Significance Statement
Higher serum phosphate and fibroblast growth factor-23 (FGF23) levels are potential modifiable risk factors to prevent cardiovascular disease in CKD. Studies evaluating intestinal phosphate binders found modest efficacy for lowering phosphate and FGF23 levels during short-term follow-up in CKD. In their randomized, placebo-controlled trial in 205 participants with stage 3b/4 CKD, the authors evaluated the effects of nicotinamide (an inhibitor of active intestinal phosphate transport), the phosphate binder lanthanum carbonate, or both, versus placebo over 12 months. They found that neither drug, alone or together, reduced serum phosphate or FGF23. Participants taking lanthanum carbonate had reductions in urinary phosphate, however gastrointestinal symptoms limited adherence. Secondary on-treatment analyses suggest that blocking intestinal phosphate absorption may lower FGF23, suggesting potential opportunities for future studies using novel therapies with better tolerability.
Keywords: kidney disease, mineral metabolism, fibroblast growth factor, phosphate, clinical trial, pilot study
Abstract
Background
Higher serum phosphate and fibroblast growth factor-23 (FGF23) levels may be modifiable to prevent cardiovascular disease in CKD. Short-term studies have reported modest efficacy in phosphate and FGF23 reduction with intestinal phosphate binders in CKD.
Methods
To investigate effects of lanthanum carbonate (LC; a phosphate binder) and/or nicotinamide (NAM; an inhibitor of active intestinal phosphate transport) on serum phosphate and FGF23 in stage 3b/4 CKD, we conducted a randomized trial among individuals with eGFR 20–45 ml/min per 1.73 m2 to NAM (750 mg twice daily) plus LC (1000 mg thrice daily), NAM plus LC placebo, LC plus NAM placebo, or double placebo for 12 months. Dual primary end points were change from baseline in serum phosphate and intact FGF23 concentrations.
Results
Mean eGFR for the 205 participants was 32ml/min per 1.73 m2. At baseline, serum phosphate was 3.7 mg/dl and median FGF23 was 99 pg/ml (10th, 90th percentiles: 59, 205). Mean rates of change in phosphate increased slightly over 12 months in all groups and did not differ significantly across arms. Similarly, percent changes in FGF23 per 12 months increased for all arms except LC plus placebo, and did not differ significantly across arms. Gastrointestinal symptoms limited adherence. Adverse events rates were similar across arms.
Conclusions
LC and/or NAM treatment did not significantly lower serum phosphate or FGF23 in stage 3b/4 CKD over 12 months. Although these agents appeared safe, intestinal symptoms limited adherence. Reducing phosphate and FGF23 in nondialysis CKD will require new approaches.
Persons with CKD are at high risk of cardiovascular disease and mortality.1 Although mechanisms remain uncertain, alterations in mineral metabolism represent potential causal intermediates. Reduction in GFR is associated with altered phosphate homeostasis,2 and even modest phosphate elevations within the normal laboratory range are associated with arterial calcification and cardiovascular disease events.3,4 Higher phosphate and reduced GFR also stimulate upregulation of counter-regulatory hormones, including fibroblast growth factor-23 (FGF23).5 FGF23, in turn, stimulates urinary phosphate excretion in an attempt to normalize serum phosphate concentrations in the face of lower GFR. Although this may be advantageous in the short-term, chronic exposure to higher FGF23 may induce left ventricular hypertrophy and increases risk of heart failure.6 Thus, there is considerable interest in lowering both serum phosphate and FGF23 concentrations in persons with CKD.
Prior studies targeting phosphate and FGF23 primarily used intestinal phosphate binders. Although these agents lower intestinal phosphate absorption,7 they also upregulate the active phosphate transporter sodium phosphate cotransporter 2b (Npt2b) in the small intestine.8 As such, use of binders in isolation may lower phosphate absorption acutely, but may promote relative hyperabsorption through enhanced Npt2b expression when binders are not present in the intestinal lumen. This may limit the efficacy of intestinal phosphate binders in CKD.9
When administered in high doses, nicotinamide (also known as vitamin B3) inhibits Npt2b expression in vitro and in animal models.10 Several studies demonstrate that nicotinamide lowers serum phosphate concentrations in patients with ESRD.11–17 Treating Npt2b knockout mice with intestinal phosphate binders potentiated their phosphate and FGF23 lowering effects,8 leading us to hypothesize that the effects of phosphate binders and nicotinamide could be additive. Whether, in patients with CKD who are not on dialysis, nicotinamide lowers serum phosphate and FGF23 concentrations, and whether the putative effect is potentiated by coadministration of an intestinal phosphate binder is unknown.
The CKD Optimal Management with Binders and Nicotinamide (COMBINE) trial is a randomized, double-blind, placebo-controlled trial designed to test the efficacy and safety of nicotinamide and lanthanum carbonate, in isolation or in combination, in persons with CKD stage 3b–4.9 The dual primary efficacy end points were rates of change in serum phosphate and FGF23 per 12 months of follow-up. Although effects were hypothesized to be evident within 3 months, participants were treated for 12 months to assess tolerability and safety, and to determine if the approach tested in the COMBINE trial may be suitable for longer-term use in phase 3 trials. We hypothesized that randomization to combined treatment with nicotinamide and lanthanum carbonate would reduce serum phosphate and FGF23 concentrations relative to double placebo, and would be well tolerated by patients with CKD. Further, we hypothesized that treatment with nicotinamide or lanthanum carbonate monotherapy would also decrease rate of declines in phosphate and FGF23 relative to double placebo, but that the magnitude would be intermediate to that observed in the dual active therapy arm.
Methods
Study Design and Oversight
The COMBINE trial was designed as a four-arm, parallel group, double-blind, randomized, controlled trial. Persons were recruited from CKD clinics at seven centers across the United States (Denver, CO; San Diego, CA; Chicago, IL; Evanston, IL; Salt Lake City, UT [two clinical centers]; and Washington, DC). The Cleveland Clinic served as the coordinating center. The design and rationale of the trial have been published previously,9 and the full protocol is available online (Supplemental Protocol).
The steering committee designed the study, gathered the data in collaboration with clinical sites, made decisions to submit the manuscript for publication, and takes responsibility for the fidelity of the study’s compliance with the protocol. The writing committee wrote the first and all subsequent drafts of the manuscript and takes responsibility for the completeness and accuracy of the data and analysis. Shire Pharmaceuticals donated lanthanum carbonate and placebo, and Endurance Products Company donated nicotinamide and placebo. These entities played no role in the design, conduct, or analysis of the study, drafting of the manuscript, or decisions about submission for publication. An independent data safety monitoring board monitored study progress and safety events. The study was approved by the institutional review boards at each center. An Investigational New Drug exemption was granted by the Food and Drug Administration, and the study was registered with Clinicaltrials.gov under identifier NCT02258074.
The trial was designed to enroll 200 participants. When this randomization goal was met, five additional participants had already met eligibility criteria, consented, and had entered the run-in phase. These participants were considered eligible resulting in a final sample of 205.
Study Participants
Inclusion criteria required an eGFR between 20 and 45 ml/min per 1.73 m2 by the CKD Epidemiology Collaboration creatinine equation,18 serum phosphate concentration ≥2.8 mg/dl, platelet count ≥125,000/mm3, ability to provide informed consent, and no plans to relocate from the area for the next 12 months. Key exclusion criteria included known allergy to nicotinamide or lanthanum carbonate, use of cinacalcet, recent (within 14 days) initiation or change in dose of activated vitamin D medications, parathyroid hormone (PTH) concentrations over five-fold the upper limit of normal (ULN), alcohol use (>14 drinks/wk) or liver disease (clinical or imaging diagnosis of cirrhosis, or liver function tests over two times the ULN), creatinine kinase over two-fold the ULN, current use of nicotinamide or intestinal phosphate binders, severe anemia (hemoglobin <9 g/dl), and hypoalbuminemia (<2.5 g/dl). A complete list of inclusion and exclusion criteria is provided within the study protocol (Supplemental Protocol).
Randomization and Intervention
Eligible participants began a 2-week run-in period during which they took placebo for both nicotinamide and lanthanum carbonate. Participants who achieved 80% compliance with both placebos for one or more of the run-in visits remained eligible. Venous blood samples and 24-hour urine collections were obtained twice at least 1 week apart during run-in, and participants received educational material encouraging low dietary phosphate intake. The protocol encouraged afternoon study visits to facilitate blood draws at the peak of the serum phosphate diurnal rhythm.19 Randomization was in equal proportions to the four arms and was stratified by clinical site. Randomization was performed using randomly permuted blocks of size 4 and 8. These were generated by computer in equal proportions to the four arms and stratified by clinical site. All randomization sequences were stored on a server at the data coordinating center. As each participant was randomized, the randomization program directly chose the next assignment in each sequence with no human interaction. Randomization was concealed to participants, investigators, and staff, and treatment was communicated to study staff through identification of prespecified treatment kits that were identified by barcodes and were otherwise indistinguishable with regards to containing either active treatment or placebo.
During the first month after randomization, participants took nicotinamide 750 mg or placebo daily, and lanthanum carbonate 500 mg or placebo thrice daily with meals. Subsequently, doses were titrated up to nicotinamide or placebo 750 mg twice daily, and lanthanum carbonate or placebo 1000 mg thrice daily with meals. Participants were seen monthly through month 3, and again at 6, 9, 11, and 12 months after randomization. At each visit, participants reported any interval emergency room visits, hospitalizations, or health-related procedures, and completed standardized questionnaires on gastrointestinal symptoms. Study personnel contacted participants by phone midway between the month 3 and 6 visits, and between the month 6 and 9 visits to encourage compliance and reinitiate or uptitrate medications if appropriate. When gastrointestinal symptoms were limiting compliance, doses of study medications could be titrated down or discontinued at the discretion of the site investigator. All efforts were made to decrease or discontinue one medication or placebo rather than both, and to reinitiate study medication if symptoms abated at the next phone or in-person contact.
Serum phosphate was measured at the central laboratory at each visit, and doses of both study medications/placebo were decreased by 50% if they were <2.8 mg/dl. If serum phosphate concentrations had fallen outside 1.5–6.0 mg/dl, study medications would have been permanently discontinued; this did not occur. Initiation of dialysis, transplantation, and pregnancy also mandated permanent discontinuation of study medications. Validated symptom questionnaires were administered at each visit to capture self-reported severity of gastrointestinal and other ailments. These included several that were thought to be potentially linked to the study treatment: severe bruising, bleeding, severe diarrhea, severe nausea, heartburn, flushing, and hives.
Study Outcomes
The dual primary end points were the slope of change per 12 months in serum phosphate and FGF23 concentrations. Venous blood samples were taken twice, 1 week apart, during the run-in period. Phosphate and FGF23 were measured in both specimens, and results were averaged to define each participant’s baseline. We measured postrandomization phosphate and FGF23 at months 1, 2, 3, 6, 9, and 12. Serum phosphate was measured by colorimetry centrally at Spectra Clinical Research of Rockleigh, New Jersey. The lower limit of detection was 0.1 mg/dl, and the laboratory’s acceptable coefficients of variation were 2.7%. EDTA plasma samples were centrifuged, decanted, and frozen to −80°C within 2 hours of collection. Specimens were shipped on dry ice monthly to the central FGF23 laboratory at the University of Washington, where measurements were made using an intact ELISA assay (Kainos, Japan). FGF23 measurements were made en bloc, when all participants’ specimens from across the protocol had been delivered, to minimize the influence of laboratory drift on study results. The lower limit of detection was 3.0 pg/ml, and the interassay coefficients of variation were 4.7%–10.5% across the analytic range.
Safety and tolerability were evaluated as additional end-points. Tolerability was assessed as percent that ceased taking one or both of their study medications/placebos and remained off of study medications for the rest of follow-up. Safety was assessed as the percentage that experienced a lab-related or symptom-related adverse event as captured by questionnaire. Severe adverse events including hospitalizations, deaths, transplantation or the initiation of dialysis were also compared.
Statistical Analyses
Baseline characteristics were evaluated as means±SD, medians with percentiles, or frequencies, as appropriate. When more than one baseline quantity was captured, we analyzed the last measure. We analyzed mineral metabolism and kidney function parameters in the intent-to-treat manner using two-slope, linear, mixed-effects models. Given their right-skewed distributions, FGF23, PTH, and urine albumin-to-creatinine ratio (ACR) were log-transformed before analysis, and resulting coefficients were transformed to represent percentage changes per time period (year or month) from baseline in the geometric mean. We hypothesized that treatment effects for all outcomes would manifest by month 3. We therefore modeled this initial “acute” stage separately from the succeeding “chronic” stage (months 3–12). The slopes for the two stages were weighted by their proportional time spans to produce a composite measure for the 12-month rates of change.20
For serum phosphate and FGF23, changes within the three active treatment arms were compared with double placebo, resulting in six comparisons. We therefore prespecified a two-sided significance level of 0.05/6=0.0083. Each mixed-effect model incorporated random intercepts, while treating study period and treatment as fixed effects. When analyzing treatment effects on eGFR, values of ten were imputed for protocol visits after participants started dialysis. We performed a set of post hoc analyses for serum phosphate and FGF23, first restricting values to those collected before participants permanently discontinued active treatment. We also compared treatment effects on acute phase slopes and on differences from baseline to average chronic phase values (using linear regression for the latter tests). Potential modifying effects of high (>4.0 mg/dl) versus other (≤4.0 mg/dl) baseline phosphate levels on phosphate trends between treatments were explored using group interaction effects. In an a priori, prespecified secondary analysis, we tested overall effects of the presence/absence of the treatments (factorial design) for all markers. When interactions by treatment arm had been excluded, we explored persons treated with lanthanum carbonate (either the nictoinamide and lanthanum double active arm [N-L] or the nicotinamide placebo/lanthanum active arm [p-L]) versus all others (nicotinamide active and lanthanum placebo [N-p] and double placebo [p-p]) and persons treated with nicotinamide (N-L and N-p) versus all others (p-L and p-p).
We investigated potential bias from informative patterns of missing serum phosphate and FGF23 data using multiple imputation. Ten values were imputed for each missing data point using a Markov chain Monte Carlo algorithm, assuming multivariate normality. Statistical inferences from the multiply imputed data were performed using the standard adjustments to SEMs of Little and Robin. All modeling above was adjusted for sex,21 clinical center, and (except for eGFR) baseline eGFR.
On the basis of observed changes in serum phosphate in a 6-month pilot study of across-group differences of 0.42 mg/dl in serum phosphate, assuming a within-group SD of change in phosphate over time of 0.55 mg/dl,22 and a significance level of 0.0083, we calculated 43 participants per group would yield 80% power. Prior unpublished data also demonstrated a within-treatment group SD of 18.65 pg/ml for change in FGF23 levels. This led to an estimated effect size of 14.3 pg/ml to be detected with 80% power, given the same 43 participants per group with the set α level. Overall, we targeted 50 participants per arm, assuming a 14% annual attrition rate.
Percentages of participants within treatment arms who permanently discontinued study medications or who experienced certain prespecified adverse events were compared using Fisher exact tests. Unless otherwise specified, a significance level of 0.05 was used without adjustment for multiple comparisons. Statistical analyses were performed with SAS software, version 9.4 (SAS Institute) and R software (2018; R Core Team).
Results
Participant Characteristics
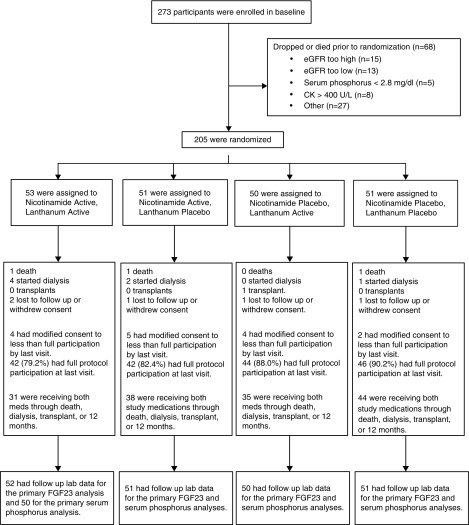
Between March 5, 2015 and December 17, 2016, 273 individuals consented to participate. Sixty-eight were excluded, predominantly because of eGFR values outside of the eligibility range. Two hundred five individuals were randomized (Figure 1), and all but one provided follow-up laboratory data. Among the 205 participants, the mean (±SD) age was 69±12 years, 38% were women, and 34% were nonwhite. Mean eGFR was 32±7 ml/min per 1.73 m2 and median ACR was 0.14 g/g (10th, 90th percentile: 0.01, 1.35). The mean serum phosphate was 3.7±0.6 mg/dl, and median intact FGF23 was 99 pg/ml (10th, 90th percentile: 59, 205). Baseline characteristics were well balanced across the four randomized treatment arms (Table 1).
Figure 1.
CONsolidated Standards of Reporting Trials diagram depicting consent, randomization, and dropout in the COMBINE trial. CK, creatine kinase.
Table 1.
Baseline characteristics by treatment arm in the COMBINE trial
| Characteristic | All | N-L | N-p | p-L | p-p |
|---|---|---|---|---|---|
| n=205 | n=53 | n=51 | n=50 | n=51 | |
| Age, yr, ±SD | 69±12 | 69±12 | 70±12 | 67±13 | 69±10 |
| Women, n (%) | 77 (38) | 20 (38) | 17 (33) | 22 (44) | 18 (35) |
| Race, n (%) | |||||
| White | 136 (66) | 35 (66) | 37 (73) | 32 (64) | 32 (63) |
| Black | 52 (25) | 13 (25) | 9 (18) | 14 (28) | 16 (31) |
| Other | 17 (8) | 5 (9) | 5 (10) | 4 (8) | 3 (6) |
| Diabetes, n (%) | 112 (55) | 31 (58) | 23 (45) | 33 (66) | 25 (49) |
| SBP, mm Hg, ±SD | 129±17 | 126±15 | 130±15 | 129±17 | 129±21 |
| DBP, mm Hg, ±SD | 71±12 | 70±11 | 72±13 | 71±12 | 72±13 |
| BMI, kg/m2, ±SD | 31.9±7.3 | 33.2±8.5 | 32.5±8.1 | 30.9±6.3 | 31.0±6.1 |
| eGFR, ml/min per 1.73 m2, ±SD | 32±7 | 32±7 | 32±7 | 33±8 | 32±7 |
| Urine ACR, g/ga | 0.14 [0.01, 1.35] | 0.14 [0.01, 1.09] | 0.06 [0.01, 1.42] | 0.17 [0.01, 1.65] | 0.17 [0.01, 1.29] |
| Calcitriol use, n (%) | 41 (20) | 8 (15) | 13 (26) | 10 (20) | 10 (20) |
| Serum phosphate, mg/dl, ±SD | 3.7±0.6 | 3.6±0.5 | 3.8±0.5 | 3.7±0.6 | 3.6±0.5 |
| Serum FGF23, pg/mla | 99 [59, 205] | 104 [57, 188] | 104 [61, 192] | 95 [49, 188] | 96 [63, 225] |
| Serum calcium, mg/dl, ±SD | 9.5±0.5 | 9.5±0.5 | 9.5±0.5 | 9.5±0.5 | 9.5±0.5 |
| Intact PTH, pg/ml, ±SD | 121±85 | 121±89 | 116±80 | 123±76 | 125±95 |
| 24 h urine phosphate, mg/d, ±SD | 668±253 | 657±265 | 713±235 | 659±268 | 644±245 |
| 24 h urine phosphate-to-creatinine, mg/g, ±SD | 462±132 | 447±146 | 487±126 | 452±121 | 463±131 |
N-L, nicotinamide active/lanthanum carbonate active; N-p, nicotinamide active/lanthanum carbonate placebo; p-L, nicotinamide placebo/lanthanum carbonate active; p-p, nicotinamide placebo/lanthanum carbonate placebo; SBP, systolic BP; DBP, diastolic BP; BMI, body mass index.
Median [10th, 90th percentile].
Rates of Change in Phosphate and FGF23
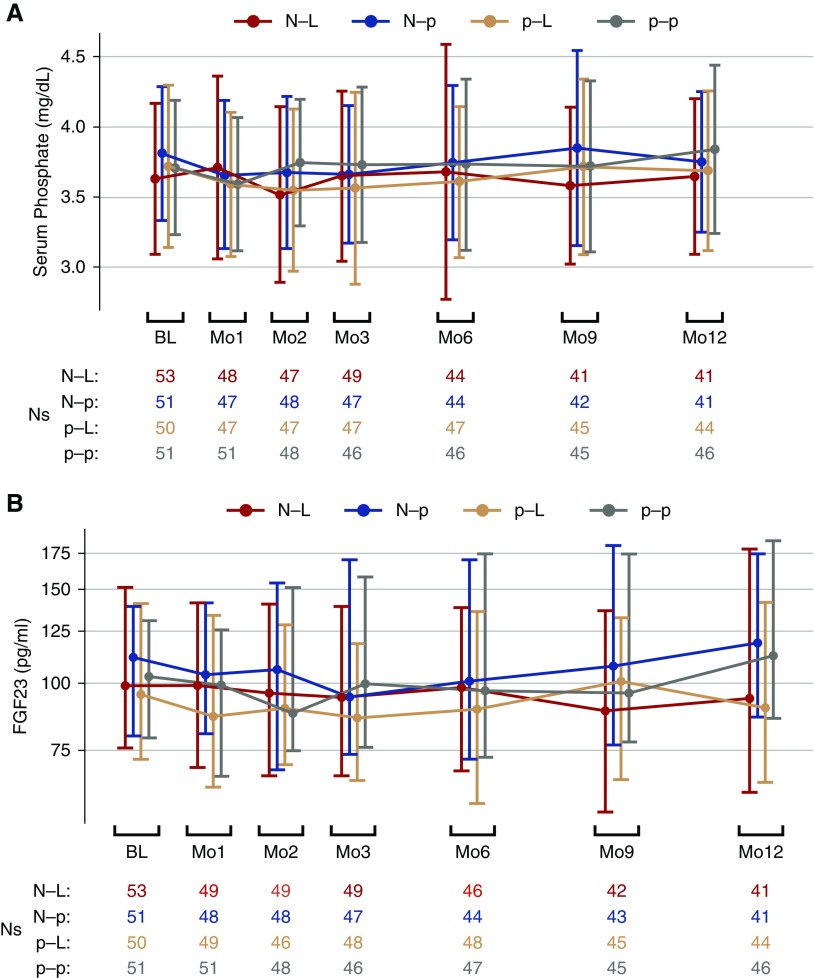
Over the 12-month protocol, there was no significant difference in the rates of change in serum phosphate (P values all ≥0.50) or FGF23 (P values all ≥0.05) in any of the three active treatment arms relative to the p-p group (Figure 2, Table 2). Results were similar when examining the first 3 months after randomization except that there was a trend toward declining serum phosphate in the p-L group relative to the p-p group (–0.043 versus +0.019 mg/dl per month; P=0.02; Supplemental Table 1). Results were also similar when we compared the average serum phosphate and FGF23 concentrations at months 3, 6, 9, and 12 versus baseline (Supplemental Table 2), in subgroup analysis among those with serum phosphate concentrations >4.0 mg/dl at baseline, and when multiple imputation was utilized to evaluate potential effects of missing follow-up data (data not shown).
Figure 2.
Changes in serum phosphate and FGF23 over 12 months in response to nicotinamide and lanthanum carbonate in nondialysis CKD. (A) Changes in serum phosphate by treatment arm in the COMBINE trial are presented as means with error bars reflecting ±1 SD. (B) Changes in intact FGF23 (B) by treatment arm are presented as medians; error bars depict interquartile ranges. N-L, nicotinamide active/lanthanum carbonate active; N-p, nicotinamide active/lanthanum carbonate placebo; p-L, nicotinamide placebo/lanthanum carbonate active; p-p, nicotinamide placebo/lanthanum carbonate placebo.
Table 2.
Change in serum phosphate and FGF23 over 12 months in the COMBINE trial
| Trt | (Na) Observed Concentrationsb | Adjusted Estimates of Treatment Effectsc,d |
P Valuee versus p-p | ||
|---|---|---|---|---|---|
| Baseline | Month 3 | Month 12 | Change per Yearf (95% CI) | ||
| Phosphate, mg/dl | |||||
| N-L | (53) 3.63±0.54 | (49) 3.65±0.61 | (41) 3.65±0.56 | 0.06 (−0.08 to 0.20) | 0.50 |
| N-p | (51) 3.81±0.48 | (47) 3.66±0.49 | (41) 3.75±0.50 | 0.12 (−0.03 to 0.27) | 0.95 |
| p-L | (50) 3.72±0.58 | (47) 3.56±0.68 | (44) 3.69±0.57 | 0.06 (−0.09 to 0.21) | 0.52 |
| p-p | (51) 3.71±0.48 | (46) 3.73±0.55 | (46) 3.84±0.60 | 0.12 (−0.01 to 0.26) | — |
| FGF23, pg/mld | |||||
| N-L | (53) 103.1 [57.5, 210.5] | (49) 95.4 [45.6, 197.9] | (41) 97.4 [45.6, 250.5] | 4.7% (−5.6% to 15.1%) | 0.21 |
| N-p | (51) 111.9 [64.4, 202.8] | (47) 94.3 [62.6, 225.7] | (41) 119.7 [62.6, 285.5] | 19.3% (8.5% to 30.3%) | 0.45 |
| p-L | (50) 97.1 [49.8, 187.8] | (48) 88.0 [47.5, 178.5] | (44) 92.6 [49.1, 200.1] | −0.3% (−10.9% to 10.4%) | 0.05 |
| p-p | (51) 102.9 [63.9, 229.4] | (46) 102.5 [61.7, 328.2] | (46) 114.8 [55.0, 287.3] | 13.8% (4.3% to 23.3%) | — |
Trt, randomized treatment group; p-p, nicotinamide placebo/lanthanum carbonate placebo; 95% CI, 95% confidence interval; N-L, nicotinamide active/lanthanum carbonate active; N-p, nicotinamide active/lanthanum carbonate placebo; p-L, nicotinamide placebo/lanthanum carbonate active; —, reference category.
Descriptive statistics for observed concentrations and estimated treatment effects derived using all available data.
Phosphate results are mean±SD, FGF23 results are median [10th, 90th percentiles].
Results of mixed-effects analyses controlling for the baseline level of the factor analyzed, sex, eGFR, and clinical center.
FGF23 values were log-transformed before analysis. Estimated treatment effects expressed as percentage change per month (from baseline) in the geometric mean.
P values <0.008 reflect statistical significance due to multiple comparisons.
Twelve-month changes were composed from the average of any participant slopes from the chronic phase (months 3–12) and any participant slopes from the acute phase (baseline to month 3), weighting by the two periods’ relative duration.
In a secondary analysis that was prespecified a priori in the protocol, we tested for interactions by treatment arm and explored participants treated with lanthanum carbonate versus all others (irrespective of nicotinamide or placebo assignment) and participants treated with nicotinamide versus all others (irrespective of lanthanum assignment). No interactions by treatment arm on changes in phosphate or FGF23 were observed (P interactions 0.97 and 0.96). In stratified analysis, there were no relationships of assignment to a lanthanum carbonate or nicotinamide containing arm with changes in phosphate. However, persons randomized to an arm containing lanthanum carbonate had 14% lower FGF23 concentrations over 12 months than those randomized to arms not containing lanthanum carbonate (Table 3).
Table 3.
Change in serum phosphate and FGF23 in participants treated in lanthanum treated versus untreated and nicotinamide treated versus untreated participants
| Trt | Change per Year (95% CI)a | Factorial Interaction P Valueb | ||
|---|---|---|---|---|
| Within Trt | LT versus LU | P Valuec | ||
| Serum phosphate, mg/dl | ||||
| LT | 0.059 (−0.04 to 0.16) | −0.06 (−0.20 to 0.08) | 0.40 | 0.97 |
| LU | 0.120 (0.02 to 0.22) | |||
| NT | 0.089 (−0.01 to 0.19) | −0.002 (−0.14 to 0.14) | 0.98 | |
| NU | 0.091 (−0.01 to 0.19) | |||
| FGF23, pg/ml | ||||
| LT | 2.3% (−5.2% to 9.9%) | −14.0% (−24.2% to −3.7%) | 0.008 | 0.96 |
| LU | 16.5% (9.3% to 23.8%) | |||
| NT | 12.1% (4.4% to 19.9%) | 5.2% (−5.4% to 15.9%) | 0.33 | |
| NU | 6.9% (−0.4% to 14.2%) | |||
Trt, randomized treatment group; 95% CI, 95% confidence interval; LT, lanthanum carbonate treated; LU, lanthanum carbonate untreated; NT, nicotinamide treated; NU, nicotinamide untreated.
FGF23 values were log-transformed before analysis. Estimated treatment effects expressed as percentage change per month (from baseline) in the geometric mean.
Result for the general question: does the effect of either treatment depend on presence/absence of other treatment?
Results of mixed-effects analyses controlling for the baseline level of the factor analyzed, sex, eGFR, and clinical center.
Safety and Tolerability
The percentage of patients who experienced any laboratory or symptom related adverse events were similar across treatment arms (P=0.71; Table 4). The number of individuals reaching ESRD and hospitalizations were numerically higher in the N-L double active group, but the number of events was few and statistically similar. There was one death each in the N-L, N-p, and p-p groups, and none in the p-L group.
Table 4.
Number experiencing adverse events by treatment arm in the COMBINE trial
| Adverse Event | N-L (n=53) | N-p (n=51) | p-L (n=50) | p-p (n=51) |
|---|---|---|---|---|
| Any laboratory or symptom-related adverse eventa | 33 | 30 | 29 | 26 |
| Prespecified laboratory adverse events | ||||
| Serum phosphate ≥5.9 mg/dl | 1 | 0 | 1 | 0 |
| Serum phosphate <1.5 mg/dl | 1 | 0 | 0 | 0 |
| Platelet <100,000/mm2 | 1 | 0 | 0 | 2 |
| Liver function abnormalities | 0 | 0 | 0 | 0 |
| Creatine kinase >800 U/L | 1 | 0 | 0 | 0 |
| Prespecified symptom-related adverse events (questionnaire) | ||||
| Nausea (severe) | 11 | 4 | 9 | 4 |
| Heartburn (severe) | 2 | 0 | 3 | 4 |
| Diarrhea (severe) | 12 | 8 | 5 | 7 |
| Flushing (Y/N) | 1 | 4 | 5 | 4 |
| Hives (Y/N) | 1 | 9 | 5 | 0 |
| Bruising (Y/N) | 20 | 21 | 16 | 18 |
| Bleeding (Y/N) | 10 | 10 | 13 | 7 |
| Serious adverse events | ||||
| Reached ESRD (dialysis or transplant) | 4 | 2 | 1 | 1 |
| Hospitalizations | 17 | 13 | 14 | 13 |
| Deaths | 1 | 1 | 0 | 1 |
N-L, nicotinamide active/lanthanum carbonate active; N-p, nicotinamide active/lanthanum carbonate placebo; p-L, nicotinamide placebo/lanthanum carbonate active; p-p, nicotinamide placebo/lanthanum carbonate placebo; Y/N, yes/no.
P=0.71 (Fisher exact test).
During the study, 42% of participants in the N-L arm discontinued either drug as compared with 14% in the p-p arm. Study drug discontinuations were intermediate in the N-p and p-L arms (25% and 30%, respectively; Table 5). These rates differed significantly across arms (P=0.02). Gastrointestinal symptoms and pill burden were the most common reasons given for discontinuations, and when pill burden was reported as the primary cause, it was often accompanied by gastrointestinal symptoms. Although pill burden was most frequently reported in the N-L arm (11%), only one participant (2%) in the p-p arm discontinued study drugs giving this reason, despite identical pill burden across arms.
Table 5.
Study drug tolerability over 12 months in the COMBINE trial
| Study Drug Tolerability Event | N-L | N-p | p-L | p-p |
|---|---|---|---|---|
| (n=53) | (n=51) | (n=50) | (n=51) | |
| Stopped either druga | 22 (42%) | 13 (25%) | 15 (30%) | 7 (14%) |
| Stopped nicotinamide or placebo | 19 (36%) | 12 (24%) | 8 (16%) | 5 (10%) |
| Stopped lanthanum or placebo | 21 (40%) | 12 (24%) | 15 (30%) | 6 (12%) |
| Stopped both medications | 18 (34%) | 11 (22%) | 8 (16%) | 4 (8%) |
| Primary reason given for study medication discontinuation | ||||
| Gastrointestinal symptoms | 5 (9%) | 4 (8%) | 6 (12%) | 1 (2%) |
| Pill burden | 6 (11%) | 3 (6%) | 2 (4%) | 1 (2%) |
| Pill burden and gastrointestinal symptoms | 5 (9%) | 2 (4%) | 1 (2%) | 1 (2%) |
| Increasing comorbidity | 5 (9%) | 1 (2%) | 0 | 3 (6%) |
| Hyperphosphatemia | 1 (2%) | 0 | 1 (2%) | 0 |
| Nonadherence/stopped attending study visits | 3 (6%) | 5 (10%) | 5 (10%) | 1 (2%) |
| Withdrawal of consent | 2 (4%) | 0 | 0 | 1 (2%) |
| Loss to follow-up | 0 | 0 | 1 (2%) | 0 |
Data reflect number of patients and percentage of study arm. N-L, nicotinamide active/lanthanum carbonate active; N-p, nicotinamide active/lanthanum carbonate placebo; p-L, nicotinamide placebo/lanthanum carbonate active; p-p, nicotinamide placebo/lanthanum carbonate placebo.
P=0.02 (Fisher exact test).
Rates of Change in Other Parameters of Mineral Metabolism and Kidney Function
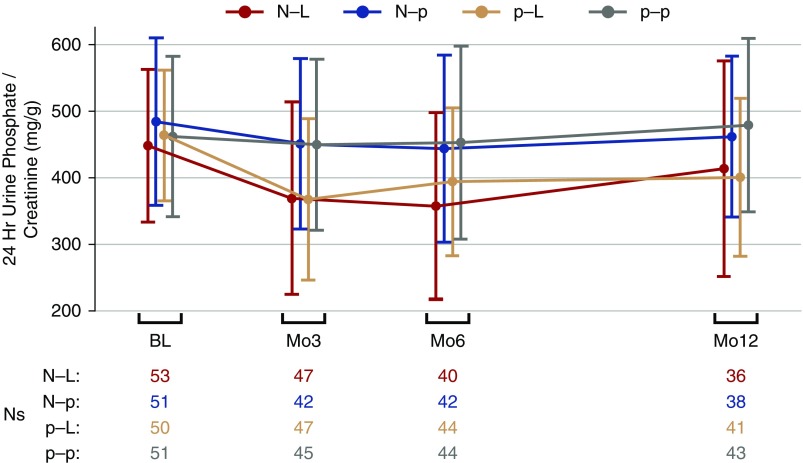
Figure 3 depicts the rates of change in 24-hour urine phosphate-to-creatinine ratio during the trial. Participants in p-L arm had a 60.0 mg/g mean reduction in urine phosphate-to-creatinine ratio over 12 months, compared with a 10.7 mg/g mean increase per 12 months in the p-p arm (P=0.009; Table 6). A similar decline in urine phosphate-to-creatinine ratio was observed in the N-L group; however, the comparison to the p-p arm did not reach statistical significance (P=0.13). The changes were evident by month 3 and both the N-L and p-L groups were statistically significantly different from the p-p arm at that time (Supplemental Table 1). Associations between urine phosphate-to-creatinine ratio and the dual primary end points showed that, through month 3, each 10% decrease per month in urine phosphate-to-creatinine ratio was associated with a 3.1% decrease per month in FGF23 (95% confidence interval, −4.8% to −1.3%; P<0.001). Results were similar over 12 months. No association was observed between the rates of changing urine phosphate-to-creatinine and serum phosphate concentrations during either time period (P≥0.25). There were also no significant differences in the 12-month gradients for PTH or eGFR among any of the active treatment arms relative to p-p; however, calcium and urine ACR increased at modestly higher rates in the N-p arm relative to the p-p arm (Table 6).
Figure 3.
Change in 24-hour urine phosphate-to-creatinine ratio over 12 months in response to nicotinamide and lanthanum carbonate in nondialysis CKD. Changes in 24-hour urine phosphate-to-creatinine ratio in milligrams per gram by treatment arm in the COMBINE trial, presented as means with error bars reflecting ±1 SD. N-L, nicotinamide active/lanthanum carbonate active; N-p, nicotinamide active/lanthanum carbonate placebo; p-L, nicotinamide placebo/lanthanum carbonate active; p-p, nicotinamide placebo/lanthanum carbonate placebo.
Table 6.
Changes in other mineral metabolism and kidney function parameters over 12 months in the COMBINE trial
| Trt | (Na) Observed Concentrationsb | Adjusted Estimates of Treatment Effectsc |
P Value versus p-p | ||
|---|---|---|---|---|---|
| Baseline | Month 3 | Month 12 | Change per Yeard (95% CI) | ||
| 24 h urine phosphate-to-creatinine, mg/g | |||||
| N-L | (53) 448±114 | (47) 369±145 | (36) 413±162 | −32.3 (−76.5 to 11.9) | 0.13 |
| N-p | (51) 484±126 | (42) 451±128 | (38) 461±121 | −26.5 (−64.8 to 11.8) | 0.15 |
| p-L | (50) 463±98 | (47) 367±121 | (41) 400±118 | −60.0 (−101.0 to −19.0) | 0.009 |
| p-p | (51) 461±120 | (45) 449±128 | (43) 478±130 | 10.7 (−22.3 to 43.7) | — |
| PTH, pg/mle | |||||
| N-L | (53) 92.5 [35.0, 222.0] | (48) 97.5 [31, 238] | (41) 108 [48, 209] | 4.4% (−11.6% to 20.6%) | 0.29 |
| N-p | (50) 109.5 [42.0, 184.8] | (46) 77.0 [35, 264] | (40) 85 [33, 241] | −0.1% (−13.6% to 13.5%) | 0.10 |
| p-L | (50) 105.3 [40.8, 240.3] | (47) 85.0 [49, 246] | (42) 90 [41, 223] | 2.4% (−10.2% to 15.2%) | 0.15 |
| p-p | (51) 101.0 [47.0, 218.0] | (46) 100.0 [50, 245] | (45) 111 [53, 298] | 15.4% (3.4% to 27.4%) | — |
| Serum calcium, mg/dl | |||||
| N-L | (53) 9.50±0.49 | (49) 9.69±0.50 | (41) 9.64±0.52 | 0.04 (−0.09 to 0.16) | 0.39 |
| N-p | (51) 9.44±0.43 | (47) 9.65±0.45 | (41) 9.65±0.59 | 0.18 (0.04 to 0.32) | 0.02 |
| p-L | (50) 9.48±0.46 | (47) 9.59±0.51 | (44) 9.55±0.54 | 0.04 (−0.06 to 0.15) | 0.29 |
| p-p | (51) 9.50±0.51 | (46) 9.48±0.51 | (46) 9.46±0.53 | −0.03 (−0.13 to 0.06) | — |
| eGFR, ml/min per 1.73 m2 | |||||
| N-L | (53) 31.6±7.5 | (49) 30.8±8.1 | (41) 31.5±9.6 | −1.67 (−3.71 to 0.38) | 0.56 |
| N-p | (51) 31.8±7.9 | (47) 29.9±9.0 | (41) 29.2±8.8 | −3.76 (−5.53 to −2.00) | 0.29 |
| p-L | (50) 32.2±7.4 | (47) 31.6±9.0 | (44) 31.7±10.2 | −0.80 (−2.35 to 0.75) | 0.16 |
| p-p | (51) 32.3±9.1 | (46) 30.2±10.7 | (46) 29.3±12.1 | −2.45 (−4.14 to −0.77) | — |
| Urine ACR, mg/ge | |||||
| N-L | (53) 100 [12, 1123] | (47) 83 [10, 2030] | (41) 125 [15, 1182] | 54.0% (22.1% to 86.8%) | 0.14 |
| N-p | (51) 56 [8, 1341] | (45) 104 [7, 3380] | (39) 101 [9, 2680] | 63.0% (34.0% to 92.6%) | 0.04 |
| p-L | (50) 176 [10, 1789] | (48) 309 [7, 2398] | (44) 252 [16, 2697] | 26.7% (−3.1% to 57.3%) | 0.85 |
| p-p | (51) 181 [9, 1387] | (46) 195 [6, 1896] | (42) 123 [8, 2452] | 22.9% (−2.4% to 48.7%) | — |
| Fractional excretion of phosphate | |||||
| N-L | (53) 25.4±7.8 | (47) 22.3±9.7 | (36) 24.2±10.0 | −0.45 (−2.75 to 1.85) | 0.18 |
| N-p | (51) 26.4±8.2 | (42) 27.5±10.1 | (38) 28.4±12.0 | 2.04 (−1.17 to 5.26) | 0.85 |
| p-L | (50) 25.5±6.2 | (47) 21.6±7.8 | (41) 23.2±9.4 | −2.07 (−4.58 to −0.43) | 0.03 |
| p-p | (51) 27.2±10.9 | (45) 27.7±10.7 | (43) 29.6±11.3 | 1.68 (−0.45 to 3.82) | — |
Trt, randomized treatment group; p-p, nicotinamide placebo/lanthanum carbonate placebo; 95% CI, 95% confidence interval; N-L, nicotinamide active/lanthanum carbonate active; N-p, nicotinamide active/lanthanum carbonate placebo; p-L, nicotinamide placebo/lanthanum carbonate active; —, reference category.
Descriptive statistics for observed concentrations and estimated treatment effects derived using all available data.
Data are mean±SD for all variables except PTH and urine ACR, which are shown as median [10th, 90th percentiles].
Results of mixed-effects analyses controlling for the baseline level of the factor analyzed, sex, eGFR, and clinical center.
Twelve-month changes were composed from the average of any participant slopes from the chronic phase (months 3–12) and any participant slopes from the acute phase (baseline to month 3), weighting by the two periods’ relative duration.
FGF23 and urine ACR values were log-transformed before analysis. Estimated treatment effects expressed as percentage change per month (from baseline) in the geometric mean.
Post hoc, we conducted an analysis of participants who remained “on protocol,” censoring participants when they discontinued study medications, rather than by intent-to-treat. This was done to gauge whether drug efficacy may have been affected by compromised tolerability. We found no significant changes in serum phosphate across arms compared with p-p. The magnitude of the rate of decline of FGF-23 and 24-hour urine phosphate-to-creatinine ratio were larger in the on-protocol analyses (Supplemental Table 3).
Discussion
We tested whether nicotinamide and lanthanum carbonate, alone or in combination, may provide a useful strategy to lower phosphate and FGF23 concentrations in persons with CKD stage 3b–4. Our primary results indicate that these agents, in isolation or in combination, do not produce greater 12-month declines in phosphate or FGF23 relative to placebo. Although these medications appeared safe, discontinuations were substantially more common in active treatment arms than in the double placebo arm, despite close monitoring and repeated efforts to maintain adherence.
Although intestinal phosphate binders are effective in lowering phosphate concentrations in ESRD patients, prior clinical trials in patients with CKD stages 3–4 have demonstrated either no change,23–26 or small magnitude changes in serum phosphate relative to placebo, on the order of 0.2–0.3 mg/dl.7,27 Unlike patients with ESRD, individuals with CKD stages 3–4 likely have sufficient kidney function to overcome reductions in intestinal phosphate absorption by compensatory reductions in renal phosphate excretion without major changes in serum phosphate. In addition, binders increase active sodium phosphate transporters,8,28 which can be blocked by high-dose nicotinamide.29,30 Thus, we hypothesized that combined treatment with an intestinal phosphate binder and nicotinamide may synergistically lower phosphate and FGF23.8 However, using 24-hour urine phosphate excretion as a marker of intestinal absorption, nicotinamide did not reduce 24-hour urine phosphate excretion relative to placebo.31 In contrast, participants randomized to either of the two arms with active lanthanum carbonate showed diminishing urine phosphate excretion relative to placebo. Contrary to our expectation of synergy between nicotinamide and lanthanum carbonate, we observed no additive effect on urine phosphate excretion when the two agents were coadministered. These data suggest that nicotinamide had little or no effect on phosphate absorption in the participants with CKD evaluated in this study.
We observed a 14% discontinuation rate in the p-p arm compared with 42% in the N-L arm; participants randomized to arms with one active drug had intermediate discontinuation rates. Both gastrointestinal complaints and participants’ decisions to stop attending study visits were more common in the active treatment arms compared with p-p, despite close contact with study personnel (at least every 6 weeks) and repeated efforts to re-initiate study medications and manage drug-related symptoms during the trial. These findings have important implications. Differential discontinuations across arms suggest that symptoms related to the study medications were responsible for many of the discontinuations. Although pill burden was often reported by participants, it was an infrequent reason for discontinuation in the p-p arm despite identical pill burden across arms, and pill burden often was accompanied by gastrointestinal symptoms when given as the reason for discontinuation. Further, although discontinuation rates were high, we believe the very close engagement and efforts of investigators and staff to promote adherence and uptitrate whenever possible provides a realistic perspective of the side-effect burden of the study medications in patients with CKD who are generally otherwise asymptomatic. This intervention for 12 months or longer appears unlikely to be a viable treatment option for patients with CKD in routine clinical care. Regarding the trial efficacy results, the high discontinuation rates in active treatment arms may have biased the efficacy results toward the null hypothesis. This raises the question of how to interpret the null results of this trial. Was the underlying biologic premise that we hypothesized incorrect, or were its interventions insufficient to demonstrate effects?
To further assess the biologic premises, we conducted several additional analyses. An analysis that was prespecified in the study protocol compared person treated in arms containing lanthanum carbonate (N-L plus p-L) versus arms that did not contain lanthanum (N-p and p-p), and similarly nicotinamide containing versus noncontaining arms. This analysis demonstrated a 14% reduction in FGF23 in lanthanum-treated patients over 1 year. A post hoc, on-protocol analyses evaluated the potential efficacy of the interventions among individuals who tolerated the study medications, recognizing the potential bias in these exploratory analyses. In these analyses, there was an 8% annual reduction in FGF23 in the p-L arm, compared with a 14% annual increase in the p-p arm. In intent-to-treat analysis, individuals in the arms with active lanthanum carbonate had greater declines in urine phosphate and the degree of lowering over time was significantly related to the gradient of FGF23 reduction. Collectively, these findings suggest that treatments which limit intestinal phosphate absorption may lower FGF23. However, because adherence to the protocol was low likely related to drug-related symptoms, novel approaches distinct from those tested here will be required in future studies.
Strengths of this study include its multicenter, randomized, double-blind design and its 12-month protocol. The longer follow-up was specifically designed to allow assessment of relatively long-term tolerability in addition to acute and chronic effects. The availability of multiple 24-hour urine collections and other measures of mineral metabolism provides insights into mechanistic pathways, and close monitoring and frequent contact were in place to facilitate maximum adherence.
The trial also has important limitations. Because six equally important comparisons needed to be evaluated, sample size and power calculations used an α level of 0.0083, a high bar to meet for statistical differences. Regardless, even at an α level of 0.05, our interpretation would have been similar. The majority of study participants had phosphate concentrations within the normal laboratory range, as is common in CKD stage 3b–4. Whether results generalize to patients with CKD with overt hyperphosphatemia is unknown; however, results were similar in subgroup analysis among those with serum phosphate >4.0 mg/dl at baseline. Nicotinamide inhibits active phosphate transport in the intestine, yet recent studies suggest that passive paracellular phosphate flux is also regulated.32 The relative contribution of passive versus active transport in humans is unknown. We measured intact FGF23, but recent discoveries highlight distinct biology in regulation relative to C-terminal fragments.33,34 Future studies are required to evaluate C-terminal FGF23, and the potential implications of iron metabolism and inflammation on any differences in results.
In conclusion, in persons with CKD stage 3b–4, treatment with nicotinamide, lanthanum carbonate, or both together did not significantly lower serum phosphate or FGF23. Although these medications appeared safe in patients with CKD, they were not well tolerated. Future studies targeting intestinal phosphate absorption for FGF23 lowering appear warranted; however, novel strategies that combine greater efficacy and tolerability will be required.
Disclosures
Dr. Ix reports grants from NIDDK during the conduct of the study and grants from Baxter International outside the submitted work. Dr. Isakova reports other from Shire, personal fees from Bayer, personal fees from Eli Lilly, outside the submitted work. Dr. Raphael reports grants from NIH, during the conduct of the study. Dr. Cheung reports grants from NIDDK, during the conduct of the study. Dr. Sprague reports grants from NIH, during the conduct of the study; grants and personal fees from Vifor, grants and personal fees from Opko, grants and personal fees from Amgen, grants from Reata, outside the submitted work. Dr. Fried reports other from NIDDK, during the conduct of the study. Dr. Gassman reports grants from NIH/NIDDK, during the conduct of the study. Dr. Block reports personal fees, nonfinancial support and other from Ardelyx, Inc., grants, personal fees and non-financial support from Keryx, Inc., outside the submitted work. Dr. Wolf reports personal fees from Keryx, during the conduct of the study; personal fees from Amgen, personal fees from Diasorin, personal fees from Akebia, personal fees from AMAG, personal fees from Ardelyx, personal fees from Lutipold, personal fees from SANOFI, grants from SHIRE, outside the submitted work.
Supplementary Material
Acknowledgments
Nicotinamide and placebo were donated by Endurance Products Company, Tigard, Oregon; and lanthanum carbonate and placebo were donated by Shire Pharmaceuticals, Wayne, Pennsylvania. The investigators wish to thank the study participants and many individuals who made the trial possible, including National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK): Flessner, J.W. Kusek, K.C. Abbott, P.L. Kimmel, S. Mendley, Fried (Steering Committee Chair); Data Coordinating Center (Cleveland Clinic): Gassman, G.J. Beck, K. Brittain, S. Sherer, B. Hu, C. Kendrick, A. Larive, K. Wiggins, J. MacKrell, V. Konig; Laboratory Medicine Research Testing Services (FGF23), University of Washington: A. Hoofnagle, K. Van Leuven, D. Landicho, H. Pflaum, J. Wallace, T. Nguyen; George Washington University: Raj, S. Sharma, A. Ramezani, M. Wing, C. Franco, A. Dumadag, S. Andrews; Northwestern University School of Medicine: Isakova, R. Mehta, M. Bradley, G. Schwartz, C. Martinez, P. Fox, A. Stump, A. Hodakowski, D. Lipiszko; NorthShore University Health System: Sprague, S. Fettman, J. Rubens, S. Rao, S. Lewis; Veterans Medical Research Foundation: Ix, D. Rifkin, U. Selamet, C. Ginsberg, E. Castro, B. Thomas, C. Fernandes, L. Frank, A. Chostner, D. Walpole; Denver Nephrology Research: Block, L. Kooienga, M. Baltazar, M. Persky, B. Shamblin, M. Chonchol, M Sedlacek, R. Hoehler, B. Farmer; University of Utah: Cheung, C. Orme, Raphael, A. Balch, T. Greene, J. Zitterkoph, M. Varner, S. Neagle, H. Buswell; George E. Wahlen Department of Veterans Affairs Medical Center, Salt Lake City, Utah: Cheung, C. Orme, Raphael, A. Balch, T. Greene, J. Zitterkoph, M. Varner, S. Neagle, H. Buswell; Duke University: Wolf, Middleton, and Data Safety and Monitory Board members D. Warnock, J. Bonventre, D. Coyne, L. Dworkin, R. Glassock, J. Hodges, A. Thompson, M. Leonard, M. Pahl, A.K. Singh, JR Landis, D. Bluemke.
Designed the study: Dr. Ix, Dr. Isakova, Dr. Gassman, Dr. Flessner, Dr. Block, and Dr. Wolf. Conducted the experiment: Dr. Ix, Dr. Isakova, Dr. Raphael, Dr. Raj, Dr. Cheung, Dr. Sprague, Dr. Block, and Dr. Wolf. Data analysis: Dr. Larive, Dr. Gassman, and Dr. Middleton. Interpreted study findings: Dr. Ix, Dr. Isakova, Dr. Larive, Dr. Raphael, Dr. Raj, Dr. Cheung, Dr. Sprague, Dr. Fried, Dr. Gassman, Dr. Middleton, Dr. Flessner, Dr. Block, and Dr. Wolf. Drafted first and subsequent versions of the manuscript: Dr. Ix. Critically revised the manuscript: Dr. Ix, Dr. Isakova, Dr. Larive, Dr. Raphael, Dr. Raj, Dr. Cheung, Dr. Sprague, Dr. Fried, Dr. Gassman, Dr. Middleton, Dr. Flessner, Dr. Block, and Dr. Wolf. Secured funding: Dr. Ix, Dr. Isakova, Dr. Raphael, Dr. Raj, Dr. Cheung, Dr. Gassman, Dr. Block, and Dr. Wolf.
This trial was sponsored by the NIDDK Pilot Clinical Trials consortium (contracts U01DK097093, U01DK099877, U01DK099924, U01DK099930, and U01DK099933) with additional support from an ancillary study grant to Isakova (R01DK102438).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Dual Inhibition of Gastrointestinal Phosphate Absorption: More Questions Than Answers,” on pages 909–910.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018101058/-/DCSupplemental.
Supplemental Protocol. Study Protocol.
Supplemental Table 1. Acute Changes in Biochemical Variables Over 3 Months.
Supplemental Table 2. Change of Biochemical Variables from Baseline to the Average of Months 3, 6, 9, and 12.
Supplemental Table 3. Changes in Biochemical Variables in Participants Who Remained on Treatment – “Per Protocol” Analysis.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, et al.: Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol 60: 200–207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominguez JR, Kestenbaum B, Chonchol M, Block G, Laughlin GA, Lewis CE, et al.: Osteoporotic Fractures in Men (MrOS) Study Research Group : Relationships between serum and urine phosphorus with all-cause and cardiovascular mortality: The Osteoporotic Fractures in Men (MrOS) Study. Am J Kidney Dis 61: 555–563, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA: Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 20: 397–404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett SM, Gunawardene SC, Bringhurst FR, Jüppner H, Lee H, Finkelstein JS: Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 21: 1187–1196, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Faul C, Amaral AP, Oskouei B, Hu M-C, Sloan A, Isakova T, et al.: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, et al.: Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiavi SC, Tang W, Bracken C, O’Brien SP, Song W, Boulanger J, et al.: Npt2b deletion attenuates hyperphosphatemia associated with CKD. J Am Soc Nephrol 23: 1691–1700, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isakova T, Ix JH, Sprague SM, Raphael KL, Fried L, Gassman JJ, et al.: Rationale and approaches to phosphate and fibroblast growth factor 23 reduction in CKD. J Am Soc Nephrol 26: 2328–2339, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginsberg C, Ix JH: Nicotinamide and phosphate homeostasis in chronic kidney disease. Curr Opin Nephrol Hypertens 25: 285–291, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi Y, Tanaka A, Nakamura T, Fukuwatari T, Shibata K, Shimada N, et al.: Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int 65: 1099–1104, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Cheng SC, Young DO, Huang Y, Delmez JA, Coyne DW: A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin J Am Soc Nephrol 3: 1131–1138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young DO, Cheng SC, Delmez JA, Coyne DW: The effect of oral niacinamide on plasma phosphorus levels in peritoneal dialysis patients. Perit Dial Int 29: 562–567, 2009 [PubMed] [Google Scholar]
- 14.El Borolossy R, El Wakeel LM, El Hakim I, Sabri N: Efficacy and safety of nicotinamide in the management of hyperphosphatemia in pediatric patients on regular hemodialysis. Pediatr Nephrol 31: 289–296, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Vasantha J, Soundararajan P, Vanitharani N, Kannan G, Thennarasu P, Neenu G, et al.: Safety and efficacy of nicotinamide in the management of hyperphosphatemia in patients on hemodialysis. Indian J Nephrol 21: 245–249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shahbazian H, Zafar Mohtashami A, Ghorbani A, Abbaspour MR, Belladi Musavi SS, Hayati F, et al.: Oral nicotinamide reduces serum phosphorus, increases HDL, and induces thrombocytopenia in hemodialysis patients: A double-blind randomized clinical trial. Nefrologia 31: 58–65, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Lenglet A, Liabeuf S, El Esper N, Brisset S, Mansour J, Lemaire-Hurtel A-S, et al. : Efficacy and safety of nicotinamide in haemodialysis patients: The NICOREN study. Nephrol Dial Transplant 32: 870–879, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.: CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ix JH, Anderson CAM, Smits G, Persky MS, Block GA: Effect of dietary phosphate intake on the circadian rhythm of serum phosphate concentrations in chronic kidney disease: A crossover study. Am J Clin Nutr 100: 1392–1397, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gassman JJ, Greene T, Wright JT Jr., Agodoa L, Bakris G, Beck GJ, et al.: Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). J Am Soc Nephrol 14[Suppl 2]: S154–S165, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ix JH, Chonchol M, Laughlin GA, Shlipak MG, Whooley MA: Relation of sex and estrogen therapy to serum fibroblast growth factor 23, serum phosphorus, and urine phosphorus: The Heart and Soul Study. Am J Kidney Dis 58: 737–745, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ix JH, Ganjoo P, Tipping D, Tershakovec AM, Bostom AG: Sustained hypophosphatemic effect of once-daily niacin/laropiprant in dyslipidemic CKD stage 3 patients. Am J Kidney Dis 57: 963–965, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Isakova T, Gutiérrez OM, Smith K, Epstein M, Keating LK, Jüppner H, et al. : Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant 26: 584–591, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chue CD, Townend JN, Moody WE, Zehnder D, Wall NA, Harper L, et al.: Cardiovascular effects of sevelamer in stage 3 CKD. J Am Soc Nephrol 24: 842–852, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira RB, Cancela ALE, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, et al.: Early control of PTH and FGF23 in normophosphatemic CKD patients: A new target in CKD-MBD therapy? Clin J Am Soc Nephrol 5: 286–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez-Parra E, Gonzalez-Casaus ML, Galán A, Martinez-Calero A, Navas V, Rodriguez M, et al. : Lanthanum carbonate reduces FGF23 in chronic kidney disease Stage 3 patients. Nephrol Dial Transplant 26: 2567–2571, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Fishbane S, Block GA, Loram L, Neylan J, Pergola PE, Uhlig K, et al.: Effects of ferric citrate in patients with nondialysis-dependent CKD and iron deficiency anemia. J Am Soc Nephrol 28: 1851–1858, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radanovic T, Wagner CA, Murer H, Biber J: Regulation of intestinal phosphate transport. I. Segmental expression and adaptation to low-P(i) diet of the type IIb Na(+)-P(i) cotransporter in mouse small intestine. Am J Physiol Gastrointest Liver Physiol 288: G496–G500, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Katai K, Tanaka H, Tatsumi S, Fukunaga Y, Genjida K, Morita K, et al. : Nicotinamide inhibits sodium-dependent phosphate cotransport activity in rat small intestine. Nephrol Dial Transplant 14: 1195–1201, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Eto N, Miyata Y, Ohno H, Yamashita T: Nicotinamide prevents the development of hyperphosphataemia by suppressing intestinal sodium-dependent phosphate transporter in rats with adenine-induced renal failure. Nephrol Dial Transplant 20: 1378–1384, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Hill KM, Martin BR, Wastney ME, McCabe GP, Moe SM, Weaver CM, et al.: Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int 83: 959–966, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King A, Siegl M, He Y, Nie B, Wang J, Koo-McCoy S, et al. : Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med 10: eaam6474, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, et al.: Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A 108: E1146–E1155, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, et al.: Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int 89: 135–146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.