Abstract
The potential adverse effects of transfusion of red blood cells after prolonged storage have been hotly debated. During refrigerated storage, red blood cells are damaged, a process known as the red blood cell “storage lesion.” We hypothesized that the delivery of a bolus of iron derived from these rapidly cleared, damaged, red blood cells is responsible for some of the adverse effects of transfusion. Iron may play a role in producing a pro-inflammatory response to transfused red blood cells, potentially through the effects of reactive oxygen species on stress pathways and inflammasome activation. Furthermore, the excess iron may impair the host’s ability to combat infection by its innate iron-withholding pathways. This symposium paper summarizes the background for the “iron hypothesis” as it relates to transfusion of red blood cells after prolonged refrigerated storage. It also includes a summary of the data from recent murine and human studies, and concludes with a discussion of several unresolved questions arising from these published studies.
Keywords: Red blood cell transfusion, Iron, Cytokines, Inflammation, Infection
Résumé
Le potentiel effet délétère de la transfusion de globules rouges après une conservation prolongée reste un sujet de débat. Durant la conservation réfrigérée, les globules rouges subissent des lésions de stockage. Nous avons posé l’hypothèse que la délivrance d’un bolus de fer, dérivé de ces globules rouges conservés et rapidement éliminés de la circulation, peut être responsable de certaines réactions indésirables imputées à la transfusion. Le fer peut jouer un rôle en induisant une réponse pro-inflammatoire, potentiellement, via les effets des espèces réactives de l’oxygène sur les voies de stress et sur l’activation des facteurs d’inflammation. De plus, un excès de fer peut diminuer la capacité de l’hôte à se défendre contre les agents infectieux par les voies innées de la prise en charge du fer. Ce manuscrit résume l’état de l’art de « l’hypothèse fer », en relation avec la transfusion de globules rouges conservés de façon prolongée dans des conditions réfrigérées. Il inclut aussi un résumé des données récentes sur le sujet, obtenues chez l’homme et sur des modèles murins, pour conclure avec une discussion sur un certain nombre de questions soulevées par ces études et non résolues à ce jour.
Keywords: Transfusion de globules rouges, Fer
1. Introduction
One “holy grail” of the practice of transfusion medicine is to manufacture and prescribe blood products as if they were pharmaceuticals. This goal entails a high degree of reproducibility, quality control, and robustness in the manufacture of the blood products, and clear medical indications and dosage requirements for their use. One clear success in this regard is the evolution in the treatment of patients with hemophilia A, progressing over the last 60 years from plasma to cryoprecipitate, to purified pooled human factor VIII, to the present use of recombinant factor VIII. Indeed, in many institutions, the latter product is maintained and distributed by the pharmacy rather than by the blood bank.
Unfortunately, to date, there has been a general lack of success in the production of a safe, pharmaceutical-like, red blood cell substitute (e.g. cross-linked human hemoglobin [1]). Nonetheless, major advances in infectious disease testing markedly reduced the risks for transfusion-transmitted infections, such as those due to hepatitis C and human immunodeficiency virus. In addition, recently, there has been renewed interest in the stability of red blood cells during refrigerated storage, and in their transfusion efficacy and safety after prolonged storage (i.e. up to 42 days). In fact, multiple retrospective studies suggest that, as compared with the transfusion of “fresh” red blood cells (e.g. less than 14 days of storage), the transfusion of “old” red blood cells (e.g. greater than 28 days of storage) is associated with multiple complications, including increases in sepsis, pneumonia, multi-organ failure, myocardial infarction, acute renal failure, thrombosis, and mortality [2]. These important studies have significant flaws [3], including the absence of a plausible pathophysiological mechanism, and their conclusions are subject to much controversy. In addition, the actual findings are often quite subtle. As one example, in the most widely discussed study, although the “in-house mortality” almost doubles in patients receiving transfusions of “old” red blood cells, this statistically-significant increase is only from 1.7 to 2.8% [4]. Thus, any proposed mechanism(s) must be able to explain these relatively subtle changes in the incidence of these types of complications. Finally, multiple randomized prospective trials are now underway to address this issue (e.g. the Red Cell Storage Duration Study [RECESS], the Age of Blood Evaluation [ABLE] study, and the Age of Red Blood Cells in Premature Infants [ARIPI] study) [5–7]. Although these are not necessarily designed to test particular pathophysiological mechanisms, it is anticipated that they will provide very useful information.
There is general agreement that, if there are negative consequences deriving from the transfusion of older, stored red blood cells, then these result from the red blood cell “storage lesion.” The latter has been studied extensively and comprises many changes to the red blood cell during storage, including changes in red blood cell shape accompanied by vesiculation, membrane loss, and lysis, decreases in cellular levels of 2,3-diphosphoglycerate, adenosine triphosphate, reduced glutathione, and nitric oxide, decreased membrane expression of CD47, and increased oxidation of cellular lipids and proteins [8]. These cellular changes also lead to increases in extracellular hemoglobin, heme, potassium, and lactate dehydrogenase, and decreases in extracellular pH [9]. The clearest consequences of the storage lesion are hemolysis ex vivo in the storage bag and decreased 24-hour red blood cell recovery in vivo post-transfusion [10]. Indeed, in the United States, these particular consequences are included in the regulations of the Food and Drug Administration, which mandate that, at outdate, red blood cell units must exhibit less than 1% hemolysis in the storage bag and lead to greater than 75% recovery of the stored red blood cells at 24 hours post-transfusion [10]. Interestingly, even in healthy recipients, the 24-hour post-transfusion recovery is often significantly less than 75% and this can even be worse in ill patients [10,11].
Although other investigators are studying the consequences of infusing free hemoglobin, microparticles, CD40 ligand, and other soluble components in the supernatant [12–18], our group focuses on the 25% or less of transfused red blood cells that are cleared from the circulation in the first 24 hours; indeed the vast majority of these effete red blood cells are cleared in the first 1–2 hours post-transfusion [11]. Because these red blood cells are believed to be primarily cleared by extravascular hemolysis, we developed the “iron hypothesis” to explain, at least some of, the adverse consequences resulting from transfusion of “old” red blood cells; that is:
acute delivery of a bolus of hemoglobin-derived iron to the mononuclear phagocyte system by clearance of a subpopulation of stored red blood cells is responsible for the harmful effects of transfusion (e.g. infection).
This hypothesis is based, in part, on calculations demonstrating that at steady state, a healthy adult human clears approximately 1 mL of (senescent) red blood cells each hour, which delivers approximately 1 mg of iron to the mononuclear phagocyte system. In contrast, if 25% of the cells in one unit of packed red blood cells were cleared in one hour, this would deliver approximately 60 mg of iron to the mononuclear phagocyte system, a 60-fold increase. We believe that this would overwhelm the cell’s iron-handling ability, leading to increased intracellular iron and the production of circulating non-transferrin-bound iron (Fig. 1). These massive changes in intracellular and extracellular iron levels suggest that iron is a central, critical mediator that can affect innate immunity and inflammation (Fig. 2), nutritional immunity (Table 1), and infection. Each of these will be briefly discussed below.
Fig. 1.
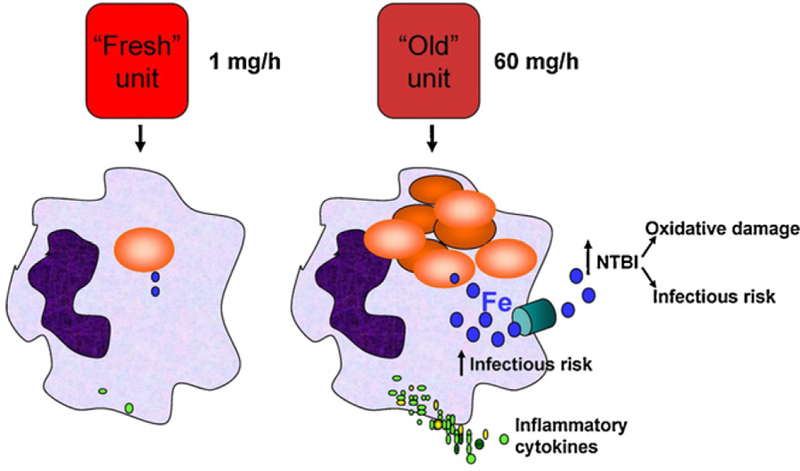
Iron hypothesis. The clearance of transfused “fresh” red blood cells should not differ from the clearance of endogenous, naturally-senescent red blood cells, leading to the clearance of approximately 1 mg of iron per hour. In contrast, if 25% of one unit of older, stored red blood cells are cleared by extravascular hemolysis in one hour, then approximately 60 mg of iron are cleared in that time. The diagram shows two macrophages, the one on the left has ingested one red blood cell; the one on the right has ingested multiple red blood cells. The iron released from hemoglobin is represented by blue-filled circles, intracellularly synthesized and secreted cytokines are represented by green-filled circles, the turquoise cylinder in the macrophage on the right represents ferroportin, the iron export channel. NTBI: non-transferrin-bound iron.
Fig. 2.
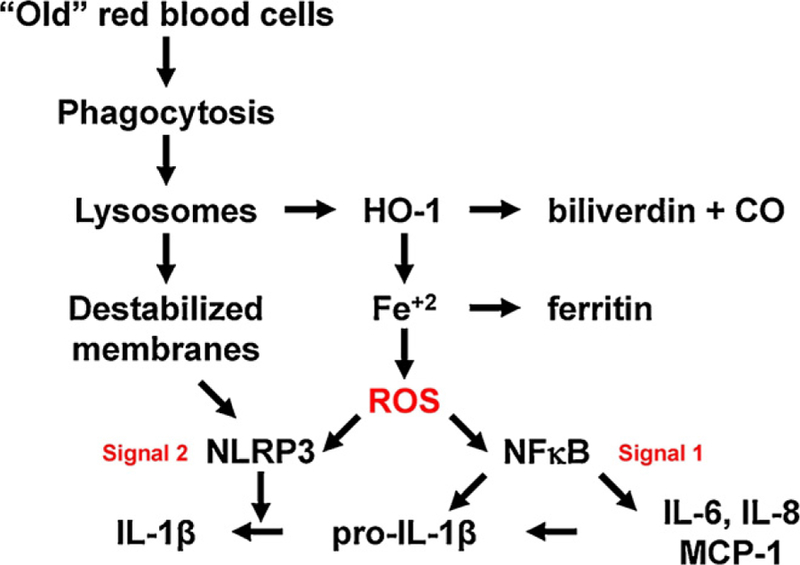
Iron, inflammasomes, and inflammation. This hypothetical model suggests a mechanism by which macrophage ingestion of an excess of red blood cells could activate NFκB, and also provide both signal 1 and signal 2 for inflammasome activation, thereby producing a pro-inflammatory cytokine response. HO-1: heme oxygenase-1; CO: carbon monoxide; ROS: reactive oxygen species; NLRP: nucleotide-binding oligomerization domain, leucine rich repeat, and pyrin domain containing proteins; IL: interleukin; MCP-1: monocyte chemoattractant protein-1.
Table 1.
Molecules and mechanisms involved in iron-related nutritional immunity.
| Host (e.g. humans) | Pathogen (e.g. bacteria or fungi) |
|---|---|
| Transferrin | Transferrin receptor |
| Lactoferrin | Non-transferrin-bound iron uptake mechanisms |
| NGAL | Heme uptake mechanisms |
| Haptoglobin | Siderophores |
| Hemopexin Ferritin | |
| NRAMP1 (intracellular iron transporter) | |
| Ferroportin (cell membrane iron transporter) | |
| Hepcidin (iron regulatory hormone) | |
| Hemopexin Ferritin |
NGAL: neutrophil gelatinase-associated lipocalin.
Research on the innate immune system, particularly with regard to membrane-bound Toll-like receptors (TLR) and cytosolic inflammasomes, has exploded in the last decade (for review, see [19,20]). This has provided new insights into the mechanisms involved in protection against foreign invaders. For example, much progress has been made in describing various molecular patterns that are associated with particular classes of microbial pathogens. Perhaps the greatest mechanistic understanding involves the interaction between one particular pathogen-associated molecular pattern, lipopolysaccharides, found on the surface of invading Gram-negative bacteria, and the TLR4 pattern-recognition receptor on the surface of host cells; this interaction orchestrates a broad array of responses aimed at protecting the host from infection.
Interestingly, these recent studies have also begun identifying the role that innate immunity plays in the host response to internal damage due to non-infectious causes. These gave rise to the concept of damage-associated molecular patterns that activate innate immunity; these include compounds such as adenosine triphosphate, cholesterol crystals, and urate crystals, the recognition of which can coordinate the host response in diverse disorders such as acetaminophen toxicity, atherosclerosis, and gout, respectively [19]. In this context, the cellular machinery coordinating the response to damage-associated molecular patterns includes the inflammasome, a cytosolic “machine” that induces inflammation, primarily through the enzymatic conversion of pro-interleukin 1-beta (pro-IL-1β) into IL-1β, with subsequent non-classical secretion of this pro-inflammatory cytokine. Presumably to prevent over-exuberant, inappropriate inflammatory responses, activation of the inflammasome pathway requires two signals [19]. Signal 1 (e.g. provided by lipopolysaccharide/TLR4-induced signal transduction) increases synthesis of pro-IL-1β and inflammasome components, such as members of the family of NLRP (nucleotide-binding oligomerization domain, leucine rich repeat, and pyrin domain containing proteins). Signal 2 induces assembly of the inflammasome complex, which includes multiple copies of a particular NLRP (e.g. NLRP3). The identity of the molecule(s) in signal 2 providing the final common pathway for inflammasome assembly is controversial, but multiple studies suggest that reactive oxygen species can serve this function [19]. This information suggests a model in which massive erythrophagocytosis of older, stored red blood cells could produce inflammation (Fig. 2); in this case, excess “free” intracellular iron produces reactive oxygen species by the Fenton reaction, which can potentially provide both signal 1 and signal 2.
“Nutritional immunity” can be considered a part of the innate immune system. With regard to iron, nutritional immunity refers to the process by which the host prevents invading pathogens from acquiring this nutrient, which is required for the growth and survival of virtually all living organisms. Evolution provided humans with multiple molecules to modify circulating iron levels and to sequester iron and iron-containing molecules (e.g. hemoglobin and heme) in tissues and fluids (Table 1). Interestingly, evolution has also endowed pathogens with multiple molecules and mechanisms for acquiring iron from the host (Table 1). This produces an ever-escalating contest between invading pathogens and the host. For example, bacteria and fungi can use siderophores (i.e. small molecules that chelate iron), which are then imported into the pathogen. To combat this mechanism, the host produces neutrophil gelatinase-associated lipocalin (NGAL), which can bind iron-containing siderophores, thereby preventing iron acquisition by the pathogen [21]. Some pathogens can then modify the siderophore (e.g. by glucosylation), which when iron-bound, can no longer be recognized by NGAL [22].
As a result of breakdown, or defects, in nutritional immunity, the host may be more susceptible to infection by “ferrophilic” pathogens. As examples, Mycobacterium tuberculosis and Salmonella typhimurium are primarily intracellular pathogens; their survival and proliferation in macrophages is greatly accelerated by iron overload [23]. Another example relevant to transfusion medicine involves Yersinia enterocolitica, which thrives in refrigerated packed red blood cells because it grows well at low temperatures and thrives in the presence of iron [24].
2. Mouse model of refrigerated storage of red blood cells
To study the adverse consequences of transfusion of older, stored red blood cells and to test the “iron hypothesis,” we developed a mouse model of refrigerated storage of leukoreduced murine red blood cells [25]. In this model, the 24-hour post-transfusion recovery of red blood cells stored for 14 days is approximately 75% with most clearance occurring in the first 1–2 hours (Table 2). In addition, using macrophage depletion by liposomal clodronate, we demonstrated that the effete red blood cells were cleared by extravascular hemolysis by the mononuclear phagocyte system. The rapid clearance of the older, stored red blood cells induced a pro-inflammatory cytokine response characterized by increases in circulating levels multiple cytokines, including IL-1β (unpublished observations), IL-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1) [26]. This inflammatory response synergized with that induced by infusing sub-clinical amounts of lipopolysaccharides to produce clinical illness. In addition, rapid clearance of the older, stored red blood cells led to the production of non-transferrin-bound iron, which correlated with enhanced proliferation in serum in vitro of ferrophilic bacteria [26]. Importantly, these effects on inflammation and iron metabolism only resulted from the acute delivery of a bolus of hemoglobin-iron to the mononuclear phagocyte system by the clearance of intact red blood cells; these effects were not seen after infusion of equivalent amounts of hemolysate, red blood cell membranes (i.e. “ghosts”), or both. Transfusion of older, stored red blood cells also had two additional clinical consequences in vivo:
explosive and fatal proliferation of S. typhimurium in a mouse model of self-limited disease with this pathogen [27];
increased alloimmunization to a foreign blood group antigen expressed on the transfused red blood cells [28].
Table 2.
Comparison of results of transfusions of older, stored red blood cells in the mouse model and with humans.
| Mice | Humans | |
|---|---|---|
| Extravascular hemolysis | Yes | Yes |
| Non-transferrin-bound iron production | Yes | Yes |
| Enhanced bacterial growth in vitro | Yes | Yes |
| Increased risk of sepsis in vivo | Yes | ???a |
| Pro-inflammatory response | Yes | Nob |
| Synergistic inflammation with underlying clinical disorder | Yes | ???c |
| Enhanced alloimmunization to blood group antigens | Yes | ??? |
Although retrospective studies suggest an increased incidence of sepsis in patients transfused older, stored red blood cells [4], this was not seen in a prospective study of healthy human volunteers [29], nor has this been formally tested in randomized, prospective clinical trials with human patients.
Based on transfusion of a single unit of packed red blood cells over two hours to healthy volunteers [29].
Although recent results suggest that pediatric cardiac surgery patients receiving red blood cell transfusions may have increased circulating levels of pro-inflammatory cytokines [30], this has not been correlated with the length of the storage interval.
3. Studies with healthy human volunteers
In parallel with the mouse model studies described above, we embarked on studies with healthy human volunteers to test the “iron hypothesis” [29]. To this end, 14 healthy adults donated the equivalent of two pre-storage leukoreduced red blood cell units by an apheresis method; they were then transfused with one “fresh” autologous unit (i.e. on day #3–7) and, subsequently, with one “old” autologous unit (i.e. on day #40–42). No adverse events, no changes in vital signs, and no transfusion reactions were seen. In contrast, transfusions with old, but not fresh, red blood cells led to statistically-significant increases in circulating levels of total and direct bilirubin, but no changes in circulating levels of haptoglobin or lactate dehydrogenase; these findings are compatible with extravascular hemolysis of a subpopulation of transfused, storage-damaged red blood cells. In addition, transfusions with old, but not fresh, red blood cells led to statistically-significant increased circulating levels of iron, transferrin saturation, non-transferrin-bound iron, and ferritin; this was accompanied by increased serum proliferation in vitro by ferrophilic bacteria. These results are very similar to those found in the mouse model. However, in contrast to the murine studies, no evidence of inflammation was seen in these healthy adults after transfusion of fresh or old red blood cells, even though multiple pro-inflammatory markers were measured.
4. Unresolved questions
Results with the mouse model and the studies with human volunteers were very similar regarding the occurrence of extravascular hemolysis and the effects on iron metabolism. However, the transfusion-induced inflammatory response seen in mice was not found in healthy adults. Several possible explanations for this difference are described, as follows:
the biology of the response to old red blood cell transfusions may simply differ in humans and rodents;
transfusion-induced inflammation may be subject to a dose response effect. Indeed, when studied in mice, a “two-unit” stored red blood cell transfusion induced a significantly greater pro-inflammatory cytokine response than a “one-unit” transfusion [26]. In contrast, each of the 14 healthy human volunteers was transfused with only one unit of older, stored red blood cells;
the extent of the transfusion-induced inflammatory response may vary based on the transfusion rate and the subsequent red blood cell clearance rate. In the published murine studies, the transfusions were performed by “IV push” and were complete within seconds [26]; in contrast, the human volunteers were transfused over the course of two hours [29];
the clinical status of the host may matter. Although post-transfusion inflammation was seen in healthy mice, but not in healthy human adults, this inflammation was greatly enhanced in mice infused with sub-clinical amounts of lipopolysaccharides. Thus, it is possible that post-transfusion inflammation will be seen in humans with particular clinical conditions. For example, preliminary results suggest that red blood cell transfusions may increase circulating IL-6 levels in children undergoing cardiac surgery [30].
In summary, the occurrence of transfusion-induced inflammation in humans may require “A lot of units in a susceptible host” (M. Gladwin, personal communication).
5. Conclusions and future directions
In conclusion, transfusions of older, stored red blood cells into mice produce adverse effects that are consistent with the “iron hypothesis.” That is, the acute clearance of a bolus of stored red blood cells induces an acute inflammatory response and a change in iron metabolism. These predispose the host to exacerbations of bacterial infection and enhanced alloimmunization to a red blood cell-encoded blood group antigen. In healthy human volunteers, transfusions of entire units of old, but not fresh, red blood cells lead to significant extravascular hemolysis, demonstrable by routine laboratory tests, and changes in iron metabolism similar to those found in the murine model. Although this may increase the risk of pathogen colonization and/or infection, no adverse effects were seen in any volunteer. In addition, in contrast to the mouse model, no evidence of post-transfusion inflammation was seen in the healthy human volunteers.
Future studies aimed at understanding whether or not transfusion-induced inflammation can occur in humans will focus on potentially susceptible patients, such as those with hemoglobinopathies (i.e. sickle cell anemia and beta-thalassemia) and acutely-ill pediatric intensive care unit patients. In addition, the potential adverse consequences of non-transferrin-bound iron production (e.g. sepsis, endothelial dysfunction with thrombosis) will be evaluated in these populations.
Acknowledgements
The authors would like to thank Dr. James Zimring and Dr. Jeanne Hendrickson of Emory University, and the members of the Laboratory of Transfusion Biology and the Division of Laboratory Medicine at Columbia University, for their efforts in obtaining the described experimental results and for advice on this topic. This work was supported in part by grants from the National Institutes of Health (R01-HL098014, U01-HD064827, K08-HL103756, UL1-RR024156), the National Blood Foundation, and a Louis V. Gerstner Scholars Award.
Footnotes
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
References
- [1].Creteur J, Vincent JL. Hemoglobin solutions. Crit Care Med 2003;31:S698–707. [DOI] [PubMed] [Google Scholar]
- [2].Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion 2011. [doi: 10.1111/j.1537-2995.2011.03466.x]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dzik W Fresh blood for everyone? Balancing availability and quality of stored RBCs. Transfus Med 2008;18:260–5. [DOI] [PubMed] [Google Scholar]
- [4].Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, et al. Duration of red-cell storage and complications after cardiac surgery. New Engl J Med 2008;358:1229–39. [DOI] [PubMed] [Google Scholar]
- [5].Steiner ME, Assmann SF, Levy JH, Marshall J, Pulkrabek S, Sloan SR, et al. Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7). Transfus Apher Sci 2010;43:107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lacroix J, Hebert P, Fergusson D, Tinmouth A, Blajchman MA, Callum J, et al. The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Transfus Med Rev 2011;25:197–205. [DOI] [PubMed] [Google Scholar]
- [7].Fergusson D, Hutton B, Hogan DL, LeBel L, Blajchman MA, Ford JC, et al. The age of red blood cells in premature infants (ARIPI) randomized controlled trial: study design. Transfus Med Rev 2009;23: 55–61. [DOI] [PubMed] [Google Scholar]
- [8].Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion 2006;46: 2014–27. [DOI] [PubMed] [Google Scholar]
- [9].Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA 2007;104:17063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion 2008;48:1053–60. [DOI] [PubMed] [Google Scholar]
- [11].Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, de Grip WJ, Bos HJ, Bosman GJ. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion 2008;48:1478–85. [DOI] [PubMed] [Google Scholar]
- [12].Spinella PC, Sparrow RL, Hess JR, Norris PJ. Properties of stored red blood cells: understanding immune and vascular reactivity. Transfusion 2011;51:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jy W, Ricci M, Shariatmadar S, Gomez-Marin O, Horstman LH, Ahn YS. Microparticles in stored red blood cells as potential mediators of transfusion complications. Transfusion 2011;51:886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roback JD, Neuman RB, Quyyumi A, Sutliff R. Insufficient nitric oxide bioavailability: a hypothesis to explain adverse effects of red blood cell transfusion. Transfusion 2011;51:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion 2011;51:844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Grimshaw K, Sahler J, Spinelli SL, Phipps RP, Blumberg N. New frontiers in transfusion biology: identification and significance of mediators of morbidity and mortality in stored red blood cells. Transfusion 2011;51: 874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Reynolds JD, Hess DT, Stamler JS. The transfusion problem: role of aberrant S-nitrosylation. Transfusion 2011;51:852–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weinberg JA, Barnum SR, Patel RP. Red blood cell age and potentiation of transfusion-related pathology in trauma patients. Transfusion 2011;51:867–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature 2012;481:278–86. [DOI] [PubMed] [Google Scholar]
- [20].Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Bio 2011;21:R488–93. [DOI] [PubMed] [Google Scholar]
- [21].Bao G, Clifton M, Hoette TM, Mori K, Deng SX, Qiu A, et al. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat Chem Biol 2010;6:602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Valdebenito M, Muller SI, Hantke K. Special conditions allow binding of the siderophore salmochelin to siderocalin (NGAL-lipocalin). FEMS Microbiol Lett 2007;277:182–7. [DOI] [PubMed] [Google Scholar]
- [23].Weinberg ED. Iron availability and infection. Biochim Biophys Acta 2009;1790:600–5. [DOI] [PubMed] [Google Scholar]
- [24].Tipple MA, Bland LA, Murphy JJ, Arduino MJ, Panlilio AL, Farmer JJ 3rd, et al. Sepsis associated with transfusion of red cells contaminated with Yersinia enterocolitica. Transfusion 1990;30:207–13. [DOI] [PubMed] [Google Scholar]
- [25].Gilson CR, Kraus TS, Hod EA, Hendrickson JE, Spitalnik SL, Hillyer CD, et al. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion 2009;49:1546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 2010;115:4284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hod EA, Hendrickson JE, Sharma S, Francis RO, Wojczyk BS, Zimring JC, et al. Transfusion of mouse RBCs stored in AS-1 induces inflammation and exacerbates bacterial sepsis. Transfusion 2010;50:S23-010D. [Google Scholar]
- [28].Hendrickson JE, Hod EA, Spitalnik SL, Hillyer CD, Zimring JC. Storage of murine red blood cells enhances alloantibody responses to an erythroid-specific model antigen. Transfusion 2010;50:642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood 2011;118:6675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cholette JM, Henrichs KF, Alfieris GM, Powers KS, Phipps R, Spinelli SL, et al. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions: results of a prospective, randomized, controlled clinical trial. Pediatr Crit Care Med 2011. [doi: 10.1097/PCC.0b013e31822f173c]. [DOI] [PMC free article] [PubMed] [Google Scholar]


