Abstract
Purpose:
To investigate the stiffening effect of localized corneal crosslinking (L-CXL) within and beyond the irradiated region in three dimensions.
Methods:
Ten porcine eyes were debrided of epithelium and incrementally soaked with 0.1% riboflavin solution. Using a customized, sharp-edged mask, half of the cornea was blocked while the other half was exposed to blue light (447 nm). The three-dimensional biomechanical properties of each cornea were then measured via Brillouin microscopy. An imaging system was used to quantify the optimal transition zone between crosslinked and non-crosslinked sections of the cornea when considering light propagation and scattering.
Results:
A broad transition zone of 610 μm in width was observed between the fully crosslinked and non-crosslinked sections, indicating the stiffening response extended beyond the irradiated region. Light propagation and the scattering induced by the riboflavin-soaked cornea accounted for a maximum of 25 and 159 + 3.2. μm respectively.
Conclusions:
The stiffening effect of localized CXL extends beyond that of the irradiated area. When considering localized CXL protocols clinically, it will be important to account for increased stiffening in surrounding regions.
Keywords: Cornea, Collagen, Crosslinking, Stiffness, Transition, Brillouin, Microscopy, Localized cross-linking, Customized cross-linking, CXL
INTRODUCTION
Corneal ectasia, the second leading cause of corneal transplantation worldwide,1 is characterized by alterations in corneal morphology due to a non-uniform decrease in the stiffness of the cornea.2–4 The US Food and Drug Administration has recently approved corneal crosslinking (CXL) to halt the ectatic process by stiffening the cornea. The procedure calls for soaking the cornea with a photosensitizer (riboflavin) followed by uniform UV-A exposure.5–7 When riboflavin is exposed to UV light in an oxygenated environment, reactive oxygen species are formed.6 This leads to the creation of additional chemical bonds throughout the corneal tissue collagen, thereby, increasing overall corneal strength.8,9,10
Localized CXL has recently been proposed to address the spatially dependent characteristics of ectasia.11–13 By limiting the irradiated area, the risk of complication and infection is expected to diminish due to less epithelial removal,14 potential haze, and stromal damage.12 Most importantly, because the stiffening is concentrated in the mechanically compromised area of the cornea, greater local topographical flattening is expected.15 However, the biomechanical properties of the cornea after localized CXL procedures have not yet been evaluated due to the lack of characterization methods capable of measuring corneal mechanical properties with three-dimensional resolution.
The purpose of this study was to noninvasively measure and characterize the spatial distribution of the modulus of the cornea following localized CXL using Brillouin microscopy and determine the magnitude and extent of the stiffening effect from CXL beyond the irradiated (UV-A exposed) region of the cornea.
METHODS
Freshly enucleated porcine eyes were obtained from a local slaughterhouse. They were kept in ice during the transportation until the start of the experiment. The experiment was completed within 8 hours after the sacrifice of the porcine. All corneas with intact epithelium were visually inspected to avoid using damaged or unclear tissue prior to any experimentation. Localized CXL was performed ex vivo by restricting illumination during CXL to half the porcine cornea using a customized mask. Localized CXL was performed using a traditional UV protocol and a protocol using blue light.
UVA Localized Corneal Crosslinking (UV L-CXL)
To illustrate the issue, we performed UV localized CXL experiment on one porcine eye after epithelial debridement followed by the administration of one drop of 0.1% riboflavin solution every 3 minutes for 20 minutes. Next, a constant UV-A (365 nm) power of 9 mW/cm2 was applied via a high-power UV Curing LED System (Thorlabs, Newton, NJ) for 10 minutes.9,16,17 As the primary purpose of the UV light experiment was to illustrate the spatial variation of CXL procedure and to minimize any potential variation in tissue hydration, an accelerated protocol was chosen and implemented. Half of the cornea was blocked from UV using a homemade, sharp-edged mask placed directly above the anterior of the cornea. During UV exposure, one drop of riboflavin solution was administered to the cornea at 5-minute intervals. The UV L-CXL methods are outlined in Table 1. Using Brillouin Microscopy, we analyzed the biomechanical properties of the sample in three dimension and calculated the transition zone.
Table 1:
CXL Methods
| Parameter | Variable (UVA Light) | Variable (Blue Light) |
|---|---|---|
| Treatment target | Ectasia | Ectasia |
| Fluence (total) (J/cm2) | 5.4 | 18 |
| Soak time and interval (minutes) | 20 (q3) | 20 (q3) |
| Intensity (mW) | 9 | 15 |
| Treatment time (minutes) | 10 | 20 |
| Epithelium status | Off | Off |
| Chromophore | Riboflavin | Riboflavin |
| Chromophore carrier | Dextran | Dextran |
| Chromophore osmolarity | Iso-osmolar | Iso-osmolar |
| Chromophore concentration | 0.1% | 0.1% |
| Light source | UVA (365nm) UV Curing LED System (Thorlabs, Newton, NJ) | Blue (447nm) Diode Laser (Opto Engine LLC, Salt Lake City, UT) |
| Irradiation mode (interval) | Continuous | Continuous |
| Protocol modifications | Blocking mask | Blue laser light, Blocking mask |
| Protocol abbreviation in manuscript | UV L-CXL (Localized) | Blue Light L-CXL (Localized) |
Blue Light / Localized Corneal Collagen Cross-Linking (Blue Light CXL / L-CXL)
Because the UV-A lamp is an extended source emitting incoherent light, it was reasonable to assume that radiation could not be confined to sharp transitions; thus, we utilized a blue laser and validated a CXL protocol using blue light.
For the blue light localized CXL experiment, 10 porcine eyes had epithelial debridement followed by the administration of one drop of 0.1% riboflavin solution every 3 minutes for 20 minutes. Similar to previous experiments performing blue light CXL,18,19 the cornea was then exposed to 15 mW/cm2 blue light (447 nm) radiation for 20 minutes via a blue diode laser light source (Opto Engine LLC, Salt Lake City, UT). During blue light exposure, one drop of riboflavin solution was applied at 5-minute intervals. In a similar manner to UV L-CXL, the blue light procedure was localized using the blocking mask. The Blue Light L-CXL methods are outlined in Table 1.
Using Brillouin Microscopy, we analyzed the biomechanical properties of each sample and calculated each transition zone. We aligned the transition zones of the corneas at 50% respective normalized stiffness and averaged across the depth axis.
Brillouin Microscopy
A confocal Brillouin microscope was utilized with similar configuration of previous studies9,20–23 but at a different incident wavelength. Briefly, a 660 nm laser with an optical power of 15 mW was focused into the sample by a 40x objective lens with numerical aperture of 0.6 (Olympus), a transverse resolution of ~1 μm, and depth resolution of ~2 μm. The scattered light, collected through the same objective, was coupled into a single mode fiber and delivered to a two-stage virtually imaged-phase-array (VIPA) spectrometer featuring an electron multiplying charge coupled device (EMCCD) camera (Andor, IXon Du-897). Each Brillouin spectrum was acquired in 0.15 seconds. To quantify the Brillouin shift at each sample location, raw spectra from the camera were fitted using a Lorentzian function and calibrated using the known frequency shifts of water and glass.
From the Brillouin frequency shift, the local mechanical properties of the cornea can be estimated using the following relationship:
where M’ is the Brillouin-derived longitudinal modulus, is the measured Brillouin frequency shift, is the refractive index of the material, is the wavelength of the incident photons, and is the density of the material. The spatially varying ratio of was approximated to the constant value of 0.57 g/cm3 based on literature values24,18; we estimate this to introduce a 0.3% uncertainty throughout the cornea.25,26 Previously, we have validated the strong relationship (R > 0.9) between Brillouin-derived modulus and the Young’s Modulus found via gold-standard stress-strain compression tests on porcine corneas.9
Calculation of Transition Zones
The primary metric quantified in this study was the transition zone between the fully irradiated and non-irradiated corneal areas. A sharp-edged mask was used throughout the protocols to define the two sections. While several different parameters were evaluated, including light intensity and Brillouin modulus, a common computation of the transition zone was conducted on each respective image.
To calculate the transition zone, the light intensity was averaged along the perpendicular axis normal to the transition edge and normalized using the equation:
where In is the normalized intensity, I is the average measured intensity, and Imin and Imax are the intensities at the lowest and greatest plateau respectively. We calculated the transition zone as the distance between 10% and 90% of the maximum value. Only the anterior third of the cornea was used for analysis of the transition zone of CXL procedures as that was the region primarily affected by localized crosslinking.9,27 For Brillouin maps, Brillouin modulus was used in place of light intensity throughout the calculation of transition zone.
Biomechanical Testing
To validate the blue-light protocol, we compared the Young’s Modulus of control and blue light-crosslinked buttons punched from the same respective cornea. 8 porcine corneas were dissected. Two 5 mm central corneal disc samples were created (Integra Miltex Disposable Biopsy Punch) from each cornea. Both disc samples were treated with one drop of 0.1% riboflavin solution every 3 minutes for 20 minutes. One of the two samples was set aside as the control while the other sample was fully exposed to 15 mW/cm2 blue light radiation for 20 minutes. During the irradiation process, hydration of both the control and exposed discs was maintained via the application of 1 drop of riboflavin solution at 5-minute intervals.
Next, mechanical properties of each sample were tested using a Microsquisher compressive stress-strain instrument (model: MT G2) and the associated Squisherjoy software (Cellscale, Waterloo, Ontario). The instrument directly compressed the corneal samples using a 6 mm x 6 mm plate attached to a 0.5588 mm diameter microbeam. To obtain the Young’s modulus of a sample, the Stress (Force/Area) vs Strain (Displacement/Thickness) curve was obtained and the slope of the linear segment of the curve following the sleek strain was quantified. For each sample, we reported the modulus at 1.5–4.5% strain.
Measurement of light propagation from blue light L-CXL setup
In order to isolate the transition zone generated by the blue light source alone, using the Blue Light L-CXL setup, we replaced the corneal sample with a complementary metal–oxide–semiconductor (CMOS) camera (Mightex Systems, Toronto, Ontario). The camera has a 1280 × 1024 monochrome resolution with 5.2 μm per pixel and 50 ms exposure time. To quantify the light exposure at the varying depths of the cornea, we placed the camera at a distance 0, 500, and 1000 μm from the mask; 0 μm to represent the anterior surface and 1000 μm to represent the posterior edge at the estimated maximum porcine corneal thickness. At the three distances, respective images were captured and the light transition zone was calculated.
Measurement of light scattering within the cornea
Light scattering within the cornea could result in riboflavin outside of the directly irradiated area becoming excited, thereby, crosslinking surrounding cornea that is otherwise covered by the mask. To measure light scattering throughout the L-CXL procedure, we mimicked the Blue Light L-CXL setup at three conditions. First, we replaced the cornea with a CMOS camera at a distance 3 mm from the mask. Respective images (n = 7) were taken and transition zones calculated based on measured light intensity. Following each image capture, we placed a 5 mm punched porcine cornea disc on top of the CMOS camera. Under the same conditions, images were captured and analyzed.
Finally, we subjected punched discs (n = 4) to one drop 0.1% riboflavin solution every 3 minutes for 20 minutes. We then placed the cornea on the camera, captured respective images, and similarly analyzed the riboflavin/cornea transition zone.
Statistical Analysis
For gold-standard biomechanical analysis, the Young’s Modulus of samples extracted from the same cornea were calculated as previously indicated and compared using a Wilcoxon Signed Rank Test. To compare the corneal scattering-induced transition zones to their respective controls, we performed a Wilcoxon Signed Rank test. To compare the scattering-induced transition zones of riboflavin-soaked corneas to virgin corneas and cornea-omitted controls, a Mann-Whitney U test was performed.
RESULTS
Figure 1a shows the Brillouin map of a UV-induced, locally crosslinked cornea. Brillouin microscopy revealed two distinct lateral regions within the cornea that extend the entire depth: a fully crosslinked section and a non-crosslinked section. The two regions primarily varied in anterior stiffness. Importantly, these sections were separated by a remarkably broad transition zone. Figure 1c, a transverse profile of the normalized Brillouin shift, shows an evident transition zone between the crosslinked and non-crosslinked sections.
Figure 1.


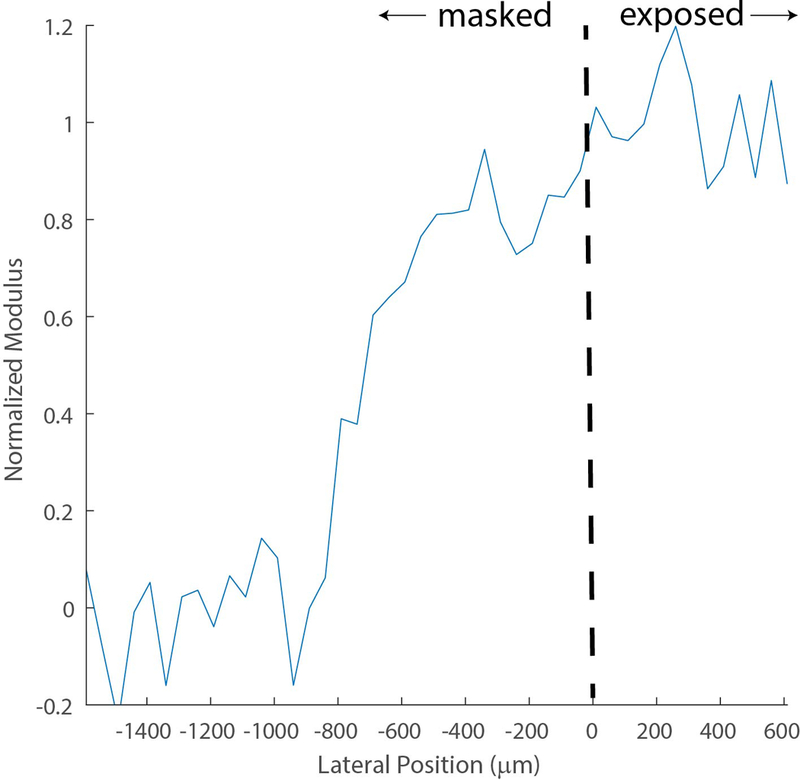
a: Representative Brillouin map (4000 μm x 700 μm) produced with MATLAB software (color map: jet) depicting the Brillouin shifts of a UV-induced, locally crosslinked porcine cornea positioned anterior up. The dotted line illustrates the edge of the UV blocking mask, differentiating the blocked and exposed sections of the cornea. A higher Brillouin shift correlates to a higher Brillouin modulus. b: The normalized Brillouin shift versus lateral position. Similar to 1a, the dotted line illustrates the edge of the UV blocking mask. The transition zone was taken as 10–90% normalized shift.
Figure 2 shows the crosslinking capabilities of the blue light protocol. Specifically, Figure 2a displays representative Brillouin maps of control and blue light-crosslinked corneas. To validate the stiffening effects of the blue-light protocol, we compared the Microsquisher®-derived Young’s Modulus of control and blue light-crosslinked buttons punched from the same respective cornea. As summarized in Figure 2b, the blue light-crosslinked samples showed significantly higher Young’s Modulus (p ≤ 0.05) than the untreated controls, yielding a modulus of 3972.2 + 1048 Pa vs 1419.7 + 312.8 Pa respectively (180% the Young’s Modulus of their respective controls).
Figure 2.
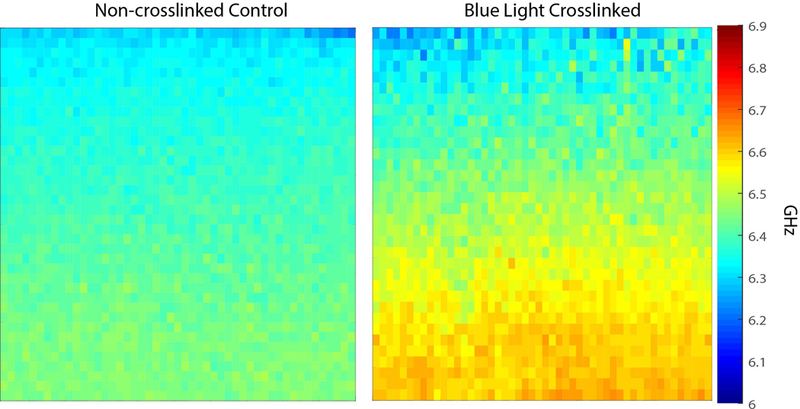
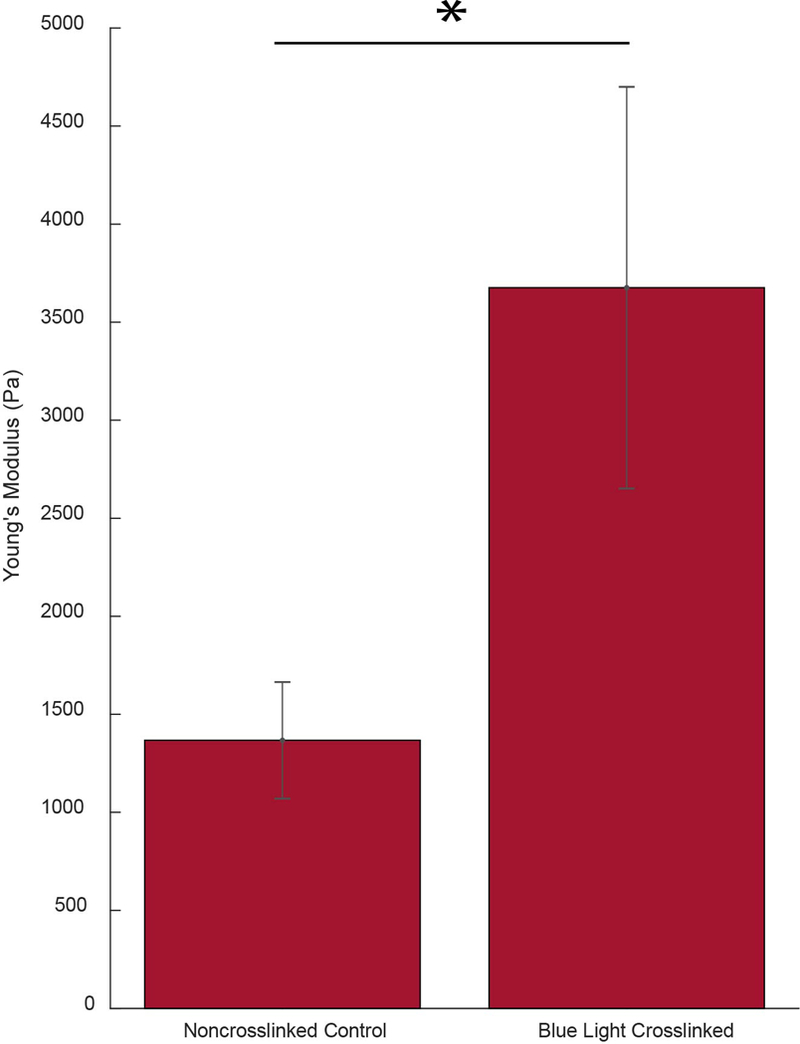
a: Representative Brillouin maps (4000 μm x 700 μm) produced with MATLAB software (color map: jet) of both a virgin (control) as well as a blue light-crosslinked porcine cornea positioned anterior up. The crosslinked cornea was exposed to 15 mW/cm2 blue light for 20 minutes. b: Bar graph comparing the elasticity, found via Microsquisher® compression, of control corneal punches and crosslinked punches. Error bars represent SEM for each condition (* = p < 0.05).
Figure 3 quantifies the transition zone induced strictly from the blue light L-CXL optical setup. The transition zone broadened as distance between camera and mask increased from 0 to 500 and 1000 μm, mimicking the anterior, central, and posterior sections of the porcine cornea, respectively. At its broadest, the transition zone due to illumination was still less than 25 μm
Figure 3:
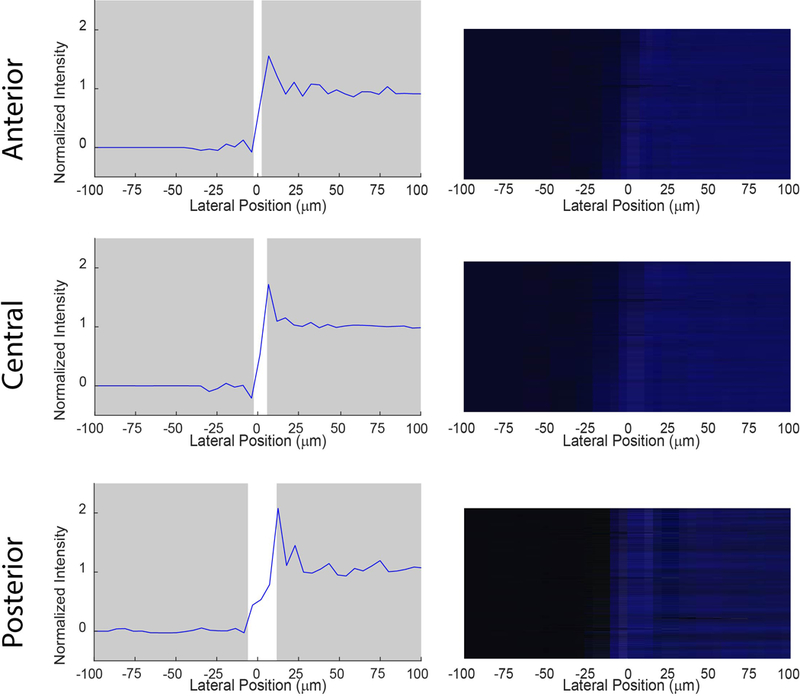
Transition zone and representative light intensity image at anterior (0 μm), central (500 μm), and posterior (1000 μm) depths from the blocking mask generated from the blue light alone. The normalized light intensity versus lateral position was analyzed using Mightex CMOS-captured images at 0, 500, and 1000 μm between the mask and camera; representing the sharpest possible transition zones through air. Point 0 μm on the horizontal axis was located at 50% normalized intensity. The transition zone at any depth did not exceed 25 μm.
Figure 4 shows the average normalized transition zone in the blue light crosslinked porcine eyes of 610 μm extending beyond the mask surface
Figure 4.
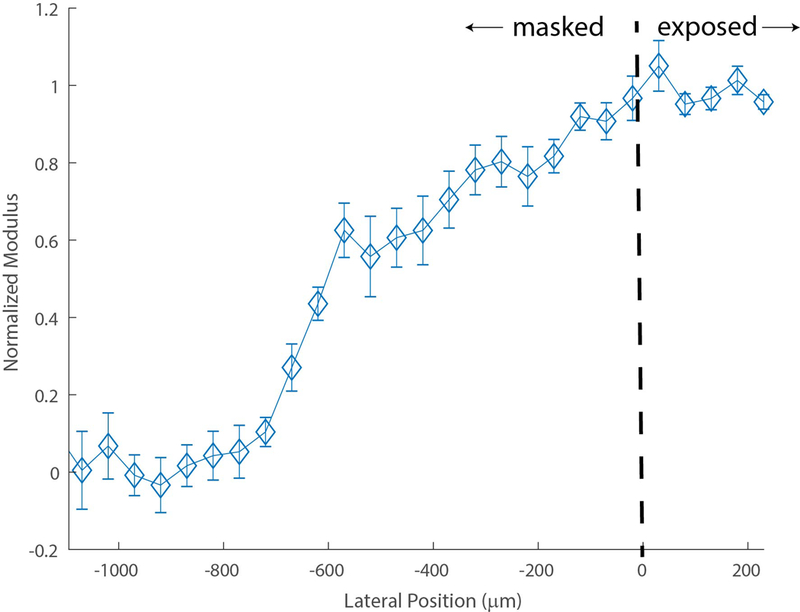
Averaged normalized transition zone of (n = 10) porcine corneas post blue light L-CXL. The dotted line illustrates the edge of the UV blocking mask, differentiating the blocked and exposed sections of the cornea. The average transition zone was calculated between the 10% and 90% of the maximum plateau of normalized shift. Error bars represent SEM for each position.
Figure 5 quantifies the scattering-induced transition zones throughout the cornea during blue light L-CXL. Through just air, therefore without any corneal scattering, a sharp transition zone of 37 + 8.9 μm was found. This is consistent with Fig. 3 as we had to increase distance between the mask and camera from 1 to 3 mm which caused the slight increase in transition zone. In an untreated cornea, the transition zone significantly increased to 102 + 17.2 μm (p ≤ 0.01). The transition zone of the cornea increased even further due to the presence of riboflavin, reaching 159 + 3.2 μm; a significantly increased transition zone compared to the untreated cornea condition (p ≤ 0.05
Figure 5.

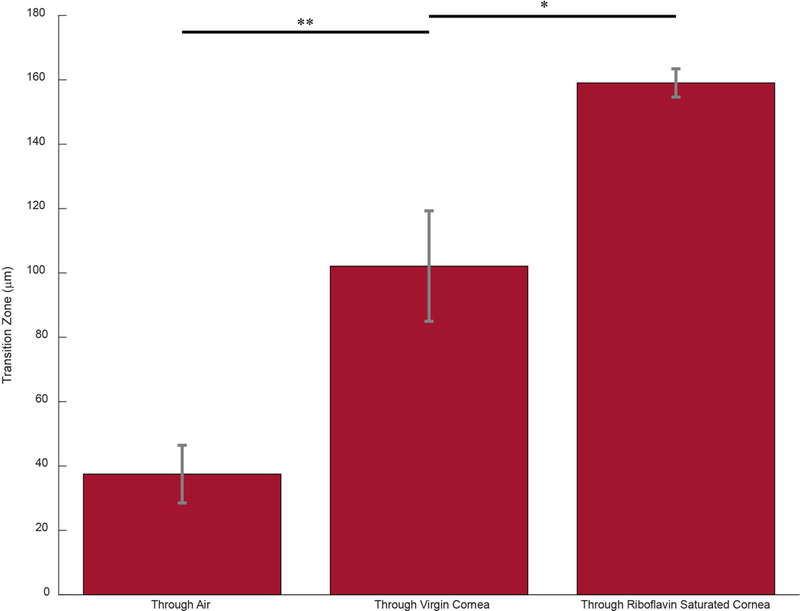
a: Representative graphs of the normalized light intensity versus lateral position from the 0 point at 50% intensity as the light traveled through air, a virgin cornea, and a riboflavin-soaked cornea to quantify light scattering propagation in each environment b: Bar graph representing the average transition zone as light traveled through three distinct environments. Error bars represent SEM for each condition (* = p < 0.05, ** = p <0.01)
A summary of the transition zone findings can be found in the Table 2.
Table 2:
Transition zones observed from each protocol
| Protocol | Average Transition Zone μm) |
|---|---|
| Blue Light L-CXL | 610 |
| Light Propagation | 25 |
| Virgin Corneal Scattering | 102 |
| Riboflavin Soaked Corneal Scattering | 159 |
DISCUSSION
In this study, we characterized the spatially-varying stiffness of the cornea following L-CXL. Biomechanical imaging analysis via Brillouin Microscopy demonstrated two distinct regions; a crosslinked area and a non-crosslinked area. The shift versus depth profile of the crosslinked section mimicked that of a uniformly crosslinked cornea.28 As expected, the crosslinked section markedly differed from the non-crosslinked section in the anterior region.9,18,28 The two sections were separated by a broad transition zone. Therefore, the crosslinking effect extended greater than the area exposed to light.
To investigate if the effect was optically induced, we replaced the UV light source with a blue laser to optimize sharpness and set the CXL procedure to replicate the stiffening results of UV crosslinking. Under localized protocol, we observed a transition zone of over 600 μm even though the blocking mask produced an illumination transition zone through air of less than 25 μm. The broad zone within locally crosslinked corneas must be due to an intrinsic characteristic of the cornea or CXL procedure.
The experiments conducted were on ex vivo porcine corneas. With time, ex vivo corneas become increasingly opaque, thereby, becoming more susceptible to light scattering. In order to validate our findings for future in vivo settings, we measured the scattering effect of the porcine corneas. We found that, at a 3 mm distance from the mask, the corneal transition zone was significantly greater than the illumination transition zone through air. The scattering induced solely by the porcine corneas contributed to roughly 100 μm transition zone and increased further to over 150 μm after the corneas were soaked in riboflavin. In vivo corneas have lower natural scattering property,29,30 therefore, our results represent an upper bound of the transition zone one may find in vivo.
Blue light was used in this experimental set up. At 15 mW/cm2 for 20 minutes, the blue light stiffened the corneas to 180% the Young’s Modulus of their respective controls. This can thus be considered a suitable model to simulate the stiffening of standard CXL protocols performed using UV.31 Clinical application calls for columnized UVA exposure. A clinically-used, columnated UV system, creating a well-confined source of light, should produce similar results to that of our setup. Here, we used a coherent laser for illumination understanding it to be an ideal optical scenario regarding the transition zone. As expected, the blue coherent light allowed us to obtain sharp confinement of the illumination.
After considering of all these different factors, we estimated less than 250 μm of the 610 μm transition zone being due to light-related effects. Therefore, as summarized in the Table, a large portion of the transition zone remains unaccounted by light phenomena. A number of factors could be contributing to this effect. The area of crosslinking depends on the distribution of riboflavin concentration during light exposure, absorbed photons of light, and oxygen exposure to activated riboflavin.5–8,32 Throughout the 20 minutes of light exposure, there exists a diffusion of oxygen radicals and/or hydrogen peroxide,33,34 both of which contribute to crosslinking. Therefore, any diffusion of such reactants could result in the extended crosslinking area. Furthermore, the cornea becomes increasingly turbid throughout crosslinking,35,36 accounting for increased scattering of light as the procedure is performed. Light scattering throughout the process of L-CXL could result in the excitation of riboflavin molecules outside of the irradiated area, subsequently leading to crosslinking within the transition zone.
Localized crosslinking protocols have garnered added attention recently.11–13 Seiler and colleagues employed localized crosslinking with treatments with customized maximal irradiation ranging from 5.4 J/cm2 up to 10 J/cm2 centered on the maximum posterior elevation in concentric rings and found improved maximal flattening and corneal regularization at one year but unknown long term stabilization effects.11 Cassagne and colleagues performed topography-guided CXL in zonal patterns with total irradiance ranging from 5.4 J/cm2 in regions surrounding the cone to 15 J/cm2 on top of the cone using 30 mW/cm2 pulsed ultraviolet-A irradiance and found significant improvements in CDVA, Maximum keratometry (Kmax), mean keratometry in the inferior part of the cornea (I index), a demarcation line that was more pronounced in maximally treated areas but less pronounced in surrounding regions at one year.12 Nordström and colleagues performed asymmetrical treatment zone centered on the area of maximum corneal steepness with treatment energies ranging from 7.2 J/cm2 to 15.0 J/cm2 and found improved visual acuity, spherical refractive error, and K max as compared to uniformly applied CXL using 5.4 J/cm2 pulsed fluence.13
None of these protocols were based on any specific corneal stiffness alteration but rather on the general concept of applying greater irradiation leading to greater stiffness in the maximal cone region and less in surrounding regions. As localized CXL protocols become more sophisticated over time and specific corneal stiffening outcomes are targeted, the biomechanical impact on the transition zone will need to be taken into account during treatment planning.
In conclusion, we found a broad transition zone of approximately 600 μm between the fully crosslinked and non-crosslinked sections of the cornea in localized CXL procedures. Therefore, there exists a stiffening effect which extends beyond the irradiated area. We quantified the contribution of light propagation through the cornea, finding it only partially describes the extent of the transition zone. Finally, we validated our transition zones were not due to ex vivo scattering artifacts and, therefore, are a result of intrinsic corneal properties. When conducting localized CXL clinically, it will be important to account for increased stiffening outside of the irradiated area.
Acknowledgments
Financial Support: Supported in part by NIH grant R01 EY028666 and an unrestricted departmental grant to the USC Roski Eye Institute from Research to Prevent Blindness, Inc.
Footnotes
Financial Disclosures:
FH: FH - Shareholder/investor for EMAGine AG (Zug, Switzerland), consultant for GroupAdvance Consulting GmbH (Zug, Switzerland), exclusive patent owner for PCT patent/application (corneal apparatus used for CXL and chromophore for CXL application), recipient of travel funds from Light for Sight Foundation (Zurich, Switzerland), directed research funds from Light for Sight Foundation (Zurich, Switzerland), Schwind Eye Tech Solutions (Kleinostheim, Germany), VELUX Foundation (Søborg, Denmark), Gelbert Foundation (Geneva, Switzerland), and in-kind financial contribution for research materials from SOOFT Italia (Montegiorgio, Italy).
None of the other authors have any financial disclosures for this topic
REFERENCES
- [1].Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, Thuret G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmology. 2016; 134(2): 167. [DOI] [PubMed] [Google Scholar]
- [2].Scarcelli G, Besner S, Pineda R, Kalout P, Yun SH. In Vivo Biomechanical Mapping of Normal and Keratoconus Corneas. JAMA Ophthalmology. 2015; 133(4): 480–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ruberti JW, Sinha Roy A, Roberts CJ. Corneal Biomechanics and Biomaterials. Annual Review of Biomedical Engineering. 2011; 13(1): 269–295. [DOI] [PubMed] [Google Scholar]
- [4].Roberts CJ, Dupps WJ Jr. Biomechanics of corneal ectasia and biomechanical treatments. Journal of Cataract and Refractive Surgery. 2014; 40(6): 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kymionis GD, Portaliou DM, Diakonis VF, Kounis GA, Panagopoulou SI, Grentzelos MA. Corneal Collagen Cross-linking With Riboflavin and Ultraviolet-A Irradiation in Patients With Thin Corneas. American Journal of Ophthalmology. 2012; 153(1): 24–28. [DOI] [PubMed] [Google Scholar]
- [6].Richoz O, Hammer A, Tabibian D, Gatzioufas Z, Hafezi F. The Biomechanical Effect of Corneal Collagen Cross-Linking (CXL) With Riboflavin and UV-A is Oxygen Dependent. Translational Vision Science & Technology. 2013; 2(7): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a–induced collagen crosslinking for the treatment of keratoconus. American Journal of Ophthalmology. 2003. 135(5): 620–627. [DOI] [PubMed] [Google Scholar]
- [8].Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin–ultraviolet-A-induced cross-linking. Journal of Cataract & Refractive Surgery. 2003; 29(9): 1780–1785. [DOI] [PubMed] [Google Scholar]
- [9].Webb JN, Su JP, Scarcelli G. Mechanical outcome of accelerated corneal crosslinking evaluated by Brillouin microscopy. Journal of Cataract & Refractive Surgery. 2017; 43(11): 1458–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang Y, Conrad AH, Conrad GW. Effects of ultraviolet-A and riboflavin on the interaction of collagen and proteoglycans during corneal cross-linking. Journal of Biological Chemistry. 2011; 286(15): 13011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Seiler TG, Fischinger I, Koller T, Zapp D, Frueh BE, Seiler T. Localized Corneal Cross-linking: One-Year Results. American Journal of Ophthalmology. 2016; 166: 14–21. [DOI] [PubMed] [Google Scholar]
- [12].Cassagne M, Pierné K, Galiacy SD, Asfaux-Marfaing MP, Fournié P, Malecaze F. Localized Topography-Guided Corneal Collagen Cross-linking for Keratoconus. Journal of Refractive Surgery. 2017; 33(5): 290–297. [DOI] [PubMed] [Google Scholar]
- [13].Nordström M, Schiller M, Fredriksson A, Behndig A. Refractive improvements and safety with topography-guided corneal crosslinking for keratoconus: 1-year results. British Journal of Ophthalmology. 2017; 101: 920–5. [DOI] [PubMed] [Google Scholar]
- [14].Sachdev GS, Sachdev M. Recent advances in corneal collagen cross-linking. Indian Journal of Ophthalmology. 2017; 65(9): 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sinha Roy A, Dupps WJ Jr. Patient-specific computational modeling of keratoconus progression and differential responses to collagen cross-linking. Investigative Ophthalmology & Visual Science, 2011; 52(12): 9174–9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wernli J, Schumacher S, Spoerl E, Mrochen M. The efficacy of corneal crosslinking shows a sudden decrease with very high intensity UV light and short treatment time. Investigative Ophthalmology & Visual Science 2013; 54: 1176–1180. [DOI] [PubMed] [Google Scholar]
- [17].Hammer A, Richoz O, Arba Mosquera S, Tabibian D, Hoogewoud F, Hafezi F. Corneal biomechanical properties at different corneal cross-linking (CXL) irradiances. Investigative Ophthalmology & Visual Science. 2014; 55: 2881–2884 [DOI] [PubMed] [Google Scholar]
- [18].Scarcelli G, Pineda R, Yun SH. Brillouin optical microscopy for corneal biomechanics. Investigative Ophthalmology & Visual Science. 2012; 53: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kashiwabuchi RT, Quinto GG, Castro-Combs J, Behrens A. Corneal collagen cross-linking with riboflavin/lumiflavin and blue light (5450nm) to reduce posterior lamellar dislocation in endothelium keratoplasty. Investigative Ophthalmology & Visual Science. 2009; 50(13): 549519516002 [Google Scholar]
- [20].Scarcelli G, Polacheck WJ, Nia HT, Patel K, Grodzinsky AJ, Kamm RD, Yun SH. Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy. Nature Methods. 2015; 12: 1132–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Scarcelli G, Yun SH. Confocal Brillouin microscopy for three dimensional mechanical imaging. Nature Photonics. 2007; 9: 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Scarcelli G, Yun SH. Multistage VIPA etalons for high-extinction parallel Brillouin spectroscopy. Optics Express. 2011; 19: 10913–10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Berghaus KV, Yun SH, Scarcelli G. High speed sub-GHz spectrometer for Brillouin scattering analysis. Journal of Visualized Experiments. 2015; 53468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kikkawa Y, Hirayama K. Uneven swelling of the corneal stroma. Investigative Ophthalmology. 1970; 9: 735–741. [PubMed] [Google Scholar]
- [25].Aldahlawi NH, Hayes S, O’Brart DPS, Meek KM. Standard versus accelerated riboflavin–ultraviolet corneal collagen crosslinking: resistance against enzymatic digestion. Journal of Cataract Refractive Surgery. 2015; 41: 1989–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McCall AS, Kraft S, Edelhauser HF, Kidder GW, Lundquist RR, Bradshaw HE, Dedeic Z, Dionne MJC, Clement EM, Conrad GW. Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA). Investigative Ophthalmology & Visual Science. 2010; 51: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wollensak G, Spoerl E, Wilsch M, Seiler T. Keratocyte Apoptosis After Corneal Collagen Cross-linking Using Riboflavin/UVA Treatment. Cornea. 2004; 23(1): 43–49 [DOI] [PubMed] [Google Scholar]
- [28].Scarcelli G, Kling S, Quijano E, Pineda R, Marcos S, Yun SH. Brillouin microscopy of collagen crosslinking: noncontact depth-dependent analysis of corneal elastic modulus. Investigative Ophthalmology & Visual Science, 2013; 54(2): 1418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Olsen T Light scattering from the human cornea. Investigative Ophthalmology & Visual Science, 1982; 23(1): 81–6. [PubMed] [Google Scholar]
- [30].Spadea L, Maraone G, Verboschi F, Vingolo EM, Tognetto D. Effect of corneal light scatter on vision: a review of the literature. International Journal of Ophthalmology. 2016; 9(3): 459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Seifert J, Hammer CM, Rheinlaender J, Sel S, Scholz M, Paulsen F, Schäffer TE. Distribution of Young’s modulus in porcine corneas after riboflavin/UVA-induced collagen cross-linking as measured by atomic force microscopy. PloS One. 2014; 9(1): 88186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Roberts CJ, Dupps WJ Jr, Downs JC. Biomechanics of the eye. Wayne, PA: Kugler Publications; 2018. [Google Scholar]
- [33].Wilson G, Riley MV. Does topical hydrogen peroxide penetrate the cornea. Investigative Ophthalmology & Visual Science. 1993; 34(9): 2752–60. [PubMed] [Google Scholar]
- [34].Chalmers RL. A review of the metabolism of hydrogen peroxide by external ocular structures. International Contact Lens Clinic. 1995; 22(7–8): 143–147. [Google Scholar]
- [35].Beckman Rehnman J, Janbaz CC, Behndig A, Lindén C. Spatial distribution of corneal light scattering after corneal collagen crosslinking. Journal of Cataract & Refractive Surgery. 2011; 37(11): 1939–1944. [DOI] [PubMed] [Google Scholar]
- [36].Wollensak G, Wilsch M, Spoerl E, Seiler T. Collagen Fiber Diameter in the Rabbit Cornea After Collagen Crosslinking by Riboflavin/UVA. Cornea. 2004; 23(5): 503–507. [DOI] [PubMed] [Google Scholar]


