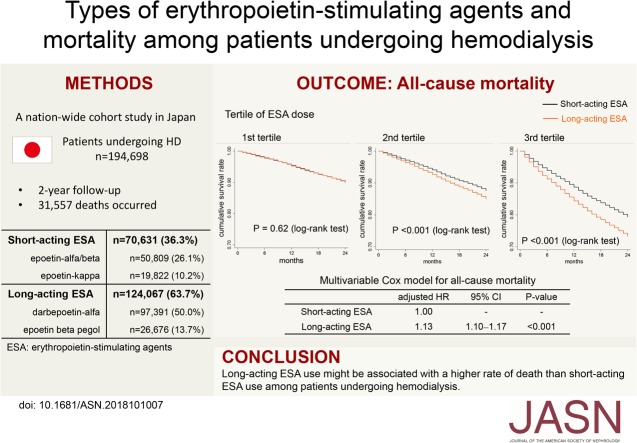
Significance Statement
Although both short-acting and long-acting erythropoietin-stimulating agents (ESAs) are used to treat anemia in patients undergoing hemodialysis, the relative effects on survival of these ESA types are unknown. In this nationwide, registry-based cohort study enrolling 194,698 patients on hemodialysis, the authors found that long-acting ESA users showed a 13% higher rate of death than short-acting ESA users (P<0.001) during the 2-year follow-up period. The difference in risk was pronounced among patients receiving high doses of ESA, for whom the adjusted 2-year number needed to harm for death was 30.8. Survival of long-acting ESA users who achieved more optimal hemoglobin levels was inferior to that of short-acting ESA users. Among patients on hemodialysis, long-acting ESA use might be associated with an increased rate of death compared with short-acting ESA use.
Keywords: erythropoietin-stimulating agents, mortality, hemodialysis
Visual Abstract
Abstract
Background
Despite the widespread use of erythropoietin-stimulating agents (ESAs) to treat anemia in patients undergoing hemodialysis, the relative mortality risks associated with use of different types of ESAs are unknown.
Methods
To compare the mortality risk associated with use of short-acting ESAs versus long-acting ESAs, we conducted a nationwide cohort study of 194,698 hemodialysis patients in Japan who received either a short-acting (epoetin α/β or epoetin κ) or a long-acting (darbepoetin or epoetin β pegol) ESA. Study outcomes were 2-year all-cause and cause-specific mortality. In addition to Cox proportional hazards models, we performed an instrumental variable analysis in which facility-level long-acting ESA prescription rates were taken as the instrumental variable.
Results
During the 2-year follow-up period, 31,557 deaths occurred. In a multivariable Cox model, long-acting ESA users had a 13% higher rate of deaths compared with short-acting ESA users, a significant difference (P<0.001). Similar results were obtained in other analyses. This difference in risk was pronounced among patients receiving high doses of ESA (for whom the adjusted 2-year number needed to harm for death was 30.8). Long-acting ESA use was associated with an increased rate of death from cardiovascular diseases, infection, and malignancies. In the instrumental variable analysis, long-acting ESA users remained at a significantly higher risk of death. Compared with anemic (hemoglobin 9.0–9.9 g/dl) short-acting ESA users, long-acting ESA users who achieved more optimal hemoglobin levels (10.0–10.9 g/dl) showed a higher mortality rate.
Conclusions
Among patients undergoing hemodialysis, use of long-acting ESAs might be associated with a higher risk of death than use of short-acting ESAs.
Anemia is common in patients with CKD and is associated with an increased risk of death and hospitalization.1,2 Erythropoiesis-stimulating agents (ESAs) have revolutionized the therapeutic strategy of anemia in CKD, improving physical activity, cognitive function, and quality of life, as well as reducing the need for blood transfusion.3–7 Despite the efficacy of ESAs for treating anemia, however, randomized trials over the past two decades have failed to demonstrate a survival benefit of attempting to achieve near-normal hemoglobin levels by ESAs.8–11 Instead, these studies witnessed an increased risk of vascular thrombosis, cardiovascular events, and death with higher hemoglobin targets. A post hoc analysis of the Correction of Hemoglobin and Outcomes in Renal Insufficiency trial reported that high doses of ESA, but not the high hemoglobin target, may be responsible for the increased risk of myocardial infarction, heart failure, and mortality.12 Accordingly, the Kidney Disease Improving Global Outcomes guideline insists on the importance of balancing the potential benefits and harms of ESA therapy.13
In contrast to the issue regarding the toxicity of high-dose ESAs, no studies have yet clarified which type of ESA might be superior to another with respect to survival. Given different pharmacologic properties, the prognostic effect may vary among different types of ESA. In the management of anemia in CKD, it is critically important to recognize a potential interclass difference in ESAs with respect to mortality. In this nationwide cohort study of patients undergoing hemodialysis, we aimed to compare the risk of all-cause and cause-specific mortality across different types of ESA users.
Methods
Database and Patient Selection
All data used in this study were obtained from the Japanese Society for Dialysis Therapy (JSDT) Renal Data Registry (JRDR) database. The detailed protocol of JRDR has been described elsewhere.14 Since 1968, the JSDT has been conducting an annual survey of dialysis facilities and individual patients undergoing dialysis therapy across Japan by sending questionnaires to the facilities, and prospectively compiling demographic and clinical data (the latest data as of December 31 in each year). This is an unbiased complete census of patients on dialysis in Japan. The questionnaire was filled out by medical staffs in each facility. Given that the response rate to the questionnaire was 99% in 2012 (4233 per 4279 facilities providing maintenance dialysis therapy in Japan), the database included nearly all dialysis facilities in Japan. Data on the type of ESA were collected for the first time in 2012 and, therefore, the datasets of 2012, 2013, and 2014 were combined (24-month follow-up). Survey items were reported elsewhere.14
We included patients who (1) were aged 18 years or older, (2) received in-center hemodialysis or hemodiafiltration, and (3) were treated with ESAs. Patients receiving multiple types of ESA were excluded. The study protocol was approved by the Ethics Committee of the JSDT.
Baseline Covariates
The following information was obtained from the database in 2012: age, sex, body mass index, region of each facility (47 prefectures in Japan), facility size (the number of patients in each facility), predialysis systolic and diastolic BP, the primary kidney disease (diabetes or nondiabetes), hemodialysis vintage, duration of dialysis treatment (hours per week), types and dose of ESA, single-pool Kt/V, laboratory measurements (predialysis albumin, urea nitrogen, C-reactive protein [CRP], hemoglobin, ferritin, transferrin saturation, calcium, phosphate, and intact and whole parathyroid hormone [PTH] levels), and past history of cardiovascular diseases (myocardial infarction, cerebral infarction, cerebral hemorrhage, and amputation of the extremities). If serum albumin levels were <4.0 g/dl, serum calcium levels were corrected as follows: corrected calcium (mg/dl) = measured calcium (mg/dl)+(4.0–serum albumin [g/dl]). Whole PTH levels were multiplied by 1.7 to obtain an equivalent value for intact PTH. Erythropoietin resistance index (ERI) was calculated as follows: ERI=ESA dose/(body wt [kg]×hemoglobin [g/dl]). To compare ESA doses and ERI across different types of ESA, they were standardized to the mean of 0 and SD of 1 for each type of ESA.
We included the following facility-based indicators in our analysis: (1) the within-facility proportion of patients with serum calcium, phosphate, and intact PTH levels within the Japanese guideline’s target range15; (2) the within-facility proportion of patients with a single-pool Kt/V ≥1.2; and (3) a dialysate endotoxin level of <0.1 EU/ml. Dialysate endotoxin levels were measured at least once a year in 96.2% of facilities and once a month in 76.3% of facilities. The highest endotoxin level of the year was reported.
Outcomes
We collected data on the month of death as well as causes of death from the databases in 2013 and 2014. Classification codes of the causes of death have been previously reported.14 Cardiovascular deaths were divided into either death from cardiac diseases (heart failure, ischemic heart diseases, arrhythmia, and valvular heart disease) or strokes (cerebral infarction, cerebral hemorrhage, and subarachnoid hemorrhage). Noncardiovascular deaths were divided into either deaths from infectious diseases or malignancy. The causes of death were ascertained essentially by a review of patients’ medical records by questionnaire respondents, mainly site physicians.
Statistical Analyses
For each baseline variable, the standardized difference in the mean or proportion between two groups was calculated; a value <0.1 was considered to denote a negligible difference between groups.
Survival analyses were performed using the Cox proportional hazards models. Multivariable models were adjusted for the following covariates: model 1 adjusted for age, sex, body mass index, predialysis systolic BP, duration and frequency of dialysis treatment, dialysis vintage, single-pool Kt/V, diabetes mellitus, a past history of cardiovascular diseases, laboratory data (albumin, urea nitrogen, CRP, hemoglobin, ferritin, calcium, phosphate, and PTH), and ERI. These covariates were chosen because they are known to be related to patients’ prognosis and/or they could potentially confound the relationship between ESA types and mortality risk. Model 2 was adjusted for covariates in model 1 and facility indicators. Because the choice of ESA could be largely influenced by each facility’s preference, this model was adjusted for the facility indicators including the facility-level status of calcium, phosphate, and PTH, in addition to these parameters at patient-level, to account for different clinical practice patterns across facilities. Model 3 was adjusted for a propensity score and facility indicators. The propensity score, which represents a probability of receiving long-acting ESA, was created from a logistic regression model including all covariates in model 1. Using the inverse of the propensity score, the Cox model (model 2) was weighted by inverse probability of treatment weighting (IPTW). Finally, a propensity-score matching was performed using a nearest-neighbor 1:1 matching. The propensity-score matched participants were compared using a multivariable Cox model (model 2). In Japan, <1% of dialysis patients undergo kidney transplantation annually. Because of a quite low rate of transplantation, we did not censor patients at kidney transplantation.
As a sensitivity analysis, multiple imputation was performed in the multivariable Cox model (model 1). The missing values of all covariates were imputed by fully conditional chained equations (Stata command “mi impute chained”), including the types of ESA; accordingly, patients with missing data on the type of ESA, who were excluded from the rest of the study, were included in this particular analysis. All covariates in model 1 were used for imputation. Those covariates that were remarkably skewed (CRP, PTH, ferritin, and dialysis vintage) were log-transformed to normalize the distribution before multiple imputation. We created five imputed datasets which were analyzed separately and combined using Rubin rules.
Subgroup analyses were performed for all baseline covariates. A product term between ESA types and each covariate was entered into the Cox model (model 2) to evaluate the potential interaction on the rate of death. Given the potential importance of the effect modification by ESA dose and ERI, we depicted Kaplan–Meier survival curves comparing the long- and short-acting ESA users for each tertile of ESA dose and ERI. The adjusted 2-year number needed to harm (NNH) for death was calculated from the reciprocal of the adjusted absolute risk difference (ARD) between the long- and short-acting ESA users of each tertile of ESA dose and ERI. The adjusted ARD was derived from two hypothetical predicted probabilities for death estimated from a multivariable logit model16; one was the predicted probability if all patients had received the long-acting ESA, and the other was the predicted probability if all patients had received the short-acting ESA. These predicted probabilities were then averaged over the whole subjects. The adjusted ARD was the difference in the average predicted probabilities. All covariates in model 2 were included in the multivariable logit model as their original values.
Furthermore, we analyzed the mortality rates of long- and short-acting ESA users across different hemoglobin categories (9.0−9.9, 10.0−10.9, and 11.0−11.9 g/dl) in order to compare the prognosis of patients achieving target hemoglobin levels under one type of ESA to that of anemic patients treated with another type of ESA.
For the comparison of the four types of ESA, IPTW was calculated as an inverse of the probability of receiving the type of ESA a patient actually received by using a multinomial logistic regression model including all independent variables included in model 1.
We performed an instrumental variable analysis to provide estimates that would not be biased even by unmeasured confounders. A facility-level preference for the long-acting ESA over short-acting ESA was treated as the instrumental variable on the basis of the following assumptions: (1) the facility-level preference strongly predicts a type of ESA prescribed for a patient, (2) the facility-level preference is unrelated to patients’ characteristics, and (3) the facility-level preference is related to the outcomes only through the types of ESA. The facility-level preference was quantified by an adjusted facility-level prescription rate of the long-acting ESA. This was estimated by a mixed-effects logistic regression in which a dependent variable was the type of ESA prescribed for a patient and independent variables were covariates in model 2, with a facility-level random effect taken into account. The intercept for the random effect represents the predicted level of the long-acting ESA use at each facility adjusted by the above-mentioned covariates (the long-acting ESA proportion).17 Discrimination ability of the long-acting ESA proportion for the type of ESA prescribed for an individual patient was assessed by the area under the receiver operating characteristic curve in univariate logistic regression analysis. For the instrumental variable analysis, a two-stage least squares regression with adjustment for covariates in model 2 was conducted to estimate an ARD for the 2-year mortality between the long- and short-acting ESA users. The performance of a two-stage least squares regression in a binary outcome setting is well comparable with that of alternative instrumental variable approaches appropriate for a binary outcome, such as a two-stage logistic regression and a generalized method of moments approach, especially in large sample sizes like our study.18–20 We also examined an association between the long-acting ESA proportion and rate of death by a Cox model (model 2). In these analyses, only dialysis clinics with >50 patients were included.
We have summarized the statistical models used in this study in Supplemental Table 1.
All reported P values were two-sided, and values <0.05 were considered statistically significant. All statistical analyses were performed using Stata 13 (StataCorp., College Station, TX).
Results
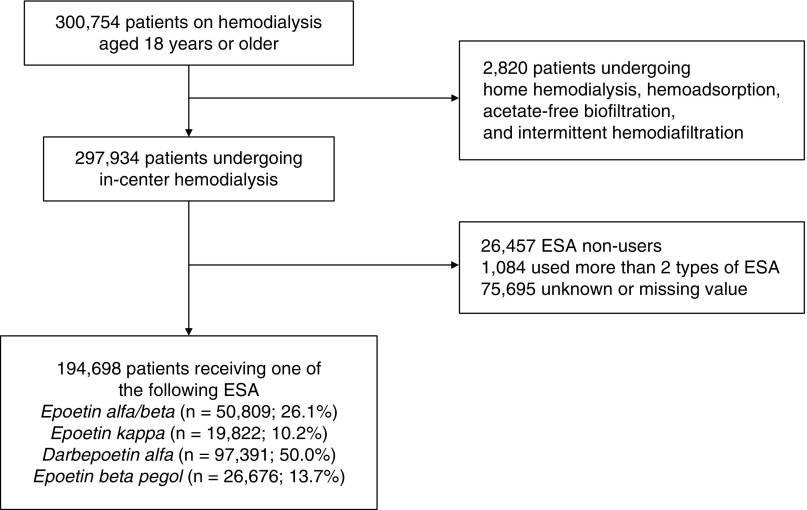
The process of patient selection is shown in Figure 1. Among a total of 300,754 patients undergoing dialysis therapy, those who received in-center hemodialysis were enrolled in this study (n=297,934). We excluded patients who did not receive ESA (n=26,457) and those who received more than one type of ESA (n=1084). Although data on the type of ESA prescribed were missing for 75,695 patients, we confirmed that demographic and clinical characteristics between patients with and without these data were not substantially different, with standardized differences of <0.1 for all covariates (Supplemental Table 2). The remaining 194,698 patients were included in the subsequent analyses. The proportion of patients using each type of ESA was as follows: epoetin (EPO)-α/β, n=50,809 (26.1%); EPO-κ, n=19,822 (10.2%); darbepoetin-α (DPO), n=97,391 (50.0%); and epoetin-β pegol (continuous erythropoiesis receptor activator [CERA]), n=26,676 (13.7%). Dialysate endotoxin levels were <0.1 EU/ml in 90.8% of all facilities.
Figure 1.
A total of 194,698 patients receiving ESA were enrolled in this study.
Comparison Between Short- and Long-Acting ESA
Patients were divided into two groups; short-acting (EPO-α/β and -κ) and long-acting (DPO and CERA) ESA users. These two groups were comparable across all baseline characteristics with standardized differences of <0.1 (Table 1).
Table 1.
Baseline characteristics according to long- and short-acting ESA users
| Characteristic | No. of Missing Value (%) | Total, |
Long-Acting ESA Users |
Short-Acting ESA Users |
Standardized Difference |
|---|---|---|---|---|---|
| n=194,698 | n=124,067 | n=70,631 | |||
| Age, yr | 5 (<0.1) | 67.5 (12.4) | 67.7 (12.3) | 67.2 (12.5) | 0.04 |
| Men, % | 0 | 61.6 | 61.6 | 61.5 | 0.002 |
| Body mass index, kg/m2 | 16,822 (8.6) | 22.3 (4.2) | 22.2 (4.2) | 22.3 (4.3) | 0.02 |
| HD vintage, yr | 41 (<0.1) | 6 [3–11] | 6 [3–11] | 6 [3–11] | 0.01 |
| HD duration, h/wk | 1349 (0.7) | 11.7 (1.8) | 11.7 (1.8) | 11.8 (1.8) | 0.06 |
| Single-pool Kt/V | 11,129 (5.7) | 1.43 (0.32) | 1.43 (0.32) | 1.43 (0.32) | 0.02 |
| Predialysis sBP, mm Hg | 9298 (4.8) | 152.1 (24.0) | 152.1 (24.1) | 152.1 (23.8) | 0.001 |
| Predialysis dBP, mm Hg | 9577 (4.9) | 77.6 (14.4) | 77.5 (14.5) | 77.7 (14.4) | 0.01 |
| Urea nitrogen, mg/dl | 1299 (0.7) | 61.9 (16.1) | 61.8 (16.2) | 62.1 (15.8) | 0.02 |
| Albumin, g/dl | 3886 (2.0) | 3.63 (0.58) | 3.61 (0.65) | 3.66 (0.42) | 0.09 |
| CRP, mg/dl | 31,955 (16.4) | 0.1 [0.1–0.4] | 0.1 [0.1–0.5] | 0.1 [0.1–0.4] | 0.06 |
| Albumin-adjusted Ca, mg/dl | 1389 (0.7) | 9.22 (0.75) | 9.22 (0.75) | 9.23 (0.75) | 0.009 |
| Phosphate, mg/dl | 1477 (0.8) | 5.17 (1.44) | 5.18 (1.45) | 5.15 (1.42) | 0.02 |
| Intact PTH, pg/ml | 13,236 (6.8) | 125 [66–205] | 125 [66–205] | 126 [66–206] | 0.002 |
| Hemoglobin, g/dl | 2366 (1.2) | 10.5 (1.2) | 10.5 (1.2) | 10.5 (1.1) | 0.02 |
| Ferritin, ng/ml | 18,121 (9.3) | 80 [34–169] | 77 [33–163] | 86 [36–179] | 0.05 |
| TSAT, % | 44,246 (22.7) | 26.5 (24.3) | 26.7 (26.7) | 26.2 (19.3) | 0.02 |
| Diabetes mellitus, % | 0 | 37.9 | 38.0 | 37.9 | 0.002 |
| Past history of MI, % | 23,115 (11.9) | 9.2 | 9.2 | 9.1 | 0.004 |
| Past history of CH, % | 23,672 (12.2) | 5.7 | 5.7 | 5.7 | 0.002 |
| Past history of CI, % | 21,867 (11.2) | 17.9 | 17.8 | 18.0 | 0.003 |
| Past history of amputation, % | 22,802 (11.7) | 3.3 | 3.5 | 3.1 | 0.02 |
Data presented as mean (SD) or median [interquartile range]. Standardized difference of <0.1 denotes a negligible difference between groups. HD, hemodialysis; sBP, systolic BP; dBP, diastolic BP; Ca, calcium; TSAT, transferrin saturation; MI, myocardial infarction; CH, cerebral hemorrhage; CI, cerebral infarction.
Over the 2-year follow-up period, 31,557 deaths occurred (9513 cardiovascular deaths and 22,044 noncardiovascular deaths). In an unadjusted Cox model, the long-acting ESA users had a 20% higher rate of all-cause death than short-acting ESA users (2-year mortality risk: 17.2% versus 14.6%) (Table 2). This association was slightly attenuated but remained significant after adjustment for demographic and clinical factors, facility indicators (Table 2; model 2), and a propensity score (Table 2; model 3) and in the Cox model weighted by IPTW (Table 2; IPTW). The results were materially unchanged after further adjusting for facilities’ region and facility size (adjusted hazard ratio [HR], 1.13; 95% confidence interval [95% CI], 1.09 to 1.16; P<0.001). When missing values were imputed by multiple imputation, the use of long-acting ESA was still associated with an increased rate of death (adjusted HR, 1.08; 95% CI, 1.05 to 1.10; P<0.001).
Table 2.
Cox proportional hazards models for the rate of all-cause and cause specific mortality
| Model | n | HR | 95% CI | P Value | |
|---|---|---|---|---|---|
| All-cause death | |||||
| Unadjusted | Short-acting ESA | 70,631 | 1.00 | — | — |
| Long-acting ESA | 124,067 | 1.20 | 1.17 to 1.23 | <0.001 | |
| Model 1 | Short-acting ESA | 42,740 | 1.00 | — | — |
| Long-acting ESA | 75,458 | 1.14 | 1.10 to 1.17 | <0.001 | |
| Model 2 | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.13 | 1.10 to 1.17 | <0.001 | |
| Model 3 | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.11 | 1.08 to 1.15 | <0.001 | |
| IPTW | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.13 | 1.09 to 1.17 | <0.001 | |
| PS-matched | Short-acting ESA | 42,473 | 1.00 | — | — |
| Long-acting ESA | 42,473 | 1.12 | 1.08 to 1.16 | <0.001 | |
| Cardiovascular death | |||||
| Unadjusted | Short-acting ESA | 70,631 | 1.00 | — | — |
| Long-acting ESA | 124,067 | 1.18 | 1.14 to 1.23 | <0.001 | |
| Model 1 | Short-acting ESA | 42,740 | 1.00 | — | — |
| Long-acting ESA | 75,458 | 1.12 | 1.07 to 1.18 | <0.001 | |
| Model 2 | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.12 | 1.06 to 1.17 | <0.001 | |
| Model 3 | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.10 | 1.04 to 1.15 | <0.001 | |
| IPTW | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.12 | 1.06 to 1.18 | <0.001 | |
| PS-matched | Short-acting ESA | 42,473 | 1.00 | — | — |
| Long-acting ESA | 42,473 | 1.11 | 1.05 to 1.18 | <0.001 | |
| Death from cardiac disease | |||||
| Unadjusted | Short-acting ESA | 70,631 | 1.00 | — | — |
| Long-acting ESA | 124,067 | 1.18 | 1.13 to 1.23 | <0.001 | |
| Model 1 | Short-acting ESA | 42,740 | 1.00 | — | — |
| Long-acting ESA | 75,458 | 1.11 | 1.05 to 1.18 | <0.001 | |
| Model 2 | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.11 | 1.05 to 1.17 | <0.001 | |
| Model 3 | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.09 | 1.03 to 1.15 | 0.004 | |
| IPTW | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.11 | 1.05 to 1.17 | <0.001 | |
| PS-matched | Short-acting ESA | 42,473 | 1.00 | — | — |
| Long-acting ESA | 42,473 | 1.09 | 1.02 to 1.16 | 0.008 | |
| Death from strokes | |||||
| Unadjusted | Short-acting ESA | 70,631 | 1.00 | — | — |
| Long-acting ESA | 124,067 | 1.20 | 1.10 to 1.31 | <0.001 | |
| Model 1 | Short-acting ESA | 42,740 | 1.00 | — | — |
| Long-acting ESA | 75,458 | 1.16 | 1.04 to 1.30 | 0.007 | |
| Model 2 | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.16 | 1.04 to 1.29 | 0.009 | |
| Model 3 | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.15 | 1.03 to 1.28 | 0.01 | |
| IPTW | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.16 | 1.04 to 1.30 | 0.008 | |
| PS-matched | Short-acting ESA | 42,473 | 1.00 | — | — |
| Long-acting ESA | 42,473 | 1.20 | 1.06 to 1.36 | 0.003 | |
| Noncardiovascular death | |||||
| Unadjusted | Short-acting ESA | 70,631 | 1.00 | — | — |
| Long-acting ESA | 124,067 | 1.21 | 1.17 to 1.25 | <0.001 | |
| Model 1 | Short-acting ESA | 42,740 | 1.00 | — | — |
| Long-acting ESA | 75,458 | 1.14 | 1.10 to 1.19 | <0.001 | |
| Model 2 | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.14 | 1.10 to 1.19 | <0.001 | |
| Model 3 | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.12 | 1.08 to 1.17 | <0.001 | |
| IPTW | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.14 | 1.09 to 1.19 | <0.001 | |
| PS-matched | Short-acting ESA | 42,473 | 1.00 | — | — |
| Long-acting ESA | 42,473 | 1.12 | 1.07 to 1.17 | <0.001 | |
| Death from infectious diseases | |||||
| Unadjusted | Short-acting ESA | 70,631 | 1.00 | — | — |
| Long-acting ESA | 124,067 | 1.25 | 1.19 to 1.32 | <0.001 | |
| Model 1 | Short-acting ESA | 42,740 | 1.00 | — | — |
| Long-acting ESA | 75,458 | 1.15 | 1.07 to 1.23 | <0.001 | |
| Model 2 | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.14 | 1.07 to 1.22 | <0.001 | |
| Model 3 | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.11 | 1.04 to 1.19 | 0.002 | |
| IPTW | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.14 | 1.06 to 1.22 | <0.001 | |
| PS-matched | Short-acting ESA | 42,473 | 1.00 | — | — |
| Long-acting ESA | 42,473 | 1.10 | 1.01 to 1.19 | 0.02 | |
| Death from malignancy | |||||
| Unadjusted | Short-acting ESA | 70,631 | 1.00 | — | — |
| Long-acting ESA | 124,067 | 1.26 | 1.17 to 1.37 | <0.001 | |
| Model 1 | Short-acting ESA | 42,740 | 1.00 | — | — |
| Long-acting ESA | 75,458 | 1.15 | 1.04 to 1.27 | 0.009 | |
| Model 2 | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.14 | 1.03 to 1.26 | 0.01 | |
| Model 3 | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.15 | 1.04 to 1.27 | 0.008 | |
| IPTW | Short-acting ESA | 42,612 | 1.00 | — | — |
| Long-acting ESA | 75,291 | 1.14 | 1.03 to 1.26 | 0.02 | |
| PS-matched | Short-acting ESA | 42,473 | 1.00 | — | — |
| Long-acting ESA | 42,473 | 1.14 | 1.01 to 1.28 | 0.03 | |
—, not applicable; PS, propensity score.
Model 1 adjusted for age, sex, body mass index, predialysis systolic BP, dialysis duration, dialysis vintage, single-pool Kt/V, diabetes mellitus, a past history of cardiovascular diseases, laboratory data (albumin, urea nitrogen, CRP, hemoglobin, ferritin, albumin-adjusted calcium, phosphate, and PTH), and standardized ERI. Model 2 adjusted for covariates in model 1 plus facility indicators. Model 3 adjusted for a PS plus facility indicators. IPTW and PS-matched analyses were adjusted for covariates in model 2.
In a propensity-score matched cohort, baseline characteristics were nearly identical between the two groups (Supplemental Table 3). The long-acting ESA users exhibited a 12% higher rate of all-cause death than short-acting ESA users in this matched cohort (Table 2; propensity score–matched).
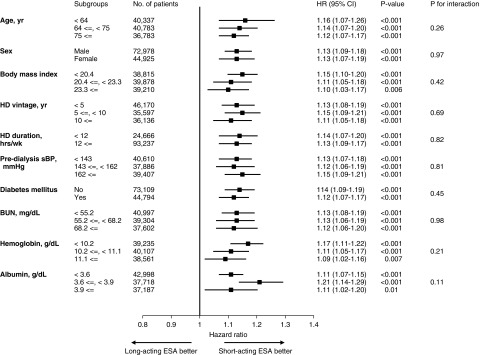
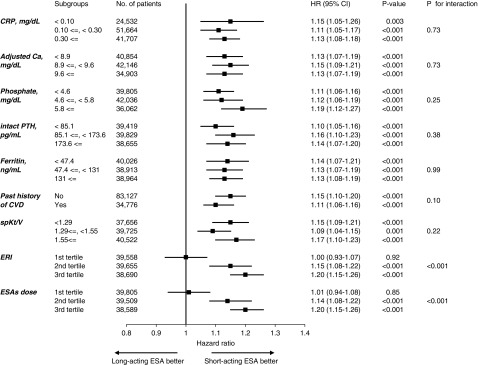
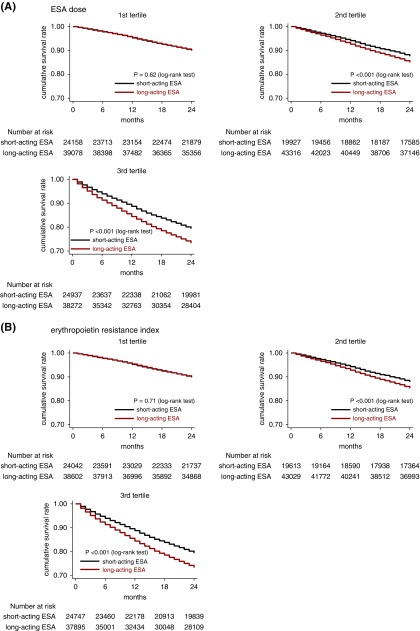
The use of long-acting ESA was associated with an increased rate of death across most of the subgroups, whereas a significant effect modification was identified by the dose of ESA and ERI (Figures 2 and 3). The adjusted HRs (95% CIs) for all-cause deaths in the long-acting ESA users were 1.01 (0.94 to 1.08), 1.14 (1.08 to 1.22), and 1.20 (1.15 to 1.26) in the first to third tertile of ESA dose, respectively (Pinteraction<0.001); and 1.00 (0.93 to 1.07), 1.15 (1.08 to 1.22), and 1.20 (1.15 to 1.26) in the first to third tertile of ERI, respectively (Pinteraction<0.001) (Figure 2). The adjusted 2-year NNHs for death were 2000, 64.1, and 30.8 in the first to third tertile of ESA doses, respectively; and 2000, 62.5, and 30.8 in the first to third ERI tertile, respectively. The average dose of each ESA is summarized in Supplemental Table 4.
Figure 2.
Long-acting ESA use associates with a higher rate of death across all subgroups except for the 1st tertile of ERI and ESA dose. Models are adjusted for covariates in model 2 (age, sex, body mass index, predialysis systolic BP, dialysis duration, dialysis vintage, single-pool Kt/V, diabetes mellitus, a past history of cardiovascular diseases, laboratory data [albumin, urea nitrogen, CRP, hemoglobin, ferritin, albumin-adjusted calcium, phosphate, and PTH], standardized ERI, and facility indicators). Bar represents 95% CI. Ca, calcium; CVD, cardiovascular disease; HD, hemodialysis; sBP, systolic BP.
Figure 3.
Difference in overall survival rate between short- and long-acting ESA users is larger in higher tertiles of ESA dose and ERI. Patients were stratified by (A) tertile of ESA dose and (B) tertile of ERI.
The long-acting ESA use was associated with an increased rate of cardiovascular death, including deaths from cardiac diseases and strokes (Table 2). The rate of noncardiovascular death, and in particular death due to malignancy and infectious diseases, was also elevated in the long-acting ESA users (Table 2).
We compared the mortality rate between short- and long-acting ESA with different hemoglobin categories (Table 3). Compared with anemic patients treated with short-acting ESA (hemoglobin levels of 9.0−9.9 g/dl), patients treated with long-acting ESA who achieved the optimal hemoglobin level (10.0−10.9 g/dl) had a 9% higher rate of all-cause death.
Table 3.
HRs for all-cause death across hemoglobin categories and types of ESAs
| Hemoglobin, g/dl | Short-Acting ESA | Long-Acting ESA | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| 9.0–9.9 | 1.00 | — | — | 1.17 | 1.10 to 1.25 | <0.001 |
| 10.0–10.9 | 0.97 | 0.89 to 1.06 | 0.50 | 1.09 | 1.01 to 1.19 | 0.03 |
| 11.0–11.9 | 0.98 | 0.86 to 1.12 | 0.79 | 1.10 | 0.97 to 1.25 | 0.14 |
Model adjusted for covariates in model 2 (age, sex, body mass index, predialysis systolic BP, dialysis duration, dialysis vintage, single-pool Kt/V, diabetes mellitus, a past history of cardiovascular diseases, laboratory data [albumin, urea nitrogen, CRP, ferritin, albumin-adjusted calcium, phosphate, and PTH], standardized ERI, and facility indicators). —, not applicable.
At baseline, 44.7% of facilities prescribed long-acting ESA and 13.8% prescribed short-acting ESA for more than 80% of ESA users in their facilities. In the instrumental variable analysis, the F-statistic in the first stage of the two-stage least squares regression was 35,549 (>10), indicating that the instrumental variable (the facility-level proportion of long-acting ESA users) strongly predicted the type of ESA prescribed for an individual patient. Similarly, the area under the receiver operating characteristic curve of the instrumental variable for the prediction of the type of ESA was quite high (0.86). The second stage of the two-stage least squares regression revealed a higher risk of death in long-acting ESA users compared with short-acting ESA users (ARD, 1.4%; 95% CI, 0.6% to 2.3%; P=0.001). Additionally, the higher facility-level long-acting ESA proportion was associated with an increased rate of death (HR, 1.01 per 10% increase in the long-acting ESA proportion] 95% CI, 1.00 to 1.02; P=0.01).
Comparison of the Four Types of ESA
Patients were divided into four groups: (1) EPO-α/β, (2) EPO-κ, (3) DPO, and (4) CERA. Baseline characteristics were comparable across four groups, with the exception of ferritin levels, which were slightly higher in EPO-κ users and lower in DPO users (Supplemental Table 5).
In an unadjusted Cox model, the rate of all-cause death was the highest among DPO users, followed by CERA and EPO-κ users, with the rate being the lowest among EPO-α/β users (Supplemental Table 6). This trend remained significant in the multivariable Cox model and in the IPTW model (Supplemental Table 6). DPO users had the highest rate of death from cardiac diseases and malignancy. The rate of death from stroke was the highest among CERA users.
Discussion
In this nationwide cohort study of patients undergoing hemodialysis in Japan, the long-acting ESA use was associated with an increased rate of cardiovascular and noncardiovascular mortality compared with short-acting ESA use. The magnitude of this association was large enough to be clinically relevant particularly among patients receiving high doses of ESA (NNH, 30.8) and among patients with high ERI (NNH, 30.8). The strength of the association was not substantially altered after adjustment for relevant clinical factors in different statistical models including propensity score–matching and IPTW probably because the baseline characteristics were quite similar between long- and short-acting ESA users; this implies that the clinical decision in the choice of ESA depended largely on facility’s preference, rather than on a patient’s characteristics, given the very high F-statistic in the two-stage least squares regression analysis. This setting is a unique opportunity to perform the instrumental variable analysis, which can deal with even unmeasured confounders, showing that long-acting ESA use was indeed linked to a poorer prognosis. Taken together, although our study was observational, the risk of bias attributable to the choice of ESA was considered to be low.
The interclass difference among ESA in terms of the risk of death has not been clarified. A meta-analysis of randomized trials of patients with advanced CKD reported that the mortality risk was not significantly different between DPO and EPO-α users (odds ratio for DPO, 1.33; 95% CI, 0.88 to 2.01).21 This study, however, was inconclusive owing to a low statistical power and a short follow-up period. Another network meta-analysis also reported an uncertain difference in the risk of death between DPO and EPO users (relative risk for DPO, 1.35; 95% CI, 0.84 to 2.15) and between DPO and CERA users (relative risk for DPO, 0.89; 95% CI, 0.53 to 1.51); this meta-analysis was, again, on the basis of trials with a limited sample size and a high risk of bias.22 It was reported that the mortality rate did not differ among patients in facilities predominantly using DPO and EPO; however, patients in DPO facilities showed a 16% increased rate of myocardial infarction and a 10% increased rate of composite outcome (stroke, myocardial infarction, and cardiovascular mortality), although this was not statistically significant.23 This study was different from ours in that (1) only patients on incident hemodialysis were enrolled and (2) the study population was highly selective (restricted to independent and hospital-based, nonchained facilities). Our 2-year nationwide cohort study involved a much larger sample size that allowed us to show a higher risk of death among long-acting ESA users.
The pharmacologic differences between long- and short-acting ESA might explain our findings. The long t1/2 and increased biologic activity of DPO and CERA provide better maintenance of hemoglobin levels. However, these properties might cause harm if they exert a sustained and intense stimulus on nonerythroid cells, considering that erythropoietin receptors are widely expressed across diverse cell types. For example, erythropoietin stimulates erythropoietin receptors on endothelial cells,24 promoting their proliferation and angiogenesis25–27 and may exacerbate atherosclerotic lesions28 Erythropoietin is also involved in maturation of megakaryocytes.29,30 Administration of ESA enhances platelet aggregation in hemodialysis patients31 thus may induce a prothrombotic state. In the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT), the risk of stroke was doubled in DPO compared with placebo.11 We speculate that these detrimental effects are more strongly elicited by long-acting ESA since they are given at longer intervals with higher single doses than short-acting ESA, resulting in higher peak concentrations. Further studies are needed to clarify the difference in the atherogenic potential between short- and long-acting ESA.
Erythropoietin receptors are known to be expressed on various types of tumor cells.32 It has been reported that ESA could promote proliferation, growth, and metastasis of cancer cells.33 A meta-analysis of randomized trials of patients with malignancies revealed an increased risk of death among patients receiving ESA.34 In the TREAT, the risk of death from cancer was higher among patients treated with DPO than among patients receiving a placebo when patients had a prior history of malignancy.11 We found that the rate of malignancy-related death was elevated among long-acting ESA users. Although patients with malignancies often exhibit resistance to ESA therapy thus requiring high doses of ESA, this result may imply that we should take care when prescribing high doses of long-acting ESA for these patients as it may lead to a poor prognosis without efficiently increasing hemoglobin levels.
We unexpectedly found that the long-acting ESA use was associated with a higher rate of infection-related death. Interestingly, experimental studies revealed immuno-suppressive effects of erythropoietin.35,36 Therefore, aggressive use of ESA might increase the susceptibility to infectious diseases. Our study adds novel information regarding the possible detrimental effect of long-acting ESA on the clinical course of patients with infectious diseases.
Among the four types of ESA, the mortality rate was the highest in DPO users and the lowest in EPO-α/β users. Although the underlying mechanisms are unknown, the prognosis of patients receiving EPO-κ, a biosimilar product of EPO-α, was slightly worse than that of those receiving EPO-α/β.
Notably, the prognosis of the long-acting ESA users whose hemoglobin levels were within the optimal range (10.0−10.9 g/dl) was still inferior to that of anemic short-acting ESA users (hemoglobin levels of 9.0−9.9 g/dl). This result suggests that the potential superiority of long-acting ESA over short-acting ESA in increasing hemoglobin levels does not necessarily outweigh the detrimental effects of long-acting ESA with respect to mortality. Although researchers have long investigated the optimal target level of hemoglobin, our findings shed light on the idea that the prognosis also depends on how to achieve that target.
Because of the observational study design, the results of this study do not allow any causal inference. However, our findings fulfill most of the Hill criteria for causality: (1) strength (the small NNH in the high ESA dose subgroup), (2) temporality (administration of ESA preceded death), (3) dose-response relationship (the risk difference became larger with higher ESA doses), (4) plausibility (experimental studies showed harmful effects of ESA), (5) consistency (the previous studies reported a trend toward poorer prognosis for long-acting ESA users;21–23 our result was consistent in a variety of statistical models), and (6) analogy (DPO increased the risk of stroke and death from malignancy in the previous randomized trial11). Taken together, the causality of our findings might be plausible despite the observational study design.
There are several limitations in our study. Our database contained missing data, especially with regard to the type of ESA prescribed. However, no substantial differences were found in baseline characteristics between those with and without these data. Furthermore, the multiple imputation approach did not materially alter the results. Therefore, bias arising from the data missing is considered to be minimum. A quality control survey was not performed thus we could not assure data accuracy. Baseline variables were derived only from the 2012 database and were on the basis of a single measurement; thus, we could not account for time-varying alterations in clinical characteristics and laboratory data. In particular, the type of ESA prescribed could be changed during the study period, although the crossover of the drugs is expected to bias the results toward null. We did not have information about iron supplementation therapy; thus, it was not accounted for in the multivariable Cox models. However, the instrumental variable analysis, which showed a robust relationship between long-acting ESA use and increased mortality, could deal with such unmeasured confounders as iron supplementation. This study included only patients on hemodialysis in Japan; thus, the external validity to other ethnicity and other populations, such as nondialysis and patients on peritoneal dialysis, is uncertain. Finally, the instrumental variable of our study, i.e., the facility-level preference for the long-acting ESA, might relate to the outcome through pathways other than the long-acting ESA use. In particular, because ESA is reimbursed under the bundled payment in Japan, the choice of ESA may be driven by cost-consciousness, which might affect patients’ prognosis. However, this unlikely explains the worse prognosis of long-acting ESA users given the relative expensiveness of long-acting ESA compared with short-acting ones. In Japan, the cost of dialysis treatment is covered almost entirely by the public health insurance system, therefore, patients’ socioeconomic status could have little effect on the choice of ESA.
In conclusion, we analyzed the data from nearly 200,000 patients on hemodialysis, demonstrating that the use of long-acting ESA, especially at high doses, was associated with a worse clinical prognosis than that of short-acting ESA. This result was consistent in a variety of statistical models including the instrumental variable method. Compared with long-acting ESA users, short-acting ESA users exhibited favorable outcomes even when their hemoglobin levels were lower. As this was an observational study, future well powered, randomized trials should ascertain the mortality risk attributed to long-acting ESA use. The external validity to other countries than Japan should also be examined. Our findings highlight the importance of the selection of ESA in the management of anemia in patients on hemodialysis.
Disclosures
Dr. Sakaguchi has received grants and honorariums from Chugai Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., and Kyowa Hakko Kirin Co., Ltd. Dr. Hamano has received grants and honorariums from Chugai Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., and Kyowa Hakko Kirin Co., Ltd., and does consultancy for Kyowa Hakko Kirin Co., Ltd. Dr. Wada has nothing to disclose. Dr. Masakane received honorariums from Kyowa Hakko Kirin Co., Ltd., Chugai Pharmaceutical Co., Ltd., and Kissei Pharmaceutical Co., Ltd.; there are no other relationships or activities that could appear to have influenced the submitted work.
Supplementary Material
Acknowledgments
The authors wish to express heartfelt appreciation to the Japanese Society for Dialysis Therapy, the principal investigators of all prefectures, and all of the personnel and patients at the institutions participating in this survey.
Dr. Sakaguchi and Dr. Hamano designed the study. Dr. Wada collected data and prepared the dataset. Dr. Sakaguchi analyzed the data and made the figures, Dr. Sakaguchi, Dr. Hamano, and Dr. Masakane drafted and revised the paper. All authors approved the final version of the manuscript.
The contents and opinions in this paper are those of the authors only and do not reflect those of the Japanese Society for Dialysis Therapy.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Erythropoiesis-Stimulating Agents and Mortality,” on pages 907–908.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018101007/-/DCSupplemental.
Supplemental Table 1. Statistical models used in this study.
Supplemental Table 2. Comparison of baseline characteristics between patients with and without missing data on the types of ESA.
Supplemental Table 3. Comparison of baseline characteristics after propensity score-matching.
Supplemental Table 4. The average dose of ESA in each ESA dose tertile.
Supplemental Table 5. Baseline characteristics according to four types of ESA.
Supplemental Table 6. Comparison of mortality rate across the four types of ESA.
References
- 1.Ma JZ, Ebben J, Xia H, Collins AJ: Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 10: 610–619, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Xia H, Ebben J, Ma JZ, Collins AJ: Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol 10: 1309–1316, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW: Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med 316: 73–78, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Evans RW, Rader B, Manninen DL; Cooperative Multicenter EPO Clinical Trial Group : The quality of life of hemodialysis recipients treated with recombinant human erythropoietin. JAMA 263: 825–830, 1990 [PubMed] [Google Scholar]
- 5.Grimm G, Stockenhuber F, Schneeweiss B, Madl C, Zeitlhofer J, Schneider B: Improvement of brain function in hemodialysis patients treated with erythropoietin. Kidney Int 38: 480–486, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Marsh JT, Brown WS, Wolcott D, Carr CR, Harper R, Schweitzer SV, et al.: rHuEPO treatment improves brain and cognitive function of anemic dialysis patients. Kidney Int 39: 155–163, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, et al.: Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 111: 992–1000, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, et al.: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al.: CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, et al.: CREATE Investigators : Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al.: TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, et al.: Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 74: 791–798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.KDIGO : KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2: 279–335, 2012 [Google Scholar]
- 14.Nakai S, Hanafusa N, Masakane I, Taniguchi M, Hamano T, Shoji T, et al.: An overview of regular dialysis treatment in Japan (as of 31 December 2012). Ther Apher Dial 18: 535–602, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Guideline Working Group, Japanese Society for Dialysis Therapy : Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther Apher Dial 12: 514–525, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Norton EC, Miller MM, Kleinman LC: Computing adjusted risk ratios and risk differences in Stata. Stata J 13: 492–509, 2013 [Google Scholar]
- 17.Tentori F, Albert JM, Young EW, Blayney MJ, Robinson BM, Pisoni RL, et al.: The survival advantage for haemodialysis patients taking vitamin D is questioned: Findings from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 24: 963–972, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Schneeweiss S, Setoguchi S, Brookhart A, Dormuth C, Wang PS: Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ 176: 627–632, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rassen JA, Schneeweiss S, Glynn RJ, Mittleman MA, Brookhart MA: Instrumental variable analysis for estimation of treatment effects with dichotomous outcomes. Am J Epidemiol 169: 273–284, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Judkins DR, Porter KE: Robustness of ordinary least squares in randomized clinical trials. Stat Med 35: 1763–1773, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Wilhelm-Leen ER, Winkelmayer WC: Mortality risk of darbepoetin alfa versus epoetin alfa in patients with CKD: Systematic review and meta-analysis. Am J Kidney Dis 66: 69–74, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer SC, Saglimbene V, Mavridis D, Salanti G, Craig JC, Tonelli M, et al.: Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: A network meta-analysis. Cochrane Database Syst Rev (12): CD010590, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winkelmayer WC, Chang TI, Mitani AA, Wilhelm-Leen ER, Ding V, Chertow GM, et al.: Longer-term outcomes of darbepoetin alfa versus epoetin alfa in patients with ESRD initiating hemodialysis: A quasi-experimental cohort study. Am J Kidney Dis 66: 106–113, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, et al.: Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A 91: 3974–3978, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anagnostou A, Lee ES, Kessimian N, Levinson R, Steiner M: Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc Natl Acad Sci U S A 87: 5978–5982, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haller H, Christel C, Dannenberg L, Thiele P, Lindschau C, Luft FC: Signal transduction of erythropoietin in endothelial cells. Kidney Int 50: 481–488, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Ribatti D, Presta M, Vacca A, Ria R, Giuliani R, Dell’Era P, et al.: Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood 93: 2627–2636, 1999 [PubMed] [Google Scholar]
- 28.Janmaat ML, Heerkens JL, de Bruin AM, Klous A, de Waard V, de Vries CJ: Erythropoietin accelerates smooth muscle cell-rich vascular lesion formation in mice through endothelial cell activation involving enhanced PDGF-BB release. Blood 115: 1453–1460, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Ishibashi T, Koziol JA, Burstein SA: Human recombinant erythropoietin promotes differentiation of murine megakaryocytes in vitro. J Clin Invest 79: 286–289, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakaguchi M, Kawakita M, Matsushita J, Shibuya K, Koishihara Y, Takatsuki K: Human erythropoietin stimulates murine megakaryopoiesis in serum-free culture. Exp Hematol 15: 1028–1034, 1987 [PubMed] [Google Scholar]
- 31.Cases A, Escolar G, Reverter JC, Ordinas A, Lopez-Pedret J, Revert L, et al.: Recombinant human erythropoietin treatment improves platelet function in uremic patients. Kidney Int 42: 668–672, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Debeljak N, Solár P, Sytkowski AJ: Erythropoietin and cancer: The unintended consequences of anemia correction. Front Immunol 5: 563, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Todaro M, Turdo A, Bartucci M, Iovino F, Dattilo R, Biffoni M, et al.: Erythropoietin activates cell survival pathways in breast cancer stem-like cells to protect them from chemotherapy. Cancer Res 73: 6393–6400, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al.: Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: A meta-analysis of randomised trials. Lancet 373: 1532–1542, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Nairz M, Schroll A, Moschen AR, Sonnweber T, Theurl M, Theurl I, et al.: Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-κB-inducible immune pathways. Immunity 34: 61–74, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cravedi P, Manrique J, Hanlon KE, Reid-Adam J, Brody J, Prathuangsuk P, et al.: Immunosuppressive effects of erythropoietin on human alloreactive T cells. J Am Soc Nephrol 25: 2003–2015, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.