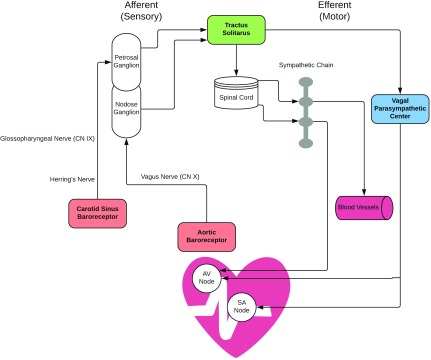
Baroreceptors are pressure sensors on specialized neurons that specifically detect stretch within arterial walls, which occurs when BP increases. When baroreceptors are stretched, they transmit afferent (sensory) signals to the nucleus tractus solitarii in the medulla. These signals inhibit sympathetic outflow and increase parasympathetic output specifically to the heart, thereby triggering vasodilation and lowering BP by decreasing both heart rate and cardiac contractility (Figure 1). Conversely, sudden hypotension decreases firing of the barosensing neurons within the carotid sinus, leading to increased sympathetic outflow, parasympathetic withdrawal, and elevations of BP. Located primarily in the carotid sinus and the aortic arch, these specialized mechanosensory receptors are also sparsely found in some walls of large caliber arteries of the thoracic cavity. Initial descriptions of baroreceptor reflexes date back >80 years. Using canine animal models,1 circulatory function was assessed after clamping or denervating the carotid sinus.2,3 These studies revealed that, by impairing baroreceptors, animals demonstrate increased BP with enhanced variability and were unable to compensate appropriately to changes in BP elicited through changes in posture, physical activity, or other stimuli. Despite decades of research, the precise molecular identity of the baroreceptor has remained elusive. The most popular theory is that the baroreceptors are ion channels located in nerve terminals within arterial structures; however, none of the examined channels have entirely accounted for baroreceptor mechanosensing.
Figure 1.
The arterial baroreflex controls arterial pressure and heart rate via connections between peripheral sensory ganglia and the nucleus tractus solitarius in the medulla. These afferent signals inhibit sympathetic outflow and increase parasympathetic output specifically to the heart and blood vessels, thereby triggering vasodilation and lowering BP by decreasing both heart rate and cardiac contractility. AV, atrioventricular; CN, cranial nerve; SA, sinoatrial.
The recent study published in Science by Zeng et al.4 demonstrates for the first time that the ion channels PIEZO1 and PIEZO2 coordinately contribute to the baroreflex in mice. These authors found expression of either PIEZO1 or PIEZO2 in some cell bodies of the nodose and petrosal ganglia from the vagus and glossopharnyngeal nerves, respectively.4 After retrograde cellular labeling, fluorescent in situ hybridization determined that PIEZO1 and PIEZO2 transcripts are sparsely expressed in the nodose-petrosal ganglion and axons within the carotid sinus. Using target-specific Phox2bCre+ mice (expressed in peripheral neurons) crossed with floxed Piezo knockout mice, the contribution of PIEZO1, PIEZO2, or PIEZO1/PIEZO2 deletion to baroreceptor reflex function was examined. Knockout of either PIEZO1 or PIEZO2 did not alter BP or heart rate response to phenylephrine or sodium nitroprusside. Double knockout (dKO) of PIEZO1/PIEZO2 resulted in abolished baroreceptor response to phenylephrine or sodium nitroprusside, which was measured by changes in systolic BP and heart rate. However, the response intervals shown seemed delayed relative to the extremely rapid adjustments that would be expected—the delayed response is seen in both wild-type and dKO mice in contrast to historical data. This may represent a methodologic error, because the authors state in the supplemental text that subsequent recordings were more rapid as expected. At rest, dKO mice exhibited increased systolic BP and heart rate during the dark phase (where activity is higher) compared with wild-type littermates. dKO mice also had significantly greater BP variability, demonstrating the absent spontaneous baroreceptor sensitivity. This indicates inability to respond to small alterations in BP. Although these data represent a critical and novel mechanism for interrogation of baroreceptor function, additional research is required to evaluate the precise consequences of PIEZO1/2 channels in mediating baroreceptor reflex. For example, to rule out compensatory pathophysiologic changes that may occur due to deletion of these channels during development, follow-up studies using conditional knockdown of these channels in animals during adult life may provide definitive evidence. Phox2bCre+ is not specific to barosensing neurons; thus, it is unclear if there are unexpected effects of PIEZO deletion in other affected nerves. Patients with baroreflex failure show extreme BP surges during psychologic and physiologic stress. Furthermore, they are exquisitely hypersensitive to vasoactive drugs (approximately ten- to 20-fold).5 In contrast, in this mouse model, BPs during the active phase and phenylephrine sensitivity were only mildly elevated, suggesting that PIEZOs may not be the sole baroreceptors. Follow-up studies will be needed to evaluate if other ion channels will synergize with PIEZOs to contribute to the baroreflex.
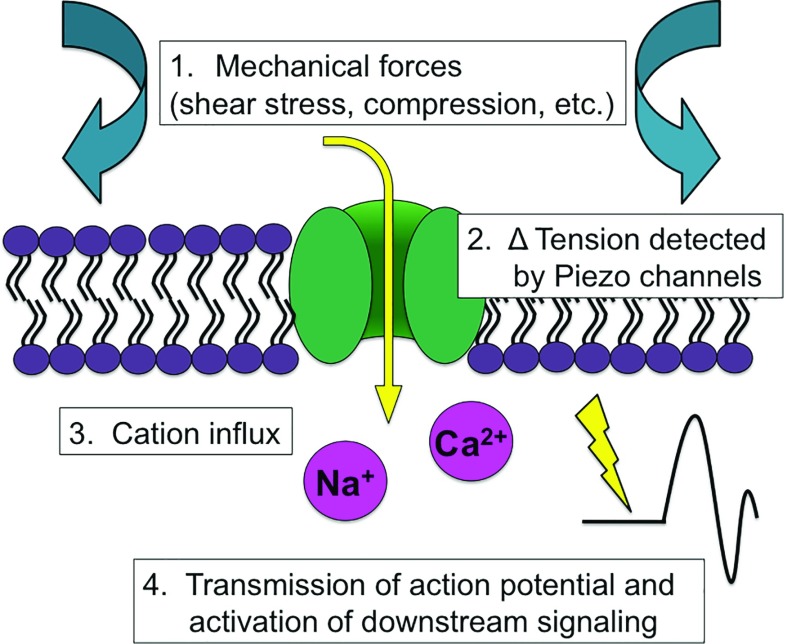
PIEZO1 and PIEZO2 were ideal candidates for assessing baroreceptor function, because a wide array of mechanosensing processes has been ascribed to these excitatory ion channels (Figure 2).6 These include cell volume regulation in erythrocytes, pressure sensing in the lungs, and cardiovascular system development. The first identification of the PIEZO1 gene was in β-amyloid plaque-associated astrocytes,7 later to be determined to be mechanically activated cation channels.8 PIEZO1 and PIEZO2 are largely evolutionarily conserved among vertebrates. PIEZO1 is mainly expressed in lung, bladder, skin, colon, kidney, and cardiovascular tissues, whereas PIEZO2 is highly enriched in dorsal root ganglia, with lower levels in lung and bladder. Additional research determined all sites of PIEZO1 and PIEZO2 expression to be involved in mechanotransmission. Activated by pressure, PIEZO channels transmit mechanical stimuli into electrical activity. On the basis of structure and biophysical studies, it is believed that PIEZO channels respond to deformation of cell membrane and thus, can sense membrane tension, rendering them exquisitely responsive to mechanical force.9 It should be noted that, although it seems likely that PIEZO channels are responsive to mechanical strain, this has not been conclusively demonstrated. Instead, this study examines events downstream of the initial stimulus of cell membrane deformation.
Figure 2.
Mechanical forces alter cation influx via PIEZO channels. Shear stress or compression change surface tension in the cell membrane to activate the PIEZO channel, which in turn, leads to increased intracellular cations and activation of downstream signaling.
These findings are particularly relevant to human hypertensive disorders and may be translatable to diseases caused by known mutations of PIEZO channels. However, the expression pattern of PIEZO1/2 in humans may differ from that seen in mice, which may limit direct translation of these findings. Attesting to the wide range of mechanosensitive processes involving PIEZO channels, genetic alterations in either PIEZO1 or PIEZO2 cause diverse pathologic phenotypes. Both loss-of-function and gain-of-function gene mutations are described for both PIEZO1 and PIEZO2 (reviewed in ref. 10), including lymphatic dysplasia, xerocytosis, arthrogryposis, and muscle atrophy. Although the ability of the baroreceptor reflex to modulate long-term BP is still debated, dysfunction of baroreceptor sensitivity is associated with labile or resistant hypertension as well as predictive of heart failure and increased morbidity and mortality due to vascular complications. The discovery of Zeng et al.4 that PIEZO1 and PIEZO2 are indispensable for the baroreflex is thought provoking. For the first time, we now have molecular targets to study this important physiologic function. To that end, a small synthetic molecule termed Yoda1 was found to elicit the same downstream cellular responses as does activation of PIEZO1 (but not PIEZO2) by mechanical stretch.6 Observations in carefully conducted animal experiments and patients with resistant arterial hypertension suggest that the baroreflex can mediate sustained changes in BP.11,12 Moreover, knowledge of baroreceptor-sensing physiology may lead to development of targeted therapeutics that modify baroreceptor function in patients with labile or resistant hypertension. This work also has important implications for the renal community. In patients with CKD, baroreceptor sensitivity is reduced and may contribute to hypertension, impaired heart rate variability, and sudden cardiac death. Future studies of PIEZO channels can thus potentially further define the contribution of baroreceptors to long-term BP regulation and increased sympathetic nerve activity in patients with CKD.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Brown RV, Hilton JG: The effectiveness of the baroreceptor reflexes under different anesthetics. J Pharmacol Exp Ther 118: 198–203, 1956 [PubMed] [Google Scholar]
- 2.De Burgh Daly M: A method for eliciting baroreceptor reflexes from the isolated carotid sinus. J Physiol 128: 33–5P, 1955 [PubMed] [Google Scholar]
- 3.De Daly M B, Luck CP: The effects of carotid sinus baroreceptor reflexes on pulmonary arterial pressure and pulmonary blood flow in the dog. J Physiol 143: 343–368, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng WZ, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, et al.: PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 362: 464–467, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan J, Shannon JR, Black BK, Costa F, Ertl AC, Furlan R, et al.: Malignant vagotonia due to selective baroreflex failure. Hypertension 30: 1072–1077, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Ranade SS, Syeda R, Patapoutian A: Mechanically activated ion channels. Neuron 87: 1162–1179, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satoh K, Hata M, Takahara S, Tsuzaki H, Yokota H, Akatsu H, et al.: A novel membrane protein, encoded by the gene covering KIAA0233, is transcriptionally induced in senile plaque-associated astrocytes. Brain Res 1108: 19–27, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al.: Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330: 55–60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haselwandter CA, MacKinnon R: Piezo’s membrane footprint and its contribution to mechanosensitivity. eLife 7: pii:e41968, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alper SL: Genetic diseases of PIEZO1 and PIEZO2 dysfunction. Curr Top Membr 79: 97–134, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, et al.: Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: Results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J Am Coll Cardiol 58: 765–773, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Thrasher TN: Baroreceptors, baroreceptor unloading, and the long-term control of blood pressure. Am J Physiol Regul Integr Comp Physiol 288: R819–R827, 2005 [DOI] [PubMed] [Google Scholar]