Selection Process.
HMO indicates health maintenance organization.
Identifying Bladder Cancer Treatments
We identified patients who underwent radical cystectomy alone or in combination with radiation and/or chemotherapy using procedure codes and Current Procedural Terminology codes (eTable 1 in the Supplement) from the Medicare claims data. Receipt of trimodal therapy was defined as patients who underwent transurethral resection of the bladder tumor followed by radiotherapy and chemotherapy with bladder cancer–specific J codes within 180 days following diagnosis in the absence of a concomitant code for radical cystectomy.17 When available in Medicare claims, we calculated the total number of radiotherapy fractions by counting the number of unique Current Procedural Terminology codes for radiation delivery using a window from the start of radiation delivery until 90 days thereafter.18 To avoid duplicate counting of services rendered with respective expenditures, we used a combination of patient identification, Medicare claim dates, and Healthcare Common Procedure Coding System codes to remove duplicate records.19
Study Covariates
From SEER, we abstracted patient’s clinical and sociodemographic information. Education was defined according to the proportion of residents in a patient’s Zip code who have completed high school diploma. A Zip code–level median household income was used to define income quartiles. Both education and income information were obtained from the 2000 Census and the 2008-2012 American Community Survey.17,20 Comorbidities were assessed from the Medicare claims data using the Klabunde modification of the Charlson comorbidity index during the year prior to cancer diagnosis.21
Outcomes
Patients were followed up from the diagnosis date (ie, transurethral resection of the bladder tumor immediately preceding cystectomy or chemotherapy/radiation) for 1 year or until death to determine the total costs of care. All Medicare expenditures (inpatient, outpatient, and physician services) within 1 year of diagnosis were summed to obtain total costs.11 Categorized inpatient and outpatient costs were derived from Medicare Provider and Analysis Review and Outpatient Standard Analytic Files with carrier claims files, respectively. We adopted the methods used by the Health Care Cost Institute to categorize total 1-year costs into major services, such as hospitalization, medication expenses (intravenous medications), radiology, and pathology/laboratory.19,22,23,24 All costs were inflated to the 2017 US dollar using the medical component of consumer price index (CPI).14,25 To test the robustness of the study findings, costs were also inflated using alternative cost inflation methods, namely the overall component of CPI as well as the gross domestic product implicit price deflator. Secondary outcomes included overall and cancer-specific survival.
Statistical Analysis
We constructed a logistic regression model to predict the probability of treatment (trimodal therapy vs radical cystectomy) as a function of sociodemographic (age at diagnosis, sex, race/ethnicity, marital status, census region, income, and education) and clinical characteristics (comorbidity, cancer stage, cancer grade, and year of diagnosis). We derived inverse probability treatment weights (IPTW) for each patient as the inverse of the propensity score for patients undergoing radical cystectomy and the inverse of 1 minus the propensity score for patients undergoing radical trimodal therapy. Differences observed in baseline characteristics between the radical cystectomy and trimodal therapy groups were adjusted using IPTW-weighted propensity score models.26 A standardized difference of less than 0.10 between treatment groups indicated a balance in covariates. No collinearity was observed during the IPTW model building except for income and education, and income was included in the final model. No significant interaction was observed between age and comorbidity. The SAS procedure Proc Logistic was used to derive propensity scores and stddiff macro (SAS Institute) was used to calculate standardized differences.27
Due to the skewed nature of health care costs, medians and interquartile ranges were used to describe 90-day, 180-day, and 365-day costs. Unadjusted median costs between radical cystectomy and trimodal therapy were compared using the Hodges-Lehmann (H-L) estimator. We used the Basu and Manning method15 to compare costs between the 2 treatment approaches to account for skewed values in costs in the presence of censoring and death and to distinguish between survival and intensity effects. The survival effect is the association of an independent variable with costs because of differences in survival between patients who underwent radical cystectomy vs trimodal therapy. The intensity effect estimates the association of an independent variable with costs because of differences in accumulated resource use for patients who remained alive. The costs estimator used the IPTW cohort working with the assumptions of continuous distributions of death and censoring and separated the marginal association of the 2 treatments with total costs into a portion generated by affecting survival and another by affecting the rates of cost accumulation if alive.15 To estimate the dollar amount of excess spending attributed to trimodal therapy, we extrapolated the adjusted mean differences in 1-year costs associated with trimodal therapy to an analogous group of patients with bladder cancer in 2017.11,28,29
We performed the following sensitivity analyses. First, because of the expanding use of neoadjuvant chemotherapy, we compared a subset of patients who received neoadjuvant chemotherapy followed by radical cystectomy with those who received trimodal therapy in terms of 1-year costs. Second, we examined Medicare claims for patients who were continuously enrolled in Medicare Part D (prescription drug coverage) from 2007 to 2011. The cost analysis included total Part D costs and out-of-pocket costs for prescription drugs. For all sensitivity analyses, we used the medical component of CPI.
Kaplan-Meier survival curves compared survival between radical cystectomy and trimodal therapy. Inverse probability of treatment–weighted adjusted Cox proportional hazards regression, as well as Fine and Gray competing risk regression models, were constructed to compare overall survival and cancer-specific survival, respectively. We also compared survival by the stage of diagnosis and number of radiotherapy fraction. All statistical tests were 2-sided, and all analyses were performed using SAS, version 9.4 (SAS Institute) and Stata software, version 13 (StataCorp). Statistical significance was set at P < .05.
Results
The study cohort consisted of 2963 patients eligible for analysis (Table 1). Overall, 2235 (75.4%) and 728 (24.6%) of those with identified muscle-invasive bladder cancer underwent radical cystectomy and trimodal therapy, respectively. Twenty-five patients (3.4%) underwent salvage cystectomy following trimodal therapy. In the IPTW unweighted population, a significantly higher proportion of patients who underwent cystectomy were younger, women, and had none to fewer comorbidities compared with those who underwent trimodal therapy (all standardized difference, >0.10). Patients undergoing trimodal therapy were more likely to have had tumor stage classification T2 bladder cancer (standardized difference = 0.51). In the IPTW weighted cohort, all variables were well balanced (standardized difference, <0.10). There was no significant difference in the mean (SD) time from diagnosis to treatment for either radical cystectomy or trimodal therapy (93 [76] days vs 97 [129] days; P = .42).
Table 1. Patient Demographics and Clinical Characteristics Before and After Inverse Probability Treatment Weighting.
| Characteristic, No. (%) | Total, No. | Unweighted | Weighted | ||||
|---|---|---|---|---|---|---|---|
| Study Population, No. (%) | Standardized Difference | Study Population, No. (%) | Standardized Difference | ||||
| Radical Cystectomy | Trimodal Therapy | Radical Cystectomy | Trimodal Therapy | ||||
| Demographics | 2963 (100) | 2235 (75.4) | 728 (24.6) | 2957 (49.5) | 3020 (50.5) | ||
| Age group, y | |||||||
| 66-69 | 584 (19.7) | 483 (21.6) | 101 (13.9) | −0.204 | 587 (19.9) | 632 (20.9) | 0.027 |
| 70-74 | 790 (26.7) | 639 (28.6) | 151 (20.7) | −0.183 | 792 (26.8) | 842 (27.9) | 0.025 |
| 75-79 | 845 (28.5) | 642 (28.7) | 203 (27.9) | −0.019 | 845 (28.6) | 842 (27.9) | −0.015 |
| 80-85 | 744 (25.1) | 471 (21.1) | 273 (37.5) | 0.367 | 733 (24.8) | 704 (23.3) | −0.035 |
| Sex | |||||||
| Male | 1930 (65.1) | 1412 (63.2) | 518 (71.2) | 0.171 | 1914 (64.7) | 1870 (61.9) | −0.058 |
| Female | 1033 (34.9) | 823 (36.8) | 210 (28.8) | −0.171 | 1043 (35.3) | 1150 (38.1) | 0.058 |
| Race/ethnicity | |||||||
| White | 2591 (87.4) | 1955 (87.5) | 636 (87.4) | −0.003 | 2584 (87.4) | 2606 (86.3) | −0.033 |
| African American | 129 (4.4) | 92 (4.1) | 37 (5.1) | 0.046 | 128 (4.3) | 144 (4.8) | 0.021 |
| Hispanic | 98 (3.3) | 76 (3.4) | 22 (3.0) | −0.022 | 100 (3.4) | 123 (4.1) | 0.036 |
| Other | 145 (4.9) | 112 (5.0) | 33 (4.5) | −0.022 | 145 (4.9) | 147 (4.9) | −0.001 |
| Marital status | |||||||
| Single | 423 (14.3) | 316 (14.1) | 107 (14.7) | 0.016 | 425 (14.40 | 448 (14.8) | 0.013 |
| Married | 1836 (62.0) | 1410 (63.1) | 426 (58.5) | −0.094 | 1830 (61.9) | 1849 (61.2) | −0.013 |
| Unknown | 704 (23.8) | 509 (22.8) | 195 (26.8) | 0.093 | 702 (23.8) | 723 (23.9) | 0.004 |
| Census region | |||||||
| West | 1204 (40.6) | 906 (40.5) | 298 (40.9) | 0.008 | 1197 (40.5) | 1213 (40.2) | −0.007 |
| Northeast | 673 (22.7) | 516 (23.1) | 157 (21.6) | −0.037 | 675 (22.8) | 723 (23.9) | 0.027 |
| Midwest | 357 (12.0) | 254 (11.4) | 103 (14.1) | 0.084 | 358 (12.1) | 379 (12.5) | 0.014 |
| South | 729 (24.6) | 559 (25.0) | 170 (23.4) | −0.039 | 727 (24.6) | 705 (23.3) | −0.029 |
| Median household income, $ | |||||||
| ≤42 992 | 660 (22.3) | 487 (21.8) | 173 (23.8) | 0.047 | 660 (22.3) | 672 (22.3) | −0.002 |
| 42 993-56 188 | 749 (25.3) | 579 (25.9) | 170 (23.4) | −0.059 | 748 (25.3) | 757 (25.1) | −0.006 |
| 56 189-73 827 | 761 (25.7) | 553 (24.7) | 208 (28.6) | 0.087 | 760 (25.7) | 788 (26.1) | 0.009 |
| ≥73 828 | 793 (26.8) | 616 (27.6) | 177 (24.3) | −0.074 | 789 (26.7) | 803 (26.6) | −0.002 |
| Educational level, %a | |||||||
| ≤20.58 | 797 (26.9) | 606 (27.1) | 191 (26.2) | −0.020 | 789 (26.7) | 791 (26.2) | −0.011 |
| 20.59-27.36 | 732 (24.7) | 546 (24.4) | 186 (25.5) | 0.026 | 733 (24.8) | 788 (26.1) | 0.031 |
| 27.37-34.83 | 697 (23.5) | 513 (23.0) | 184 (25.3) | 0.054 | 699 (23.6) | 701 (23.2) | −0.010 |
| ≥34.84 | 737 (24.9) | 570 (25.5) | 167 (22.9) | −0.060 | 736 (24.9) | 740 (24.5) | −0.010 |
| Comorbidity score | |||||||
| 0 | 1602 (54.1) | 1254 (56.1) | 348 (47.8) | −0.167 | 1597 (54.0) | 1655 (54.8) | 0.016 |
| 1 | 802 (27.1) | 606 (27.1) | 196 (26.9) | −0.004 | 798 (27.0) | 773 (25.6) | −0.032 |
| 2 | 317 (10.7) | 216 (9.7) | 101 (13.9) | 0.131 | 316 (10.7) | 330 (10.9) | 0.009 |
| ≥3 | 242 (8.2) | 159 (7.1) | 83 (11.4) | 0.148 | 245 (8.3) | 262 (8.6) | 0.013 |
| Cancer stage | |||||||
| II | 1377 (46.5) | 903 (40.4) | 474 (65.1) | 0.511 | 1366 (46.2) | 1341 (44.4) | −0.035 |
| III | 792 (26.7) | 689 (30.8) | 103 (14.1) | −0.408 | 792 (26.8) | 827 (27.4) | 0.013 |
| IV | 794 (26.8) | 643 (28.8) | 151 (20.7) | −0.187 | 799 (27.0) | 852 (28.2) | 0.026 |
| Cancer grade | |||||||
| Low | 120 (4.0) | 95 (4.3) | 25 (3.4) | −0.043 | 120 (4.1) | 112 (3.7) | −0.018 |
| High | 2746 (92.7) | 2082 (93.2) | 664 (91.2) | −0.073 | 2736 (92.5) | 2799 (92.7) | 0.007 |
| Unknown | 97 (3.3) | 58 (2.6) | 39 (5.4) | 0.142 | 101 (3.4) | 109 (3.6) | 0.009 |
| Year of diagnosis | |||||||
| 2002 | 299 (10.1) | 233 (10.4) | 66 (9.1) | −0.046 | 302 (10.2) | 319 (10.6) | 0.013 |
| 2003 | 303 (10.2) | 241 (10.8) | 62 (8.5) | −0.077 | 307 (10.4) | 319 (10.6) | 0.006 |
| 2004 | 332 (11.2) | 257 (11.5) | 75 (10.3) | −0.038 | 328 (11.1) | 329 (10.9) | −0.007 |
| 2005 | 343 (11.6) | 260 (11.6) | 83 (11.4) | −0.007 | 346 (11.7) | 368 (12.2) | 0.015 |
| 2006 | 311 (10.5) | 224 (10.0) | 87 (12.0) | 0.062 | 310 (10.5) | 289 (9.6) | −0.030 |
| 2007 | 283 (9.6) | 219 (9.8) | 64 (8.8) | −0.035 | 281 (9.5) | 257 (8.5) | −0.033 |
| 2008 | 286 (9.7) | 220 (9.8) | 66 (9.1) | −0.027 | 284 (9.6) | 287 (9.5) | −0.004 |
| 2009 | 262 (8.8) | 192 (8.6) | 70 (9.6) | 0.036 | 260 (8.8) | 281 (9.3) | 0.015 |
| 2010 | 278 (9.4) | 199 (8.9) | 79 (10.9) | 0.065 | 272 (9.2) | 302 (10.0) | 0.027 |
| 2011 | 266 (9.0) | 190 (8.5) | 76 (10.4) | 0.066 | 266 (9.0) | 269 (8.9) | −0.003 |
Educational level: the percentage of residents who had at least 4 years of college education.
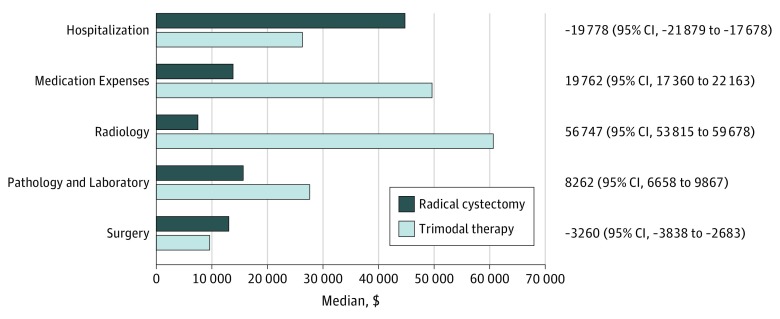
eFigure 1 in the Supplement shows the median cumulative Medicare costs over the 1-year period. Baseline 1-year costs were slightly higher for patients undergoing trimodal therapy compared with radical cystectomy ($9167 vs $7905; median difference, $1518; H-L 95% CI, $776-$2315). Table 2 demonstrates median unadjusted costs associated with each treatment in the 2017 US dollar value. Mean costs are reported in eTable 2 in the Supplement. The median total costs associated with trimodal therapy were significantly higher than those with radical cystectomy in 90 days ($83 754 vs $68 692; median difference, $11 805; H-L 95% CI, $7745-$15 864), 180 days ($187 162 vs $109 078; median difference, $62 370; H-L 95% CI, $55 581-$69 160), and 365 days after diagnosis ($289 142 vs $148 757; median difference, $109 027; H-L 95% CI, $98 692-$119 363). One-year costs using the medical component of CPI and the gross domestic product implicit price deflator are shown in eTable 3 in the Supplement. Radical cystectomy resulted in significantly higher inpatient costs (90 days, $25 407 vs $7144; 180 days, $36 885 vs $12 479; and 365 days, $47 382 vs $20 038; all P < .001). Outpatient costs were significantly lower for radical cystectomy (90 days, $36 071 vs $70 680; 180 days, $57 425 vs $163 816; and 365 days, $78 233 vs $255 280; all P < .001). These findings persisted across all disease stages (eTable 4 in the Supplement). Costs were further categorized according to services within 1 year of diagnosis (Figure 2; eTable 5 in the Supplement). Among patients who underwent radical cystectomy, hospitalization was associated with higher costs ($44 014 vs $25 577; median difference, −$19 778; H-L 95% CI, −$21 879 to −$17 678); however, radical cystectomy was associated with lower medication expense ($13 013 vs $49 587; median difference, $19 762; H-L 95% CI, $17 360-$22 163), pathology/laboratory ($15 535 vs 26 966; median difference, $8262; H-L 95% CI, $6658-$9867), and radiology costs ($7476 vs $60 599; median difference, $56 747; H-L 95% CI, $53 815-$59 687). The costs for all categories are reported in eTable 5 in the Supplement.
Table 2. Medicare Costs (USD) Associated With Radical Cystectomy and Trimodal Therapy Following Bladder Cancer Diagnosis.
| No. of Days | Median (IQR), $ | Hodges-Lehmann Estimate (95% CI)a | |||||
|---|---|---|---|---|---|---|---|
| Radical Cystectomy | Trimodal Therapy | ||||||
| Total | Inpatient | Outpatient | Total | Inpatient | Outpatient | ||
| 90 db | 68 692 (44 912-98 871) | 25 406 (7542-41 297) | 36 071 (21 690-59 521) | 83 754 (50 974-129 299) | 7144 (0-17 662) | 70 680 (36 656-115 582) | 11 805 (7745-15 864) |
| 180 db | 109 078 (71 368-170 788) | 36 885 (24 028-58 854) | 57 425 (33 237-109 114) | 187 162 (126 905-261 817) | 12 479 (0-31 780) | 163 816 (108 624-232 342) | 62 370 (55 581-69 160) |
| 365 db | 148 757 (87 282-252 518) | 47 382 (30 128-76 547) | 78 233 (42 781-173 847) | 289 142 (197 649-409 655) | 20 038 (6501-45 975) | 255 280 (168 008-372 602) | 109 027 (98 692-119 363) |
Abbreviations: IQR, interquartile range; USD, US dollar.
Hodges-Lehmann median difference in total costs (trimodal therapy minus radical cystectomy).
Radical cystectomy vs trimodal therapy total; inpatient and outpatient P values all <.001.
Figure 2. Costs According to Categories of Services Associated With Bladder Cancer Diagnosis Within 1 Year of Diagnosis.
Hodges-Lehmann median difference in total costs with 95% confidence intervals for trimodal therapy minus radical cystectomy. Medical services include professional services and temporary procedures. The inpatient and outpatient P values were all <.001.
The cumulative incremental effects for total Medicare payments within 1 year from the time of diagnosis are shown in Table 3. Trimodal therapy resulted in significantly increased unadjusted mean costs compared with radical cystectomy ($289 142 vs $148 757; P < .001). These findings persisted in adjusted incremental effects analyses taking into account survival ($7080; 95% CI, $3098-$11 103) and intensity ($129 854; 95% CI, $115 793-$145 299) effects of trimodal therapy vs radical cystectomy. Extrapolating adjusted mean cost differences figures to an analogous group of patients with bladder cancer in 2017 would result in an excess spending of approximately $468 million associated with trimodal therapy.
Table 3. Cumulative Incremental Effects on Total Medicare Costs Within 1 Year From Bladder Cancer Diagnosisa.
| Treatment | Total Medicare Costs, $ | |||
|---|---|---|---|---|
| Unadjusted Costs, Mean (SD) | Adjusted Incremental Effects (95% CI) | |||
| Overall | Survival | Intensity | ||
| Radical cystectomy | 190 934 (176 657) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Trimodal therapy | 323 608 (367 675) | 136 935 (122 131-152 115) | 7080 (3098-11 103) | 129 854 (115 793-145 299) |
95% Confidence intervals were estimated via nonparametric bootstrapping using 1000 samples with replacement and individuals as clusters.
Of the 2235 patients who underwent radical cystectomy, 252 (11.3%) received neoadjuvant chemotherapy (eTable 6 in the Supplement). The costs associated with neoadjuvant chemotherapy followed by radical cystectomy were significantly greater than trimodal therapy (median, $292 930 vs $289 142; H-L median, $24 897; 95% CI, $5243-$44 551).
Of 1092 patients (36.9%) with bladder cancer from 2007 to 2011, 477 (43.7%) were continuously enrolled in Medicare Part D (eTable 7 in the Supplement). Medicare Part D costs associated with trimodal therapy (123 patients [25.8%]) were significantly higher than radical cystectomy (354 patients [74.2%]; median, $2822 vs $2110; H-L median, $667; 95% CI, $219-$1114). Out-of-pocket prescription drug costs were not significantly different for trimodal therapy vs radical cystectomy (median, $400 vs $349; H-L median, $15; 95% CI, −$50 to $80).
eFigure 2 in the Supplement illustrates the adjusted overall and cancer-specific survival rates for radical cystectomy vs trimodal therapy. After IPTW, patients after trimodal therapy had significantly decreased overall survival (hazard ratio [HR], 1.54; 95% CI, 1.39-1.71) and cancer-specific survival rates (HR, 1.51; 95% CI, 1.40-1.63). Both cancer-specific and overall survival outcomes were significantly worse following trimodal therapy compared with radical cystectomy for patients with cT2 and T3 disease (eTable 8 in the Supplement). Among the subset of patients with radiotherapy fraction claims data available (707 [23.9%]), the median fractions delivered was 27 (interquartile range, 12-37). Compared with patients who underwent radical cystectomy, patients who received fewer than 27 fractions of radiotherapy had far worse overall survival rates (HR, 1.59; 95% CI, 1.41-1.79) than those receiving 27 fractions or more (HR, 1.22;, 95% CI, 1.09-1.37).
Discussion
Survival among patients with bladder cancer decreases exponentially once the tumor invades the bladder muscle.2 Approximately half of patients with muscle-invasive bladder cancer undergo definitive treatment, which includes surgery for fewer than 20%.17,30 Bladder-sparing therapies, including trimodal therapy, are increasingly used despite the lack of a randomized clinical trial–based evidence and conflicting data from observational studies.2,4,5,9 Assessing the costs of cancer care has become particularly relevant in recent years because of the rapidly increasing costs of cancer treatment.11,31,32 In this study, we observed significantly increased total costs associated with trimodal therapy, which would result in an excess spending of $468 million compared with radical cystectomy for patients who received a diagnosis of bladder cancer in 2017. Moreover, we found that patients who underwent trimodal therapy vs radical cystectomy had worse survival rates.
Our study revealed several findings. First, trimodal therapy was significantly associated with higher 90-day, 180-day, and 365-day costs. These findings persisted across all disease stages, highlighting the substantial costs associated with bladder cancer care. Moreover, while radical cystectomy had higher inpatient costs, we observed that the high attributable outpatient costs associated with trimodal therapy were the main driver for the total cost difference observed between the treatment approaches. Specifically, the most frequent (and substantial) categories associated with increased costs for trimodal therapy were radiology procedures and medication expenses. Conversely, increased costs associated with radical cystectomy preceded by neoadjuvant chemotherapy rather than trimodal therapy were attributed to hospitalization and medication expenses.
Second, given the current health care climate and the growing emphasis on value-based cancer care, our findings of varied costs of care while accounting for survival are timely in the context of health care spending. The cost of cancer care is expected to increase from $158 billion to $174 billion from 2010 to 2020, largely driven by increasing cancer incidence, improved survival outcomes, costlier treatments, and an expanding health care market.33 Economic models have estimated that cost of care for bladder cancer will increase from $4 billion in 2010 to nearly $6 billion in 2020.33 Treatment strategies that yield improved clinical outcomes at a lower cost are crucial in containing the rising cost of cancer care.33 While current health economic approaches assign a higher value to cost-effectiveness analyses, our cost calculations might assist health care clinicians and patients to select more effective and less costly approaches when feasible.34 The Institute of Medicine has advocated for limiting the use of advanced technology modalities (eg, robotic surgery, radiotherapy, and advanced imaging) given the lack of proven benefits in an effort to reduce unnecessary expenditures in health care.31 Moreover, there is growing concern regarding the challenges of costly anticancer drugs and mechanisms being explored to help control rising costs.35,36,37 While parity laws in more than 43 states did not markedly aggravate national health care spending, out-of-pocket costs for oral anticancer drugs continue to rise.37 Medicare covers approximately two-thirds of patients with cancer with regulations regarding pricing decisions. Our findings of increased costs attributed to medication expenses and outpatient services among Medicare beneficiaries highlight the further need for cost containment.
Third, trimodal therapy was associated with increased costs after considering survival and intensity effects. Health care costs and utilization analyses have the inherent limitation of censoring bias, with an inability to observe all patients until the end treatment or death.15 Propensity-score matching and IPTW attempt to match patients according to known confounders; however, these methods cannot by themselves distinguish between the association of covariates with survival and the intensity of use that jointly determine costs.15 Using robust statistical methods and considering survival and intensity effects, we observed an increased mean first-year cost of $136 935 (95% CI, $122 131-$152 115) per patient treated with trimodal therapy than radical cystectomy.
Limitations
Our findings must be considered within the context of the study design. First, patients enrolled in this study were age 66 to 85 years old. While most patients with bladder cancer receive their diagnosis in their 60s or later, our findings may not be generalizable to younger patients. Second, we did not capture clinician and hospital-level data and cannot comment on observed differences according to these predictors. We used previously accepted methods to capture total Medicare costs and cannot determine costs directly associated with bladder cancer using administrative claims data.11 Third, while we attempted to control for the inherent selection bias and other confounders in this retrospective study using an IPTW-weighted model, we acknowledge the limitations in using such a study design, such as unmeasured confounding. Fourth, we used IPTW while considering informative censoring of the cost outcome as well as the incremental effects of survival and intensity of treatment. Despite using these methods with descriptive information of attributed cost categories, this article compares costs and is not a cost-effectiveness study, which would incorporate quality-of-life years gained. Moreover, with increased adoption of robotic surgery, our findings may underestimate costs of surgery in the modern era. Finally, we added costs up to 365 days from diagnosis date and further long-term “global cost of care” assessments are needed.38
Conclusions
In a population-based cohort, trimodal therapy was associated with increased costs and decreased survival compared with radical cystectomy. Differences in costs were largely attributed to medication and radiology expenses that were associated with trimodal therapy; when considering the effects of survival and intensity of use, there was a mean excess spending of $136 935 per patient per year for trimodal therapy compared with radical cystectomy. Extrapolating this mean cost difference to the total US population resulted in an excess spending of $468 million for trimodal therapy compared with radical cystectomy for patients who received a diagnosis in 2017. Patients who underwent radical cystectomy who received neoadjuvant chemotherapy had increased costs compared with trimodal therapy. In addition to cost containment for cancer care, and in particular anticancer medications, these findings suggest the need for improved survival and quality-of-life years gained to justify using trimodal therapy to manage bladder cancer.
eFigure 1. Median Cumulative Medicare Costs within One Year of Bladder Cancer Diagnosis
eFigure 2. Adjusted Kaplan-Meier Curves of Overall (A) and Cancer-Specific (B) Survival According to Treatment
eTable 1. International Classification of Diseases–Version 9 (ICD-9) and Common Procedural Terminology (CPT) Codes Used to Identify Patients with Bladder Cancer
eTable 2. Mean ±SD Medicare Costs (USD) Associated with Radical Cystectomy and Trimodal Therapy Following Bladder Cancer Diagnosis
eTable 3. Calculation of One-Year Medicare Costs (USD) Using the Medical Component of Consumer Price Index and Gross Domestic Product Implicit Price Deflator Methods
eTable 4. Median Medicare Costs (USD) within One-Year from Clinical Diagnosis According to Clinical Stage
eTable 5. Costs (USD) According to Categories of Clinical Services Associated with Bladder Cancer Diagnosis within One-Year of Diagnosis
eTable 6. One-Year Medicare Costs (USD) among Patients who Received Neoadjuvant Chemotherapy and Radical Cystectomy versus Trimodal Therapy
eTable 7. One-Year Medicare Costs (USD) among Patients Continuously Enrolled in Medicare Part A + B, Medicare Part D, and Out-of-Pocket Prescription Drugs (Part of Medicare Part D)
eTable 8. Proportional Hazards Regression Model of Predictors for Overall and Cancer-Specific Survival According to Clinical Stage and Total number of radiotherapy fractions received
References
- 1.Alfred Witjes J, Lebret T, Compérat EM, et al. . Updated 2016 EAU Guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):-. doi: 10.1016/j.eururo.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 2.Clark PE, Agarwal N, Biagioli MC, et al. ; National Comprehensive Cancer Network (NCCN) . Bladder cancer. J Natl Compr Canc Netw. 2013;11(4):446-475. doi: 10.6004/jnccn.2013.0059 [DOI] [PubMed] [Google Scholar]
- 3.Chang SS, Bochner BH, Chou R, et al. . Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol. 2017;198(3):552-559. doi: 10.1016/j.juro.2017.04.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gakis G, Efstathiou J, Lerner SP, et al. ; International Consultation on Urologic Disease-European Association of Urology Consultation on Bladder Cancer 2012 . ICUD-EAU International Consultation on Bladder Cancer 2012: radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63(1):45-57. doi: 10.1016/j.eururo.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 5.Mitin T, George A, Zietman AL, et al. . Long-term outcomes among patients who achieve complete or near-complete responses after the induction phase of bladder-preserving combined-modality therapy for muscle-invasive bladder cancer: a pooled analysis of NRG oncology/RTOG 9906 and 0233. Int J Radiat Oncol Biol Phys. 2016;94(1):67-74. doi: 10.1016/j.ijrobp.2015.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gofrit ON, Nof R, Meirovitz A, et al. . Radical cystectomy vs. chemoradiation in T2-4aN0M0 bladder cancer: a case-control study. Urol Oncol. 2015;33(1):19.e1-19.e5. doi: 10.1016/j.urolonc.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni GS, Hermanns T, Wei Y, et al. . Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol. 2017;35(20):2299-2305. doi: 10.1200/JCO.2016.69.2327 [DOI] [PubMed] [Google Scholar]
- 8.Williams SB, Shan Y, Jazzar U, et al. . Comparing survival outcomes and costs associated with radical cystectomy and trimodal therapy for older adults with muscle-invasive bladder cancer. JAMA Surg. 2018;153(10):881-889. doi: 10.1001/jamasurg.2018.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahn DB, Handorf EA, Ghiraldi EM, et al. . Contemporary use trends and survival outcomes in patients undergoing radical cystectomy or bladder-preservation therapy for muscle-invasive bladder cancer. Cancer. 2017;123(22):4337-4345. doi: 10.1002/cncr.30900 [DOI] [PubMed] [Google Scholar]
- 10.Seisen T, Sun M, Lipsitz SR, et al. . Comparative effectiveness of trimodal therapy versus radical cystectomy for localized muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2017;72(4):483-487. doi: 10.1016/j.eururo.2017.03.038 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen PL, Gu X, Lipsitz SR, et al. . Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29(12):1517-1524. doi: 10.1200/JCO.2010.31.1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Accordino MK, Wright JD, Vasan S, et al. . Use and costs of disease monitoring in women with metastatic breast cancer. J Clin Oncol. 2016;34(24):2820-2826. doi: 10.1200/JCO.2016.66.6313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leow JJ, Cole AP, Seisen T, et al. . Variations in the costs of radical cystectomy for bladder cancer in the USA. Eur Urol. 2017;S0302-2838(17)30640-1. [DOI] [PubMed] [Google Scholar]
- 14.Hu JC, Chughtai B, O’Malley P, et al. . Perioperative outcomes, health care costs, and survival after robotic-assisted versus open radical cystectomy: a national comparative effectiveness study. Eur Urol. 2016;70(1):195-202. doi: 10.1016/j.eururo.2016.03.028 [DOI] [PubMed] [Google Scholar]
- 15.Basu A, Manning WG. Estimating lifetime or episode-of-illness costs under censoring. Health Econ. 2010;19(9):1010-1028. doi: 10.1002/hec.1640 [DOI] [PubMed] [Google Scholar]
- 16.Weir HK, Johnson CJ, Mariotto AB, et al. . Evaluation of North American Association of Central Cancer Registries’ (NAACCR) data for use in population-based cancer survival studies. J Natl Cancer Inst Monogr. 2014;2014(49):198-209. doi: 10.1093/jncimonographs/lgu018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams SB, Huo J, Chamie K, et al. . Underutilization of radical cystectomy among patients diagnosed with clinical stage T2 muscle-invasive bladder cancer. Eur Urol Focus. 2017;3(2-3):258-264. doi: 10.1016/j.euf.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 18.Shirvani SM, Jiang J, Gomez DR, Chang JY, Buchholz TA, Smith BD. Intensity modulated radiotherapy for stage III non-small cell lung cancer in the United States: predictors of use and association with toxicities. Lung Cancer. 2013;82(2):252-259. doi: 10.1016/j.lungcan.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Health Cost Institute 2016 Health care cost and utilization report: analytic methodology. https://www.healthcostinstitute.org/images/pdfs/HCCI_2016_Methodology_v1.0_1.23.18.pdf. Accessed November 26, 2018.
- 20.US Census Bureau Zip code census file for SEER-Medicare patients. https://healthcaredelivery.cancer.gov/seer-cahps/medicare/zipcode.census.file.pdf. Accessed November 19, 2018.
- 21.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258-1267. doi: 10.1016/S0895-4356(00)00256-0 [DOI] [PubMed] [Google Scholar]
- 22.Avritscher EB, Cooksley CD, Grossman HB, et al. . Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006;68(3):549-553. doi: 10.1016/j.urology.2006.03.062 [DOI] [PubMed] [Google Scholar]
- 23.Svatek RS, Hollenbeck BK, Holmäng S, et al. . The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol. 2014;66(2):253-262. doi: 10.1016/j.eururo.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 24.Kondalsamy-Chennakesavan S, Bouman C, De Jong S, et al. . Clinical audit in gynecological cancer surgery: development of a risk scoring system to predict adverse events. Gynecol Oncol. 2009;115(3):329-333. doi: 10.1016/j.ygyno.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 25.Cipriano LE, Romanus D, Earle CC, et al. . Lung cancer treatment costs, including patient responsibility, by disease stage and treatment modality, 1992 to 2003. Value Health. 2011;14(1):41-52. doi: 10.1016/j.jval.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang D, Dalton JE A unified approach to measuring the effect size between two groups using SAS. http://support.sas.com/resources/papers/proceedings12/335-2012.pdf. Accessed November 26, 2018.
- 28.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 29.Noone AMHN, Krapcho M, Miller D, et al. SEER Cancer Statistics Review, 1975-2015. https://seer.cancer.gov/csr/1975_2015/. Accessed June 20, 2018.
- 30.Gore JL, Litwin MS, Lai J, et al. ; Urologic Diseases in America Project . Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst. 2010;102(11):802-811. doi: 10.1093/jnci/djq121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith GL, Ganz PA, Bekelman JE, et al. . Promoting the appropriate use of advanced radiation technologies in oncology: summary of a national cancer policy forum workshop. Int J Radiat Oncol Biol Phys. 2017;97(3):450-461. doi: 10.1016/j.ijrobp.2016.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocque G, Blayney DW, Jahanzeb M, et al. . Choosing wisely in oncology: are we ready for value-based care? J Oncol Pract. 2017;13(11):e935-e943. doi: 10.1200/JOP.2016.019281 [DOI] [PubMed] [Google Scholar]
- 33.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128. doi: 10.1093/jnci/djq495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnipper LE, Davidson NE, Wollins DS, et al. . Updating the American Society of Clinical Oncology Value Framework: revisions and reflections in response to comments received. J Clin Oncol. 2016;34(24):2925-2934. doi: 10.1200/JCO.2016.68.2518 [DOI] [PubMed] [Google Scholar]
- 35.Lichter AS. From choosing wisely to using wisely: increasing the value of cancer care through clinical research. J Clin Oncol. 2018;36(14):1387-1388. doi: 10.1200/JCO.2018.78.4264 [DOI] [PubMed] [Google Scholar]
- 36.Gross CP, Gluck AR. Soaring cost of cancer treatment: moving beyond sticker shock. J Clin Oncol. 2018;36(4):305-307. doi: 10.1200/JCO.2017.76.0488 [DOI] [PubMed] [Google Scholar]
- 37.Dusetzina SB, Huskamp HA, Winn AN, Basch E, Keating NL. Out-of-pocket and health care spending changes for patients using orally administered anticancer therapy after adoption of state parity laws. JAMA Oncol. 2018;4(6):e173598. doi: 10.1001/jamaoncol.2017.3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porter ME, Lee TH. From volume to value in health care: the work begins. JAMA. 2016;316(10):1047-1048. doi: 10.1001/jama.2016.11698 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Median Cumulative Medicare Costs within One Year of Bladder Cancer Diagnosis
eFigure 2. Adjusted Kaplan-Meier Curves of Overall (A) and Cancer-Specific (B) Survival According to Treatment
eTable 1. International Classification of Diseases–Version 9 (ICD-9) and Common Procedural Terminology (CPT) Codes Used to Identify Patients with Bladder Cancer
eTable 2. Mean ±SD Medicare Costs (USD) Associated with Radical Cystectomy and Trimodal Therapy Following Bladder Cancer Diagnosis
eTable 3. Calculation of One-Year Medicare Costs (USD) Using the Medical Component of Consumer Price Index and Gross Domestic Product Implicit Price Deflator Methods
eTable 4. Median Medicare Costs (USD) within One-Year from Clinical Diagnosis According to Clinical Stage
eTable 5. Costs (USD) According to Categories of Clinical Services Associated with Bladder Cancer Diagnosis within One-Year of Diagnosis
eTable 6. One-Year Medicare Costs (USD) among Patients who Received Neoadjuvant Chemotherapy and Radical Cystectomy versus Trimodal Therapy
eTable 7. One-Year Medicare Costs (USD) among Patients Continuously Enrolled in Medicare Part A + B, Medicare Part D, and Out-of-Pocket Prescription Drugs (Part of Medicare Part D)
eTable 8. Proportional Hazards Regression Model of Predictors for Overall and Cancer-Specific Survival According to Clinical Stage and Total number of radiotherapy fractions received