Abstract
Mitochondrial bioenergetics require the coordination of two different and independent genomes. Mutations in either genome will affect mitochondrial functionality and produce different sources of cellular stress. Depending on the kind of defect and stress, different tissues and organs will be affected, leading to diverse pathological conditions. There is no curative therapy for mitochondrial diseases, nevertheless, there are strategies described that fight the various stress forms caused by the malfunctioning organelles. Here, we will revise the main kinds of stress generated by mutations in mitochondrial genes and outline several ways of fighting this stress.
Keywords: mitochondrial dysfunction, cellular stress, mitochondrial pathology, therapy
MITOCHONDRIA AND CELL METABOLISM
Mitochondria play a pivotal role in eukaryotic metabolism. They catabolise redox equivalents, derived from nutrient uptake, and use them to provide the bulk of cellular energy in the form of ATP. The oxidative phosphorylation system (OXPHOS) is responsible for this energy production and it is composed of five multi-oligomeric complexes present in the inner mitochondrial membrane. Transfer of electrons through complexes I to IV reduce molecular oxygen to water. This process is coupled to proton pumping from the matrix to the intermembrane space (IMS), while the return of protons to the matrix through the F1Fo ATPase generates ATP 1. However, an inefficient flow of electrons through the respiratory chain complexes would partially reduce oxygen and produce reactive oxygen species (ROS) like superoxide and hydrogen peroxide. At low concentrations, these molecules act as second messengers and can activate gene transcription and trigger cellular responses, like cellular growth, production of cellular antioxidants or stimulation of mitochondrial biogenesis 2,3. However, once a certain threshold is exceeded, these molecules may incite oxidative damage in the form of mitochondrial DNA (mtDNA) alterations or lipid peroxidation, generating cellular stress that leads to aging or cell death.
In addition, mitochondria are involved in many other key cellular functions. Dissipation of the proton gradient by uncoupling proteins (UCPs) generates heat instead of energy and this plays an important role in exposure to cold or hibernation 4. Calcium (Ca2+) uptake inside mitochondria is mediated by the mitochondrial calcium uniporter (MCU). Although the complex has a low affinity for Ca2+, the transport takes place due to the high concentration of Ca2+ (>10 μM) present in micro domains located in the contact sites between endoplasmic reticulum (ER) and mitochondria 5. The mitochondrial Ca2+ uptake not only shapes the cytosolic Ca2+ dynamics, which is crucial for muscle contraction, exocytosis and gene transcription, but also modulates at least three dehydrogenases of the Krebs cycle, thus regulating energy metabolism. Finally, Ca2+ overload in mitochondria regulates apoptosis due to formation of the permeable transition pore (PTP) and release of cytochrome c from the IMS 6. Mitochondria are involved in the biogenesis and maturation of different cofactors, like heme, biotin or iron-sulfur (Fe/S) clusters. Despite the chemical simplicity of Fe/s clusters, their biosynthesis requires more than two dozen proteins in eukaryotes and takes place both in mitochondria and the cytosol 7. Alterations in these mechanisms are linked to severe neurodegenerative, metabolic or haematological diseases 8.
Since mitochondria take part in many different metabolic processes, mitochondrial malfunction can affect numerous aspects of the cell. As a consequence, various forms of cellular stress are generated, leading to a large variety of pathological conditions. Here, we review different forms of cellular stress caused by mitochondrial malfunction and the strategies used to fight this stress.
MITOCHONDRIAL DEFECTS
Mitochondria have retained their own genome, the mtDNA. This small, circular, double-stranded DNA is located in the mitochondrial matrix in all cell types, and can be found with copy numbers that range from several to thousands of copies. In human, the mtDNA encodes 13 polypeptides of the respiratory chain, as well as for part of the translation machinery, required for the synthesis of these polypeptides within mitochondria: two ribosomal RNAs (mt-rRNAs) and 22 transfer RNAs (mt-tRNAs) 9. The remaining mitochondrial proteins (approx. 99%) are encoded in the nucleus, synthesized on the cytosolic ribosomes and imported into mitochondria. Therefore, we will distinguish between mitochondrial malfunction caused by mutations in the mtDNA and those caused by mutations of nuclear genes encoding mitochondrial proteins.
Alterations in the mtDNA
Some features of mtDNA make it especially sensitive to oxidative damage and mutation. Firstly, mtDNA has no introns, so every single nucleotide carries information essential for protein coding; mtDNA is naked, there are no histone proteins protecting it from damage; and although DNA repair systems do exist in mitochondria, their mechanisms and extent are poorly understood 10, therefore mutations usually remain and are transmitted to the next generation until they are removed by selection11. Moreover, the proximity to the respiratory chain, a ROS producing source, increases the risk of potential damage. For all these reasons, the mutational rate of the mitochondrial genome is much higher than that of the nuclear genome 12.
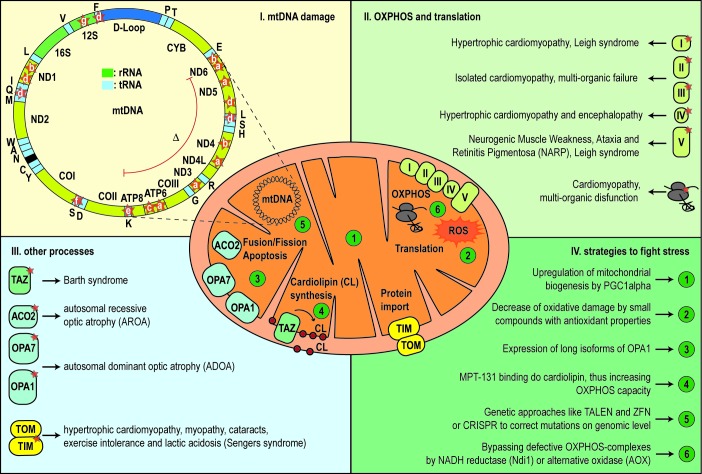
Pathological changes in the mtDNA can appear as point mutations in protein coding sequences, mt-tRNAs or even mt-rRNAs. In addition, major rearrangements of mtDNA, like deletions or insertions/duplications, are a cause of disease. Due to the fact that every cell contains a variable number of mtDNA molecules, mutations can be present in homoplasmy (all copies share the same mtDNA genotype) or heteroplasmy (only a population of DNA is mutated). The level of heteroplasmy of a mutation is a critical determinant of the cellular stress of a certain tissue or organ and has a major role in the disease phenotype. Finally, a reduction of mtDNA copies (depletion syndrome) can also hamper energy production and generate cellular stress (see Table 1, Figure 1) 12.
Figure 1. FIGURE 1: Mitochondrial dysfunctions are related to mutations in mtDNA and defects in nuclear encoded mitochondrial proteins.
(I) Overview of mutations within the mtDNA.
(II) The majority of mitochondrial defects based on a malfuntion of OXPHOS complexes and mitochondrial translation.
(III) Defects in other processes, like mitochondrial fusion and fission or lipid homeostasis, leads to different mitochondrial diseaes.
(IV) Different strategies to figth the diverse forms of mitochondrial stress. (a: Leigh Syndrome LS; b: Leber Hereditary Optic Neuropathy LHON; c: Neurogenic Muscle Weakness, Ataxia and Retinitis Pigmentosa NARP; d: Mitochondrial Encephalomyopathy, Lactid Acidosis and Stroke-like Episodes MELAS; e: Myoclonic Epilepsy and Ragged Red Fiber Disease MERRF; f: Sensorineural Hearing Loss SNHL; g: mitochondrial non-syndromic Hearing Loss; (: Kearns Sayre Syndrome KSS).
Alterations in the mitochondrial proteins encoded in the nucleus
Due to the diverse cellular roles that mitochondria fulfill, there are many mitochondrial processes that cause a pathology when disturbed. In the last years, massive sequencing approaches have significantly increased the number of known mutations implicated in mitochondrial diseases. Examples of this are defects in: factors involved in the biogenesis or integrity of respiratory chain complexes, those that regulate mtDNA maintenance, proteins required for transcription of mt-mRNA elements involved in translation of mtDNA encoded proteins, regulators of lipid metabolism, factors involved in cellular signalling and even enzymes of the Krebs cycle (see Table 2).
CELLULAR EFFECTS ON DIFFERENT TISSUES
Typically, mitochondrial disorders have been divided between those presenting with multiple symptoms, usually known as syndromes, and those characterized by tissue specific phenotypes. It remains to be addressed, which factors determine the tissue-specificity of mitochondrial diseases. However, to better address the different kinds of stress caused by mitochondrial distress, we will describe them classified by tissues/organs and give some examples of alterations that cause these problems.
Sensory organs
Hearing loss is one of the most prevalent sensory disorders 61. Genetic factors are thought to account for more than half of congenital and childhood-onset hearing loss, including mutations in mtDNA 62 and mitochondrial nuclear genes like the heme A biogenesis factor COX10 63,64 or the AAA protease responsible for complex III assembly BSC1L 65,66.
Mutations in the 12S mt-rRNA (m.1555A>G and m.1494 C>T) have been associated with aminoglycoside-induced ototoxicity and mitochondrial non-syndromic hearing loss. Studies using mitochondrial cybrids derived from Hela cells and lymphoblasts have shown that these mutations affect the integrity and fidelity of the mitochondrial ribosome, therefore causing decreased mitochondrial translation, either in the presence or the absence of aminoglycosides, and resulting in a cell growth defect 67,68.
However, a study using osteosarcoma 143B derived cybrids showed no effect on mitochondrial translation after aminoglycoside treatment 69.
This discrepancy, the phenotypic differences between asymptomatic relatives and patients all harbouring the same mutational load and the fact that only in some cases the defect arose upon antibiotic treatment, raised the search for modifying factors of aminoglycoside induced ototoxicity within the nuclear genetic background 70. Indeed, no negative effect was observed after aminoglycoside treatment in primate cells from the Cercophiteciade family where the m.1494 C>T was fixed as the wild-type allele and cells carried a compensating mutation in mitochondrial ribosomal protein S12 (MRPS12) 71, whereas primate cells from orangutan carrying the m.1555A>G mutation and no MRP mutation showed a drastic effect after antibiotic treatment 72. In addition, this biochemical effect has been linked to stress signalling. Cybrids carrying the m.1555A>G mutation showed hypermethylation of the mitochondrial ribosome, disturbed mitochondrial translation and assembly of the respiratory chain, resulting in increased production of ROS. Enhanced superoxide levels are sensed by AMPK, which signals further to E2F1, activating pro-apoptotic signalling in the cell. This induction seems to be tissue-specific, happens mainly in the inner ear and may explain the specific hearing defect observed in the presence of this particular mutation 73 (see Table 1).
Table 1.
TABLE 1. Types of mitochondrial disease caused by mitochondrial encoded genes.
| Mitochondrial defects - mitochondrial encoded (mtDNA) 60 | |||
| Disease | Coding | Mutation | Reference |
| Kearns Sayre Syndrome (KSS) | ND3, ND4, ND4L, ND5, COX3, ATP6, ATP8, tRNALeu, tRNASer, tRNAHis, tRNAArg, tRNAGly, tRNALys | Δ4977 (5 kb deletion) | 13,14 |
| Leigh Syndrome (LS) | ATP6 | m.8993T>C | 15 |
| m.9176T>G | 16 | ||
| ND3 | m.10158T>C | 17,18,19 | |
| ND4 | m.11777C>A | 20,15 | |
| ND5 | m.12706T>C | 22 | |
| ND6 | m.14459G>A | 23,24 | |
| m.14487T>C | 25,26 | ||
| Leber Hereditary Optic Neuropathy (LHON) | ND4 | m.11778G>A | 27 |
| ND1 | m.3460G>A | 28,29 | |
| ND6 | m.14484T>C | 30,31,32 | |
| Neurogenic Muscle Weakness, Ataxia and Retinitis Pigmentosa (NARP) | ATP6 | m.8993T>G | 33,34 |
| Mitochondrial Encephalomyopathy, Lactid Acidosis and Stroke-like Episodes (MELAS) | ND1 | m.3697C>A | 35 |
| ND5 | m.13513G>A | 36 | |
| m.13514A>G | 37 | ||
| tRNAPhe | m.583G>A | 38,39 | |
| tRNALeu (UUR) | m.3243A>G | 40 | |
| m.3256C>T | 41,42 | ||
| m.3271T>C | 43,44,45 | ||
| m.3291T>C | 46 | ||
| tRNAGln | m.4332G>A | 47 | |
| Myoclonic Epilepsy and Ragged Red Fiber Disease (MERRF) | tRNALys | m.8344A>G | 48,49 |
| m.8356T>C | 50,51,52 | ||
| m.8363G>A | 53 | ||
| Sensorineural Hearing Loss (SNHL) | tRNASer | m.7445A>G | 54 |
| m.7511T>C | 55 | ||
| Deafness (DEAF) | 12s rRNA | m.1494C>T | 56 |
| m.1555A>G | 57,58,59 | ||
Eye complications are also frequently found to be associated with mitochondrial dysfunction 74 and can be divided into primary and secondary. Primary afflictions are caused by genetic defects, whereas secondary afflictions are produce by hypertensive angiopathy of the retinal arteries, or diabetic retinopathy in mitochondrial diseases with diabetes 75. Mitochondrial optic neuropathies have been associated with mutations in mtDNA and in nuclear genes. The most frequent eye disorder due to mtDNA mutation is Leber´s hereditary optic neuropathy (LHON) 76. LHON usually affects young male adults and is characterised by mostly bilateral subacute or acute, painless, loss of central vision, with decreased colour vision77. There are three main mtDNA mutations that underly the majority of LHON cases and all of them are found in complex I genes: m.11778G > A in the ND4 gene, m.3460G > A in the ND1 gene, and m.14484T > C in the ND6 gene (see Table 1). In addition, these mutations are usually present in homoplasmy, indicating that probably other factors are involved in the development of the disorder. The molecular mechanisms underlying LHON are not yet fully understood. There have been some risks factors proposed, like specific mitochondrial haplogroups, smoking, alcohol consumption, and the use of some antibiotics. Differences in mitochondrial mass have been also postulated to play a role in the incomplete manifestation of the disease. LHON mutation carriers with no pathological phenotype have significantly higher mtDNA copy number in leukocytes than affected carriers. By comparing fibroblasts from unaffected and affected mutation carriers, along with controls, it was shown that unaffected carriers have increased mitochondrial transcripts, respiratory chain proteins and enzyme activities compared to controls and affected carriers. Therefore, increased mitochondrial mass may play a protective role in LHON and compensate for complex I dysfunction 78. In addition, males seem to be more affected because of the lack of protective effects from estrogen. Indeed, a study using cybrids carrying LHON mtDNA mutations showed that the addition of estradiol increased mitochondrial biogenesis and decreased ROS production by enhancing the activity of detoxifying enzymes like SOD2, leading to a decrease in apoptosis 79 (see Table 1).
The most common eye afflictions associated with nDNA mutations are autosomal dominant optic atrophy (ADOA), most frequently due to mutations in the Dynamin-like GTPase OPA1, and autosomal recessive optic atrophy (AROA), which has been mainly associated with mutations in the aconitate hydratase ACO2, or the uncharacterised transmembrane protein TMEM126A (OPA7) 76. ADOA is clinically characterised by bilaterally symmetric progressive deterioration of the central visual acuity. Approximately 60-70% of ADOA cases are caused by genetic alterations in OPA1, other genes implicated in this pathology are OPA2 80, OPA3 81, OPA4 82, OPA5 83, OPA8 84 and WFS1 85 (see Table 1). OPA1 is a protein with eight different isoforms, processed by the mitochondrial metallochaperones YME1L and OMA1 86,87,88. The best-known function of OPA1 is for inner mitochondrial fusion during mitochondrial dynamics. In addition, OPA1 is involved in the remodelling of cristae by tethering inter-cristae membranes and proper function of the protein is required for maintaining cristae structure 89. OPA1 mutations cause defective mitochondrial fusion and altered cristae structure, leading to direct effects on mitochondrial bioenergetics, including a decreased mitochondrial membrane potential and ATP synthesis and increased ROS production 90. Interestingly, deletion of YME1L in murine heart, which alters OPA1 processing and function in a tissue-specific way, causes dilated cardiomyopathy (Figure 1) 91.
AROA presents with progressive impairment of visual capacity. The defect could either be spontaneously recovered or may lead to bilateral and progressive blindness 77. Mutations in ACO2, affect the mitochondrial tricarboxylic acid cycle and therefore mitochondrial energy supply is depleted in patients 92. Although there have been several AROA patients with mutations in TMEM126A, the exact function of the protein and therefore the molecular mechanism underlying optic atrophy has yet to be determined 93,94,95.
Heart
Cardiac muscle has a high energetic demand, therefore cardiac complications are frequent among mitochondrial diseases. One of the most common cardiac afflictions present in these pathologies is cardiomyopathy, which is estimated to occur in 20-40% of children with mitochondrial disease 4,96,97. However, other symptoms like arrhythmia, conduction defects, pulmonary hypertension, dilated aortic root, pericardial effusion or coronary heart disease can also be developed as consequence of mitochondrial malfunction 5,98.
Mitochondrial cardiomyopathies are characterised by abnormal myocardial structure or function that results from genetic defects that impair the mitochondrial respiratory chain 6,98. Hypertrophic cardiomyopathy is the most common form, present in more than 50% of cases 7,96, but other forms, like dilated, restrictive, histiocytoid and left ventricular non-compactation cardiomyopathies can also be found among these patients 8,99.
As described before (see above), genetic defects affecting the integrity of respiratory chain complexes, mitochondrial translation, maintenance of mtDNA, lipid metabolism and other metabolic pathways inside mitochondria might lead to cardiac disease. Several important perturbations have been described in subunits or factors required for the proper assembly of respiratory chain complexes. In general, mutations in these proteins cause an impairment of respiration and ATP production, increased ROS production and finally, cellular stress derived from a bioenergetics impairment. To date, pathological mutations have been found in 26 structural subunits of complex I 9,100, that together with mutations in assembly factors represent around 30% of childhood mitochondrial diseases 11,101. Complex I defects can be present with isolated cardiomyopathy or together with multi-organic failure. Mutations in subunits of complex II or III have also been associated with different types of cardiomyopathy 12,102,103,104. Of special interest are defects of the cytochrome c oxidase, caused by mutations in assembly factors and in nuclear-encoded structural subunits. Mutations in the complex IV assembly factors COX10 and COX15 have been associated with hypertrophic cardiomyopathy 16,105. Both assembly factors are involved in the biosynthesis of heme A, the prosthetic group of the cytochrome c oxidase. Mutations in COX6B1, have been associated with cardiomyopathy and encephalopathy and showed decreased levels of the mature cytochrome c oxidase complex in patient-derived tissues and cells 12,106,107.
Although COX6B1 was thought to be a loosely interacting structural subunit of complex IV, studies have postulated Cox12 (yeast homolog of COX6B1) to be involved in the delivery of copper to Cox2, together with other metallochaperones like Sco1, Sco2 and Coa6 61,108. Indeed, mutations in human SCO2 have been mainly associated with cardioencephalopathy 62,109,110,111, whereas mutations in SCO1 have been associated with hepatic failure and encephalopathy 67,68,112,113, as well as cardiomyopathy 64,114. Both proteins contain a CXXXC that is able to coordinate copper. They bind to apo-COX2 and deliver two copper atoms to the CuA center. Both enzymes have different but cooperative functions and disruptions in their function impair the maturation of cytochrome c oxidase 70,115. In addition, the SCO1 and SCO2 proteins are involved in regulating cellular copper homeostasis 71,116. Recently, it has been shown that SCO1 keeps the copper transporter CTR1 in the plasma membrane, this function being essential for the development of adult myocardium in mice 72,117. Mutations in COA6 have been described in infants with hypertrophic cardiomyopathy and combined complex I and IV, or isolated complex IV deficiency in the heart 73,118,119. COA6 is required for cytochrome c oxidase assembly 74,120,121. It is involved in the insertion of copper into COX2 and it has been described to interact with SCO2 75,122 and SCO1 76,123 after the translocation of the COX2 C-terminal domain into the IMS by COX18 124. However, why disturbance of copper metabolism, or ultimately of the cytochrome c oxidase, specifically affects the heart remains unclear (see Table 2, Figure 1).
Table 2.
TABLE 2. Mitochondrial defects caused by nuclear encoded genes.
| Mitochondrial defects - nuclear encoded (nDNA) 60 | ||||||
| OXPHOS (structural proteins and assembly factors) | ||||||
| Complex I | Complex II | Complex III | Complex IV | Complex V | ||
| NDUFS1 | NDUFS2 | SDH-A | CYC1 | COX4I1 | COX4I2 | ATP5E |
| NDUFS3 | NDUFS4 | SDH-B | UQCRC2 | COX5A | COX6B1 | ATP5A1 |
| NDUFS6 | NDUFS7 | SDH-C | UQCRB | COX6A1 | COX8A | ATP8A2 |
| NDUFS8 | NDUFB3 | SDH-D | UQCRQ | COX7B | COX15 | ATPAF2 |
| NDUFB9 | NDUFB10 | SDHAF1 | BCS1L | SURF1 | COX20 | TMEM70 |
| NDUFB11 | NDUFV1 | SDHF2 | LYRM7 | SCO1 | COA3 | |
| NDUFA2 | NDUFA9 | UQCC2 | SCO2 | COA5 | ||
| NDUFA10 | NDUFA11 | UQCC3 | COX10 | COA6 | ||
| NDUFA12 | NDUFA13 | COX14 | COA7 | |||
| NDUFAF1 | NDUFAF2 | PET100 | LRPPRC | |||
| NDUFAF3 | NDUFAF4 | APOPT1 | FASTKD2 | |||
| NDUFAF5 | NDUFAF6 | TACO1 | ||||
| NUBPL | FOXRED | |||||
| ACAD9 | ||||||
| mtDNA maintenance | ||||||
| POLG | POLG2 | ANT1 | MPV17 | OPA1 | MFN2 | C10ORF2 |
| FBXL4 | FBXL4 | RRM2B | SUCLA2 | SUCLG1 | TK2 | TFAM |
| MGME1 | ||||||
| Mitochondrial Import | ||||||
| DDP | DNAJC19 | |||||
| Mitochondrial Protein Synthesis | ||||||
| AARS2 | CARS2 | DARS2 | EARS2 | FARS2 | GARS2 | HARS2 |
| IARS2 | KARS | LARS | LARS2 | NARS2 | PARS2 | RARS2 |
| SARS2 | TARS2 | VARS2 | YARS2 | EFG1 | TSFM | TUFM |
| GTPBP3 | MTFMT | MTO1 | TRMT5 | TRMT10C | TRMU | GFM1 |
| GFM2 | C12orf65 | RMND1 | MRPL3 | MRPS7 | MRPL12 | MRPS16 |
| MRPS22 | MRPL44 | PUS1 | ||||
| Iron Homeostasis | ||||||
| FRDA | ABCB7 | GLRX5 | ISCU | BOLA3 | NFU1 | ISCA2 |
| IBA57 | LYRM4 | LYRM7 | FDXL1 | |||
| Coenzyme Q10 biogenesis | ||||||
| COQ2 | COQ4 | COQ5 | COQ6 | COQ7 | COQ9 | APTX |
| PDSS1 | PDSS2 | CABC1 | ||||
| Mitochondrial quality control | ||||||
| SPG7 | AFG3L2 | |||||
| Mitochondrial Integrity | ||||||
| DLP1 | TAZ1 | RMRP | ||||
| Mitochondrial Metabolism | ||||||
| PDHA1 | ETHE1 | ATAD3 | ||||
Mutations in the mitochondrial translation machinery have also been associated with cardiomyopathy. Primary defects produce a decreased synthesis of mitochondrial polypeptides, but ultimately also impair mitochondrial bioenergetics and cause cellular stress. Mutations in the 16S mt-rRNA, and the m.1555A>G mutation in the 12S mt-rRNA, have been associated with hypertrophic and restrictive cardiomyopathy 78,125,126. Mutations in ribosomal proteins (MRPL3 and MRPL44) and the translation elongation factor (TSFM) can cause cardiomyopathy, together with multi-organic disease 79,127,128,129,130. Finally, defects in mitochondrial tRNAs can be linked to isolated cardiomyopathy or multi-organic disfunction 131,132,133 (see Table 1 and Table 2).
Alterations of lipid metabolism inside mitochondria can also be a determinant for cardiac disease. Barth syndrome is an x-linked autosomal recessive disease, characterized by cardiomyopathy, skeletal myopathy, neutropenia, growth retardation, and 3-methylglutaconic acidurea 80,134,135,136 . This disorder is caused by mutations in the Tafazzin protein, TAZ1, a mitochondrial acyl-transferase involved in the biogenesis of cardiolipin (CL), a phospholipid almost exclusively found in the inner mitochondrial membrane 81,137. The adequate presence of CL is required for structural stability of many critical protein complexes in the mitochondrial membrane and it is therefore essential for many mitochondrial processes ranging from protein import, cristae morphology, function of the respiratory chain or cell stress signaling 82,136. Interestingly, oxidation of CL causes loss of interaction with cytochrome c, a pre-requisite for triggering apoptosis. Oxidized CL has been found to be involved in the opening of the mitochondrial permeability transition pore (MPTP). In addition, CL is exposed to the outer mitochondrial membrane during apoptosis, where it is used as a binding platform for pro-apoptotic factors. Therefore, CL homeostasis plays an important role in cardiomyocyte programmed death upon ischaemia or reperfusion (Figure 1) 83,136.
Mutations in another lipid related enzme, the acylglycerol kinase AGK, have been associated with hypertrophic cardiomyopathy, myopathy, cataracts, exercise intolerance and lactic acidosis (Sengers syndrome). AGK was recently described as a component of the carrier protein translocase of the inner membrane (TIM22) 84,138,139, meaning that a defective import of carrier proteins alters mitochondrial metabolism and may disturb the function of the heart.
Neurological disorders
Similar to previously described organs and tissues and due to the high energy demands, neurological complications are commonly linked to mitochondrial disfunction. Indeed, some of the most known mitochondrial syndromes caused by abnormalities in the mtDNA present with drastic neurological symptoms: Kearns-Sayre syndrome (KSS), a multisystem disorder with progressive external ophthalmoplegia, pigmentary retinopathy, heart block and frequently other signs like ataxia, dementia or endocrine problems is associated with single deletions of mtDNA 140. MELAS (Mitochondrial Myopathy, Encephalopathy, Lactic Acidosis and Stroke-like Episodes) is caused in 80% of the cases by the m.3243A>G mutation in the tRNALEU(UUR) gene, although there have been other mutations described in protein coding genes 35,36,40,80,136,141. MERRF (Myoclonic Epilepsy and Ragged-Red Fiber), which usually also presents with cerebellar ataxia is mainly caused by mutations in the tRNAlys gene (m8344A>G, m8356T>C, m.8363G>A), being the m.8344A>G the most frequent of them 49,51,53,142. The previously described defects affect the gene expression machinery of the mitochondrial genome and will generally affect mitochondrial protein synthesis, moreover an increased ROS production has been described in cybrids carrying the MELAS m.3243A>G mutation or the KSS associated common deletion Δ4977 89,143. In addition, there are mutations described in protein coding genes or in mitochondrial nuclear genes that would only affect individual complexes of the respiratory chain: NARP (Neuropathy, Ataxia and Retinitis Pigmentosa) has been mainly associated to the m.8933T>G/C mutation in the complex V subunit mt-ATP6 33. NARP patient derived cells were also found to have increased ROS production and decreased levels of ATP production 143. Leigh syndrome is a progressive neurometabolic disorder that usually presents with seizures, hypotonia, fatigue, nystagmus, poor reflexes, eating and swallowing difficulties, breathing problems, poor motor function, and ataxia. This unique mitochondrial disorder is found to be caused by both mutations in the mtDNA and the nDNA. Mutations in many different genes have been identified to be the origin of Leigh syndrome, including mtDNA subunits of the complex I, IV and V, mt-tRNAs, nuclear encoded subunits of complex I, IV and II, the pyruvate dehydrogenase complex, or some assembly factors of the cytochrome c oxidase (SURF1, SCO1, SCO2, COX10, COX15) or complex III (BSC1L) 144. Cells derived from patients with 3 different complex I mutations and Leigh syndrome exhibited increased ROS production 143,145 (see Table 1 and Table 2, Figure 1).
As already mentioned, many more genes are being identified as the reason behind mitochondrial dysfunction. Defects in mtDNA maintenance may result into defective mtDNA replication and lead to quantitative loss of mtDNA (mtDNA depletion) or qualitative one (mtDNA deletion). Downstream perturbation of mitochondrial protein synthesis will final lead to a bioenergetics defect. MPV17 is a mitochondrial inner membrane protein involved in maintenance of mtDNA. It is believed to be involved in the import of deoxynucleotides into mitochondria. Pathogenic variants in MPV17 have been reported to cause hepatocerebral mtDNA depletion syndrome with liver failure, de velopment delay and other neurological manisfestations 146,147. In addition, infantile Navajo neuropathy (NNH), a neurohepatological disorder prevalently present among Navajo children in the southwestern of USA has been found to be caused by mutations in MPV17 148. A recent report analysing new pathological variants in MPV17 showed that most patients exhibited a single or combined respiratory chain complex activity decrease 149 (see Table 2).
Aminoacyl-tRNA synthetases (ARS), are a family of proteins encoded in the nucleus and present in either the cytosol or mitochondria that ensure the proper conjugation of an amino acid with its cognate tRNA molecules. All mt-ARS are synthesized in the cytosol, imported to mitochondria due to an N-terminal targeting sequence (presequence) which is cleaved upon translocation to the matrix. Pathogenic variants of mt-ARS will affect mitochondrial translation and have been implicated in human neurological disorders of the brain, spinal cord and motor neurons in addition to other symptoms. Some of the most typical presentations are leukoencephalopathy with involvement of the brainstem and spinal cord and high lactate due to mt-aspartyl-tRNA synthetase (DARS2) mutations 150, leukoencephalopathy with thalamus and brainstem involvement and high lactate, caused by mt-glutamyl- tRNA synthetase (EARS2 151). However, there are other mt-ARS mutations which may also produce white matter lesions. The similar symptoms shown by ARS mutations may imply a shared mechanism of disease, however such a mechanism has not been yet demonstrated. Among the possible molecular reasons are: a reduced aminoacylation activity, altered dimerization, mislocalization, gain of function though pathogenic interactions and loss of noncanonical function 152 (see Table 2).
NEW STRATEGIES TO FIGHT MITOCHONDRIAL DERIVED STRESS
Nowadays there is no actual treatment for mitochondrial diseases. Nevertheless, in the last years a number of therapeutic strategies have been proposed, mainly in animal models. They can be classified into those acting on common pathways, and therefore applicable to different diseases, and those which aim to ameliorate a particular disorder (Figure 1) 153.
Those tissues or organs affected by decreased ATP production, and therefore impaired bioenergetics, can benefit from increased mitochondrial mass and activity. The transcriptional co-activator peroxisome proliferator activated receptor-1alpha (PGC1alpha) is the master regulator of mitochondrial biogenesis. It increases the activity of several transcription factors, like the nuclear respiratory factors (NR1 and NR2), thereby controlling the expression of OXPHOS related genes. In addition, PGC1alpha interacts with the peroxisomal proliferator activator receptors (PPARs), which regulate the expression of fatty acid oxidation genes 154. PGC1alpha is activated either by deacetylation by Sirt1, or phosphorylation by AMPK, both of which can be modulated pharmacologically 155.Under physiological conditions, PCG1alpha shows its highest expression levels in the heart, and mouse models lacking this protein have shown a normal cardiac function in unstimulated conditions. However, an impaired cardiac function was observed during certain stress conditions, like intense exercise or aortic constriction. Thus, the physiological role of PCG1alpha seems to be in fighting cellular stress 156.
Another possible strategy is to bypass the block in the respiratory chain from specific complex defects. In such a way, electrons would flow again and reduce ROS production. Concomitantly, unaffected complexes would pump protons across the inner membrane and increase ATP production. The yeast Saccharomyces cerevisiae NADH reductase (Ndi1), which transfers electrons from NADH to coenzyme Q (CoQ), has been used to bypass CI defects 157. In a similar approach, the alternative oxidase (AOX), which transfers electrons from CoQ to molecular oxygen in different organisms, has been used to bypass CIII and IV defects in cell culture 158 and to ameliorate to different extent respiratory defects in fly models 159,160. The enzyme has been successfully expressed in murine models 161, however correction of respiratory chain defects has not been shown yet in vivo in mammals.
As previously described (see above), the dynamin-like GTPAse OPA1 is required for proper mitochondrial shaping. Regulating fission and fusion helps fight mitochondrial malfunction. Increasing the expression of long isoforms of OPA1 improves respiration efficiency by enhancing supercomplex assembly and protects in vivo from many insults, such as ischemia/reperfusion, denervation/induced muscle atrophy, and OXPHOS deficiency 89,162,163.
In order to cope with increased oxidative damage generated in damaged mitochondria, different small molecules with antioxidant properties have been tested. Some examples, like Idebenone, lipoic acid, or Coenzyme Q10 , directly transfer electrons to the respiratory chain and bypass defective complexes. Others, like EPI-743 and RP103, enhance the biogenesis of glutathione, an important cellular antioxidant. KH176, can reduce altered cellular ROS levels and protect OXPHOS deficient cells against redox stress by targeting the Thioredoxin/Peroxiredoxin system 164. MTP-131 is a member of the Szeto‐Schiller (SS) peptide family and binds to CL. It increases OXPHOS capacity and improves the way mitochondria respond to metabolic changes. L-Arginine, a donor of nitric oxide, which thus regulates vascular tone, was shown to induce an improvement in aerobic capacity and muscle metabolism in models for mitochondrial disease 165.
Finally, genetic approaches can be used to correct mutations at a genomic level. Mitochondrially targeted restriction endonucleases have been used to shift heteroplasmy levels in cell lines with mutations in mtDNA and in heteroplasmic mice. Introduction of TALE and zinc finger nucleases (TALEN and ZFN) enabled the addition of specificity to the nucleases so that mutant DNA molecules could be selected for by directing unspecific restriction enzyme (FokI) to appropriate specific sequence assembling ZFN or TALE modules 166,167. However, this approach requires very large constructs that do not so easily fit into adeno-associated viruses (AVV) vectors. The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) system is a bacterial immune system that has been modified for genome engineering. Due to the simplicity and adaptability of this technique, CRISPR has quickly displaced the previously established TALENs or ZFNs for genome engineering. CRISPR consists of two elements: a guide RNA (gRNA) and a non-specific CRISPR-associated endonuclease (Cas9). The gRNA is a short synthetic RNA composed of a "scaffold", necessary for Cas9 binding, and a 20 nt targeting sequence that is specific to the gene of interest 168. CRISPR was originally employed to knock-out target genes, but it has also been used to chromosomally modify or tag proteins, and to activate or repress target genes. However, the viability of this approach to target mitochondrial genes, mainly because of the requirement of a reliable nucleotide import system into mitochondria is not yet clear 169.
CONCLUDING REMARKS
Mitochondrial diseases show very complex and various clinical presentations. Because of the dual genetic origin of mitochondrial proteins, the number of genes susceptible of causing a mitochondrial pathology is large. Thus, the diagnostic process of mitochondrial diseases is usually complicated and very long, and in many cases although there is a clear suspicion of a mitochondrial defect, the final defect behind the phenotype remains undercover 170.
Despite the heterogeneity of the diseases and the genetic defects the final kind of cellular stresses are similar. In the most cases, there is a bioenergetic defect or an increased production of ROS. It remains unclear why although many genetic defects are present in the whole body, only certain tissues are affected. There may be different mechanisms to cope with mitochondrial stress, which may be tissue specific 73. Indeed, disease models of mtDNA replication machinery failure have been linked to imbalance of the cellular dNTPs pool and consequently to increased glutathione biogenesis through de novo serine biogenesis. This metabolic switch was proposed to be a specific and rapid response to cellular stress/mtDNA damage in skeletal muscle and heart 171. Together with this pathway, transcriptional response and mitochondrial unfolded protein response constitute the integrated mitochondrial stress response (ISRmt), which is controlled by the metabolic signalling regulator mTORC1 in muscle. However, long-term activation of cellular stress responses may be detrimental since chronic upregulation of anabolism contributes to mitochondrial myopathy pathogenesis 172. In addition, there is a certain multifactorial component in mitochondrial diseases. In some cases, ancient mt-rRNAs mutations rendered no adverse phenotype unless some environmental factors were used 72. Therefore, the different incorporation and tolerance of tissues and organs for different xenobiotics may be different. Population variation plays also an important role in enhancing/ diminishing mitochondrial-related phenotypes, like population polymorphisms in mt-rRNAs and side effects derived from antibiotic treatment 173. Nevertheless, a deeper investigation will be required to understand tissue specificity of mitochondrial diseases.
There have been several approaches proposed to treat mitochondrial diseases, however their application to the clinics is still a challenge 153. Nevertheless, the discovery of new genome editing tools and the development of stem cell technologies will provide open new avenues of possibilities for the treatment of mitochondrial diseases.
Acknowledgments
We are indebted to Sylvie Callegari for critical reading of the manuscript and to Peter Rehling and Alexander Schendzielorz for fruitful discussion.
Abbreviations
- ADOA
autosomal dominant optic atrophy
- AROA
autosomal recessive optic atrophy
- ARS
aminoacyl-tRNA synthetase
- CL
cardiolipin
- CRISPR
clustered regularly interspaced short palindromic repeats
- LHON
Leber’s hereditary optic neuropathy
- mt
mitochondrial
- OXPHOS
oxidative phosphorylation
- ROS
reactive oxygen species
References
- 1.Mick DU, Dennerlein S, Wiese H, Reinhold R, Pacheu-Grau D, Lorenzi I, Sasarman F, Weraarpachai W, Shoubridge EA, Warscheid B, Rehling P. MITRAC links mitochondrial protein translocation to respiratory-chain assembly and translational regulation. Cell. 2012;151(7):1528–1541. doi: 10.1016/j.cell.2012.11.053. [DOI] [PubMed] [Google Scholar]
- 2.Yoboue ED, Devin A. Reactive oxygen species-mediated control of mitochondrial biogenesis. Int J Cell Biol. 2012;2012:403870. doi: 10.1155/2012/403870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid Med Cell Longev. 2016;2016:1245049. doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busiello RA, Savarese S, Lombardi A. Mitochondrial uncoupling proteins and energy metabolism. Front Physiol. 2015;6:36. doi: 10.3389/fphys.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penna E, Espino J, De Stefani D, Rizzuto R. The MCU complex in cell death. Cell calcium. 2018;69:73–80. doi: 10.1016/j.ceca.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Raffaello A, Mammucari C, Gherardi G, Rizzuto R. Calcium at the Center of Cell Signaling: Interplay between Endoplasmic Reticulum, Mitochondria, and Lysosomes. Trends Biochem Sci. 2016;41(12):1035–1049. doi: 10.1016/j.tibs.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lill R. Function and biogenesis of iron-sulphur proteins. Nature. 2009;460(7257):831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- 8.Cardenas-Rodriguez M, Chatzi A, Tokatlidis K. Iron-sulfur clusters: from metals through mitochondria biogenesis to disease. J Biol Inorg Chem. 2018;23(4):509–520. doi: 10.1007/s00775-018-1548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montoya J, López-Pérez MJ, Ruiz-Pesini E. Mitochondrial DNA transcription and diseases: past, present and future. Biochim Biophys Acta. 2006;1757(9-10):1179–1189. doi: 10.1016/j.bbabio.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Zinovkina LA. Mechanisms of Mitochondrial DNA Repair in Mammals. Biochemistry. 2018;83(3):233–249. doi: 10.1134/S0006297918030045. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Pesini E, Mishmar D, Brandon M, Procaccio V, Wallace DC. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science. 2004;303(5655):223–226. doi: 10.1126/science.1088434. [DOI] [PubMed] [Google Scholar]
- 12.Montoya J, López-Gallardo E, Herrero-Martín MD, Martínez-Romero I, Gómez-Durán A, Pacheu D, Carreras M, Díez-Sánchez C, López-Pérez MJ, Ruiz-Pesini E. Diseases of the human mitochondrial oxidative phosphorylation system. Adv Exp Med Biol. 2009;652(Chapter 5):47–67. doi: 10.1007/978-90-481-2813-6_5. [DOI] [PubMed] [Google Scholar]
- 13.Zeviani M, Moraes CT, DiMauro S, Nakase H, Bonilla E, Schon EA, Rowland LP. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1988;38(8):1339–1339. doi: 10.1212/WNL.38.8.1339. [DOI] [PubMed] [Google Scholar]
- 14.Lestienne P, Ponsot G. KEARNS-SAYRE SYNDROME WITH MUSCLE MITOCHONDRIAL DNA DELETION. The Lancet. 1988;331(8590):885. doi: 10.1016/S0140-6736(88)91632-7. [DOI] [PubMed] [Google Scholar]
- 15.de Vries DD, van Engelen BGM, Gabreëls FJM, Ruitenbeek W, van Oost BA. A second missense mutation in the mitochondrial ATPase 6 gene in Leigh's syndrome. Ann Neurol. 2004;34(3):410–412. doi: 10.1002/ana.410340319. [DOI] [PubMed] [Google Scholar]
- 16.Carrozzo R, Murray J, Santorelli FM, Capaldi RA. The T9176G mutation of human mtDNA gives a fully assembled but inactive ATP synthase when modeled in Escherichia coli. FEBS Lett. 2000;486(3):297–299. doi: 10.1016/S0014-5793(00)02244-4. [DOI] [PubMed] [Google Scholar]
- 17.Crimi M, Papadimitriou A, Galbiati S, Palamidou P, Fortunato F, Bordoni A, Papandreou U, Papadimitriou D, Hadjigeorgiou GM, Drogari E, Bresolin N, Comi GP. A New Mitochondrial DNA Mutation in ND3 Gene Causing Severe Leigh Syndrome with Early Lethality. Pediatric Res. 2004;55(5):842–846. doi: 10.1203/01.PDR.0000117844.73436.68. [DOI] [PubMed] [Google Scholar]
- 18.McFarland R, Kirby DM, Fowler KJ, Ohtake A, Ryan MT, Amor DJ, Fletcher JM, Dixon JW, Collins FA, Turnbull DM, Taylor RW, Thorburn DR. De novo mutations in the mitochondrial ND3 gene as a cause of infantile mitochondrial encephalopathy and complex I deficiency. Ann Neurol. 2004;55(1):58–64. doi: 10.1002/ana.10787. [DOI] [PubMed] [Google Scholar]
- 19.Lebon S, Chol M, Benit P, Mugnier C, Chretien D, Giurgea I, Kern I, Girardin E, Hertz-Pannier L, de Lonlay P, Rötig A, Rustin P, Munnich A. Recurrent de novo mitochondrial DNA mutations in respiratory chain deficiency. J Med Genet. 2003;40(12):896–899. doi: 10.1136/jmg.40.12.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deschauer M, Bamberg C, Claus D, Zierz S, Turnbull DM, Taylor RW. Late-onset encephalopathy associated with a C11777A mutation of mitochondrial DNA. Neurology. 2003;60(8):1357–1359. doi: 10.1212/01.WNL.0000055869.99975.4B. [DOI] [PubMed] [Google Scholar]
- 21.Komaki H, Akanuma J, Iwata H, Takahashi T, Mashima Y, Nonaka I, Goto Y-I. A novel mtDNA C11777A mutation in Leigh syndrome. Mitochondrion. 2003;2(4):293–304. doi: 10.1016/S1567-7249(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 22.Taylor RW, Morris AA, Hutchinson M, Turnbull DM. Leigh disease associated with a novel mitochondrial DNA ND5 mutation. Eur J Hum Genet. 2002;10(2):141–144. doi: 10.1038/sj.ejhg.5200773. [DOI] [PubMed] [Google Scholar]
- 23.Kirby DM, Kahler SG, Freckmann ML, Reddihough D, Thorburn DR. Leigh disease caused by the mitochondrial DNA G14459A mutation in unrelated families. Ann Neurol. 2000;48(1):102–104. doi: 10.1002/1531-8249(200007)48:1<102::AID-ANA15>3.3.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 24.Jun AS, Brown MD, Wallace DC. A mitochondrial DNA mutation at nucleotide pair 14459 of the NADH dehydrogenase subunit 6 gene associated with maternally inherited Leber hereditary optic neuropathy and dystonia. Proc Natl Acad Sci U S A. 1994;91(13):6206–6210. doi: 10.1073/pnas.91.13.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solano A, Roig M, Vives-Bauza C, Hernandez-Peña J, Garcia-Arumi E, Playan A, López-Pérez MJ, Andreu AL, Montoya J. Bilateral striatal necrosis associated with a novel mutation in the mitochondrial ND6 gene. Ann Neurol. 2003;54(4):527–530. doi: 10.1002/ana.10682. [DOI] [PubMed] [Google Scholar]
- 26.Ugalde C, Triepels RH, Coenen MJH, Van Den Heuvel LP, Smeets R, Uusimaa J, Briones P, Campistol J, Majamaa K, Smeitink JAM, Nijtmans LGJ. Impaired complex I assembly in a Leigh syndrome patient with a novel missense mutation in the ND6 gene. Ann Neurol. 2003;54(5):665–669. doi: 10.1002/ana.10734. [DOI] [PubMed] [Google Scholar]
- 27.Wallace D, Singh G, Lott M, Hodge J, Schurr T, Lezza A, Elsas L, Nikoskelainen E. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 28.Howell N, Bindoff LA, McCullough DA, Kubacka I, Poulton J, Mackey D, Taylor L, Turnbull DM. Leber hereditary optic neuropathy: identification of the same mitochondrial ND1 mutation in six pedigrees. Am J Hum Genet. 1991;49(5):939–950. [PMC free article] [PubMed] [Google Scholar]
- 29.Huoponen K, Vilkki J, Aula P, Nikoskelainen EK, Savontaus ML. A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am J Hum Genet. 1991;48(6):1147–1153. [PMC free article] [PubMed] [Google Scholar]
- 30.Brown MD, Voljavec AS, Lott MT, MacDonald I, Wallace DC. Leber's hereditary optic neuropathy: a model for mitochondrial neurodegenerative diseases. FASEB J. 1992;6(10):2791–2799. doi: 10.1096/fasebj.6.10.1634041. [DOI] [PubMed] [Google Scholar]
- 31.Howell N, Kubacka I, Xu M, McCullough DA. Leber hereditary optic neuropathy: involvement of the mitochondrial ND1 gene and evidence for an intragenic suppressor mutation. Am J Hum Genet. 1991;48(5):935–942. [PMC free article] [PubMed] [Google Scholar]
- 32.Johns DR, Neufeld MJ, Park RD. An ND-6 mitochondrial DNA mutation associated with Leber hereditary optic neuropathy. Biochem Biophys Res Commun. 1992;187(3):1551–1557. doi: 10.1016/0006-291x(92)90479-5. [DOI] [PubMed] [Google Scholar]
- 33.Holt IJ, Harding AE, Petty RK, Morgan-Hughes JA. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990;46(3):428–433. [PMC free article] [PubMed] [Google Scholar]
- 34.Harding AE, Holt IJ, Sweeney MG, Brockington M, Davis MB. Prenatal diagnosis of mitochondrial DNA8993 T----G disease. Am J Hum Genet. 1992;50(3):629–633. [PMC free article] [PubMed] [Google Scholar]
- 35.Kirby DM, McFarland R, Ohtake A, Dunning C, Ryan MT, Wilson C, Ketteridge D, Turnbull DM, Thorburn DR, Taylor RW. Mutations of the mitochondrial ND1 gene as a cause of MELAS. J Med Genet. 2004;41(10):784–789. doi: 10.1136/jmg.2004.020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santorelli FM, Tanji K, Kulikova R, Shanske S, Vilarinho L, Hays AP, DiMauro S. Identification of a novel mutation in the mtDNA ND5 gene associated with MELAS. Biochem Biophys Res Commun. 1997;238(2):326–328. doi: 10.1006/bbrc.1997.7167. [DOI] [PubMed] [Google Scholar]
- 37.Corona P, Antozzi C, Carrara F, D'Incerti L, Lamantea E, Tiranti V, Zeviani M. A novel mtDNA mutation in the ND5 subunit of complex I in two MELAS patients. Ann Neurol. 2001;49(1):106–110. doi: 10.1002/1531-8249(200101)49:1<106::aid-ana16>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 38.Darin N, Kollberg G, Moslemi A-R, Tulinius M, Holme E, Grönlund MA, Andersson S, Oldfors A. Mitochondrial myopathy with exercise intolerance and retinal dystrophy in a sporadic patient with a G583A mutation in the mt tRNA(phe) gene. Neuromuscul Disord. 2006;16(8):504–506. doi: 10.1016/j.nmd.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Hanna MG, Nelson IP, Morgan-Hughes JA, Wood NW. MELAS: a new disease associated mitochondrial DNA mutation and evidence for further genetic heterogeneity. J Neurol Neurosurg Psychiatry. 1998;65(4):512–517. doi: 10.1136/jnnp.65.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goto Y, Nonaka I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- 41.Sato W, Hayasaka K, Shoji Y, Takahashi T, Takada G, Saito M, Fukawa O, Wachi E. A mitochondrial tRNA(Leu)(UUR) mutation at 3,256 associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). Biochem Mol Biol Int. 1994;33(6):1055–1061. [PubMed] [Google Scholar]
- 42.Moraes CT, Ciacci F, Bonilla E, Jansen C, Hirano M, Rao N, Lovelace RE, Rowland LP, Schon EA, DiMauro S. Two novel pathogenic mitochondrial DNA mutations affecting organelle number and protein synthesis. Is the tRNA(Leu(UUR)) gene an etiologic hot spot? J Clin Invest. 1993;92(6):2906–2915. doi: 10.1172/JCI116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashi J, Ohta S, Takai D, Miyabayashi S, Sakuta R, Goto Y, Nonaka I. Accumulation of mtDNA with a mutation at position 3271 in tRNA(Leu)(UUR) gene introduced from a MELAS patient to HeLa cells lacking mtDNA results in progressive inhibition of mitochondrial respiratory function. Biochem Biophys Res Commun. 1993;197(3):1049–1055. doi: 10.1006/bbrc.1993.2584. [DOI] [PubMed] [Google Scholar]
- 44.Sakuta R, Goto Y, Horai S, Nonaka I. Mitochondrial DNA mutations at nucleotide positions 3243 and 3271 in mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes: a comparative study. J Neurol Sci. 1993;115(2):158–160. doi: 10.1016/0022-510x(93)90219-o. [DOI] [PubMed] [Google Scholar]
- 45.Goto Y, Nonaka I, Horai S. A new mtDNA mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS). Biochim Biophys Acta. 1991;1097(3):238–240. doi: 10.1016/0925-4439(91)90042-8. [DOI] [PubMed] [Google Scholar]
- 46.Goto YI, Tsugane K, Tanabe Y, Nonaka I, Horai S. A New Point Mutation at Nucleotide Pair-3291 of the Mitochondrial Transfer-RNALeu(Uur) Gene in a Patient with Mitochondrial Myopathy, Encephalopathy, Lactic-Acidosis, and Stroke-Like Episodes (MELAS). Biochem Biophys Res Commun. 1994;202(3):1624–1630. doi: 10.1006/bbrc.1994.2119. [DOI] [PubMed] [Google Scholar]
- 47.Bataillard M, Chatzoglou E, Rumbach L, Sternberg D, Tournade A, Laforet P, Jardel C, Maisonobe T, Lombes A. Atypical MELAS syndrome associated with a new mitochondrial tRNA glutamine point mutation. Neurology. 2001;56(3):405–407. doi: 10.1212/WNL.56.3.405. [DOI] [PubMed] [Google Scholar]
- 48.Shoffner JM, Lott MT, Lezza AM, Seibel P, Ballinger SW, Wallace DC. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- 49.Wallace DC, Zheng XX, Lott MT, Shoffner JM, Hodge JA, Kelley RI, Epstein CM, Hopkins LC. Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell. 1988;55(4):601–610. doi: 10.1016/0092-8674(88)90218-8. [DOI] [PubMed] [Google Scholar]
- 50.Masucci JP, Davidson M, Koga Y, Schon EA, King MP. In vitro analysis of mutations causing myoclonus epilepsy with ragged-red fibers in the mitochondrial tRNA(Lys)gene: two genotypes produce similar phenotypes. Mol Cell Biol. 1995;15(5):2872–2881. doi: 10.1128/MCB.15.5.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeviani M, Muntoni F, Savarese N, Serra G, Tiranti V, Carrara F, Mariotti C, DiDonato S. A MERRF/MELAS overlap syndrome associated with a new point mutation in the mitochondrial DNA tRNA(Lys) gene. Eur J Hum Genet. 1993;1(1):80–87. doi: 10.1159/000472390. [DOI] [PubMed] [Google Scholar]
- 52.Silvestri G, Moraes CT, Shanske S, Oh SJ, DiMauro S. A new mtDNA mutation in the tRNA(Lys) gene associated with myoclonic epilepsy and ragged-red fibers (MERRF). Am J Hum Genet. 1992;51(6):1213–1217. [PMC free article] [PubMed] [Google Scholar]
- 53.Ozawa M, Nishino I, Horai S, Nonaka I, Goto Y-I. Myoclonus epilepsy associated with ragged-red fibers: A G-to-A mutation at nucleotide pair 8363 in mitochondrial tRNALys in two families. Muscle Nerve. 1997;20(3):271–278. doi: 10.1002/(SICI)1097-4598(199703)20:3<T271::AID-MUS2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 54.Reid FM, Vernham GA, Jacobs HT. A novel mitochondrial point mutation in a maternal pedigree with sensorineural deafness. Human Mut. 1994;3(3):243–247. doi: 10.1002/humu.1380030311. [DOI] [PubMed] [Google Scholar]
- 55.Sue CM, Tanji K, Hadjigeorgiou G, Andreu AL, Nishino I, Krishna S, Bruno C, Hirano M, Shanske S, Bonilla E, Fischel-Ghodsian N, DiMauro S, Friedman R. Maternally inherited hearing loss in a large kindred with a novel T7511C mutation in the mitochondrial DNA tRNA(Ser(UCN)) gene. Neurology. 1999;52(9):1905–1908. doi: 10.1212/WNL.52.9.1905. [DOI] [PubMed] [Google Scholar]
- 56.Hema Bindu L, Reddy PP. Genetics of aminoglycocide-induced and prelingual non-syndromic mitochondrial hearing impairment: A review. Int J Audiol. 2009;47(11):702–707. doi: 10.1080/14992020802215862. [DOI] [PubMed] [Google Scholar]
- 57.Fischel-Ghodsian N, Prezant TR, Bu X, Öztas S. Mitochondrial ribosomal RNA gene mutation in a patient with sporadic aminoglycoside ototoxicity. Am J Otolaryngol. 1993;14(6):399–403. doi: 10.1016/0196-0709(93)90113-L. [DOI] [PubMed] [Google Scholar]
- 58.Hutchin T, Haworth I, Higashi K, Fischel-Ghodsian N, Stoneking M, Saha N, Arnos C, Cortopassi G. A molecular basis for human hypersensitivity to aminoglycoside antibiotics. Nucleic Acids Research. 1993;21(18):4174–4179. doi: 10.1093/nar/21.18.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prezant TR, Agapian JV, Bohlman MC, Bu X, Oztas S, Qiu WQ, Arnos KS, Cortopassi GA, Jaber L, Rotter JI. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet. 1993;4(3):289–294. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- 60.Lott MT, Leipzig JN, Derbeneva O, Xie HM, Chalkia D, Sarmady M, Procaccio V, Wallace DC. mtDNA Variation and Analysis Using Mitomap and Mitomaster. Curr Protoc Bioinformatics. 2013;44(1):1.23.1–1.2326. doi: 10.1002/0471250953.bi0123s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kral A, O'Donoghue GM. Profound deafness in childhood. N Engl J Med. 2010;363(15):1438–1450. doi: 10.1056/NEJMra0911225. [DOI] [PubMed] [Google Scholar]
- 62.Mutai H, Watabe T, Kosaki K, Ogawa K, Matsunaga T. Mitochondrial mutations in maternally inherited hearing loss. BMC Med Genet. 2017;18(1):32. doi: 10.1186/s12881-017-0389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pitceathly RDS, Taanman J-W, Rahman S, Meunier B, Sadowski M, Cirak S, Hargreaves I, Land JM, Nanji T, Polke JM, Woodward CE, Sweeney MG, Solanki S, Foley AR, Hurles ME, Stalker J, Blake J, Holton JL, Phadke R, Muntoni F, Reilly MM, Hanna MG, UK10K Consortium. COX10 mutations resulting in complex multisystem mitochondrial disease that remains stable into adulthood. JAMA Neurol. 2013;70(12):1556–1561. doi: 10.1001/jamaneurol.2013.3242. [DOI] [PubMed] [Google Scholar]
- 64.Antonicka H, Leary SC, Guercin G-H, Agar JN, Horvath R, Kennaway NG, Harding CO, Jaksch M, Shoubridge EA. Mutations in COX10 result in a defect in mitochondrial heme A biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated COX deficiency. Hum Mol Genet. 2003;12(20):2693–2702. doi: 10.1093/hmg/ddg284. [DOI] [PubMed] [Google Scholar]
- 65.Hinson JT, Fantin VR, Schönberger J, Breivik N, Siem G, McDonough B, Sharma P, Keogh I, Godinho R, Santos F, Esparza A, Nicolau Y, Selvaag E, Cohen BH, Hoppel CL, Tranebjaerg L, Eavey RD, Seidman JG, Seidman CE. Missense mutations in the BCS1L gene as a cause of the Björnstad syndrome. N Engl J Med. 2007;356(8):809–819. doi: 10.1056/NEJMoa055262. [DOI] [PubMed] [Google Scholar]
- 66.Fernandez-Vizarra E, Bugiani M, Goffrini P, Carrara F, Farina L, Procopio E, Donati A, Uziel G, Ferrero I, Zeviani M. Impaired complex III assembly associated with BCS1L gene mutations in isolated mitochondrial encephalopathy. Hum Mol Genet. 2007;16(10):1241–1252. doi: 10.1093/hmg/ddm072. [DOI] [PubMed] [Google Scholar]
- 67.Guan MX, Fischel-Ghodsian N, Attardi G. A biochemical basis for the inherited susceptibility to aminoglycoside ototoxicity. Hum Mol Genet. 2000;9(12):1787–1793. doi: 10.1093/hmg/9.12.1787. [DOI] [PubMed] [Google Scholar]
- 68.Iglesias E, Llobet L, Pacheu-Grau D, Gómez-Durán A, Ruiz-Pesini E. Cybrids for Mitochondrial DNA Pharmacogenomics. Drug Development Research. 2012;73(8):453–460. doi: 10.1002/ddr.21037. [DOI] [Google Scholar]
- 69.Giordano C, Pallotti F, Walker WF, Checcarelli N, Musumeci O, Santorelli F, d'Amati G, Schon EA, DiMauro S, Hirano M, Davidson MM. Pathogenesis of the deafness-associated A1555G mitochondrial DNA mutation. Biochem Biophys Res Commun. 2002;293(1):521–529. doi: 10.1016/S0006-291X(02)00256-5. [DOI] [PubMed] [Google Scholar]
- 70.Pacheu-Grau D, Gómez-Durán A, López-Pérez MJ, Montoya J, Ruiz-Pesini E. Mitochondrial pharmacogenomics: barcode for antibiotic therapy. Drug Discov Today. 2010;15(1-2):33–39. doi: 10.1016/j.drudis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 71.Emperador S, Pacheu-Grau D, Bayona-Bafaluy MP, Garrido-Pérez N, Martín-Navarro A, López-Pérez MJ, Montoya J, Ruiz-Pesini E. An MRPS12 mutation modifies aminoglycoside sensitivity caused by 12S rRNA mutations. Front Genet. 2014;5:469. doi: 10.3389/fgene.2014.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pacheu-Grau D, Gómez-Durán A, López-Gallardo E, Pinós T, Andreu AL, López-Pérez MJ, Montoya J, Ruiz-Pesini E. "Progress" renders detrimental an ancient mitochondrial DNA genetic variant. Hum Mol Genet. 2011;20(21):4224–4231. doi: 10.1093/hmg/ddr350. [DOI] [PubMed] [Google Scholar]
- 73.Raimundo N, Song L, Shutt TE, McKay SE, Cotney J, Guan M-X, Gilliland TC, Hohuan D, Santos-Sacchi J, Shadel GS. Mitochondrial stress engages E2F1 apoptotic signaling to cause deafness. Cell. 2012;148(4):716–726. doi: 10.1016/j.cell.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Finsterer J, Zarrouk-Mahjoub S, Daruich A. The Eye on Mitochondrial Disorders. J Child Neurol. 2016;31(5):652–662. doi: 10.1177/0883073815599263. [DOI] [PubMed] [Google Scholar]
- 75.Zhu Y, Gu X, Xu C. A Mitochondrial DNA A8701G Mutation Partly Associated with Maternally Inherited Hypertension and Dilated Cardiomyopathy in a Chinese Pedigree. Chin Med J. 2016;129(15):1890. doi: 10.4103/0366-6999.186656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leruez S, Amati-Bonneau P, Verny C, Reynier P, Procaccio V, Bonneau D, Milea D. Mitochondrial dysfunction affecting visual pathways. Rev Neurol. 2014;170(5):344–354. doi: 10.1016/j.neurol.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 77.Finsterer J, Mancuso M, Pareyson D, Burgunder J-M, Klopstock T. Mitochondrial disorders of the retinal ganglion cells and the optic nerve. Mitochondrion. 2017 doi: 10.1016/j.mito.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Giordano C. Efficient mitochondrial biogenesis drives incomplete penetrance in Leber's hereditary optic neuropathy. Brain. 2014;137(Pt 2):335–353. doi: 10.1093/brain/awt343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giordano C, Montopoli M, Perli E, Orlandi M, Fantin M, Ross-Cisneros FN, Caparrotta L, Martinuzzi A, Ragazzi E, Ghelli A, Sadun AA, d'Amati G, Carelli V. Oestrogens ameliorate mitochondrial dysfunction in Leber's hereditary optic neuropathy. Brain. 2011;134(Pt 1):220–234. doi: 10.1093/brain/awq276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katz BJ, Zhao Y, Warner JEA, Tong Z, Yang Z, Zhang K. A family with X-linked optic atrophy linked to the OPA2 locus Xp11.4-Xp11.2. Am J Med Genet A. 2006;140(20):2207–2211. doi: 10.1002/ajmg.a.31455. [DOI] [PubMed] [Google Scholar]
- 81.Sergouniotis PI, Perveen R, Thiselton DL, Giannopoulos K, Sarros M, Davies JR, Biswas S, Ansons AM, Ashworth JL, Lloyd IC, Black GC, Votruba M. Clinical and molecular genetic findings in autosomal dominant OPA3-related optic neuropathy. Neurogenetics. 2015;16(1):69–75. doi: 10.1007/s10048-014-0416-y. [DOI] [PubMed] [Google Scholar]
- 82.Kerrison JB, Arnould VJ, Ferraz Sallum JM, Vagefi MR, Barmada MM, Li Y, Zhu D, Maumenee IH. Genetic heterogeneity of dominant optic atrophy, Kjer type: Identification of a second locus on chromosome 18q12.2-12.3. Arch Ophthalmol. 1999;117(6):805–810. doi: 10.1001/archopht.117.6.805. [DOI] [PubMed] [Google Scholar]
- 83.Barbet F, Hakiki S, Orssaud C, Gerber S, Perrault I, Hanein S, Ducroq D, Dufier J-L, Munnich A, Kaplan J, Rozet J-M. A third locus for dominant optic atrophy on chromosome 22q. J Med Genet. 2005;42(1):e1. doi: 10.1136/jmg.2004.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carelli V, Schimpf S, Fuhrmann N, Valentino ML, Zanna C, Iommarini L, Papke M, Schaich S, Tippmann S, Baumann B, Barboni P, Longanesi L, Rugolo M, Ghelli A, Alavi MV, Youle RJ, Bucchi L, Carroccia R, Giannoccaro MP, Tonon C, Lodi R, Cenacchi G, Montagna P, Liguori R, Wissinger B. A clinically complex form of dominant optic atrophy (OPA8) maps on chromosome 16. Hum Mol Genet. 2011;20(10):1893–1905. doi: 10.1093/hmg/ddr071. [DOI] [PubMed] [Google Scholar]
- 85.Eiberg H, Hansen L, Kjer B, Hansen T, Pedersen O, Bille M, Rosenberg T, Tranebjaerg L. Autosomal dominant optic atrophy associated with hearing impairment and impaired glucose regulation caused by a missense mutation in the WFS1 gene. J Med Genet. 2006;43(5):435–440. doi: 10.1136/jmg.2005.034892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, Langer T. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol. 2014;204(6):919–929. doi: 10.1083/jcb.201308006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baker MJ, Lampe PA, Stojanovski D, Korwitz A, Anand R, Tatsuta T, Langer T. Stress-induced OMA1 activation and autocatalytic turnover regulate OPA1-dependent mitochondrial dynamics. EMBO J. 2014;33(6):578–593. doi: 10.1002/embj.201386474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou J-C, Westermann B, Rugarli EI, Langer T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187(7):1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pernas L, Scorrano L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu Rev Physiol. 2016;78:505–531. doi: 10.1146/annurev-physiol-021115-105011. [DOI] [PubMed] [Google Scholar]
- 90.Kao S-H, Yen M-Y, Wang A-G, Yeh Y-L, Lin A-L. Changes in Mitochondrial Morphology and Bioenergetics in Human Lymphoblastoid Cells With Four Novel OPA1 Mutations. Invest Ophthalmol Vis Sci. 2015;56(4):2269–2278. doi: 10.1167/iovs.14-16288. [DOI] [PubMed] [Google Scholar]
- 91.Wai T, García-Prieto J, Baker MJ, Merkwirth C, Benit P, Rustin P, Rupérez FJ, Barbas C, Ibañez B, Langer T. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science. 2015;350(6265):aad0116. doi: 10.1126/science.aad0116. [DOI] [PubMed] [Google Scholar]
- 92.Metodiev MD, Gerber S, Hubert L, Delahodde A, Chretien D, Gérard X, Amati-Bonneau P, Giacomotto M-C, Boddaert N, Kaminska A, Desguerre I, Amiel J, Rio M, Kaplan J, Munnich A, Rötig A, Rozet J-M, Besmond C. Mutations in the tricarboxylic acid cycle enzyme, aconitase 2, cause either isolated or syndromic optic neuropathy with encephalopathy and cerebellar atrophy. J Med Genet. 2014;51(12):834–838. doi: 10.1136/jmedgenet-2014-102532. [DOI] [PubMed] [Google Scholar]
- 93.Hanein S, Perrault I, Roche O, Gerber S, Khadom N, Rio M, Boddaert N, Jean-Pierre M, Brahimi N, Serre V, Chretien D, Delphin N, Fares-Taie L, Lachheb S, Rötig A, Meire F, Munnich A, Dufier J-L, Kaplan J, Rozet J-M. TMEM126A, encoding a mitochondrial protein, is mutated in autosomal-recessive nonsyndromic optic atrophy. Am J Hum Genet. 2009;84(4):493–498. doi: 10.1016/j.ajhg.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Désir J, Coppieters F, Van Regemorter N, De Baere E, Abramowicz M, Cordonnier M. TMEM126A mutation in a Moroccan family with autosomal recessive optic atrophy. Mol Vis. 2012;18:1849–1857. [PMC free article] [PubMed] [Google Scholar]
- 95.Meyer E, Michaelides M, Tee LJ, Robson AG, Rahman F, Pasha S, Luxon LM, Moore AT, Maher ER. Nonsense mutation in TMEM126A causing autosomal recessive optic atrophy and auditory neuropathy. Mol Vis. 2010;16:650–664. [PMC free article] [PubMed] [Google Scholar]
- 96.Scaglia F, Towbin JA, Craigen WJ, Belmont JW, Smith EO, Neish SR, Ware SM, Hunter JV, Fernbach SD, Vladutiu GD, Wong L-JC, Vogel H. Clinical spectrum, morbidity, and mortality in 113 pediatric patients with mitochondrial disease. Pediatrics. 2004;114(4):925–931. doi: 10.1542/peds.2004-0718. [DOI] [PubMed] [Google Scholar]
- 97.Holmgren D, Wåhlander H, Eriksson BO, Oldfors A, Holme E, Tulinius M. Cardiomyopathy in children with mitochondrial disease; clinical course and cardiological findings. Eur Heart J. 2003;24(3):280–288. doi: 10.1016/s0195-668x(02)00387-1. [DOI] [PubMed] [Google Scholar]
- 98.El-Hattab AW, Scaglia F. Mitochondrial Cardiomyopathies. Front Cardiovasc Med. 2016;3:25. doi: 10.3389/fcvm.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Finsterer J, Kothari S. Cardiac manifestations of primary mitochondrial disorders. Int J Cardiol. 2014;177(3):754–763. doi: 10.1016/j.ijcard.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 100.Rodenburg RJ. Mitochondrial complex I-linked disease. Biochim Biophys Acta. 2016;1857(7):938–945. doi: 10.1016/j.bbabio.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 101.Brunel-Guitton C, Levtova A, Sasarman F. Mitochondrial Diseases and Cardiomyopathies. Can J Cardiol. 2015;31(11):1360–1376. doi: 10.1016/j.cjca.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 102.Alston CL, Ceccatelli Berti C, Blakely EL, Oláhová M, He L, McMahon CJ, Olpin SE, Hargreaves IP, Nolli C, McFarland R, Goffrini P, O'Sullivan MJ, Taylor RW. A recessive homozygous p.Asp92Gly SDHD mutation causes prenatal cardiomyopathy and a severe mitochondrial complex II deficiency. . Hum Genet. 2015;134(8):869–879. doi: 10.1007/s00439-015-1568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andreu AL, Checcarelli N, Iwata S, Shanske S, DiMauro S. A missense mutation in the mitochondrial cytochrome b gene in a revisited case with histiocytoid cardiomyopathy. Pediatr Res. 2000;48(3):311–314. doi: 10.1203/00006450-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 104.Carossa V, Ghelli A, Tropeano CV, Valentino ML, Iommarini L, Maresca A, Caporali L, La Morgia C, Liguori R, Barboni P, Carbonelli M, Rizzo G, Tonon C, Lodi R, Martinuzzi A, De Nardo V, Rugolo M, Ferretti L, Gandini F, Pala M, Achilli A, Olivieri A, Torroni A, Carelli V. A novel in-frame 18-bp microdeletion in MT-CYB causes a multisystem disorder with prominent exercise intolerance. Hum Mutat. 2014;35(8):954–958. doi: 10.1002/humu.22596. [DOI] [PubMed] [Google Scholar]
- 105.Antonicka H, Mattman A, Carlson CG, Glerum DM, Hoffbuhr KC, Leary SC, Kennaway NG, Shoubridge EA. Mutations in COX15 produce a defect in the mitochondrial heme biosynthetic pathway, causing early-onset fatal hypertrophic cardiomyopathy. Am J Hum Genet. 2003;72(1):101–114. doi: 10.1086/345489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abdulhag UN, Soiferman D, Schueler-Furman O, Miller C, Shaag A, Elpeleg O, Edvardson S, Saada A. Mitochondrial complex IV deficiency, caused by mutated COX6B1, is associated with encephalomyopathy, hydrocephalus and cardiomyopathy. Eur J Hum Genet. 2015;23(2):159–164. doi: 10.1038/ejhg.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Massa V, Fernandez-Vizarra E, Alshahwan S, Bakhsh E, Goffrini P, Ferrero I, Mereghetti P, D'Adamo P, Gasparini P, Zeviani M. Severe infantile encephalomyopathy caused by a mutation in COX6B1, a nucleus-encoded subunit of cytochrome c oxidase. Am J Hum Genet. 2008;82(6):1281–1289. doi: 10.1016/j.ajhg.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ghosh A, Pratt AT, Soma S, Theriault SG, Griffin AT, Trivedi PP, Gohil VM. Mitochondrial disease genes COA6, COX6B and SCO2 have overlapping roles in COX2 biogenesis. Hum Mol Genet. 2016;25(4):660–671. doi: 10.1093/hmg/ddv503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jaksch M, Horvath R, Horn N, Auer DP, Macmillan C, Peters J, Gerbitz KD, Kraegeloh-Mann I, Muntau A, Karcagi V, Kalmanchey R, Lochmuller H, Shoubridge EA, Freisinger P. Homozygosity (E140K) in SCO2 causes delayed infantile onset of cardiomyopathy and neuropathy. Neurology. 2001;57(8):1440–1446. doi: 10.1212/wnl.57.8.1440. [DOI] [PubMed] [Google Scholar]
- 110.Mobley BC, Enns GM, Wong L-J, Vogel H. A novel homozygous SCO2 mutation, p.G193S, causing fatal infantile cardioencephalomyopathy. . Clin Neuropathol. 2009;28(2):143–149. doi: 10.5414/npp28143. [DOI] [PubMed] [Google Scholar]
- 111.Papadopoulou LC, Sue CM, Davidson MM, Tanji K, Nishino I, Sadlock JE, Krishna S, Walker W, Selby J, Glerum DM, Coster RV, Lyon G, Scalais E, Lebel R, Kaplan P, Shanske S, De Vivo DC, Bonilla E, Hirano M, DiMauro S, Schon EA. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat Genet. 1999;23(3):333–337. doi: 10.1038/15513. [DOI] [PubMed] [Google Scholar]
- 112.Leary SC, Antonicka H, Sasarman F, Weraarpachai W, Cobine PA, Pan M, Brown GK, Brown R, Majewski J, Ha KCH, Rahman S, Shoubridge EA. Novel mutations in SCO1 as a cause of fatal infantile encephalopathy and lactic acidosis. Hum Mutat. 2013;34(10):1366–1370. doi: 10.1002/humu.22385. [DOI] [PubMed] [Google Scholar]
- 113.Valnot I, Osmond S, Gigarel N, Mehaye B, Amiel J, Cormier-Daire V, Munnich A, Bonnefont JP, Rustin P, Rötig A. Mutations of the SCO1 gene in mitochondrial cytochrome c oxidase deficiency with neonatal-onset hepatic failure and encephalopathy. Am J Hum Genet. 2000;67(5):1104–1109. doi: 10.1016/S0002-9297(07)62940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stiburek L, Vesela K, Hansikova H, Hulkova H, Zeman J. Loss of function of Sco1 and its interaction with cytochrome c oxidase. Am J Physiol Cell Physiol. 2009;296(5):C1218–C1226. doi: 10.1152/ajpcell.00564.2008. [DOI] [PubMed] [Google Scholar]
- 115.Leary SC, Kaufman BA, Pellecchia G, Guercin G-H, Mattman A, Jaksch M, Shoubridge EA. Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum Mol Genet. 2004;13(17):1839–1848. doi: 10.1093/hmg/ddh197. [DOI] [PubMed] [Google Scholar]
- 116.Leary SC, Cobine PA, Kaufman BA, Guercin G-H, Mattman A, Palaty J, Lockitch G, Winge DR, Rustin P, Horvath R, Shoubridge EA. The human cytochrome c oxidase assembly factors SCO1 and SCO2 have regulatory roles in the maintenance of cellular copper homeostasis. Cell Metab. 2007;5(1):9–20. doi: 10.1016/j.cmet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 117.Baker ZN, Jett K, Boulet A, Hossain A, Cobine PA, Kim B-E, Zawily El AM, Lee L, Tibbits GF, Petris MJ, Leary SC. The mitochondrial metallochaperone SCO1 maintains CTR1 at the plasma membrane to preserve copper homeostasis in the murine heart. Hum Mol Genet. 2017;26(23):4617–4628. doi: 10.1093/hmg/ddx344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Calvo SE, Compton AG, Hershman SG, Lim SC, Lieber DS, Tucker EJ, Laskowski A, Garone C, Liu S, Jaffe DB, Christodoulou J, Fletcher JM, Bruno DL, Goldblatt J, DiMauro S, Thorburn DR, Mootha VK. Molecular Diagnosis of Infantile Mitochondrial Disease with Targeted Next-Generation Sequencing. Sci Transl Med. 2012;4(118):118ra10–118ra10. doi: 10.1126/scitranslmed.3003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baertling F, A M van den Brand M, Hertecant JL, Al-Shamsi A, P van den Heuvel L, Distelmaier F, Mayatepek E, Smeitink JA, Nijtmans LGJ, Rodenburg RJT. Mutations in COA6 cause cytochrome c oxidase deficiency and neonatal hypertrophic cardiomyopathy. Hum Mutat. 2015;36(1):34–38. doi: 10.1002/humu.22715. [DOI] [PubMed] [Google Scholar]
- 120.Ghosh A, Trivedi PP, Timbalia SA, Griffin AT, Rahn JJ, Chan SSL, Gohil VM. Copper supplementation restores cytochrome c oxidase assembly defect in a mitochondrial disease model of COA6 deficiency. Hum Mol Genet. 2014;23(13):3596–3606. doi: 10.1093/hmg/ddu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vögtle F-N, Burkhart JM, Rao S, Gerbeth C, Hinrichs J, Martinou J-C, Chacinska A, Sickmann A, Zahedi RP, Meisinger C. Intermembrane space proteome of yeast mitochondria. Mol Cell Proteomics. 2012;11(12):1840–1852. doi: 10.1074/mcp.M112.021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pacheu-Grau D, Bareth B, Dudek J, Juris L, Vögtle F-N, Wissel M, Leary SC, Dennerlein S, Rehling P, Deckers M. Cooperation between COA6 and SCO2 in COX2 maturation during cytochrome c oxidase assembly links two mitochondrial cardiomyopathies. Cell Metab. 2015;21(6):823–833. doi: 10.1016/j.cmet.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 123.Stroud DA, Maher MJ, Lindau C, Vögtle F-N, Frazier AE, Surgenor E, Mountford H, Singh AP, Bonas M, Oeljeklaus S, Warscheid B, Meisinger C, Thorburn DR, Ryan MT. COA6 is a mitochondrial complex IV assembly factor critical for biogenesis of mtDNA-encoded COX2. Hum Mol Genet. 2015;24(19):5404–5415. doi: 10.1093/hmg/ddv265. [DOI] [PubMed] [Google Scholar]
- 124.Bourens M, Barrientos A. Human mitochondrial cytochrome c oxidase assembly factor COX18 acts transiently as a membrane insertase within the subunit 2 maturation module. J Biol Chem. 2017;292(19):7774–7783. doi: 10.1074/jbc.M117.778514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Z, Song Y, Li D, He X, Li S, Wu B, Wang W, Gu S, Zhu X, Wang X, Zhou Q, Dai Y, Yan Q. The novel mitochondrial 16S rRNA 2336T>C mutation is associated with hypertrophic cardiomyopathy. J Med Genet. 2014;51(3):176–184. doi: 10.1136/jmedgenet-2013-101818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Santorelli FM, Tanji K, Manta P, Casali C, Krishna S, Hays AP, Mancini DM, DiMauro S, Hirano M. Maternally inherited cardiomyopathy: an atypical presentation of the mtDNA 12S rRNA gene A1555G mutation. Am J Hum Genet. 1999;64(1):295–300. doi: 10.1086/302188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ahola S, Isohanni P, Euro L, Brilhante V, Palotie A, Pihko H, Lönnqvist T, Lehtonen T, Laine J, Tyynismaa H, Suomalainen A. Mitochondrial EFTs defects in juvenile-onset Leigh disease, ataxia, neuropathy, and optic atrophy. Neurology. 2014;83(8):743–751. doi: 10.1212/WNL.0000000000000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Distelmaier F, Haack TB, Catarino CB, Gallenmüller C, Rodenburg RJ, Strom TM, Baertling F, Meitinger T, Mayatepek E, Prokisch H, Klopstock T. MRPL44 mutations cause a slowly progressive multisystem disease with childhood-onset hypertrophic cardiomyopathy. Neurogenetics. 2015;16(4):319–323. doi: 10.1007/s10048-015-0444-2. [DOI] [PubMed] [Google Scholar]
- 129.Galmiche L, Serre V, Beinat M, Assouline Z, Lebre A-S, Chretien D, Nietschke P, Benes V, Boddaert N, Sidi D, Brunelle F, Rio M, Munnich A, Rötig A. Exome sequencing identifies MRPL3 mutation in mitochondrial cardiomyopathy. Human Mut. 2011;32(11):1225–1231. doi: 10.1002/humu.21562. [DOI] [PubMed] [Google Scholar]
- 130.Emperador S, Bayona-Bafaluy MP, Fernández-Marmiesse A, Pineda M, Felgueroso B, López-Gallardo E, Artuch R, Roca I, Ruiz-Pesini E, Couce ML, Montoya J. Molecular-genetic characterization and rescue of a TSFM mutation causing childhood-onset ataxia and nonobstructive cardiomyopathy. Eur J Hum Genet. 2016;25(1):153–156. doi: 10.1038/ejhg.2016.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giordano C, Perli E, Orlandi M, Pisano A, Tuppen HA, He L, Ierinò R, Petruzziello L, Terzi A, Autore C, Petrozza V, Gallo P, Taylor RW, d'Amati G. Cardiomyopathies due to homoplasmic mitochondrial tRNA mutations: morphologic and molecular features. Hum Pathol. 2013;44(7):1262–1270. doi: 10.1016/j.humpath.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 132.Goldstein JD, Shanske S, Bruno C, Perszyk AA. Maternally inherited mitochondrial cardiomyopathy associated with a C-to-T transition at nucleotide 3303 of mitochondrial DNA in the tRNA(Leu(UUR)) gene. Pediatr Dev Pathol. 1999;2(1):78–85. doi: 10.1007/s100249900094. [DOI] [PubMed] [Google Scholar]
- 133.Alila-Fersi O, Tabebi M, Maalej M, Belguith N, Keskes L, Mkaouar-Rebai E, Fakhfakh F. First description of a novel mitochondrial mutation in the MT-TI gene associated with multiple mitochondrial DNA deletion and depletion in family with severe dilated mitochondrial cardiomyopathy. Biochem Biophys Res Commun. 2018;497(4):1049–1054. doi: 10.1016/j.bbrc.2018.02.173. [DOI] [PubMed] [Google Scholar]
- 134.Barth PG, Valianpour F, Bowen VM, Lam J, Duran M, Vaz FM, Wanders RJA. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): an update. Am J Med Genet A. 2004;126A(4):349–354. doi: 10.1002/ajmg.a.20660. [DOI] [PubMed] [Google Scholar]
- 135.Barth PG, Van den Bogert C, Bolhuis PA, Scholte HR, van Gennip AH, Schutgens RB, Ketel AG. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): respiratory-chain abnormalities in cultured fibroblasts. J Inherit Metab Dis. 1996;19(2):157–160. doi: 10.1007/bf01799418. [DOI] [PubMed] [Google Scholar]
- 136.Dudek J, Maack C. Barth syndrome cardiomyopathy. Cardiovasc. 2017;Res. doi: 10.1093/cvr/cvx014. [DOI] [PubMed] [Google Scholar]
- 137.Xu Y, Malhotra A, Ren M, Schlame M. The enzymatic function of tafazzin. J Biol Chem. 2006;281(51):39217–39224. doi: 10.1074/jbc.M606100200. [DOI] [PubMed] [Google Scholar]
- 138.Vukotic M, Nolte H, König T, Saita S, Ananjew M, Krüger M, Tatsuta T, Langer T. Acylglycerol Kinase Mutated in Sengers Syndrome Is a Subunit of the TIM22 Protein Translocase in Mitochondria. Mol Cell. 2017;67(3):471–483. doi: 10.1016/j.molcel.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 139.Kang Y, Stroud DA, Baker MJ, De Souza DP, Frazier AE, Liem M, Tull D, Mathivanan S, McConville MJ, Thorburn DR, Ryan MT, Stojanovski D. Sengers Syndrome-Associated Mitochondrial Acylglycerol Kinase Is a Subunit of the Human TIM22 Protein Import Complex. Mol Cell. 2017;67(3):457–470. doi: 10.1016/j.molcel.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 140.Moraes CT, DiMauro S, Zeviani M, Lombes A, Shanske S, Miranda AF, Nakase H, Bonilla E, Werneck LC, Servidei S. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med. 1989;320(20):1293–1299. doi: 10.1056/NEJM198905183202001. [DOI] [PubMed] [Google Scholar]
- 141.Manfredi G, Schon EA, Moraes CT, Bonilla E, Berry GT, Sladky JT, DiMauro S. A new mutation associated with MELAS is located in a mitochondrial DNA polypeptide-coding gene. Neuromuscul Disord. 1995;5(5):391–398. doi: 10.1016/0960-8966(94)00079-o. [DOI] [PubMed] [Google Scholar]
- 142.Shoffner JM, Lott MT, Lezza AM, Seibel P, Ballinger SW, Wallace DC. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990;61(6):931–937. doi: 10.1201/b15310-58. [DOI] [PubMed] [Google Scholar]
- 143.Nissanka N, Moraes CT. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018;592(5):728–742. doi: 10.1002/1873-3468.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lake NJ, Compton AG, Rahman S, Thorburn DR. Leigh syndrome: One disorder, more than 75 monogenic causes. Ann Neurol. 2016;79(2):190–203. doi: 10.1002/ana.24551. [DOI] [PubMed] [Google Scholar]
- 145.Wojtala A, Karkucinska-Wieckowska A, Sardao VA, Szczepanowska J, Kowalski P, Pronicki M, Duszynski J, Wieckowski MR. Modulation of mitochondrial dysfunction-related oxidative stress in fibroblasts of patients with Leigh syndrome by inhibition of prooxidative p66Shc pathway. Mitochondrion. 2017;37:62–79. doi: 10.1016/j.mito.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 146.Spinazzola A, Viscomi C, Fernandez-Vizarra E, Carrara F, D'Adamo P, Calvo S, Marsano RM, Donnini C, Weiher H, Strisciuglio P, Parini R, Sarzi E, Chan A, DiMauro S, Rötig A, Gasparini P, Ferrero I, Mootha VK, Tiranti V, Zeviani M. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat Genet. 2006;38(5):570–575. doi: 10.1038/ng1765. [DOI] [PubMed] [Google Scholar]
- 147.Spinazzola A, Santer R, Akman OH, Tsiakas K, Schaefer H, Ding X, Karadimas CL, Shanske S, Ganesh J, Di Mauro S, Zeviani M. Hepatocerebral form of mitochondrial DNA depletion syndrome: novel MPV17 mutations. Arch Neurol. 2008;65(8):1108–1113. doi: 10.1001/archneur.65.8.1108. [DOI] [PubMed] [Google Scholar]
- 148.Spinazzola A, Massa V, Hirano M, Zeviani M. Lack of founder effect for an identical mtDNA depletion syndrome (MDS)-associated MPV17 mutation shared by Navajos and Italians. Neuromuscul Disord. 2008;18(4):315–318. doi: 10.1016/j.nmd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 149.El-Hattab AW, Wang J, Dai H, Almannai M, Staufner C, Alfadhel M, Gambello MJ, Prasun P, Raza S, Lyons HJ, Afqi M, Saleh MAM, Faqeih EA, Alzaidan HI, Alshenqiti A, Flore LA, Hertecant J, Sacharow S, Barbouth DS, Murayama K, Shah AA, Lin HC, Wong L-JC. MPV17-related mitochondrial DNA maintenance defect: New cases and review of clinical, biochemical, and molecular aspects. Human Mut. 2018;39(4):461–470. doi: 10.1002/humu.23387. [DOI] [PubMed] [Google Scholar]
- 150.Miyake N, Yamashita S, Kurosawa K, Miyatake S, Tsurusaki Y, Doi H, Saitsu H, Matsumoto N. A novel homozygous mutation of DARS2 may cause a severe LBSL variant. Clin Genet. 2011;80(3):293–296. doi: 10.1111/j.1399-0004.2011.01644.x. [DOI] [PubMed] [Google Scholar]
- 151.S ahin S, Cansu A, Kalay E, Dinçer T, Kul S, Çakır ISM, Kamaşak T, Budak GYGL. Leukoencephalopathy with thalamus and brainstem involvement and high lactate caused by novel mutations in the EARS2 gene in two siblings. J Neurol Sci. 2016;365:54–58. doi: 10.1016/j.jns.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 152.Boczonadi V, Jennings MJ, Horvath R. The role of tRNA synthetases in neurological and neuromuscular disorders. FEBS Lett. 2018;592(5):703–717. doi: 10.1002/1873-3468.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Viscomi C. Toward a therapy for mitochondrial disease. Biochem Soc Trans. 2016;44(5):1483–1490. doi: 10.1042/BST20160085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88(2):611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 155.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24(1):78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 156.Villena JA. New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J. 2015;282(4):647–672. doi: 10.1111/febs.13175. [DOI] [PubMed] [Google Scholar]
- 157.Perales-Clemente E, Bayona-Bafaluy MP, Pérez-Martos A, Barrientos A, Fernández-Silva P, Enríquez JA. Restoration of electron transport without proton pumping in mammalian mitochondria. Proc Natl Acad Sci U S A. 2008;105(48):18735–18739. doi: 10.1073/pnas.0810518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dassa EP, Dufour E, Gonçalves S, Paupe V, Hakkaart GAJ, Jacobs HT, Rustin P. Expression of the alternative oxidase complements cytochrome c oxidase deficiency in human cells. EMBO Mol Med. 2009;1(1):30–36. doi: 10.1002/emmm.200900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kemppainen KK, Rinne J, Sriram A, Lakanmaa M, Zeb A, Tuomela T, Popplestone A, Singh S, Sanz A, Rustin P, Jacobs HT. Expression of alternative oxidase in Drosophila ameliorates diverse phenotypes due to cytochrome oxidase deficiency. Hum Mol Genet. 2014;23(8):2078–2093. doi: 10.1093/hmg/ddt601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Camargo AF, Chioda MM, Rodrigues APC, Garcia GS, McKinney EA, Jacobs HT, Oliveira MT. Xenotopic expression of alternative electron transport enzymes in animal mitochondria and their impact in health and disease. Cell Biol Int. 2018;42(6):664–669. doi: 10.1002/cbin.10943. [DOI] [PubMed] [Google Scholar]
- 161.Szibor M, Dhandapani PK, Dufour E, Holmström KM, Zhuang Y, Salwig I, Wittig I, Heidler J, Gizatullina Z, Gainutdinov T, German Mouse Clinic Consortium, Fuchs H, Gailus-Durner V, de Angelis MH, Nandania J, Velagapudi V, Wietelmann A, Rustin P, Gellerich FN, Jacobs HT, Braun T. Broad AOX expression in a genetically tractable mouse model does not disturb normal physiology. Dis Model Mech. 2017;10(2):163–171. doi: 10.1242/dmm.027839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Civiletto G, Varanita T, Cerutti R, Gorletta T, Barbaro S, Marchet S, Lamperti C, Viscomi C, Scorrano L, Zeviani M. Opa1 overexpression ameliorates the phenotype of two mitochondrial disease mouse models. Cell Metab. 2015;21(6):845–854. doi: 10.1016/j.cmet.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Varanita T, Soriano ME, Romanello V, Zaglia T, Quintana-Cabrera R, Semenzato M, Menabò R, Costa V, Civiletto G, Pesce P, Viscomi C, Zeviani M, Di Lisa F, Mongillo M, Sandri M, Scorrano L. The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 2015;21(6):834–844. doi: 10.1016/j.cmet.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Beyrath J, Pellegrini M, Renkema H, Houben L, Pecheritsyna S, van Zandvoort P, van den Broek P, Bekel A, Eftekhari P, Smeitink JAM. KH176 Safeguards Mitochondrial Diseased Cells from Redox Stress-Induced Cell Death by Interacting with the Thioredoxin System/Peroxiredoxin Enzyme Machinery. Sci Rep. 2018;8(1):6577. doi: 10.1038/s41598-018-24900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Koopman WJ, Beyrath J, Fung C-W, Koene S, Rodenburg RJ, Willems PH, Smeitink JA. Mitochondrial disorders in children: toward development of small-molecule treatment strategies. EMBO Mol Med. 2016;8(4):311–327. doi: 10.15252/emmm.201506131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bacman SR, Williams SL, Pinto M, Peralta S, Moraes CT. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat Med. 2013;19(9):1111–1113. doi: 10.1038/nm.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gammage PA, Rorbach J, Vincent AI, Rebar EJ, Minczuk M. Mitochondrially targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutations. EMBO Mol Med. 2014;6(4):458–466. doi: 10.1002/emmm.201303672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang G, Shimada E, Zhang J, Hong JS, Smith GM, Teitell MA, Koehler CM. Correcting human mitochondrial mutations with targeted RNA import. Proc Natl Acad Sci U S A. 2012;109(13):4840–4845. doi: 10.1073/pnas.1116792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gammage PA, Moraes CT, Minczuk M. Mitochondrial Genome Engineering: The Revolution May Not Be CRISPR-Ized. Trends Genet. 2017;9525(17):30191–30199. doi: 10.1016/j.tig.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Frazier AE, Thorburn DR, Compton AG. Mitochondrial energy generation disorders: genes, mechanisms and clues to pathology. J Biol Chem. 2017 doi: 10.1074/jbc.R117.809194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nikkanen J, Forsström S, Euro L, Paetau I, Kohnz RA, Wang L, Chilov D, Viinamäki J, Roivainen A, Marjamäki P, Liljenbäck H, Ahola S, Buzkova J, Terzioglu M, Khan NA, Pirnes-Karhu S, Paetau A, Lönnqvist T, Sajantila A, Isohanni P, Tyynismaa H, Nomura DK, Battersby BJ, Velagapudi V, Carroll CJ, Suomalainen A. Mitochondrial DNA Replication Defects Disturb Cellular dNTP Pools and Remodel One-Carbon Metabolism. Cell Metab. 2016;23(4):635–648. doi: 10.1016/j.cmet.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 172.Khan NA, Nikkanen J, Yatsuga S, Jackson C, Wang L, Pradhan S, Kivelä R, Pessia A, Velagapudi V, Suomalainen A. mTORC1 Regulates Mitochondrial Integrated Stress Response and Mitochondrial Myopathy Progression. Cell Metab. 2017;26(2):419–428. doi: 10.1016/j.cmet.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 173.Pacheu-Grau D, Gómez-Durán A, Iglesias E, López-Gallardo E, Montoya J, Ruiz-Pesini E. Mitochondrial antibiograms in personalized medicine. Hum Mol Genet. 2013;22(6):1132–1139. doi: 10.1093/hmg/dds517. [DOI] [PubMed] [Google Scholar]