Significance Statement
Developing strategies for managing coronary artery calcification (CAC) in patients with CKD remains a clinical challenge. Previous experimental studies showed that magnesium inhibits vascular calcification, whereas the uremic toxin indoxyl sulfate aggravates it. In a 2-year, open-label, randomized, controlled trial with a two-by-two factorial design, the authors investigated whether oral magnesium oxide or the oral carbon adsorbent AST-120 affected CAC progression in patients with stage 3−4 CKD with risk factors for CAC. In an interim analysis with 125 enrolled patients (96 of whom completed the trial), patients taking magnesium oxide experienced a significantly smaller percentage change in CAC score compared with controls (11.3% versus 39.5%). AST-120 was not associated with a similar significant slowing of CAC. Larger-scale trials are warranted to confirm these findings.
Keywords: magnesium oxide, coronary artery calcification, chronic kidney disease, randomized controlled trial, oral carbon adsorbent
Abstract
Background
Developing strategies for managing coronary artery calcification (CAC) in patients with CKD is an important clinical challenge. Experimental studies have demonstrated that magnesium inhibits vascular calcification, whereas the uremic toxin indoxyl sulfate aggravates it.
Methods
To assess the efficacy of magnesium oxide (MgO) and/or the oral carbon adsorbent AST-120 for slowing CAC progression in CKD, we conducted a 2-year, open-label, randomized, controlled trial, enrolling patients with stage 3−4 CKD with risk factors for CAC (diabetes mellitus, history of cardiovascular disease, high LDL cholesterol, or smoking). Using a two-by-two factorial design, we randomly assigned patients to an MgO group or a control group, and to an AST-120 group or a control group. The primary outcome was percentage change in CAC score.
Results
We terminated the study prematurely after an interim analysis with the first 125 enrolled patients (of whom 96 completed the study) showed that the median change in CAC score was significantly smaller for MgO versus control (11.3% versus 39.5%). The proportion of patients with an annualized percentage change in CAC score of ≥15% was also significantly lower for MgO compared with control (23.9% versus 62.0%). However, MgO did not suppress the progression of thoracic aorta calcification. The MgO group’s dropout rate was higher than that of the control group (27% versus 17%), primarily due to diarrhea. The percentage change in CAC score did not differ significantly between the AST-120 and control groups.
Conclusions
MgO, but not AST-120, appears to be effective in slowing CAC progression. Larger-scale trials are warranted to confirm these findings.
Coronary artery calcification (CAC) is highly prevalent and rapidly progressive among patients with CKD.1–6 CAC is suspected to increase arterial stiffness and compromise coronary flow reserve, thereby leading to cardiac ischemia.7 Indeed, CAC is strongly predictive of cardiovascular events and mortality among patients with CKD, outperforming other subclinical atherosclerosis measures such as carotid intima-media thickness and ankle-brachial index.8–12 Because the prevalence of CAC starts rising at the early stages of CKD,6 it is imperative to establish evidence targeting patients with predialysis CKD. Although hyperphosphatemia is closely associated with the progression of CAC,13–15 a previous randomized trial of patients with eGFR of 20−45 ml/min per 1.73 m2 failed to show a benefit of phosphate binders for preventing the progression of CAC.16 Developing effective strategies for managing CAC in CKD has remained a major clinical challenge.
Magnesium has recently received attention due to its inhibitory effects on vascular calcification.17 In vitro studies have revealed that magnesium inhibits high phosphate–induced calcification of vascular smooth muscle cells (VSMCs).18–28 An in vivo study using a 5/6 nephrectomized rat model confirmed that magnesium supplementation, both orally and intraperitoneally, prevented aortic calcification.29 In humans, a few pilot studies have suggested the clinical effectiveness of magnesium-containing phosphate binders on the progression of vascular calcification.30,31 In addition, randomized trials have shown that serum calcification propensity, T50, was improved by oral magnesium administration for patients with stage 3–4 CKD and by high-magnesium dialysate for patients undergoing hemodialysis.32,33 Therefore, magnesium may be useful to prevent the progression of CAC.
Uremic toxins might be another potential therapeutic target of vascular calcification. Indoxyl sulfate (IS), a protein-bound uremic toxin derived from dietary tryptophan, is shown to accelerate calcification of VSMCs.34 Oral administration of IS to Dahl salt-sensitive hypertensive rats led to severe aortic calcification.35 In patients with CKD, serum IS levels were positively correlated with aortic calcification score.36 Therefore, it can be hypothesized that reducing IS levels by oral carbon adsorbent (AST-120) might prevent the progression of CAC.
This randomized trial aimed to translate these experimental findings to a clinical setting, testing the hypothesis that oral magnesium oxide and/or AST-120 suppress the progression of CAC among patients with predialysis CKD.
Methods
Study Design
In this single-center, open-label, randomized, controlled trial with a 2-by-2 factorial design, we evaluated the efficacy of oral magnesium oxide (Yoshida Pharmaceutical, Japan) and AST-120 (Kureha Corporation, Japan), separately, on the progression of CAC during a 2-year intervention period (University Hospital Medical Information Network: 000009738). This study was conducted at the outpatient department of Osaka University Hospital, Japan, from January of 2013 to May of 2018. All patients provided written informed consent before study enrollment. This trial was conducted in accordance with the principles of the Declaration of Helsinki and the study protocol was approved by the Ethics Committee of Osaka University Hospital (Approval No. 12172). The funder (Mitsubishi Tanabe Pharma Corporation) did not participate in the trial design and conduct, or in data collection and analyses.
Study Participants
Patients were eligible if they were aged 20 years or older, were diagnosed as stage 3−4 CKD (eGFR of 15−59 ml/min per 1.73 m2), and had at least one of the following risk factors of CAC: (1) diabetes mellitus, (2) a prior history of cardiovascular disease (CVD), (3) hyper-LDL cholesterol, or (4) current smoking. eGFR was calculated using an equation for the Japanese population.37 Diabetes mellitus was defined as a fasting glucose level of ≥126 mg/dl, and/or a nonfasting glucose level of ≥200 mg/dl, and/or a hemoglobin A1c level of ≥6.5%, and/or a previous diagnosis of diabetic retinopathy, and/or use of glucose-lowering medications. A prior history of CVD included coronary artery disease, cerebral infarction, cerebral hemorrhage, and peripheral artery disease. Hyper-LDL cholesterolemia was defined as LDL cholesterol levels of ≥140 mg/dl or a use of statins. Smoking status was determined by self-reporting.
Patients were excluded if they (1) received magnesium oxide and/or AST-120 before study enrollment, or (2) underwent coronary artery stenting (which precludes CAC scoring), or (3) were expected by the physician in charge to initiate RRT within 1 year.
Randomization and Intervention
Eligible patients were randomly assigned to a magnesium oxide group or a control group in a 1:1 ratio and to an AST-120 group or a control group in a 3:2 ratio. We assigned more patients to the AST-120 group because patients in the AST-120 group were expected to be more likely to drop out due to gastrointestinal side effects of this drug. Allocation was adequately concealed by using an online-based block randomization program (block size: 10) with stratification by sex, diabetes mellitus, and menopausal status. In the magnesium oxide group, patients were initially administered 330 mg (8.3 mmol) per day of magnesium oxide (elemental magnesium: 198 mg). The doses were adjusted every 1–3 months to achieve serum magnesium levels of 2.5−3.0 mg/dl. This target was on the basis of our previous cohort study of patients on hemodialysis.38 Patients in the AST-120 group received 6 g/d of AST-120. Patients in the control group received standard therapy for CKD alone. Adherence to the study drugs was assessed by interviewing patients at every follow-up visit. A good compliance was defined as taking a prescribed dose of magnesium oxide and 6 g/d of AST-120 during the study period.
Outcome and CAC Measurements
The CAC scores were measured at study entry and at 2 years or just before the initiation of RRT. The primary outcome of this study was a percentage change in CAC scores over the study period. A secondary outcome was the proportion of patients with an annualized percentage change in CAC scores of ≥15%. The percentage change in CAC score was calculated as follows:
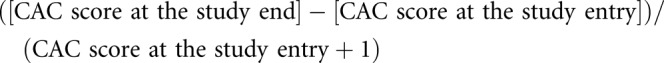 |
(the addition of 1 to the CAC score of the denominator enables the analysis of patients with a CAC score of zero).
The annualized percentage change in CAC scores is expressed as follows:
For example, if a patient had a percentage change in CAC scores of 0.44 (44%) and his follow-up time was 24 months, his annualized percentage change in CAC scores was calculated as:
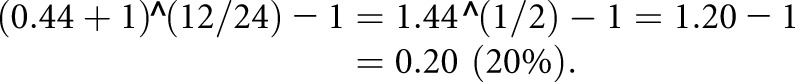 |
We defined rapid progressors as patients with annualized percentage change in CAC scores of ≥15%; this cutoff value has been widely used as a clinically meaningful progression of CAC.39–43
The methodology for the measurement of CAC score was described elsewhere.44 Computed tomography scans were performed using a multi–detector row computed tomography scanner in an independent radiology clinic (Uchida Clinic, Osaka, Japan). The CAC score, on the basis of the Agatston method,45 was measured with SmartScore version 3.5 (GE Healthcare, Japan) by a physician who was experienced in the evaluation of CAC and was blinded to patients’ information and treatment assignment. Calcifications of the descending thoracic aorta (from carina of trachea to diaphragm) were quantified with a thoracic aorta calcification score calculated by the same method as for the CAC score.
We captured all clinical events including death, cardiovascular events requiring hospitalization, and initiation of RRT.
Statistical Analyses
We estimated that a sample size of 222 patients (111 in each group) would provide a power of 90% at an overall two-sided α error level of 0.05 to detect a 30% relative reduction in the percentage change in CAC scores in the magnesium oxide group compared with the control group, given that an annual percentage change in CAC score among patients with predialysis CKD and diabetes was 14%.46 Assuming a dropout rate of approximately 10%, we planned to enroll a total of 250 patients. An interim analysis for efficacy was to be performed after at least half of the planned number of patients (i.e., 125 patients) completed the study. On the basis of the Lan−DeMets α-spending function approach (Pocock type), the stopping boundary P value at the interim analysis was <0.03; this P value as well as the sample size calculation described above was authorized by a statistician of the Ethics Committee. The interim analysis was performed by each member of the data and safety monitoring board who were independent of the execution of this trial.
Changes in laboratory parameters (serum magnesium, serum calcium, serum phosphate, fractional excretion of phosphate, plasma whole parathyroid hormone [1–84] [DS Pharma Biomedical Co., Osaka, Japan], eGFR, LDL cholesterol, hemoglobin A1c, and hemoglobin) and BP levels over the study period were compared between the study groups using a repeated measures ANOVA. Proportions of patients receiving statins and antiplatelet agents at the end of the study were compared between the groups using a chi-squared test.
The primary analysis was performed on the full analysis set which included all randomized patients who underwent the final CAC measurement. The percentage change in CAC scores was compared between the study groups using a Wilcoxon rank sum test. The proportion of patients with the annualized percentage change of ≥15% was compared using a chi-squared test.
We conducted several post hoc analyses. We assessed the efficacy of the study drugs on time-varying CAC scores using a mixed-effects linear regression model. In this analysis, CAC scores were log-transformed (i.e., ln[CAC score+1]), and an interaction term between intervention and observation time was examined. We analyzed the primary outcome after excluding patients with baseline CAC score of 0 and those with baseline CAC scores ≥1000. In subgroup analyses, patients were stratified by baseline CAC scores (<400 or ≥400) and baseline eGFR (<30 or ≥30 ml/min per 1.73 m2). As an alternative to an intention-to-treat analysis, we performed an analysis where all patients who dropped out from the study were included as rapid progressors. Similarly, we performed another analysis where all dropout patients were regarded as slow progressors. Finally, we treated all patients who dropped out from the magnesium group as rapid progressors and those who dropped out from the control group as slow progressors. As a per-protocol analysis, we included only patients who continued magnesium oxide and AST-120 (6 g/d) during the study period.
Association of the percentage change in CAC score with serum magnesium levels as well as urinary protein levels was tested by Pearson’s product moment correlation coefficient.
All P values were two-sided. A P value of <0.05 was considered statistically significant. Statistical analyses were performed using StataIC 14 (StataCorp LP, College Station, TX).
Results
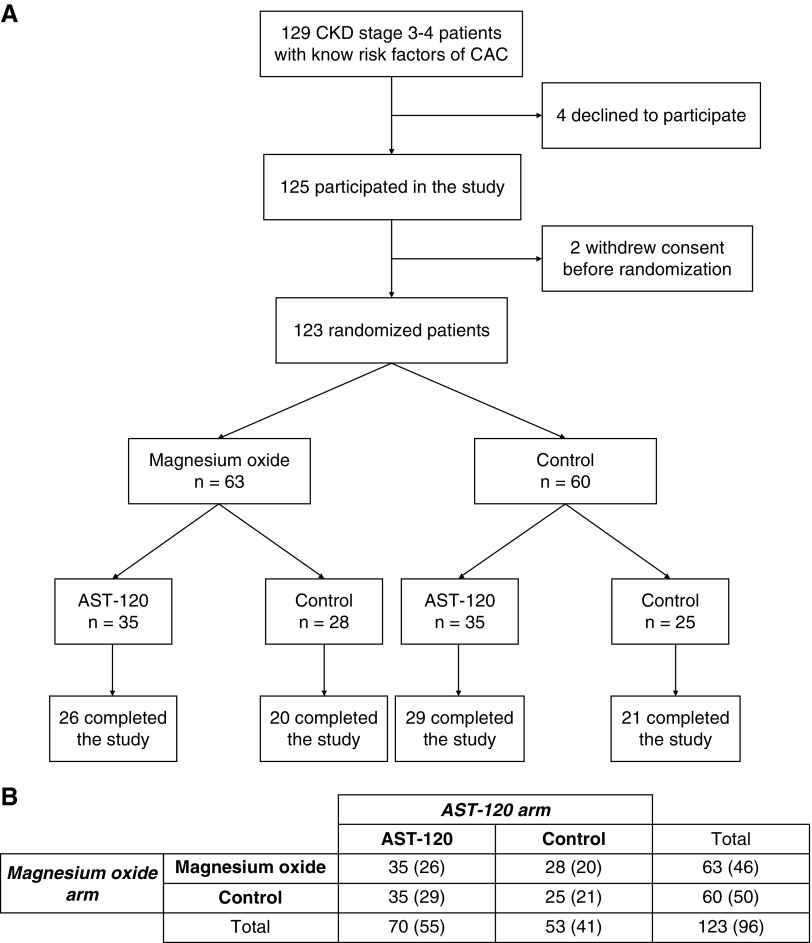
At the time of the interim analysis, a total of 145 patients were enrolled in the study; among them, 20 were still in the follow-up period. A flow chart of the first 125 enrolled (123 randomized) patients involved in the interim analysis is shown in Figure 1. More than 70% of patients had diabetes mellitus and nearly half of the patients had a past history of CVD (Table 1). The median (25th, 75th percentile) CAC score of enrolled patients was 188 (57, 807); the number of patients with CAC scores of 0−99, 100−399, 400−999, and 1000− was 44 (35.8%), 27 (22.0%), 31 (25.2%), and 21 (17.1%), respectively. The baseline characteristics were similar across the groups, except for the prevalence of current smokers which was slightly higher in the AST-120 group (Table 1). Baseline CAC scores were not significantly different between groups (P=0.54 for the magnesium oxide arm and P=0.77 for the AST-120 arm [Wilcoxon rank sum test]). No patients received any kinds of phosphate binders at baseline.
Figure 1.
A total of 123 patients underwent randomization. (A) Flow chart of the first 125 enrolled patients. (B) The numbers of patients randomized to each group (2×2 factorial design). The numbers in parentheses denote the numbers of patients who completed the study.
Table 1.
Baseline characteristics of study participants
| Characteristics | Magnesium Oxide Arm | AST-120 Arm | ||
|---|---|---|---|---|
| Magnesium oxide n=63 | Control n=60 | AST-120 n=70 | Control n=53 | |
| Age, yr | 70 (12) | 69 (11) | 69 (11) | 71 (12) |
| Female, % | 25 | 23 | 20 | 30 |
| Body mass index, kg/m2 | 23.8 (3.9) | 24.3 (4.1) | 23.8 (3.9) | 24.4 (4.1) |
| Diabetes mellitus, % | 76 | 73 | 76 | 74 |
| Past history of CVD, % | 48 | 47 | 47 | 47 |
| Past history of CHF, % | 18 | 22 | 20 | 19 |
| Current smokers, % | 13 | 12 | 16 | 8 |
| Systolic BP, mm Hg | 130 (18) | 133 (19) | 132 (18) | 130 (18) |
| Diastolic BP, mm Hg | 72 (14) | 74 (10) | 73 (13) | 73 (12) |
| Serum creatinine, mg/dl | 1.67 (0.62) | 1.63 (0.58) | 1.73 (0.64) | 1.55 (0.54) |
| eGFR, ml/min per 1.73 m2 | 35.1 (13.1) | 36.1 (13.2) | 34.9 (13.6) | 36.5 (12.4) |
| Serum calcium, mg/dl | 9.3 (0.4) | 9.2 (0.4) | 9.2 (0.4) | 9.2 (0.4) |
| Serum phosphate, mg/dl | 3.4 (0.6) | 3.4 (0.5) | 3.4 (0.6) | 3.4 (0.5) |
| Serum magnesium, mg/dl | 2.0 (0.2) | 2.1 (0.3) | 2.1 (0.2) | 2.1 (0.3) |
| Whole PTH, pg/ml | 46 (36, 82) | 46 (30, 68) | 46 (29, 76) | 44 (36, 68) |
| Serum albumin, g/dl | 3.9 (0.5) | 4.0 (0.5) | 3.9 (0.5) | 4.0 (0.5) |
| LDL cholesterol, mg/dl | 106 (30) | 102 (30) | 103 (32) | 104 (26) |
| CAC score | 266 (59, 924) | 166 (56, 630) | 169 (59, 653) | 203 (57, 907) |
Data are on the basis of all randomized patients. Continuous variables are presented as mean (SD) or median (25th, 75th percentile). CHF, congestive heart failure; PTH, parathyroid hormone.
Ninety-six patients (78%) completed the study (Figure 1). The dropout rate was slightly higher in the magnesium group (27% [17 of 63]) than in the control group (17% [10 of 60]). The reasons for dropout from the study were similar between the groups except for three cases with diarrhea in the magnesium group (Supplemental Table 1). Among those patients who completed the study, baseline characteristics were similar between the groups (Supplemental Table 2). Although most patients showed a good compliance to magnesium oxide (median [interquartile range] duration of the drug intake, 24 [24, 24] months), three patients in the magnesium group who completed the study stopped taking the drug within 1 year due to diarrhea. The mean (SD) daily dose of magnesium oxide at the end of the study was 507 (352) mg (12.7 [8.8] mmol) (elemental magnesium, 304 [211] mg). Among 55 patients in the AST-120 group who completed the study, 34 (76%) showed a good compliance (i.e., 6 g/d) and most of the other patients also continued to take the drug at lower doses (median [interquartile range] duration of drug exposure, 24 [24, 24] months; mean [SD] dose of AST-120, 5.0 [2.0] g/d).
Serum magnesium levels were significantly elevated in the magnesium group compared with the control group although only ten patients (21.7%) achieved serum magnesium levels of ≥2.5 mg/dl (Table 2). No significant differences were observed with respect to the changes in serum calcium, serum phosphate, fractional excretion of phosphate, plasma whole parathyroid hormone, or eGFR between the magnesium group and control group (Table 2) as well as between the AST-120 group and control group (Supplemental Table 3). Other laboratory data (LDL cholesterol, hemoglobin A1c, and hemoglobin), medications prescribed (statins and antiplatelet agents), and BP levels during the study period were also similar between these groups (Supplemental Table 4).
Table 2.
Change in laboratory parameters in the magnesium oxide arm
| Parameter | Allocation Group | Baseline | 1 yr | 2 yr | P Value |
|---|---|---|---|---|---|
| Serum magnesium, mg/dl | Control | 2.1 (0.3) | 2.1 (0.3) | 2.1 (0.3) | <0.001 |
| Magnesium oxide | 2.0 (0.2) | 2.2 (0.3) | 2.3 (0.4) | ||
| Serum calcium, mg/dl | Control | 9.2 (0.4) | 9.2 (0.4) | 9.2 (0.4) | 0.11 |
| Magnesium oxide | 9.3 (0.4) | 9.2 (0.4) | 9.2 (0.5) | ||
| Serum phosphate, mg/dl | Control | 3.4 (0.5) | 3.5 (0.7) | 3.5 (0.6) | 0.64 |
| Magnesium oxide | 3.5 (0.6) | 3.5 (0.7) | 3.5 (0.7) | ||
| Fractional excretion of phosphate, % | Control | 23.0 (10.8) | 23.0 (11.7) | 23.2 (11.4) | 0.10 |
| Magnesium oxide | 20.4 (12.2) | 21.3 (11.5) | 24.5 (13.5) | ||
| Plasma whole PTH, pg/ml | Control | 47 (30, 67) | 43 (34, 67) | 45 (32, 73) | 0.48 |
| Magnesium oxide | 44 (33, 64) | 45 (31, 97) | 50 (29, 82) | ||
| eGFR, ml/min per 1.73 m2 | Control | 35.6 (12.8) | 32.8 (14.8) | 33.0 (14.6) | 0.50 |
| Magnesium oxide | 35.9 (12.9) | 32.8 (13.2) | 31.2 (14.1) |
Data are on the basis of the full analysis set population and presented as mean (SD) or median (25th, 75th percentile). Differences between allocation groups are tested using a repeated-measures ANOVA. PTH, parathyroid hormone.
Efficacy End Points
Magnesium Oxide Arm
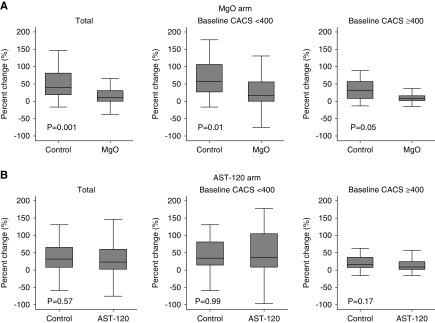
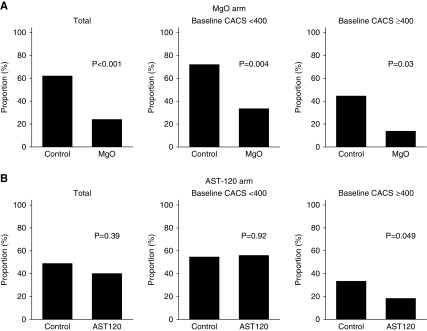
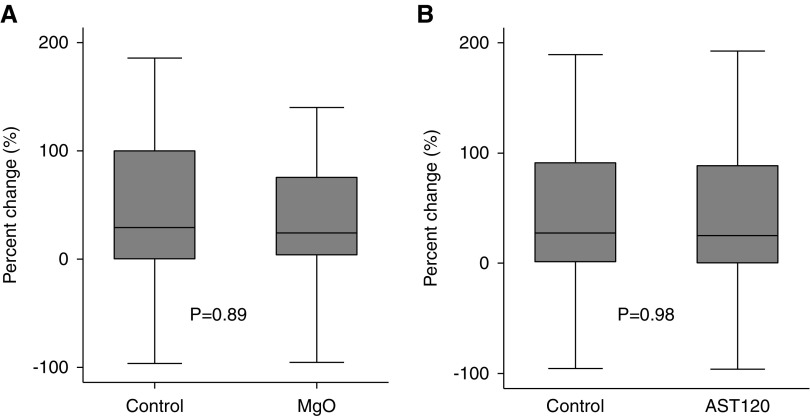
The data and safety monitoring board recommended the early termination of the trial on the basis of the results of the prespecified interim analysis which crossed the prespecified stopping boundary; the percentage change in CAC scores of the magnesium group was significantly smaller than that of the control group (median value, 11.3% versus 39.5%; P<0.001) (Figure 2A, Table 3). The interaction term between magnesium oxide and AST-120 on the percentage change in CAC scores was not significant (P for interaction=0.31). The proportion of patients with the annualized percentage change in CAC scores of ≥15% (i.e., rapid progressors) was lower in the magnesium group than that in the control group (23.9% versus 62.0%; P<0.001) (Figure 3A). In contrast, magnesium oxide did not significantly suppress the percentage change in thoracic aorta calcification scores (24.2% versus 29.0% in the magnesium group and control group, respectively; P=0.89) (Figure 4A). No significant correlation was found between the percentage change in CAC scores and that in the thoracic aorta calcification scores (P=0.84, R2<0.001).
Figure 2.
MgO, but not AST-120, retards the progression of CAC. (A) Total patients (n=96). (B) Patients with baseline CAC score <400 (n=56). (C) Patients with baseline CAC score ≥400 (n=40). Percentage changes in CAC scores are compared between groups using the Wilcoxon rank sum test. Data are on the basis of the full analysis set population. CACS, coronary artery calcification score; MgO, magnesium oxide.
Table 3.
Change in CAC scores in the magnesium oxide arm
| Subgroups | Allocation Group | n | CAC Score | Percentage Change in CAC Scores | P Value | |
|---|---|---|---|---|---|---|
| Baseline | End of the Study | |||||
| Total | Magnesium oxide | 46 | 302 (64, 924) | 375 (79, 1033) | 11.3 (0, 30.8) | 0.001 |
| Control | 50 | 166 (54, 606) | 268 (111, 816) | 39.5 (19.0, 81.3) | ||
| Baseline CAC score <1000 | Magnesium oxide | 37 | 123 (53, 547) | 165 (59, 546) | 14.3 (0, 36.7) | <0.01 |
| Control | 44 | 138 (45, 437) | 210 (75, 568) | 43.7 (20.4, 85.1) | ||
| Baseline CAC score <400 | Magnesium oxide | 24 | 67 (19, 119) | 86 (26, 159) | 17.0 (0, 55.8) | 0.01 |
| Control | 32 | 89 (24, 153) | 151 (36, 242) | 57.4 (26.8, 106.2) | ||
| Baseline CAC score ≥400 | Magnesium oxide | 22 | 942 (639, 1438) | 1045 (763, 1903) | 8.8 (1.8, 15.8) | 0.05 |
| Control | 18 | 872 (582, 1065) | 1208 (644, 2065) | 30.4 (8.0, 56.9) | ||
Data are on the basis of the full analysis set population and presented as median (25th, 75th percentile). Percentage changes in CAC scores are compared between groups using the Wilcoxon rank sum test. The median CAC scores at baseline and at the end of the study are not necessarily from the same patient. Therefore, the percentage change calculated from these two values does not correspond to the true median percentage change in CAC scores in each study group.
Figure 3.
MgO reduces the proportion of rapid progressors. Rapid progressors are defined as those patients with an annualized percentage change in CAC scores of ≥15%. (A) Total patients (n=96). (B) Patients with baseline CAC score <400 (n=56). (C) Patients with baseline CAC score ≥400 (n=40). Proportions between groups are compared using chi-squared test. Data are on the basis of the full analysis set population. CACS, coronary artery calcification score; MgO, magnesium oxide.
Figure 4.
Neither MgO nor AST-120 suppresses the progression of thoracic aorta calcification. (A) Percentage changes in thoracic aorta calcification are not significantly different between the MgO group (n=46) and control group (n=50). (B) Percentage changes in thoracic aorta calcification are not significantly different between the AST-120 group (n=55) and control group (n=41). Data are on the basis of the full analysis set population. MgO, magnesium oxide.
AST-120 Arm
The median percentage change in CAC scores was 23.1% in the AST-120 group and 31.9% in the control group (P=0.57) (Figure 2B, Supplemental Table 5). The proportion of rapid progressors was also similar between the two groups (40.0% in the AST-120 group and 48.8% in the control group; P=0.39) (Figure 3B). AST-120 did not significantly suppress the percentage change in thoracic aorta calcification scores (25.2% versus 27.4% in the AST-120 group and control group, respectively; P=0.98) (Figure 4B).
Post Hoc Analyses
In a mixed-effects linear regression model, a change in log-transformed CAC scores over the study period was significantly smaller in the magnesium group than that in the control group (P for observation time×group interaction=0.04), but no significant difference was found between the AST-120 and control group (P for observation time×group interaction=0.28).
The effect of magnesium oxide on the percentage change in CAC scores persisted after excluding five patients with baseline CAC scores of 0 (12.0% versus 39.5%; P=0.001). Similar results were obtained when patients with extraordinarily high baseline CAC scores (≥1000) were excluded (Table 3).
When patients were stratified according to their baseline CAC scores (<400 or ≥400), magnesium oxide exhibited similar effects on the percentage change in CAC scores in both subgroups (P for interaction=0.33) (Figure 2A, Table 3). Similarly, the proportion of rapid progressors was lower in the magnesium group regardless of the baseline CAC scores (Figure 3A). AST-120 did not significantly improve the percentage change in CAC scores in either of the CAC subgroups (Figure 2B). The proportion of rapid progressors was lower in the AST-120 group when patients had high baseline CAC scores (≥400) (P=0.05) (Figure 3B).
AST-120 did not affect the percentage change in CAC scores in both baseline eGFR subgroups (<30 and ≥30 ml/min per 1.73 m2) (Supplemental Table 5).
The effect of magnesium oxide on the percentage change in CAC scores was not modified by a baseline urinary protein-to-creatinine ratio (P for interaction=0.69). In the magnesium group, the percentage change in CAC scores was neither associated with a change in serum magnesium levels after magnesium supplementation (P=0.65) nor with serum magnesium levels at the end of the study (P=0.38).
In order to assess the efficacy of magnesium oxide on the basis of intention-to-treat principle, we performed an additional analysis where all patients who dropped out from the study were included as rapid progressors. In this analysis, the proportion of rapid progressors was still lower in the magnesium group compared with the control group (44.4% [28 of 63] versus 66.7% [40 of 60]; P=0.01). Similarly, the proportion of rapid progressors was lower in the magnesium group if all dropout patients were regarded as slow progressors (17.5% [11 of 63] versus 51.7% [31 of 60]; P<0.001). When all patients who dropped out from the magnesium group were treated as rapid progressors and those who dropped out from the control group were treated as slow progressors (the worst case scenario), the proportion of rapid progressors was 44.4% [28 of 63] in the magnesium group and 51.7% [31 of 60] in the control group (P=0.42). We also performed a per-protocol analysis by excluding three patients in the magnesium group who discontinued the study drug, confirming a significantly lower progression rate of CAC in the magnesium group than that in the control group (11.8% versus 39.5%; P=0.001). When including only patients who were adherent to AST-120, this drug did not significantly alter the percentage change in CAC scores (19.2% versus 31.9% in the AST-120 group and control group, respectively; P=0.38).
Clinical Events
Clinical events that occurred during the study period are summarized in Table 4. Overall, three patients died (one in the magnesium group and two in the control group). Cardiovascular events requiring hospitalization occurred in eight patients (three in the magnesium group and five in the control group). Six patients initiated RRT during the study period (two in the magnesium group and four in the control group). In the AST-120 arm, all deaths were observed in the control group, whereas all incident RRT events occurred in the AST-120 group (Supplemental Table 6).
Table 4.
Clinical events in the magnesium oxide arm
| Clinical Event | Magnesium Oxide (n=63) | Control (n=60) |
|---|---|---|
| Deaths | 1 (1.6%) | 2 (3.3%) |
| Hepatitis B virus cirrhosis (n=1) | Acute myocardial infarction (n=1) | |
| ESRD (n=1) | ||
| Cardiovascular events | 3 (4.8%) | 5 (8.3%) |
| Heart failure (n=1) | Heart failure (n=1) | |
| Percutaneous coronary intervention (n=1) | Percutaneous coronary intervention (n=1) | |
| Infectious endocarditis (n=1) | Atrioventricular block (n=1) | |
| Cerebral infarction (n=1) | ||
| Cerebral hemorrhage (n=1) | ||
| Initiation of RRT | 2 (3.2%) | 4 (6.7%) |
| Adverse events | 6 (9.5%) | 0 |
| Severe diarrhea (n=6) |
Data are on the basis of all randomized patients.
Discussion
In this randomized, controlled trial, we explored the efficacy of 2-year oral magnesium oxide and AST-120 for the progression of CAC among patients with predialysis CKD with known risk factors for vascular calcification, mainly diabetes mellitus. The interim analysis revealed that the percentage change in CAC scores was substantially reduced by magnesium oxide. Although two pilot studies of hemodialysis patients suggested a favorable effect of magnesium-containing phosphate binders on vascular calcification,30,31 our study provides the strongest evidence so far regarding the clinical benefit of magnesium for the prevention of CAC progression among patients with predialysis CKD. On the other hand, AST-120 did not exhibit a significant effect on the progression of CAC.
Our findings are in line with experimental studies showing that magnesium suppresses calcification of VSMCs through inhibiting their apoptosis, osteoblastic transdifferentiation, and Wnt/β-catenin signaling.18–28 Interestingly, some of these studies pointed out that an inhibition of the transient receptor potential melastatin 7 (TRPM7) channels, a major magnesium transporter of VSMCs, impaired the anticalcification ability of magnesium, implying a crucial role for intracellular magnesium.18,21,22,24,27 In our study, the percentage change in CAC scores was neither associated with the change in serum magnesium levels after magnesium supplementation nor with serum magnesium levels at the study end. Also, the effect of magnesium oxide was not modified by baseline urinary protein, a predictor of an increase in serum magnesium levels in response to magnesium supplementation.47 Therefore, it could be argued that the prevention of CAC progression might have been mediated by a restoration of intracellular magnesium content, but not by an increase in extracellular magnesium levels. This speculation is supported by a previous trial showing that oral magnesium supplementation for patients with diabetes mellitus impaired platelet aggregation accompanied by an increase in intracellular magnesium concentrations despite no significant alterations in extracellular magnesium levels.48 Another randomized trial showed an improvement of brachial artery flow-mediated dilation by oral magnesium supplementation especially among patients exhibiting a higher increase in intracellular magnesium concentrations.49 Considering that magnesium is the major intracellular cation, intracellular magnesium may play an essential role in terms of the prevention of vascular calcification and, therefore, intracellular magnesium concentrations might better reflect the therapeutic efficacy compared with extracellular magnesium levels.
On the other hand, an in vitro study has suggested an important role of extracellular magnesium in the inhibition of vascular calcification.28 Calciprotein particles (CPPs) in patients’ serum, a potent inducer of calcification,50,51 may be a unique therapeutic target of extracellular magnesium because magnesium is known to inhibit the maturation of CPPs.52,53 Both oral magnesium supplementation and increasing dialysate magnesium concentrations have been shown to improve serum calcification propensity, T50, a transformation time of primary to secondary CPPs in patients’ serum.32,33 Thus, increasing serum magnesium levels itself seems to have a direct clinical implication, given that a lot of cohort studies reported a negative association between serum magnesium levels and the risk of cardiovascular outcomes in CKD.38,52 In our study, the lack of a dose-response relationship between the change in serum magnesium levels and that in CAC progression may be only due to a limited statistical power. In addition, the average serum magnesium levels of the magnesium group were increased by only 0.3 mg/dl at the study end, which might not be enough to detect the potential benefit of extracellular magnesium on CAC progression.
Magnesium binds phosphate in the intestinal tract and thus is used as a phosphate binder in clinical practice.54 Therefore, it might be possible that the effect of magnesium oxide on CAC was derived from its phosphate-binding capacity. However, the phosphate-binding capacity of a magnesium salt alone is usually quite low.55 In fact, neither serum phosphate levels nor fractional excretion of phosphate was different between the magnesium group and control group during the study period. Serum phosphate levels were well within the normal range in both groups. It should be recalled that more effective phosphate binders such as lanthanum carbonate did not suppress the CAC progression of patients with non-dialysis CKD without apparent hyperphosphatemia.16 Thus, the limited phosphate-binding capacity of magnesium oxide is not likely to explain the remarkable reduction in CAC progression observed in our study.
Prevention of CAC should be initiated at an early phase of CKD because CAC progresses rapidly even before ESRD.4,6 At this stage, however, hyperphosphatemia is not usually apparent. Here, our study provides a novel therapeutic option, magnesium, for CAC among patients with predialysis CKD. Of note, we have previously reported that the prevalence of hypomagnesemia is unexpectedly high in patients with predialysis CKD.47 Thus, there should be a large, but currently overlooked, opportunity to intervene for magnesium in patients with CKD.
Although magnesium-containing drugs have been regarded to be contraindicated for patients with CKD due to a risk of hypermagnesemia, life-threatening hypermagnesemia is extremely rare in clinical practice as long as serum magnesium levels are appropriately monitored. In this study, no patients presented severe hypermagnesemia (>5.0 mg/dl). Instead, our study uncovered that oral magnesium supplementation alone was insufficient for nearly 80% of patients with CKD to increase their serum magnesium levels to >2.5 mg/dl. Although we determined the target serum magnesium range of 2.5–3.0 mg/dl according to the previous cohort study of patients on hemodialysis,38 it turned out to be difficult for patients with predialysis CKD to achieve this target by oral magnesium oxide. Magnesium oxide is commonly used as a laxative and, by its nature, is not easily absorbed in the gut. Therefore, it was difficult for some patients to increase the dose of magnesium oxide due to the occurrence of diarrhea. Because diabetes mellitus, proteinuria, and tubular injuries are known to enhance urinary magnesium wasting,47 these factors would further make it difficult to achieve the target serum magnesium levels in patients with predialysis CKD.
Contrary to the effectiveness for CAC, magnesium oxide did not retard the progression of thoracic aorta calcification. Notably, there was no significant correlation between the progression of CAC and that of the thoracic aorta calcification. It is known that risk factors for aortic calcification are not necessarily the same as those for CAC. In the Multi-Ethnic Study of Atherosclerosis, traditional risk factors such as dyslipidemia and smoking were associated with abdominal aortic calcification much more strongly than with CAC, whereas serum calcium and phosphate levels were significantly associated with CAC but not with aortic calcification.56 Similarly, among patients with eGFR of <60 ml/min per 1.73 m2, serum phosphate levels were associated with the severity of CAC but not with that of descending aorta calcification.57 Furthermore, in the randomized, controlled trial examining the efficacy of phosphate binders for patients with CKD, use of phosphate binders, especially calcium acetate, significantly increased percentage change in CAC scores but not in thoracic aorta calcium scores.16 Our study showed that the effect of magnesium is much larger for CAC than for thoracic aorta calcification. Therefore, the mineral disorders in CKD may be more dominantly related to the pathogenesis of CAC as compared with that of thoracic aortic calcification. Although the underlying mechanisms are uncertain, it is suggested that the thoracic aorta is resistant to uremic calcification compared with coronary arteries and abdominal aorta, possibly because VSMCs in the thoracic aorta are derived from neural crest which is less likely to undergo osteoblastic transdifferentiation than VSMCs in the coronary arteries and abdominal aorta, which are derived from mesenchyme.58
Accumulation of uremic toxins has been considered to contribute to the progression of vascular calcification in CKD.59 In particular, IS has been shown to exacerbate vascular calcification both in vitro and in vivo.34,35 Nevertheless, we could not find a significant effect of AST-120, which decreases the blood and tissue concentrations of uremic toxins including IS,60 on the progression of CAC. The result was similar when only patients with a good adherence to the drug were analyzed. Because the study was terminated prematurely, the statistical power might not be enough to detect a nearly 10% lower percentage change in CAC scores in the AST-120 group compared with the control group. Although we observed that AST-120 significantly reduced the proportion of rapid progressors among those with severe baseline CAC, this finding should be viewed with caution because this was an exploratory, not prespecified, subgroup analysis for the secondary outcome. Taken together, this study cannot make a definitive conclusion about the effect of AST-120 on the progression of CAC.
The major limitation of our study was a relatively small sample size and an open-label study design. The latter could be a cause of bias especially in the process of outcome measurement; however, the CAC scoring is objective and was done in a standardized manner by a trained physician who was blinded to patients’ information. Although knowledge of treatment assignment might affect physicians’ therapeutic attitude to study participants, we confirmed that the major cardiovascular risk factors such as hypertension, diabetes mellitus, anemia, and hyper-LDL cholesterolemia were treated equally between the study groups. Although interscan variability of the Agatston score was reported to be 5%–10%,42,61–63 the between-group difference in the percentage change in CAC scores in the magnesium arm (28%) is unlikely to be explained only by the interscan variability. Because of the long follow-up period and the enrollment of high-risk patients, which are certainly the strengths of this study, about one-fourth of the patients dropped out during the trial. The higher dropout rate in the magnesium group was mainly due to some cases of diarrhea. Although several patients did not take magnesium oxide sufficiently due to this side effect, we confirmed that the additional intention-to-treat and per-protocol analysis showed a consistent result emphasizing the benefit of magnesium oxide. The magnesium group had a slightly higher median baseline CAC score than the control group, which might affect the result of this study. However, after excluding patients with very high baseline CAC scores (≥1000), the baseline CAC scores became quite similar between the two groups; in this subgroup analysis, magnesium oxide still exhibited a significant benefit for preventing the progression of CAC. Because our study population was mainly composed of patients with CKD with diabetes mellitus and/or a past history of CVD, extrapolation of our findings to a low-risk population is uncertain; but it is certainly of importance to establish the effective prevention of CAC progression for such a high-risk patient group. In this regard, a similar ongoing randomized trial, MAGiCAL-CKD, involving patients with predialysis CKD regardless of cardiovascular risk factors, will provide additional and valuable information regarding the effect of magnesium supplementation on CAC.64
In conclusion, this randomized trial showed for the first time that oral magnesium oxide retarded the progression of CAC in patients with stage 3−4 CKD. This effect was seen regardless of baseline CAC severity. AST-120 did not exhibit a benefit for the CAC progression in patients with CKD. Our study provides evidence that magnesium supplementation may be a useful option for the prevention of CAC progression in CKD. Future studies should address whether magnesium improves hard outcomes such as cardiovascular events and mortality in patients with CKD.
Disclosures
Dr. K. Yamamoto, Dr. Sugimoto, and Dr. Rakugi received scholarship donations from Mitsubishi Tanabe Pharma Corporation. All authors report grant support from Mitsubishi Tanabe Pharma Corporation during the conduct of the study.
Supplementary Material
Acknowledgments
We thank the members of the data and safety monitoring board (Dr. Naohiko Fujii, Dr. Hiroaki Kawabata, and Dr. Satoshi Mikami) for their help in implementing the interim analysis.
This study was funded by Mitsubishi Tanabe Pharma Corporation.
Parts of this article were presented in abstract form at the annual meeting of the American Society of Nephrology Kidney Week, October 23–28, 2018 in San Diego, California.
Dr. Sakaguchi, Dr. Hamano, Dr. Obi, and Dr. Monden designed the study. Dr. Sakaguchi, Dr. Hamano, Dr. Obi, Dr. Monden, Dr. Oka, Dr. Yamaguchi, Dr. Matsui, Dr. Hashimoto, Dr. Matsumoto, Dr. Shimada, Dr. Takabatake, Dr. Takahashi, Dr. Kaimori, Dr. Moriyama, Dr. R. Yamamoto, Dr. Horio, Dr. K. Yamamoto, Dr. Sugimoto, Dr. Rakugi, and Dr. Isaka collected the data. Dr. Sakaguchi analyzed the data. All authors participated in data interpretation. Dr. Sakaguchi drafted the manuscript. All authors revised the manuscript and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Table of Contents
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi: 10.1681/ASN.2018111150/-/DCSupplemental.
Supplemental Table 1. Reasons for dropout from the study.
Supplemental Table 2. Baseline characteristics among patients who completed the study.
Supplemental Table 3. Change in laboratory data in the AST-120 arm.
Supplemental Table 4. Change in laboratory data, medications, and BP levels during the study period.
Supplemental Table 5. Change in CAC scores in the AST-120 arm.
Supplemental Table 6. Clinical Events in the AST-120 arm.
References
- 1.Braun J, Oldendorf M, Moshage W, Heidler R, Zeitler E, Luft FC: Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis 27: 394–401, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al.: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Larson MG, Keyes MJ, Levy D, Clouse ME, Culleton B, et al.: Kidney function is inversely associated with coronary artery calcification in men and women free of cardiovascular disease: The Framingham Heart Study. Kidney Int 66: 2017–2021, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Russo D, Corrao S, Miranda I, Ruocco C, Manzi S, Elefante R, et al.: Progression of coronary artery calcification in predialysis patients. Am J Nephrol 27: 152–158, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Kramer H, Toto R, Peshock R, Cooper R, Victor R: Association between chronic kidney disease and coronary artery calcification: The Dallas Heart Study. J Am Soc Nephrol 16: 507–513, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Kestenbaum BR, Adeney KL, de Boer IH, Ix JH, Shlipak MG, Siscovick DS: Incidence and progression of coronary calcification in chronic kidney disease: The Multi-Ethnic Study of Atherosclerosis. Kidney Int 76: 991–998, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caliskan Y, Demirturk M, Ozkok A, Yelken B, Sakaci T, Oflaz H, et al.: Coronary artery calcification and coronary flow velocity in haemodialysis patients. Nephrol Dial Transplant 25: 2685–2690, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Watanabe R, Lemos MM, Manfredi SR, Draibe SA, Canziani ME: Impact of cardiovascular calcification in nondialyzed patients after 24 months of follow-up. Clin J Am Soc Nephrol 5: 189–194, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsushita K, Sang Y, Ballew SH, Shlipak M, Katz R, Rosas SE, et al.: Subclinical atherosclerosis measures for cardiovascular prediction in CKD. J Am Soc Nephrol 26: 439–447, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Budoff MJ, Reilly MP, Yang W, Rosas SE, Rahman M, et al.:; CRIC Investigators : Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol 2: 635–643, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shantouf RS, Budoff MJ, Ahmadi N, Ghaffari A, Flores F, Gopal A, et al.: Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am J Nephrol 31: 419–425, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM: Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int 71: 438–441, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Jung HH, Kim SW, Han H: Inflammation, mineral metabolism and progressive coronary artery calcification in patients on haemodialysis. Nephrol Dial Transplant 21: 1915–1920, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Shang D, Xie Q, Ge X, Yan H, Tian J, Kuang D, et al.:; Hyperphosphatemia as an independent risk factor for coronary artery calcification progression in peritoneal dialysis patients. BMC Nephrol 16: 107, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bundy JD, Chen J, Yang W, Budoff M, Go AS, Grunwald JE, et al.:; CRIC Study Investigators : Risk factors for progression of coronary artery calcification in patients with chronic kidney disease: The CRIC study. Atherosclerosis 271: 53–60, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, et al.: Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ter Braake AD, Shanahan CM, de Baaij JHF: Magnesium counteracts vascular calcification: Passive interference or active modulation? Arterioscler Thromb Vasc Biol 37: 1431–1445, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Montezano AC, Zimmerman D, Yusuf H, Burger D, Chignalia AZ, Wadhera V, et al.: Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 56: 453–462, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Kircelli F, Peter ME, Sevinc Ok E, Celenk FG, Yilmaz M, Steppan S, et al.: Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol Dial Transplant 27: 514–521, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salem S, Bruck H, Bahlmann FH, Peter M, Passlick-Deetjen J, Kretschmer A, et al.: Relationship between magnesium and clinical biomarkers on inhibition of vascular calcification. Am J Nephrol 35: 31–39, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Louvet L, Büchel J, Steppan S, Passlick-Deetjen J, Massy ZA: Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol Dial Transplant 28: 869–878, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montes de Oca A, Guerrero F, Martinez-Moreno JM, Madueño JA, Herencia C, Peralta A, et al.: Magnesium inhibits Wnt/β-catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PLoS One 9: e89525, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louvet L, Bazin D, Büchel J, Steppan S, Passlick-Deetjen J, Massy ZA: Characterisation of calcium phosphate crystals on calcified human aortic vascular smooth muscle cells and potential role of magnesium. PLoS One 10: e0115342, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, Bai Y, Jin J, Zhang J, Zhang S, Cui L, et al.: Magnesium modulates the expression levels of calcification-associated factors to inhibit calcification in a time-dependent manner. Exp Ther Med 9: 1028–1034, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Bai Y, Zhang J, Xu J, Cui L, Zhang H, Zhang S, et al.: Magnesium prevents β-glycerophosphate-induced calcification in rat aortic vascular smooth muscle cells. Biomed Rep 3: 593–597, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louvet L, Metzinger L, Büchel J, Steppan S, Massy ZA: Magnesium attenuates phosphate-induced deregulation of a microRNA signature and prevents modulation of Smad1 and Osterix during the course of vascular calcification. BioMed Res Int 2016: 7419524, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonou T, Ohya M, Yashiro M, Masumoto A, Nakashima Y, Ito T, et al.: Magnesium prevents phosphate-induced vascular calcification via TRPM7 and Pit-1 in an aortic tissue culture model. Hypertens Res 40: 562–567, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Ter Braake AD, Tinnemans PT, Shanahan CM, Hoenderop JGJ, de Baaij JHF: Magnesium prevents vascular calcification in vitro by inhibition of hydroxyapatite crystal formation. Sci Rep 8: 2069, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diaz-Tocados JM, Peralta-Ramirez A, Rodríguez-Ortiz ME, Raya AI, Lopez I, Pineda C, et al.: Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int 92: 1084–1099, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Spiegel DM, Farmer B: Long-term effects of magnesium carbonate on coronary artery calcification and bone mineral density in hemodialysis patients: A pilot study. Hemodial Int 13: 453–459, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Tzanakis IP, Stamataki EE, Papadaki AN, Giannakis N, Damianakis NE, Oreopoulos DG. Magnesium retards the progress of the arterial calcifications in hemodialysis patients: A pilot study. Int Urol Nephrol 46:2199–2205, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Bressendorff I, Hansen D, Schou M, Silver B, Pasch A, Bouchelouche P, et al.: Oral magnesium supplementation in chronic kidney disease stages 3 and 4: Efficacy, safety, and effect on serum calcification propensity-a prospective randomized double-blinded placebo-controlled clinical trial. Kidney Int Rep 2: 380–389, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bressendorff I, Hansen D, Schou M, Pasch A, Brandi L: The effect of increasing dialysate magnesium on serum calcification propensity in subjects with end stage kidney disease: A randomized, controlled clinical trial. Clin J Am Soc Nephrol 13: 1373–1380, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Chen J, Shen Z, Gu Y, Xu L, Hu J, et al.: Indoxyl sulfate accelerates vascular smooth muscle cell calcification via microRNA-29b dependent regulation of Wnt/β-catenin signaling. Toxicol Lett 284: 29–36, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Adijiang A, Goto S, Uramoto S, Nishijima F, Niwa T: Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrol Dial Transplant 23: 1892–1901, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, et al.:; European Uremic Toxin Work Group (EUTox) : Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1551–1558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al.:; Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Isaka Y: Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int 85: 174–181, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P: Coronary artery disease: Improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology 208: 807–814, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Raggi P, Callister TQ, Shaw LJ: Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler Thromb Vasc Biol 24: 1272–1277, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, et al.; Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68: 1815–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Qunibi W, Moustafa M, Muenz LR, He DY, Kessler PD, Diaz-Buxo JA, et al.:; CARE-2 Investigators : A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: The Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis 51: 952–965, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Raggi P, Chertow GM, Torres PU, Csiky B, Naso A, Nossuli K, et al.:; ADVANCE Study Group : The ADVANCE study: A randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 26: 1327–1339, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Sakaguchi Y, Hamano T, Nakano C, Obi Y, Matsui I, Kusunoki Y, et al.: Association between density of coronary artery calcification and serum magnesium levels among patients with chronic kidney disease. PLoS One 11: e0163673, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R: Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832, 1990 [DOI] [PubMed] [Google Scholar]
- 46.Mehrotra R, Budoff M, Hokanson JE, Ipp E, Takasu J, Adler S: Progression of coronary artery calcification in diabetics with and without chronic kidney disease. Kidney Int 68: 1258–1266, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Oka T, Hamano T, Sakaguchi Y, Yamaguchi S, Kubota K, Senda M, et al. Proteinuria-associated renal magnesium wasting leads to hypomagnesemia: A common electrolyte abnormality in Chronic Kidney Disease [published online ahead of print May 22, 2018]. Nephrology Dial Transplant doi: 10.1093/ndt/gfy119 [DOI] [PubMed]
- 48.Nadler JL, Malayan S, Luong H, Shaw S, Natarajan RD, Rude RK: Intracellular free magnesium deficiency plays a key role in increased platelet reactivity in type II diabetes mellitus. Diabetes Care 15: 835–841, 1992 [DOI] [PubMed] [Google Scholar]
- 49.Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, Bairey Merz CN: Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation 102: 2353–2358, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Aghagolzadeh P, Bachtler M, Bijarnia R, Jackson C, Smith ER, Odermatt A, et al.: Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-α. Atherosclerosis 251: 404–414, 2016 [DOI] [PubMed] [Google Scholar]
- 51.Viegas CSB, Santos L, Macedo AL, Matos AA, Silva AP, Neves PL, et al.: Chronic kidney disease circulating calciprotein particles and extracellular vesicles promote vascular calcification: A role for GRP (Gla-rich protein). Arterioscler Thromb Vasc Biol 38: 575–587, 2018 [DOI] [PubMed] [Google Scholar]
- 52.Sakaguchi Y, Hamano T, Isaka Y: Effects of magnesium on the phosphate toxicity in chronic kidney disease: Time for intervention studies. Nutrients 9: E112, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasch A, Farese S, Gräber S, Wald J, Richtering W, Floege J, et al.: Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol 23: 1744–1752, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Francisco AL, Leidig M, Covic AC, Ketteler M, Benedyk-Lorens E, Mircescu GM, et al.: Evaluation of calcium acetate/magnesium carbonate as a phosphate binder compared with sevelamer hydrochloride in haemodialysis patients: A controlled randomized study (CALMAG study) assessing efficacy and tolerability. Nephrol Dial Transplant 25: 3707–3717, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robert L: Phosphate-binding magnesium salts and uses thereof. United States Patent 9,889,157. April 4, 2017
- 56.Criqui MH, Kamineni A, Allison MA, Ix JH, Carr JJ, Cushman M, et al.: Risk factor differences for aortic versus coronary calcified atherosclerosis: The multiethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol 30: 2289–2296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, et al.: Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 20: 381–387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kirsch AH, Kirsch A, Artinger K, Schabhüttl C, Goessler W, Klymiuk I, et al.: Heterogeneous susceptibility for uraemic media calcification and concomitant inflammation within the arterial tree. Nephrol Dial Transplant 30: 1995–2005, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hénaut L, Mary A, Chillon JM, Kamel S, Massy ZA: The impact of uremic toxins on vascular smooth muscle cell function. Toxins (Basel) 10: E218, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sato E, Saigusa D, Mishima E, Uchida T, Miura D, Morikawa-Ichinose T, et al.: Impact of the oral adsorbent AST-120 on organ-specific accumulation of uremic toxins: LC-MS/MS and MS imaging techniques. Toxins (Basel) 10: E19, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Achenbach S, Ropers D, Möhlenkamp S, Schmermund A, Muschiol G, Groth J, et al.: Variability of repeated coronary artery calcium measurements by electron beam tomography. Am J Cardiol 87: 210–213, A8, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Lu B, Budoff MJ, Zhuang N, Child J, Bakhsheshi H, Carson S, et al.: Causes of interscan variability of coronary artery calcium measurements at electron-beam CT. Acad Radiol 9: 654–661, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Cheng YJ, Church TS, Kimball TE, Nichaman MZ, Levine BD, McGuire DK, et al.: Comparison of coronary artery calcium detected by electron beam tomography in patients with to those without symptomatic coronary heart disease. Am J Cardiol 92: 498–503, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Bressendorff I, Hansen D, Schou M, Kragelund C, Brandi L: The effect of magnesium supplementation on vascular calcification in chronic kidney disease-a randomised clinical trial (MAGiCAL-CKD): Essential study design and rationale. BMJ Open 7: e016795, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.