Abstract
Expression of the serum- and glucocorticoid-inducible kinase 1 (SGK1) is up-regulated by several types of cell stress, such as ischemia, radiation and hyperosmotic shock. The SGK1 protein is activated by a signaling cascade involving phosphatidylinositide-3-kinase (PI3K), 3-phosphoinositide-dependent kinase 1 (PDK1) and mammalian target of rapamycin (mTOR). SGK1 up-regulates Na+/K+-ATPase, a variety of carriers including Na+-,K+-,2Cl−- cotransporter (NKCC), NaCl cotransporter (NCC), Na+/H+ exchangers, diverse amino acid transporters and several glucose carriers such as Na+-coupled glucose transporter SGLT1. SGK1 further up-regulates a large number of ion channels including epithelial Na+ channel ENaC, voltagegated Na+ channel SCN5A, Ca2+ release-activated Ca2+ channel (ORAI1) with its stimulator STIM1, epithelial Ca2+ channels TRPV5 and TRPV6 and diverse K+ channels. Furthermore, SGK1 influences transcription factors such as nuclear factor kappa-B (NF-κB), p53 tumor suppressor protein, cAMP responsive element-binding protein (CREB), activator protein-1 (AP-1) and forkhead box O3 protein (FOXO3a). Thus, SGK1 supports cellular glucose uptake and glycolysis, angiogenesis, cell survival, cell migration, and wound healing. Presumably as last line of defense against tissue injury, SGK1 fosters tissue fibrosis and tissue calcification replacing energy consuming cells.
Keywords: SGK1, glycolysis, cell survival, cell migration, angiogenesis, fibrosis and tissue calcification
INTRODUCTION
The ubiquitously expressed [1–4] serum- and glucocorticoid-inducible kinase 1 (SGK1) has originally been cloned as a gene up-regulated by serum and glucocorticoids in rat mammary tumor cells [1, 5]. The human SGK1 has been identified as a gene up-regulated by cell shrinkage [6].
SGK1 expression
Expression of SGK1 is highly variable and subject to regulation by a wide variety of triggers including hyperosmotic or isotonic cell shrinkage, dehydration, excessive glucose concentrations, mechanical stress, oxidative stress, heat shock, radiation, DNA damage, ischemia, neuronal injury and neuronal excitation [1, 3, 7–12]. SGK1 transcription is further up-regulated by several hormones and mediators including glucocorticoids, mineralocorticoids, gonadotropins, gestagens, 1,25(OH)2D3, erythropoietin, morphine, transforming growth factor β (TGFβ), interleukin-6, fibroblast and platelet-derived growth factor, thrombin, endothelin, advanced glycation end products (AGEs) and activation of peroxisome proliferator-activated receptor γ (PPARγ) [1]. Inhibitors of SGK1 expression include serum starvation, heparin, dietary iron, nucleosides and nephrilin [1]. Overall, SGK1 expression declines with age [13].
Signaling of transcriptional SGK1 regulation involves cytosolic Ca2+, cyclic AMP, stress-activated protein kinase-2 (SAPK2 or p38 MAPK kinase), protein kinase C (PKC), protein kinase RAF, big mitogen-activated protein kinase 1 (BMK1, also known as extracellular signal-regulated kinase ERK5), extracellular signal-regulated kinase 1/2 (ERK1/2), phosphatidylinositide-3-kinase (PI3K), reactive oxygen species, NADPH oxidases, nitric oxide and EWS/NOR1 (NR4A3) fusion protein [1].
The SGK1 promoter binds receptors for glucocorticoids (GR), mineralocorticoids (MR), progesterone (PR), 1,25(OH)2D3 (VDR), retinoids (RXR), farnesoids (FXR), sterol regulatory element-binding protein (SREBP), PPARγ, cAMP response element-binding protein (CREB), p53 tumor suppressor protein, Sp1 transcription factor, activator protein 1 (AP-1), activating transcription factor 6 (ATF6), heat shock factor (HSF), reticuloendotheliosis viral oncogene homolog (c-Rel), nuclear factor kappa- B (NF-kB), signal transducers and activators of transcription (STAT), TGFβ-dependent transcription factors SMAD3 and SMAD4, forkhead activin signal transducer (FAST) and the transcription factor TonE binding protein (TonEBP or NFAT5) [1].
SGK1 translation is stimulated by PI3K and requires actin polymerization [14].
SGK1 activation and its degradation
Once expressed SGK1 requires activation. Stimulators of SGK1 activity include insulin, IGF1, hepatic growth factor (HGF), follicle stimulating hormone (FSH), thrombin and corticosterone [1]. Signaling involving activation of SGK1 includes PI3K and 3-phosphoinositide (PIP3)-dependent kinase PDK1 [6]. Interaction of SGK1 and PDK1 is supported by the scaffold protein Na+/H+ exchanger regulating factor 2 (NHERF2) [3]. PIP3 is degraded and activation of SGK1 thus suppressed by the phosphatase and tensin homolog PTEN [3]. SGK1 activation further involves WNK1 (lysine deficient protein kinase 1) and mammalian target of rapamycin mTOR complex-2 (mTORC2) composed of mTOR, Rictor (rapamycin-insensitive companion of mTOR), Sin1 (stress-activated protein kinase-interacting protein 1), mLST8 and Protor-1 [1, 15–27]. SGK1 is further up-regulated by p38α MAPK, ERK5, cAMP, lithium, Ca2+-sensitive calmodulin-dependent protein kinase kinase (CaMKK), G-protein Rac1, neuronal depolarization, oxidation, hypertonicity, and fibronectin [1, 3, 6, 28].
SGK1 degradation is triggered by ubiquitination involving NEDD4-2 (neuronal precursor cells expressed developmentally down-regulated) [1, 3] and Rictor/Cullin-1 [1, 29–31]. SGK1 degradation is inhibited by glucocorticoid-induced leucine zipper protein-1 (GILZ) [32].
SGK1 kinase targets
The optimal consensus sequences for phosphorylation by SGK1 are R-X-R-X-X-(S/T)-phi and R-R-X-S/T (X = any amino acid, R = arginine, S = serine, T = threonine, phi = hydrophobic amino acid) [3, 33]. Specific SGK1 targets are N-myc down-regulated genes NDRG1 and NDRG2 [1, 3]. Other SGK1 targets are shared by other kinases including SGK and protein kinase B (PKB/Akt) isoforms [3].
SGK1 influences a variety of enzymes including ubiquitin ligase NEDD4-2, inducible nitric oxide synthase iNOS, phosphomannose mutase 2 (PMM2), phosphatidylinositol-3-phosphate-5-kinase (PIKfyve), serine/threonine kinase WNK4, ERK2 (MAPK1), mitogen-activated protein kinase/ERK kinase kinase 3 (MEKK3), stress-activated kinase (SEK1), B-Raf kinase, glycogen synthase kinase 3 (GSK-3), p53-ubiquitinating MDM2 and Notch1-IC protein degradating Fbw7 [1].
SGK1 up-regulates transcription factors such as CREB, AP-1 and NF-κB [1, 34–37]. On the other hand, SGK1 phosphorylates and thus activates NDRG1, which in turn down-regulates NF-κB signaling [1, 38]. Moreover, SGK1 down-regulates transcription factor p53 and forkhead box O3 protein (FOXO3a) [1, 39, 40].
SGK1 is a powerful regulator of several ion channels [1, 3, 41], including epithelial Na+ channel ENaC, voltage-gated Na+ channel SCN5A, renal outer medullary K+ channel ROMK1, voltage-gated K+ channels KCNE1/KCNQ1, KCNQ4, Kv1.3, Kv1.5, Kv7.2/3, Kv4.3 and hERG, the Ca2+ release-activated Ca2+ channel ORAI1 and its stimulator STIM1, transient receptor potential channels TRPV4, TRPV5 and TRPV6, kainate receptor GluR6, unselective cation channel 4F2/LAT, Cl- channels ClCka/barttin, ClC2, CFTR (Cystic fibrosis transmembrane conductance regulator) and VSOAC (volume-sensitive osmolyte and anion channel) as well as acid-sensing ion channel ASIC1 [1, 3].
SGK1 stimulates diverse carriers including Na+-,K+-,2Cl–- cotransporter NKCC2, NaCl cotransporter NCC, Na+/H+ exchangers NHE1 and NHE3, glucose carriers SGLT1, GLUT1 and GLUT4, amino acid transporters ASCT2, SN1, B(0)AT1, EAAT1, EAAT2, EAAT3, EAAT4 and EAAT5, peptide transporters PepT, Na+,dicarboxylate cotransporter NaDC-1, creatine transporter CreaT, Na+,myoinositol cotransporter SMIT as well as phosphate carriers NaPiIIa and NaPiIIb [1, 3]. Furthermore, SGK1 up-regulates the Na+/K+-ATPase and albumin uptake [1, 3].
Further targets of SGK1 include nephrin, type A natriuretic peptide receptor (NPR-A), Ca2+-regulated heat-stable protein of apparent molecular mass 24 kDa (CRHSP24), the adaptor precursor (APP) Fe65, NDRG1 and NDRG2, myosin-Vc, filamin C, microtubule-associated protein tau, Cyclin-dependent kinase inhibitor 1B (p27Kip1), and huntingtin [1, 3, 40, 42–44].
The present review discusses the role of SGK1 in the orchestration of cellular response to stress such as energy depletion. The case is made that SGK1 supports cellular energy supply by stimulation of glucose uptake and glycolysis, as well as by stimulation of angiogenesis. SGK1 supports cell survival and cell migration, a prerequisite of tissue repair. As last line of defense, SGK1 replaces energy consuming cells with extracellular matrix by stimulation of tissue fibrosis and tissue calcification. In order to limit the number of citations some of the earlier original papers have been replaced by reviews.
GLUCOSE UPTAKE AND GLYCOLYSIS
SGK1 stimulates cellular glucose uptake and thus enhances the availability of glucose for glycolysis [3]. SGK1 further stimulates the Na+/H+ ion exchanger [36] which generates an alkaline cytosolic pH, a prerequisite for an increase of glycolytic flux [1]. The up-regulation of SGK1 in ischemia thus supports energy supply by glycolysis [2, 3, 10, 45].
ANGIOGENESIS
SGK1 is required for angiogenesis during embryonic development [46] and following ischemia in the adult [47]. In myocardial ischemia, lack of SGK1 blunts the phosphorylation of SGK1 target protein NDRG1 and compromises the up-regulation of transcription factor NF-κB and its target protein, VEGF-A (vascular endothelial growth factor A). Lack of SGK1 further impairs endothelial cell (ECs) migration and tube formation in vitro, and decreases in vivo angiogenesis after myocardial infarction [47].
CELL SURVIVAL
SGK1 supports cell survival and cell proliferation of both tumor cells and neurons [1, 3, 7, 10, 48–52]. SGK1 is highly expressed in several tumors [10], including non-small cell lung cancer [53], colon cancer [10], prostate cancer [54], ovarian tumors [1], myeloma [55], and medulloblastoma [1]. SGK1 confers resistance of breast cancer cells to chemotherapy [3, 10, 56], and inhibition of SGK1 sensitizes tumor cells to cytotoxic drugs or radiation [12]. SGK1 contributes to androgen-induced growth of prostate cancer cells [2]. SGK1 counteracts the pro-apoptotic effect of membrane androgen receptors (mAR) [1] in colon carcinoma cells [57–59]. Lack of SGK1 blunts the development of spontaneous tumors in APC-deficient mice [2] and chemically-induced colonic tumors in wild-type mice [1].
SGK1 stimulates cell proliferation and inhibits cell death in part by up-regulating channels and transporters, such as the store-operated Ca2+ entry (SOCE) accomplished by ORAI1/STIM1 [1, 12, 34, 35, 60, 61]. SOCE maintains oscillations of cytosolic Ca2+ activity, which are required for depolymerization of the actin filament network, a prerequisite for cell proliferation [3, 10]. Ca2+ entry is driven by the cell membrane potential, which is generated by SGK1 sensitive K+ channels [3, 10]. The protective effect of SGK1 on neurons similarly involves, at least in part, up-regulation of ORAI1/STIM1 [51].
SGK1 further inactivates the pro-apoptotic forkhead transcription factor FOXO3A/ FKRHL1 [1], inhibits GSK-3 and up-regulates oncogenic β-catenin [3, 7], activates IKKβ with subsequent phosphorylation and degradation of the inhibitory protein IκB and translocation of NF-κB into the nucleus [10], activates the ubiquitin ligase MDM2 with subsequent MDM2-dependent ubiquitination and proteosomal degradation of pro-apoptotic transcription factor p53 [1], disrupts binding of SEK1 to JNK1 and MEKK1 [3, 10] and up-regulates Ran binding protein (RanBP), an effect affecting microtubule network and blunting taxol sensitivity of cancer cells [52, 62].
CELL MIGRATION
SGK1 is part of the machinery stimulating cell migration [47, 57, 58, 63, 64]. As shown in vascular smooth muscle cells (VSMCs) [64], the stimulation of migration by platelet-derived growth factor PDGF is paralleled by up-regulation of both, SGK1 expression and SGK1 activity [65, 66]. Genetic knockout of SGK1 decreases migration [64]. SGK1 is effective, at least in part, by up-regulation of the store-operated Ca2+ entry (SOCE), which is accomplished by the Ca2+ channel ORAI1 and its regulator STIM1. Expression of ORAI1 and STIM1 is stimulated by NF-κB, a transcription factor up-regulated by SGK1 [1, 64]. In VSMCs, SGK1 triggers nuclear translocation of transcription factor NF-κB [64].
INFLAMMATION AND FIBROSIS
SGK1 contributes to the orchestration of inflammation [52, 67–70]. The kinase is required for the interleukin-23 (IL-23)-sensitive generation of interleukin-17 (IL-17)-producing CD4+ helper T cells (TH17 cells) [71]. TH17 cells up-regulate the pro-inflammatory cytokines GM-CSF, TNF-α and interleukin-2 (IL-2) [71].
SGK1 further contributes to fibrosis in several clinical conditions, including lung fibrosis, diabetic nephropathy, glomerulonephritis, experimental nephrotic syndrome, obstructive nephropathy, cardiac remodeling, liver cirrhosis, fibrosing pancreatitis, peritoneal fibrosis, Crohn's disease and coeliac disease [1, 3, 72–75]. The expression of SGK1 is upregulated by TGFβ [3], a pivotal stimulator of fibrosis [69, 76–81]. Signaling of TGFβ includes activation of transcription factors SMAD2/3 [1], which are ubiquitinated and, thus, tagged for degradation by NEDD4L [1]. The ubiquitin ligase is inactivated by SGK1 which thus augments TGFβ action [1]. SGK1 supports inflammation and fibrosis further by activating NF-κB [3], a proinflammatory and profibrotic transcription factor [1, 82, 83]. NF-κB up-regulates connective tissue growth factor (CTGF), which in turn contributes to stimulation of cardiac remodeling and fibrosis [1, 3, 84–87], renal proteinuria and failure [88], skin aging [15], as well as fibronectin formation at hyperglycemia [1].
VASCULAR CALCIFICATION
SGK1 further participates in the orchestration of medial vascular calcification [84], which results mainly from osteo-/chondrogenic transdifferentiation of VSMCs [84]. Various triggers of VSMC osteo-/chondrogenic transdifferentiation induce a sharp increase of SGK1 expression [84]. Upregulation of SGK1 was also observed in the vasculature of rats with renal failure [89]. SGK1 increases the expression of the osteo-/chondrogenic transcription factors MSX2 and CBFA1, which in turn stimulate the expression of alkaline phosphatase ALPL [84]. The enzyme fosters vascular calcification by degrading the endogenous calcification inhibitor pyrophosphate. The effect of SGK1 on osteo-/chondrogenic transdifferentiation depends on transcriptional activity of NF-κB, a decisive regulator of vascular calcification [90, 91]. NF-κB also reduces pyrophosphate release via tristetraprolin (TTP)-mediated destabilization of ankylosis protein homolog (ANKH) mRNA [90, 91].
THE ROLE OF SGK1 IN DISEASE – CLINICAL IMPLICATIONS
A wide variety of observations point to a role of SGK1 in human pathophysiology [12]. Excessive expression and activity of SGK1 participates in the pathophysiology of diverse disorders, such as hypertension, obesity, diabetes, thrombosis, stroke, fibrosing disease, vascular calcification, infertility, autoimmune disease, and tumor growth [12,71,84]. A SGK1 gene variant (prevalence approx. 3-5% in Caucasians and approx. 10% in Africans) is associated with hypertension, stroke, obesity and type 2 diabetes [12]. Little is known about the clinical impact of SGK1 deficiency. In a SV129 genetic background, the phenotype of SGK1 knockout mice is mild and SGK1-dependent functions are apparently in large part maintained by other kinases [12]. In view of the putative role of SGK1 in neuronal survival [51], however, the possibility must be kept in mind that lack of SGK1 may accelerate the clinical course of neurodegeneration. Clearly, additional experimental and observational effort is required to define the pathophysiological impact of deranged SGK1 activity in human disease.
CONCLUSIONS
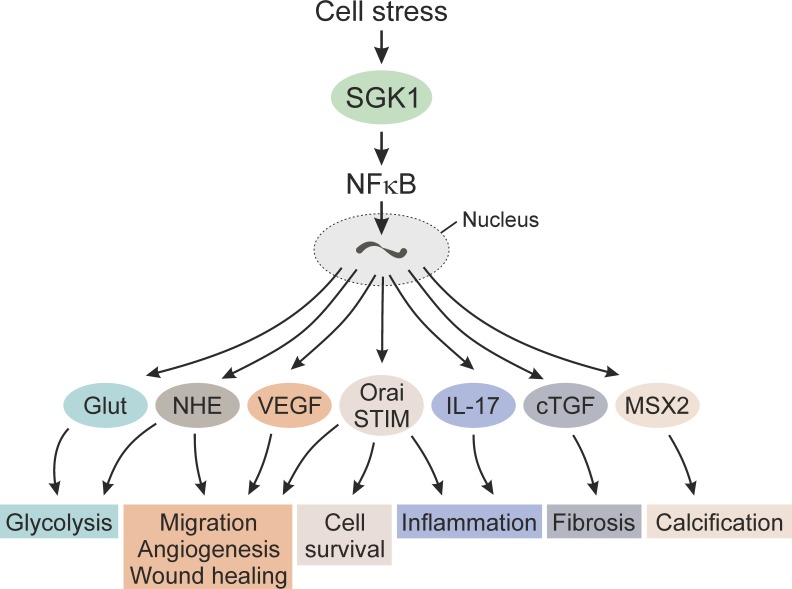
Expression of the serum- and glucocorticoid-inducible kinase SGK1 is steeply up-regulated following cell stress, such as ischemia, radiation and hyperosmotic shock. The SGK1 protein is activated by a signaling cascade involving phosphatidylinositide-3-kinase (PI3K), 3-phosphoinositide-dependent kinase 1 (PDK1) and mTOR. SGK1 is a powerful stimulator of transport across the cell membrane, such as Na+/K+-ATPase, Na+/H+ exchangers, cellular glucose uptake and ORAI1/STIM1-dependent store-operated Ca2+ entry (SOCE). SGK1 is further a powerful stimulator of transcription factors including nuclear factor κB (NF-κB; Figure 1). Upon cell stress such as energy depletion, SGK1 supports cellular glucose uptake and glycolysis, angiogenesis, cell survival, cell migration, and wound healing. If those functions fail to remove the cell stress, SGK1 initiates replacement of energy consuming cells by fibrotic and/or calcified tissue.
Figure 1. FIGURE 1: SGK1-sensitive NFκB-dependent transcription in the response to cell stress.
Please note that additional NFκB-dependent genes as well as NFκB-independent mechanisms contribute to the cellular response to stress.
Acknowledgments
The authors acknowledge the meticulous preparation of the manuscript by Lejla Subasic and the figure by Tanja Loch. Research in author's laboratories was supported by the Deutsche Forschungsgemeinschaft.
Abbreviations:
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor-kappa B
- SGK1
serum- and glucocorticoid-inducible kinase 1
- TGFβ
transforming growth factor beta
- VSMC
vascular smooth muscle cell
References
- 1.Lang F, Stournaras C. Serum and glucocorticoid inducible kinase, metabolic syndrome, inflammation, and tumor growth. Hormones. 2013;12(2):160–171. doi: 10.14310/horm.2002.1401. [DOI] [PubMed] [Google Scholar]
- 2.Lang F, Gorlach A, Vallon V. Targeting SGK1 in diabetes. Expert Opin Ther Targets. 2009;13(11):1303–1311. doi: 10.1517/14728220903260807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86(4):1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 4.Salker MS, Christian M, Steel JH, Nautiyal J, Lavery S, Trew G, Webster Z, Al-Sabbagh M, Puchchakayala G, Foller M, Landles C, Sharkey AM, Quenby S, Aplin JD, Regan L, Lang F, Brosens JJ. Deregulation of the serum- and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat Med. 2011;17(11):1509–1513. doi: 10.1038/nm.2498. [DOI] [PubMed] [Google Scholar]
- 5.Firestone GL, Giampaolo JR, O'Keeffe BA. Stimulus-dependent regulation of serum and glucocorticoid inducible protein kinase (SGK) transcription, subcellular localization and enzymatic activity. Cell Physiol Biochem. 2003;13(1):1–12. doi: 10.1159/000070244. [DOI] [PubMed] [Google Scholar]
- 6.Waldegger S, Barth P, Raber G, Lang F. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci U S A. 1997;94(9):4440–4445. doi: 10.1073/pnas.94.9.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang F, Artunc F, Vallon V. The physiological impact of the serum and glucocorticoid-inducible kinase SGK1. Curr Opin Nephrol Hypertens. 2009;18(5):439–448. doi: 10.1097/mnh.0b013e32832f125e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang F, Gorlach A. Heterocyclic indazole derivatives as SGK1 inhibitors, WO2008138448. Expert Opin Ther Pat. 2010;20(1):129–135. doi: 10.1517/13543770903365209. [DOI] [PubMed] [Google Scholar]
- 9.Lang F, Huang DY, Vallon V. SGK, renal function and hypertension. J Nephrol. 2010;23(Suppl 16):S124–129. [PMC free article] [PubMed] [Google Scholar]
- 10.Lang F, Perrotti N, Stournaras C. Colorectal carcinoma cells--regulation of survival and growth by SGK1. Int J Biochem Cell Biol. 2010;42(10):1571–1575. doi: 10.1016/j.biocel.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Lang F, Eylenstein A, Shumilina E. Regulation of Orai1/STIM1 by the kinases SGK1 and AMPK. Cell Calcium. 2012;52(5):347–354. doi: 10.1016/j.ceca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Lang F, Voelkl J. Therapeutic potential of serum and glucocorticoid inducible kinase inhibition. Expert Opin Investig Drugs. 2013;22(6):701–714. doi: 10.1517/13543784.2013.778971. [DOI] [PubMed] [Google Scholar]
- 13.Harries LW, Fellows AD, Pilling LC, Hernandez D, Singleton A, Bandinelli S, Guralnik J, Powell J, Ferrucci L, Melzer D. Advancing age is associated with gene expression changes resembling mTOR inhibition: evidence from two human populations. Mech Ageing Dev. 2012;133(8):556–562. doi: 10.1016/j.mad.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelzl L, Tolios A, Schmidt EM, Alesutan I, Walker B, Munzer P, Borst O, Gawaz M, Lang F. Translational regulation of the serum- and glucocorticoid-inducible kinase-1 (SGK1) in platelets. Biochem Biophys Res Commun. 2012;425(1):1–5. doi: 10.1016/j.bbrc.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Tsai V, Parker WE, Orlova KA, Baybis M, Chi AW, Berg BD, Birnbaum JF, Estevez J, Okochi K, Sarnat HB, Flores-Sarnat L, Aronica E, Crino PB. Fetal brain mTOR signaling activation in tuberous sclerosis complex. Cereb Cortex. 2014;24(2):315–327. doi: 10.1093/cercor/bhs310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29(21):5657–5670. doi: 10.1128/mcb.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang Z, Zhang T, Dizeyi N, Chen S, Wang H, Swanson KD, Cai C, Balk SP, Yuan X. Androgen Receptor Enhances p27 Degradation in Prostate Cancer Cells through Rapid and Selective TORC2 Activation. J Biol Chem. 2012;287(3):2090–2098. doi: 10.1074/jbc.m111.323303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall BA, Kim TY, Skor MN, Conzen SD. Serum and glucocorticoid-regulated kinase 1 (SGK1) activation in breast cancer: requirement for mTORC1 activity associates with ER-alpha expression. Breast Cancer Res Treat. 2012;135(2):469–479. doi: 10.1007/s10549-012-2161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heise CJ, Xu BE, Deaton SL, Cha SK, Cheng CJ, Earnest S, Sengupta S, Juang YC, Stippec S, Xu Y, Zhao Y, Huang CL, Cobb MH. Serum and glucocorticoid-induced kinase (SGK) 1 and the epithelial sodium channel are regulated by multiple with no lysine (WNK) family members. J Biol Chem. 2010;285(33):25161–25167. doi: 10.1074/jbc.m110.103432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyo D, Xu L, Foster DA. Phospholipase D stabilizes HDM2 through an mTORC2/SGK1 pathway. Biochem Biophys Res Commun. 2010;396(2):562–565. doi: 10.1016/j.bbrc.2010.04.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearce LR, Sommer EM, Sakamoto K, Wullschleger S, Alessi DR. Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem J. 2011;436(1):169–179. doi: 10.1042/bj20102103. [DOI] [PubMed] [Google Scholar]
- 22.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137(5):873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosner M, Dolznig H, Fuchs C, Siegel N, Valli A, Hengstschlager M. CDKs as therapeutic targets for the human genetic disease tuberous sclerosis? Eur J Clin Invest. 2009;39(12):1033–1035. doi: 10.1111/j.1365-2362.2009.02213.x. [DOI] [PubMed] [Google Scholar]
- 24.Treins C, Warne PH, Magnuson MA, Pende M, Downward J. Rictor is a novel target of p70 S6 kinase-1. Oncogene. 2010;29:1003–1016. doi: 10.1038/onc.2009.401. [DOI] [PubMed] [Google Scholar]
- 25.Thomanetz V, Angliker N, Cloetta D, Lustenberger RM, Schweighauser M, Oliveri F, Suzuki N, Ruegg MA. Ablation of the mTORC2 component rictor in brain or Purkinje cells affects size and neuron morphology. J Cell Biol. 2013;201(2):293–308. doi: 10.1083/jcb.201205030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domhan S, Schwager C, Wei Q, Muschal S, Sommerer C, Morath C, Wick W, Maercker C, Debus J, Zeier M, Huber PE, Abdollahi A. Deciphering the systems biology of mTOR inhibition by integrative transcriptome analysis. Curr Pharm Des. 2014;20(1):88–100. doi: 10.2174/138161282001140113125549. [DOI] [PubMed] [Google Scholar]
- 27.Na T, Wu G, Zhang W, Dong WJ, Peng JB. Disease-causing R1185C mutation of WNK4 disrupts a regulatory mechanism involving calmodulin binding and SGK1 phosphorylation sites. Am J Physiol Renal Physiol. 2013;304(1):F8–F18. doi: 10.1152/ajprenal.00284.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SM, Lee YJ, Yoon JJ, Kang DG, Lee HS. Effect of Poria cocos on hypertonic stress-induced water channel expression and apoptosis in renal collecting duct cells. J Ethnopharmacol. 2012;141(1):368–376. doi: 10.1016/j.jep.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 29.Gao D, Wan L, Inuzuka H, Berg AH, Tseng A, Zhai B, Shaik S, Bennett E, Tron AE, Gasser JA, Lau A, Gygi SP, Harper JW, DeCaprio JA, Toker A, Wei W. Rictor forms a complex with Cullin-1 to promote SGK1 ubiquitination and destruction. Mol Cell. 2010;39(5):797–808. doi: 10.1016/j.molcel.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao D, Wan L, Wei W. Phosphorylation of Rictor at Thr1135 impairs the Rictor/Cullin-1 complex to ubiquitinate SGK1. Protein Cell. 2010;1(10):881–885. doi: 10.1007/s13238-010-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renauld S, Tremblay K, Ait-Benichou S, Simoneau-Roy M, Garneau H, Staub O, Chraibi A. Stimulation of ENaC activity by rosiglitazone is PPARgamma-dependent and correlates with SGK1 expression increase. J Membr Biol. 2010;236(3):259–270. doi: 10.1007/s00232-010-9297-7. [DOI] [PubMed] [Google Scholar]
- 32.Soundararajan R, Wang J, Melters D, Pearce D. Glucocorticoid-induced Leucine zipper 1 stimulates the epithelial sodium channel by regulating serum- and glucocorticoid-induced kinase 1 stability and subcellular localization. J Biol Chem. 2010;285(51):39905–39913. doi: 10.1074/jbc.m110.161133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J. 1999;18(11):3024–3033. doi: 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borst O, Schmidt EM, Munzer P, Schonberger T, Towhid ST, Elvers M, Leibrock C, Schmid E, Eylenstein A, Kuhl D, May AE, Gawaz M, Lang F. The serum- and glucocorticoid-inducible kinase 1 (SGK1) influences platelet calcium signaling and function by regulation of Orai1 expression in megakaryocytes. Blood. 2012;119(1):251–261. doi: 10.1182/blood-2011-06-359976. [DOI] [PubMed] [Google Scholar]
- 35.Eylenstein A, Schmidt S, Gu S, Yang W, Schmid E, Schmidt EM, Alesutan I, Szteyn K, Regel I, Shumilina E, Lang F. Transcription factor NF-kappaB regulates expression of pore-forming Ca2+ channel unit, Orai1, and its activator, STIM1, to control Ca2+ entry and affect cellular functions. J Biol Chem. 2012;287(4):2719–2730. doi: 10.1074/jbc.m111.275925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotte A, Pasham V, Eichenmuller M, Yang W, Bhandaru M, Lang F. Influence of dexamethasone on na+/h+ exchanger activity in dendritic cells. Cell Physiol Biochem. 2011;28(2):305–314. doi: 10.1159/000331746. [DOI] [PubMed] [Google Scholar]
- 37.Terada Y, Kuwana H, Kobayashi T, Okado T, Suzuki N, Yoshimoto T, Hirata Y, Sasaki S. Aldosterone-stimulated SGK1 activity mediates profibrotic signaling in the mesangium. J Am Soc Nephrol. 2008;19(2):298–309. doi: 10.1681/asn.2007050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murakami Y, Hosoi F, Izumi H, Maruyama Y, Ureshino H, Watari K, Kohno K, Kuwano M, Ono M. Identification of sites subjected to serine/threonine phosphorylation by SGK1 affecting N-myc downstream-regulated gene 1 (NDRG1)/Cap43-dependent suppression of angiogenic CXC chemokine expression in human pancreatic cancer cells. Biochem Biophys Res Commun. 2010;396(2):376–381. doi: 10.1016/j.bbrc.2010.04.100. [DOI] [PubMed] [Google Scholar]
- 39.Dehner M, Hadjihannas M, Weiske J, Huber O, Behrens J. Wnt signaling inhibits Forkhead box O3a-induced transcription and apoptosis through up-regulation of serum- and glucocorticoid-inducible kinase 1. J Biol Chem. 2008;283(28):19201–19210. doi: 10.1074/jbc.m710366200. [DOI] [PubMed] [Google Scholar]
- 40.Sahin P, McCaig C, Jeevahan J, Murray JT, Hainsworth AH. The cell survival kinase SGK1 and its targets FOXO3a and NDRG1 in aged human brain. Neuropathol Appl Neurobiol. 2013;39(6):623–633. doi: 10.1111/nan.12023. [DOI] [PubMed] [Google Scholar]
- 41.Lang F, Shumilina E. Regulation of ion channels by the serum- and glucocorticoid-inducible kinase SGK1. FASEB J. 2013;27(1):3–12. doi: 10.1096/fj.12-218230. [DOI] [PubMed] [Google Scholar]
- 42.McCaig C, Potter L, Abramczyk O, Murray JT. Phosphorylation of NDRG1 is temporally and spatially controlled during the cell cycle. Biochem Biophys Res Commun. 2011;411(2):227–234. doi: 10.1016/j.bbrc.2011.06.092. [DOI] [PubMed] [Google Scholar]
- 43.Ohashi T, Uchida K, Uchida S, Sasaki S, Nitta K. Dexamethasone increases the phosphorylation of nephrin in cultured podocytes. Clin Exp Nephrol. 2011;15(5):688–693. doi: 10.1007/s10157-011-0479-0. [DOI] [PubMed] [Google Scholar]
- 44.Voelkl J, Castor T, Musculus K, Viereck R, Mia S, Feger M, Alesutan I, Lang F. SGK1-Sensitive Regulation of Cyclin-Dependent Kinase Inhibitor 1B (p27) in Cardiomyocyte Hypertrophy. Cell Physiol Biochem. 2015;37(2):603–614. doi: 10.1159/000430380. [DOI] [PubMed] [Google Scholar]
- 45.Rusai K, Prokai A, Szebeni B, Fekete A, Treszl A, Vannay A, Muller V, Reusz G, Heemann U, Lutz J, Tulassay T, Szabo AJ. Role of serum and glucocorticoid-regulated kinase-1 in the protective effects of erythropoietin during renal ischemia/reperfusion injury. Biochem Pharmacol. 2010;79(8):1173–1181. doi: 10.1016/j.bcp.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 46.Catela C, Kratsios P, Hede M, Lang F, Rosenthal N. Serum and glucocorticoid-inducible kinase 1 (SGK1) is necessary for vascular remodeling during angiogenesis. Dev Dyn. 2010;239(8):2149–2160. doi: 10.1002/dvdy.22345. [DOI] [PubMed] [Google Scholar]
- 47.Zarrinpashneh E, Poggioli T, Sarathchandra P, Lexow J, Monassier L, Terracciano C, Lang F, Damilano F, Zhou JQ, Rosenzweig A, Rosenthal N, Santini MP. Ablation of SGK1 impairs endothelial cell migration and tube formation leading to decreased neo-angiogenesis following myocardial infarction. PLoS One. 2013;8(11):e80268. doi: 10.1371/journal.pone.0080268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L, Wei TQ, Wang Y, Zhang J, Li H, Wang KJ. Simulated bladder pressure stimulates human bladder smooth muscle cell proliferation via the PI3K/SGK1 signaling pathway. J Urol. 2012;188(2):661–667. doi: 10.1016/j.juro.2012.03.112. [DOI] [PubMed] [Google Scholar]
- 49.Towhid ST, Liu GL, Ackermann TF, Beier N, Scholz W, Fuchss T, Toulany M, Rodemann HP, Lang F. Inhibition of colonic tumor growth by the selective SGK inhibitor EMD638683. Cell Physiol Biochem. 2013;32(4):838–848. doi: 10.1159/000354486. [DOI] [PubMed] [Google Scholar]
- 50.Baskin R, Sayeski PP. Angiotensin II mediates cell survival through upregulation and activation of the serum and glucocorticoid inducible kinase 1. Cell Signal. 2012;24(2):435–442. doi: 10.1016/j.cellsig.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pelzl L, Hauser S, Elsir B, Sukkar B, Sahu I, Singh Y, Hoflinger P, Bissinger R, Jemaa M, Stournaras C, Schols L, Lang F. Lithium Sensitive ORAI1 Expression, Store Operated Ca(2+) Entry and Suicidal Death of Neurons in Chorea-Acanthocytosis. Sci Rep. 2017;7(1):6457. doi: 10.1038/s41598-017-06451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lang F, Guelinckx I, Lemetais G, Melander O. Two Liters a Day Keep the Doctor Away? Considerations on the Pathophysiology of Suboptimal Fluid Intake in the Common Population. Kidney Blood Press Res. 2017;42(3):483–494. doi: 10.1159/000479640. [DOI] [PubMed] [Google Scholar]
- 53.Abbruzzese C, Mattarocci S, Pizzuti L, Mileo AM, Visca P, Antoniani B, Alessandrini G, Facciolo F, Amato R, D'Antona L, Rinaldi M, Felsani A, Perrotti N, Paggi MG. Determination of SGK1 mRNA in non-small cell lung cancer samples underlines high expression in squamous cell carcinomas. J Exp Clin Cancer Res. 2012;31(1):4. doi: 10.1186/1756-9966-31-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szmulewitz RZ, Chung E, Al-Ahmadie H, Daniel S, Kocherginsky M, Razmaria A, Zagaja GP, Brendler CB, Stadler WM, Conzen SD. Serum/glucocorticoid-regulated kinase 1 expression in primary human prostate cancers. Prostate. 2012;72(2):157–164. doi: 10.1002/pros.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fagerli UM, Ullrich K, Stuhmer T, Holien T, Kochert K, Holt RU, Bruland O, Chatterjee M, Nogai H, Lenz G, Shaughnessy JD, Jr., Mathas S, Sundan A, Bargou RC, Dorken B, Borset M, Janz M. Serum/glucocorticoid-regulated kinase 1 (SGK1) is a prominent target gene of the transcriptional response to cytokines in multiple myeloma and supports the growth of myeloma cells. Oncogene. 2011;30(28):3198–3206. doi: 10.1038/onc.2011.79. [DOI] [PubMed] [Google Scholar]
- 56.Sommer EM, Dry H, Cross D, Guichard S, Davies BR, Alessi DR. Elevated SGK1 predicts resistance of breast cancer cells to Akt inhibitors. Biochem J. 2013;452(3):499–508. doi: 10.1042/bj20130342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt EM, Kraemer BF, Borst O, Munzer P, Schonberger T, Schmidt C, Leibrock C, Towhid ST, Seizer P, Kuhl D, Stournaras C, Lindemann S, Gawaz M, Lang F. SGK1 sensitivity of platelet migration. Cell Physiol Biochem. 2012;30(1):259–268. doi: 10.1159/000339062. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt EM, Gu S, Anagnostopoulou V, Alevizopoulos K, Foller M, Lang F, Stournaras C. Serum- and glucocorticoid-dependent kinase-1-induced cell migration is dependent on vinculin and regulated by the membrane androgen receptor. FEBS J. 2012;279(7):1231–1242. doi: 10.1111/j.1742-4658.2012.08515.x. [DOI] [PubMed] [Google Scholar]
- 59.Gu S, Papadopoulou N, Nasir O, Foller M, Alevizopoulos K, Lang F, Stournaras C. Activation of membrane androgen receptors in colon cancer inhibits the prosurvival signals Akt/bad in vitro and in vivo and blocks migration via vinculin/actin signaling. Mol Med. 2011;17(1-2):48–58. doi: 10.2119/molmed.2010.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eylenstein A, Gehring EM, Heise N, Shumilina E, Schmidt S, Szteyn K, Munzer P, Nurbaeva MK, Eichenmuller M, Tyan L, Regel I, Foller M, Kuhl D, Soboloff J, Penner R, Lang F. Stimulation of Ca2+-channel Orai1/STIM1 by serum- and glucocorticoid-inducible kinase 1 (SGK1). FASEB J. 2011;25(6):2012–2021. doi: 10.1096/fj.10-178210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt S, Schneider S, Yang W, Liu G, Schmidt EM, Schmid E, Mia S, Brucker S, Stournaras C, Wallwiener D, Brosens JJ, Lang F. TGFbeta1 and SGK1-sensitive store-operated Ca2+ entry and Orai1 expression in endometrial Ishikawa cells. Mol Hum Reprod. 2014;20(2):139–147. doi: 10.1093/molehr/gat066. [DOI] [PubMed] [Google Scholar]
- 62.Amato R, Scumaci D, D'Antona L, Iuliano R, Menniti M, Di Sanzo M, Faniello MC, Colao E, Malatesta P, Zingone A, Agosti V, Costanzo FS, Mileo AM, Paggi MG, Lang F, Cuda G, Lavia P, Perrotti N. Sgk1 enhances RANBP1 transcript levels and decreases taxol sensitivity in RKO colon carcinoma cells. Oncogene. 2013;32(38):4572–4578. doi: 10.1038/onc.2012.470. [DOI] [PubMed] [Google Scholar]
- 63.Liu T, Yu T, Hu H, He K. Knockdown of the long non-coding RNA HOTTIP inhibits colorectal cancer cell proliferation and migration and induces apoptosis by targeting SGK1. Biomed Pharmacother. 2018;98:286–296. doi: 10.1016/j.biopha.2017.12.064. [DOI] [PubMed] [Google Scholar]
- 64.Walker-Allgaier B, Schaub M, Alesutan I, Voelkl J, Geue S, Munzer P, Rodriguez JM, Kuhl D, Lang F, Gawaz M, Borst O. SGK1 up-regulates Orai1 expression and VSMC migration during neointima formation after arterial injury. Thromb Haemost. 2017;117(5):1002–1005. doi: 10.1160/th16-09-0690. [DOI] [PubMed] [Google Scholar]
- 65.Caglayan E, Vantler M, Leppanen O, Gerhardt F, Mustafov L, Ten Freyhaus H, Kappert K, Odenthal M, Zimmermann WH, Tallquist MD, Rosenkranz S. Disruption of platelet-derived growth factor-dependent phosphatidylinositol 3-kinase and phospholipase Cgamma 1 activity abolishes vascular smooth muscle cell proliferation and migration and attenuates neointima formation in vivo. J Am Coll Cardiol. 2011;57(25):2527–2538. doi: 10.1016/j.jacc.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong W, Oguljahan B, Xiao Y, Nelson J, Hernandez L, Garcia-Barrio M, Francis SC. Serum and glucocorticoid-regulated kinase 1 promotes vascular smooth muscle cell proliferation via regulation of beta-catenin dynamics. Cell Signal. 2014;26(12):2765–2772. doi: 10.1016/j.cellsig.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lang F, Stournaras C, Alesutan I. Regulation of transport across cell membranes by the serum- and glucocorticoid-inducible kinase SGK1. Mol Membr Biol. 2014;31(1):29–36. doi: 10.3109/09687688.2013.874598. [DOI] [PubMed] [Google Scholar]
- 68.Waldegger S, Klingel K, Barth P, Sauter M, Rfer ML, Kandolf R, Lang F. h-sgk serine-threonine protein kinase gene as transcriptional target of transforming growth factor beta in human intestine. Gastroenterology. 1999;116(5):1081–1088. doi: 10.1016/s0016-5085(99)70011-9. [DOI] [PubMed] [Google Scholar]
- 69.Szebeni B, Vannay A, Sziksz E, Prokai A, Cseh A, Veres G, Dezsofi A, Gyorffy H, Szabo IR, Arato A. Increased expression of serum- and glucocorticoid-regulated kinase-1 in the duodenal mucosa of children with coeliac disease. J Pediatr Gastroenterol Nutr. 2010;50(2):147–153. doi: 10.1097/mpg.0b013e3181b47608. [DOI] [PubMed] [Google Scholar]
- 70.Binger KJ, Gebhardt M, Heinig M, Rintisch C, Schroeder A, Neuhofer W, Hilgers K, Manzel A, Schwartz C, Kleinewietfeld M, Voelkl J, Schatz V, Linker RA, Lang F, Voehringer D, Wright MD, Hubner N, Dechend R, Jantsch J, Titze J, Muller DN. High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J Clin Invest. 2015;125(11):4223–4238. doi: 10.1172/jci80919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng J, Truong LD, Wu X, Kuhl D, Lang F, Du J. Serum- and glucocorticoid-regulated kinase 1 is upregulated following unilateral ureteral obstruction causing epithelial-mesenchymal transition. Kidney Int. 2010;78(7):668–678. doi: 10.1038/ki.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov. 2012;11(10):790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bezzerides VJ, Zhang A, Xiao L, Simonson B, Khedkar SA, Baba S, Ottaviano F, Lynch S, Hessler K, Rigby AC, Milan D, Das S, Rosenzweig A. Inhibition of serum and glucocorticoid regulated kinase-1 as novel therapy for cardiac arrhythmia disorders. Sci Rep. 2017;7(1):346. doi: 10.1038/s41598-017-00413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Voelkl J, Pasham V, Ahmed MS, Walker B, Szteyn K, Kuhl D, Metzler B, Alesutan I, Lang F. Sgk1-dependent stimulation of cardiac Na+/H+ exchanger Nhe1 by dexamethasone. Cell Physiol Biochem. 2013;32(1):25–38. doi: 10.1159/000350120. [DOI] [PubMed] [Google Scholar]
- 76.Hong Y, Cao H, Wang Q, Ye J, Sui L, Feng J, Cai X, Song H, Zhang X, Chen X. MiR-22 may Suppress Fibrogenesis by Targeting TGFbetaR I in Cardiac Fibroblasts. Cell Physiol Biochem. 2016;40(6):1345–1353. doi: 10.1159/000453187. [DOI] [PubMed] [Google Scholar]
- 77.Guo Y, Dong Z, Shi Y, Wang W, Wang L, Sun J, Sun X, Tian Z, Yao J, Li Z, Cheng J, Tian Y. Sonodynamic Therapy Inhibits Fibrogenesis in Rat Cardiac Fibroblasts Induced by TGF-beta1. Cell Physiol Biochem. 2016;40(3-4):579–588. doi: 10.1159/000452571. [DOI] [PubMed] [Google Scholar]
- 78.Yu F, Yang J, Huang K, Pan X, Chen B, Dong P, Zheng J. The Epigenetically-Regulated microRNA-378a Targets TGF-beta2 in TGF-beta1-Treated Hepatic Stellate Cells. Cell Physiol Biochem. 2016;40(1-2):183–194. doi: 10.1159/000452536. [DOI] [PubMed] [Google Scholar]
- 79.Li X, Han D, Tian Z, Gao B, Fan M, Li C, Li X, Wang Y, Ma S, Cao F. Activation of Cannabinoid Receptor Type II by AM1241 Ameliorates Myocardial Fibrosis via Nrf2-Mediated Inhibition of TGF-beta1/Smad3 Pathway in Myocardial Infarction Mice. Cell Physiol Biochem. 2016;39(4):1521–1536. doi: 10.1159/000447855. [DOI] [PubMed] [Google Scholar]
- 80.Zhang F, Dang Y, Li Y, Hao Q, Li R, Qi X. Cardiac Contractility Modulation Attenuate Myocardial Fibrosis by Inhibiting TGF-beta1/Smad3 Signaling Pathway in a Rabbit Model of Chronic Heart Failure. Cell Physiol Biochem. 2016;39(1):294–302. doi: 10.1159/000445624. [DOI] [PubMed] [Google Scholar]
- 81.Feger M, Alesutan I, Castor T, Mia S, Musculus K, Voelkl J, Lang F. Inhibitory effect of NH4Cl treatment on renal Tgfss1 signaling following unilateral ureteral obstruction. Cell Physiol Biochem. 2015;37(3):955–964. doi: 10.1159/000430222. [DOI] [PubMed] [Google Scholar]
- 82.Shih VF, Tsui R, Caldwell A, Hoffmann A. A single NFkappaB system for both canonical and non-canonical signaling. Cell Res. 2011;21(1):86–102. doi: 10.1038/cr.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stone KP, Kastin AJ, Pan W. NFkB is an unexpected major mediator of interleukin-15 signaling in cerebral endothelia. Cell Physiol Biochem. 2011;28(1):115–124. doi: 10.1159/000331720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chilukoti RK, Mostertz J, Bukowska A, Aderkast C, Felix SB, Busch M, Volker U, Goette A, Wolke C, Homuth G, Lendeckel U. Effects of irbesartan on gene expression revealed by transcriptome analysis of left atrial tissue in a porcine model of acute rapid pacing in vivo. Int J Cardiol. 2013;168(3):2100–2108. doi: 10.1016/j.ijcard.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 85.Yang M, Zheng J, Miao Y, Wang Y, Cui W, Guo J, Qiu S, Han Y, Jia L, Li H, Cheng J, Du J. Serum-glucocorticoid regulated kinase 1 regulates alternatively activated macrophage polarization contributing to angiotensin II-induced inflammation and cardiac fibrosis. Arterioscler Thromb Vasc Biol. 2012;32(7):1675–1686. doi: 10.1161/atvbaha.112.248732. [DOI] [PubMed] [Google Scholar]
- 86.Das S, Aiba T, Rosenberg M, Hessler K, Xiao C, Quintero PA, Ottaviano FG, Knight AC, Graham EL, Bostrom P, Morissette MR, del Monte F, Begley MJ, Cantley LC, Ellinor PT, Tomaselli GF, Rosenzweig A. Pathological role of serum- and glucocorticoid-regulated kinase 1 in adverse ventricular remodeling. Circulation. 2012;126(18):2208–2219. doi: 10.1161/circulationaha.112.115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Voelkl J, Lin Y, Alesutan I, Ahmed MS, Pasham V, Mia S, Gu S, Feger M, Saxena A, Metzler B, Kuhl D, Pichler BJ, Lang F. Sgk1 sensitivity of Na(+)/H(+) exchanger activity and cardiac remodeling following pressure overload. Basic Res Cardiol. 2012;107(2):236. doi: 10.1007/s00395-011-0236-2. [DOI] [PubMed] [Google Scholar]
- 88.Artunc F, Amann K, Nasir O, Friedrich B, Sandulache D, Jahovic N, Risler T, Vallon V, Wulff P, Kuhl D, Lang F. Blunted DOCA/high salt induced albuminuria and renal tubulointerstitial damage in gene-targeted mice lacking SGK1. J Mol Med. 2006;84(9):737–746. doi: 10.1007/s00109-006-0082-0. [DOI] [PubMed] [Google Scholar]
- 89.Tatsumoto N, Yamada S, Tokumoto M, Eriguchi M, Noguchi H, Torisu K, Tsuruya K, Kitazono T. Spironolactone ameliorates arterial medial calcification in uremic rats: the role of mineralocorticoid receptor signaling in vascular calcification. Am J Physiol Renal Physiol. 2015;309(11):F967–979. doi: 10.1152/ajprenal.00669.2014. [DOI] [PubMed] [Google Scholar]
- 90.Voelkl J, Tuffaha R, Luong TTD, Zickler D, Masyout J, Feger M, Verheyen N, Blaschke F, Kuro OM, Tomaschitz A, Pilz S, Pasch A, Eckardt KU, Scherberich JE, Lang F, Pieske B, Alesutan I. Zinc Inhibits Phosphate-Induced Vascular Calcification through TNFAIP3-Mediated Suppression of NF-kappaB. J Am Soc Nephrol. 2018;29(6):1636–1648. doi: 10.1681/asn.2017050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao G, Xu MJ, Zhao MM, Dai XY, Kong W, Wilson GM, Guan Y, Wang CY, Wang X. Activation of nuclear factor-kappa B accelerates vascular calcification by inhibiting ankylosis protein homolog expression. Kidney Int. 2012;82(1):34–44. doi: 10.1038/ki.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]