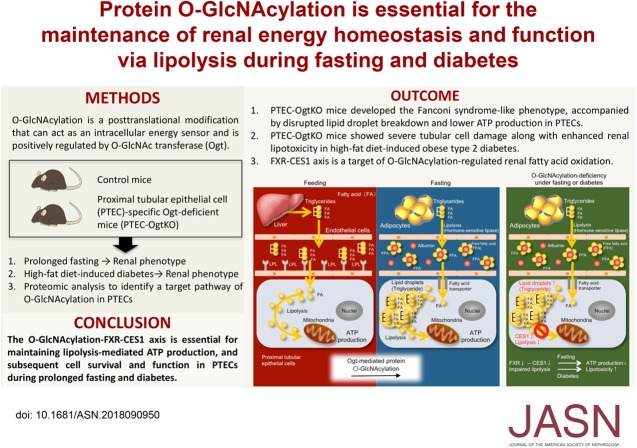
Significance Statement
Lipolysis is of particular importance for energy homeostasis in proximal tubular epithelial cells (PTECs), and it is dysregulated during the pathogenesis of diabetic kidney disease. In knockout mice lacking O-GlcNAc transferase specifically in PTECs, the authors demonstrated that protein O-GlcNAcylation, an intracellular nutrient sensing system, is essential for renal lipolysis and ATP production during prolonged fasting. They also found evidence that this novel regulatory mechanism of renal lipolysis involves farnesoid X receptor–dependent upregulation of carboxylesterase-1 and that deficiency of renal protein O-GlcNAcylation exacerbates tubulopathy in diabetic kidney disease. These findings suggest that manipulation of the renal lipolytic mechanism to overcome the effects of prolonged fasting might represent a novel therapeutic approach for diabetic kidney disease.
Keywords: diabetes mellitus, lipids, obesity, renal proximal tubule cell
Visual Abstract
Abstract
Background
Energy metabolism in proximal tubular epithelial cells (PTECs) is unique, because ATP production largely depends on lipolysis in both the fed and fasting states. Furthermore, disruption of renal lipolysis is involved in the pathogenesis of diabetic tubulopathy. Emerging evidence suggests that protein O-GlcNAcylation, an intracellular nutrient-sensing system, may regulate a number of metabolic pathways according to changes in nutritional status. Although O-GlcNAcylation in PTECs has been demonstrated experimentally, its precise role in lipolysis in PTECs is unclear.
Methods
To investigate the mechanism of renal lipolysis in PTECs—specifically, the role played by protein O-GlcNAcylation—we generated mice with PTECs deficient in O-GlcNAc transferase (Ogt). We analyzed their renal phenotypes during ad libitum feeding, after prolonged fasting, and after mice were fed a high-fat diet for 16 weeks to induce obesity and diabetes.
Results
Although PTEC-specific Ogt-deficient mice lacked a marked renal phenotype during ad libitum feeding, after fasting 48 hours, they developed Fanconi syndrome–like abnormalities, PTEC apoptosis, and lower rates of renal lipolysis and ATP production. Proteomic analysis suggested that farnesoid X receptor–dependent upregulation of carboxylesterase-1 is involved in O-GlcNAcylation’s regulation of lipolysis in fasted PTECs. PTEC-specific Ogt-deficient mice with diabetes induced by a high-fat diet developed severe tubular cell damage and enhanced lipotoxicity.
Conclusions
Protein O-GlcNAcylation is essential for renal lipolysis during prolonged fasting and offers PTECs significant protection against lipotoxicity in diabetes.
Mammalian cells use glucose, fatty acids (FAs), and ketone bodies to produce ATP, which is essential for their survival and function. The identity of the nutrient used for ATP production depends on feeding status and cell type. In most cells, glucose is the principal source of ATP in the fed state, but FAs or ketone bodies become the main source during the fasting state. Energy metabolism in kidney proximal tubular epithelial cells (PTECs) is unique, because ATP production here is thought to largely depend on lipolysis and subsequent β-oxidation, regardless of feeding status.1−4 Furthermore, a disruption of renal lipolysis is involved in the pathogenesis of tubulopathy in kidney diseases, including diabetic kidney disease (DKD).5−7 Therefore, renal lipolysis has become the focus of an emerging research field, and better understanding of renal lipolysis may contribute to the development of novel therapeutic approaches for DKD.
In general, the mechanisms by which cells take up and use FAs differ between the fed and fasting states. In the fed state, cells obtain FAs from circulating triglycerides after lipoprotein lipase (LPL)–dependent lipolysis at the endothelial surface and directly transfer FAs to mitochondria for ATP production (Supplemental Figure 1A).8 In contrast, during the fasting state, cells take up free FAs that are released from adipocytes and circulate bound to plasma albumin. After they are transported into cells, these FAs can be stored in intracellular lipid droplets and are used for ATP production after being once again liberated by intracellular lipolysis (Supplemental Figure 1B).9 Given that PTECs require continuous lipolysis for ATP production, there must be intracellular mechanisms that sense changes in nutritional status and regulate lipolytic processes in the fed and fasting states. However, little is known about the physiologic mechanism underpinning lipolysis-associated energy metabolism in PTECs.
Cells have evolved a nutrient-sensing system that involves O-GlcNAcylation, a post-translational modification. O-GlcNAcylation involves the addition of UDP-O–linked n-acetylglucosamine to proteins by O-GlcNAc transferase (Ogt).10−12 Because UDP-O–linked n-acetylglucosamine is derived from metabolites involved in FA, amino acid, glucose, and nucleotide metabolism, the extent of O-GlcNAcylation is indicative of overall intracellular nutrient status. Accumulating evidence from experimental studies conducted using tissue-specific Ogt-deficient mouse models indicates that O-GlcNAcylation is required for hepatic gluconeogenesis and lipolysis-dependent thermogenesis in brown adipose tissue during fasting.13,14 Thus, in many cells, O-GlcNAcylation may regulate a number of metabolic pathways according to changes in nutritional status. O-GlcNAcylation in PTECs has been demonstrated experimentally15−19; however, the precise role of this modification in the physiology of energy homeostasis, including in lipolysis in PTECs, has not been fully elucidated.
We hypothesized that O-GlcNAcylation is involved in the mechanism of the continuous lipolytic activity in PTECs and that its dysregulation is involved in the pathogenesis of diabetic tubulopathy. To address these hypotheses, we evaluated renal lipolysis and the renal phenotype of PTEC-specific Ogt-deficient mice exposed to a prolonged fast or with high-fat diet (HFD)–induced diabetes.
Methods
Ethics
Animal experimentation was conducted in accordance with the guidelines of the Research Center for Animal Life Science (RCALS) at Shiga University of Medical Science. All experimental protocols were approved by the Gene Recombination Experiment Safety Committee (approval number 28–12) and the RCALS at Shiga University of Medical Science (approval number 2016–8-10).
Generation of Tamoxifen-Inducible PTEC-Specific Ogt Knockout Mice
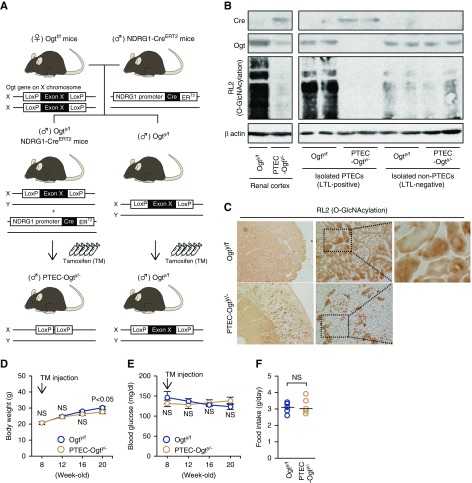
The Ogt gene resides on the X chromosome.20 Ogtf/f mice were obtained from the Jackson Laboratory (Bar Harbor, ME). This strain originated in a B6;129 background and has been backcrossed to C57BL/6 for at least ten generations. Proximal tubular epithelial cell–specific O-GlcNAc transferase knockout (PTEC-Ogty/−) mice were generated by breeding female Ogtf/f mice with male N-myc downstream-regulated gene-1 (NDRG1) promoter–derived tamoxifen (TM)-inducible CreERT2 mice with a C57BL/6 background21 (Figure 1A). Eight-week-old male Ogty/f and Ogty/f mice carrying Ndrg1CreERT2 were administered 150 mg/kg per day TM for 5 consecutive days.21 Twelve weeks after this induction, urine samples were collected from 20-week-old Ogty/f and PTEC-Ogty/− mice using a metabolic cage under both fed and 48-hour fasting conditions. Then, mice were euthanized, and renal cortical samples were collected (n=5–6).
Figure 1.
Proximal tubular epithelial cell–specific O-GlcNAc transferase knockout (PTEC-Ogty/-) mice show little developmental defect. (A) The O-GlcNAc transferase (Ogt) gene resides on the X chromosome. Female Ogtf/f and male Ogty/f mice have loxP sites inserted into exon 10 of the Ogt gene. N-myc downstream-regulated gene-1 (NDRG1) promoter–derived tamoxifen (TM)-inducible CreERT2-expressing mice were used for proximal tubular epithelial cell (PTEC)–specific Cre expression. Male Ogty/f mice carrying CreERT2 and Ogty/f mice were injected with TM for 5 consecutive days to induce Cre expression. The generated male PTEC-Ogt y/− and Ogty/f mice were used for the study. (B) Cre recombinase expression, Ogt protein expression, and protein O-GlcNAcylation, detected using an RL2 antibody, were lower in both renal cortical samples and isolated Lotus tetragolonobus lectin (LTL)–positive PTECs from PTEC-Ogty/− mice. PTECs were isolated using an anti-LTL antibody. (C) Immunostaining for renal protein O-GlcNAcylation in 20-week-old Ogty/f and PTEC-Ogty/− mice. Protein O-GlcNAcylation was lower mainly in the renal cortex of PTEC-Ogty/− mice. Original magnification, ×40 in left panels; ×200 in center panels; ×400 in right panel. (D) Change in mean body mass. PTEC-Ogty/− mice showed lower body mass gain at 20 weeks of age than control Ogty/f mice (n=5 per group). (E) Change in mean casual blood glucose concentration. There was no difference in casual blood glucose concentration between two genotypes. (F) Food intake at 20 weeks old was similar between the two genotypes. Horizontal bars indicate the median values for each group. P<0.05 was considered statistical significance.
Mouse Models of Diabetes and Atherosclerosis
Eighteen-week-old male db/db mice purchased from CLEA Japan Co. (Osaka, Japan) were used as a model of type 2 diabetes, which is characterized by overt proteinuria in the absence of severe tubulopathy. ApoE−/− mice were generated by crossbreeding male and female ApoE+/− mice.22 Their ApoE+/+ littermates were used as controls. Eight-week-old ApoE+/+ mice were fed either a normal diet or an HFD for 24 weeks to induce obesity-related microalbuminuria without severe tubulopathy. HFD-fed ApoE−/− mice were used as a model of diabetes- and atherosclerosis-associated severe tubulopathy. The normal diet (10% of total calories from fat) and HFD (60% of total calories from fat) were purchased from Research Diets (New Brunswick, NJ).
Mitochondrial Division Inhibitor Treatment Study
Eight-week-old male PTEC-Ogty/− mice were divided into two groups, vehicle and mitochondrial division inhibitor (Mdivi-1; Sigma-Aldrich, St. Louis, MO) treatment groups (n=7 each), after 4 weeks of Cre induction. Mdivi-1 (50 mg/kg per day) or vehicle was intraperitoneally administered for 3 days in 12-week-old PTEC-Ogty/− mice commencing 24 hours before the fast and continuing for the duration of the fasting period. After 48 hours of fasting, urine and kidney samples were collected.
HFD-Induced Diabetes in PTEC-Ogty/− Mice
Eight-week-old male Ogty/f mice and Ogty/f mice carrying Ndrg1CreERT2 (n=5 per group) were administered TM and started consuming the HFD. An intraperitoneal insulin tolerance test was conducted as previously described5 before euthanasia. Kidney samples were collected after 16 weeks of the dietary intervention.
Blood and Urine Analyses
Blood glucose concentrations were measured using a Glutest sensor (Sanwa Kagaku, Nagoya, Japan). Urinary albumin, amino acids, and ions were measured using standard laboratory methods.
Histologic Analyses
Three-micrometer-thick sections of frozen and paraffin-embedded fixed samples were prepared. Oil-red O staining, periodic acid–Schiff staining, hematoxylin and eosin staining, and immunohistochemistry were performed as described previously.5 The antibodies used for immunohistochemistry are listed in Supplemental Table 1. Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling staining was performed using a TACS 2 TdT DAB kit (Trevigen, Gaithersburg, MD). Quantitative analysis was performed by three independent nephrologists in a blinded fashion.23 A tubulointerstitial damage score was assigned as described previously.23
Evaluation of Mitochondrial Morphology Using Transmission Electron Microscopy
Mitochondrial structure was assessed by transmission electron microscopy examination of at least 100 mitochondria per sample at a magnification of 8000 as previously reported with some minor modifications.24 Mitochondrial size and number of mitochondria per PTEC were measured, and mitochondrial density was calculated as the number of mitochondria per square micrometer PTEC area. For aspect ratio measurements, the ratio between the major and minor axes of the ellipse equivalent to each mitochondrion was determined.
Western Blotting
Western blot analysis of proteins from the renal cortex or isolated PTECs was performed as previously described.25 The antibodies are listed in Supplemental Table 1. Scanned images of complete membranes are shown in Supplemental Figure 2.
Quantitative Real-Time PCR
Real-time PCR was performed as described previously.23 The PCR primer sets are listed in Supplemental Table 2.
Proteomic Analyses
The proteomic analysis was conducted by Medical Proteoscope (Kanagawa, Japan). Whole data are shown in Supplemental Appendix and were deposited into jPOST (identification number JPST000549) and Proteome XChange (PXID identification number PXD012456).
Snap-frozen kidney cortices from 20-week-old Ogty/f and PTEC-Ogty/− mice after 24 hours of fasting were homogenized using a pestle and sonicated in ice-cold lysis buffer (20 mM HEPES-NaOH, pH 8.0, 9 M urea, 25 mM sodium pyrophosphate, 10 mM β-glycerophosphate, and 1% vol/vol each of Phosphatase Inhibitor Cocktail solutions 2 and 3 [Sigma-Aldrich]). The lysates generated were clarified by centrifugation at 15,000×g for 10 minutes, and their protein concentrations were determined using a Bradford assay. Aliquots were subjected to a cycle of dithiothreitol reduction. After reductive alkylation, the protein solutions were diluted to achieve a urea concentration of ≤2 M with 20 mM HEPES-NaOH, and then, they were subjected to protein hydrolysis with bovine trypsin (tosyl phenylalanyl chloromethyl ketone treated; Sigma-Aldrich) at 37°C for 16 hours, with an enzyme-to-substrate ratio of 1:20 (wt/wt). Peptide samples were desalted using C18 STAGE tips and dried at low pressure. The dried samples were dissolved in a solvent consisting of water, acetonitrile, and formic acid at a volume ratio of 98:2:0.1 and diluted to 250 ng/μl. Of this, 2 μl (containing 500 ng protein) were used for liquid chromatography (LC)-mass spectrometry (MS)/MS using the following specifications and settings. Briefly, peptide separation was performed with an Ultimate 3000 RSLCnano (Thermo Fisher Scientific) containing a C18 capillary LC column (Nano HPLC Capillary Column; 75-μm internal diameter, 150-mm length, 3-μm particle size; Nikkyo Technos, Tokyo, Japan). The mobile phases consisted of formic acid, acetonitrile, and water at volume ratios of 0.1:0:100 for mobile phase A and 0.1:90:10 for mobile phase B. The peptides were continuously eluted at a rate of 350 nl/min in gradient mode: the initial proportion of 5% mobile phase B was increased to 40% B over 120 minutes, and it was subsequently increased to 95% B over the next 10 minutes. Protonated peptides in the gas phase were analyzed sequentially by MS/MS in positive ion mode consisting of a full-range scan in the mass-to-charge range of 300–1500 and subsequent product ion scans for each of the ten most intense ions in the full-scan mass spectrum.
Label-Free Quantification and Peptide Identification
Label-free relative peptide quantitation was performed by direct comparison of each MS scan profile using the Progenesis QI for proteomics software (version 2.0; Nonlinear Dynamics, Durham, NC). The normalized abundances obtained for each peptide were subjected to statistical analysis using one-way ANOVA. In this study, the detected feature was taken to be statistically significant when the P value for a given peptide was <0.05. To identify the peptide sequence, peak lists were created using Progenesis LC-MS. The MS and MS/MS data obtained were searched against mouse protein sequences (16,930 entries) in the Swiss-Prot database (January 2013) and the amino acid sequences of protein contaminants (116 entries) in The Proteome Machine Organization using MASCOT software, version 2.5 (Matrix Science, London, United Kingdom). The search parameters were enzyme, semitrypsin; maximum missed cleavage, two; peptide tolerance, ±5 ppm; MS/MS tolerance, ± 0.02 D; mass, monoisotopic mass; fixed modification, carbamidomethyl (C, +57.021 D); and variable modifications, oxidation (M, +15.099 D). The false discovery rate was estimated on a decoy database using the MASCOT software. We used a 1% false discovery rate as a cutoff for the export of results from the analysis.
Bioinformatic Analyses of Protein Expression Data
Pathway analysis of the list of data from the proteomic analysis was performed using KeyMolnet (KM Data, Tokyo, Japan). KeyMolnet is a bioinformatics integration platform that enables the analysis of specific pathways on the basis of data collected from recent papers. By importing the list of Entrez gene identifications, KeyMolnet automatically provides the corresponding molecules in the form of nodes in a network. In the various network-searching algorithms, the “interaction” search identifies molecular networks containing a group of molecules that showed differential regulation in this study. The significance was scored using following formula, in which O = the number of overlapping molecular relationships between the extracted network and the canonical pathway, V = the number of molecules displayed in the search result, C = the number of molecules belonging to specific pathways, T = the total number of molecules recorded in KeyMolnet, and X = the σ-variable that defines incidental agreements:
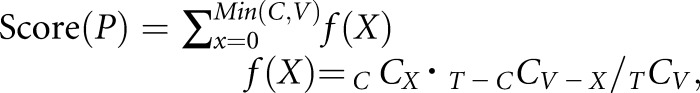 |
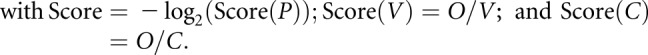 |
Isolation of Primary PTECs and Farnesoid X Receptor Agonist Treatment
Primary PTECs were isolated as described previously.26 Briefly, kidney cortices were digested in HBSS containing collagenase type II (200 U/ml; Life Technologies, Grand Island, NY) and hyaluronidase (0.2%; Wako Pure Chemical Industries, Osaka, Japan). Selection of PTECs was performed using a CELLection Biotin Binder Kit (Invitrogen, Carlsbad, CA) and biotinylated Lotus tetragonolobus agglutinin lectin (Vector Laboratories, Burlingame, CA). Isolated PTECs were resuspended in culture medium (BulletKit; Clonetics) and seeded into culture dishes.
Isolated PTECs from 20-week-old Ogty/f and PTEC-Ogty/− mice were treated with an farnesoid X receptor (FXR) agonist (GW4064; ChemScene, Monmouth Junction, NJ) or DMSO as a treatment control for 24 hours after 12 hours of starvation. Collected samples were used for detecting carboxylesterase 1 (CES1) mRNA expression.
CES1 Overexpression in PTECs
A pcDNA3 plasmid for the expression of CES1 was obtained from OriGene (Rockville, MD), and a pcDNA3-null plasmid was used as a control. Transfection in isolated PTECs of 20-week-old Ogty/f and PTEC-Ogty/− mice was performed using Lipofectamine 3000 Reagent (Invitrogen).
Fluorescence Microscopy of Isolated PTECs and Intracellular Lipid Droplets
Lipid droplets in fixed cells were visualized using boron-dipyrromethene (BODIPY) 493/503 (Invitrogen).27,28 Isolated PTECs were incubated in culture medium containing 150 μM oleic acid for 2 hours, then washed twice with PBS(−), and incubated in Krebs–Ringer buffer without glucose and oleate for 3 hours. To label lipid droplets, 200 ng/ml BODIPY 493/503 was added to the cells before imaging. Nuclei were visualized using 4′,6-diamidino-2-phenylindole.
Fluorescence analysis of CES1 protein distribution was performed as previously described29 using Alexa Fluor 594 anti-rabbit IgG as the secondary antibody. After immunostaining, cells were counterstained using BODIPY 493/503 and 4′,6-diamidino-2-phenylindole. Immunofluorescence was detected using a fluorescence microscope (BX61; Olympus, Tokyo, Japan).
Measurement of ATP Content and LPL Activity
ATP concentration in the kidney cortex and cultured cells was measured using a firefly bioluminescence kit (AMERIC-ATP Kit; Wako Pure Chemical Industries) and an intracellular ATP assay kit (Toyo Ink Group, Tokyo, Japan). Renal LPL activity was measured using an LPL activity assay kit (Cell Biolabs, Inc., San Diego, CA).
Measurement of Renal Triglyceride and Cholesterol Content
Total lipid was extracted from renal cortices using the method of Bligh and Dyer.30 Triglyceride and cholesterol contents were analyzed using the TG Assay Kit (Wako Pure Chemical Industries). Total cholesterol and free cholesterol were measured using the total cholesterol assay kit (Cell Biolabs, Inc.). Cholesteryl ester was calculated by subtracting free cholesterol from total cholesterol.
Immunoprecipitation Study
Immunoprecipitation in renal cortical samples from 20-week-old wild-type C57/BLK6J mice was conducted as previously described.31 The antibodies used in this assay are listed in Supplemental Table 1.
Statistical Analyses
The Wilcoxon rank sum test and the Kruskal–Wallis test followed by the Dunn–Bonferroni post hoc test were used for statistical comparisons of two groups and multiple groups, respectively. P<0.05 was considered to represent statistical significance.
Results
Generation of PTEC-Ogty/− Mice
The Ogt gene resides on the X chromosome.20 PTEC-Ogty/− mice were generated by crossbreeding Ogtf/f mice with NDRG1 promoter–derived TM-inducible CreERT2 mice.21 Eight-week-old male Ogty/f mice and Ogty/f mice carrying Ndrg1CreERT2 were treated with TM (Figure 1A). Effective Cre recombinase expression, Ogt deletion, and deficient protein O-GlcNAcylation in the renal cortical samples and the isolated PTECs of PTEC-Ogty/− mice were confirmed by Western blot analysis (Figure 1B) and immunostaining (Figure 1C). Low levels of Cre recombination were found in the non-PTECs isolated from the renal cortex of PTEC-Ogty/− mice (Figure 1B), suggesting that changes mediated by deficient O-GlcNAcylation in renal cortical samples should mainly reflect the modification of PTECs.
Twelve weeks after the induction with TM, body mass gain was slightly but significantly lower in PTEC-Ogty/− mice, although their casual blood glucose concentration and daily food intake were similar to those of control Ogty/f mice throughout the experimental period (Figure 1, D–F).
Functional and Histologic Abnormalities in PTEC-Ogty/− Mice
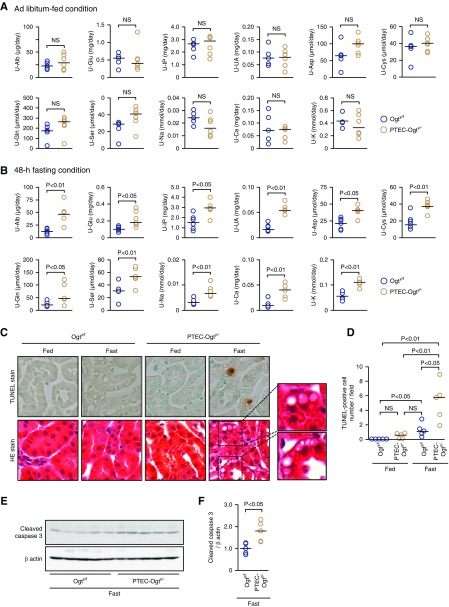
One of the principal functions of PTECs is the reabsorption of macro- and micromolecules from the urinary space. There were no significant differences in the urinary excretion of albumin, glucose, phosphate, uric acid, amino acids, and ions between the two groups during the fed state (Figure 2A). In contrast, PTEC-Ogty/− mice excreted higher concentrations of these substances in urine after 48 hours of fasting (Figure 2B). In addition to this Fanconi syndrome–like phenotype, significantly larger numbers of terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling–positive apoptotic cells and more cleavage of caspase 3 were identified in the fasted PTEC-Ogty/− mice (Figure 2, C–F) accompanied by larger numbers of vesicle-like structures in hematoxylin and eosin–stained sections (Figure 2C).
Figure 2.
Fasted proximal tubular epithelial cell–specific O-GlcNAc transferase knockout (PTEC-Ogty/-) mice develop Fanconi syndrome-like phenotype and cell apoptosis. (A) There were no significant differences in the 24-hour urinary excretion (U) of albumin (Alb); glucose (Glu); phospate (IP); uric acid (UA); some amino acids, including asparagine (Asp), cystine (Cys), glutamine (Gln), and serine (Ser); sodium (Na), calcium (Ca), and potassium (K) between 20-week-old control Ogty/f mice (n=5) and PTEC-Ogty/− mice (n=6) under ad libitum–fed condition. (B) Urinary excretions of the indicated substances in PTEC-Ogty/− mice were significantly higher than those in Ogty/f mice (n=6 per group) after a 48-hour fast. (C and D) Apoptotic cell number determined by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining was significantly higher in the fasted PTEC-Ogty/− mice (n=5 per group). Vacuolar changes in hematoxylin and eosin (HE)–stained proximal tubular epithelial cells were obvious in fasted PTEC-Ogty/− mice. Original magnification, ×400. (E and F) Cleavage of caspase 3 was higher in the fasted PTEC-Ogty/− mice (n=5 per group). Horizontal bars in the graphs of A, B, D, and F indicate the median values. P<0.05 was considered statistical significance.
Impaired Renal Lipolysis in Fasted PTEC-Ogty/− Mice
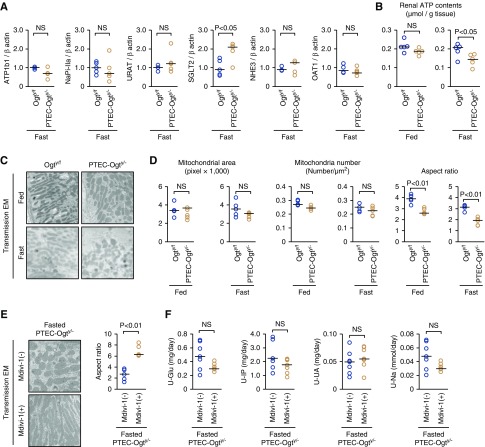
The expression of several transporters in PTECs did not differ between fasting Ogty/f and PTEC-Ogty/− mice (Figure 3A). In contrast, the ATP concentration in renal cortical samples was significantly lower in the fasted PTEC-Ogty/− mice than controls (Figure 3B), suggesting that the Fanconi syndrome–like abnormalities induced by the Ogt deficiency were mediated by ATP depletion rather than the generalized downregulation of transporters.
Figure 3.
Transporters expression and mitochondrial fission are not involved in the renal phenotype of fasted proximal tubular epithelial cell–specific O-GlcNAc transferase knockout (PTEC-Ogty/-) mice. (A) There were no significant differences in renal mRNA expression levels of the indicated transporters other than SGLT2 between 20-week-old control Ogty/f mice and PTEC-Ogty/− mice after 48 hours of fasting (n=5 each). SGLT2 expression in the fasted PTEC-Ogty/− mice was higher than in the fasted Ogty/f mice. (B) There was no significant difference in renal ATP levels between the two genotypes under the fed condition, whereas the level was significantly lower in PTEC-Ogty/− mice after 48 hours of fasting (n=5 each). (C and D) Aspect ratio of mitochondria in proximal tubular epithelial cells of PTEC-Ogty/− mice was smaller than that in Ogty/f mice, although there were no differences in mitochondria area and number. Original magnification, ×8000. (E and F) Mitochondrial division inhibitor (Mdivi-1) treatment enhanced mitochondrial fusion, but it did not improve excess urinary excretion of the indicated molecules in the fasted PTEC-Ogty/− mice at 12 weeks old (n=7 each). Original magnification, ×8000. Horizontal bars in the graphs of A, B, and D–F indicate the median values. P<0.05 was considered statistical significance. EM, electron microscopy.
Mitochondria are of great importance for ATP production. Although the mitochondrial area and numbers in PTECs were similar in Ogty/f and PTEC-Ogty/− mice, regardless of feeding status, there was a lower aspect ratio in mitochondrial morphology in the fasted PTEC-Ogty/− mice (Figure 3, C and D), suggesting an increase in mitochondrial fission. To determine whether there was a causal relationship between the renal phenotype and this mitochondrial fission, PTEC-Ogty/− mice were treated with a Drp-1 inhibitor (Mdivi-1) that blocks mitochondrial fission32 and then, fasted for 48 hours. The administration of Mdivi-1 promoted the elongation of the mitochondria (Figure 3E), but it did not ameliorate the Fanconi syndrome–like abnormalities in the fasted PTEC-Ogty/− mice (Figure 3F), suggesting that an increase in mitochondrial fission was not the primary event leading to impaired renal function in the mice.
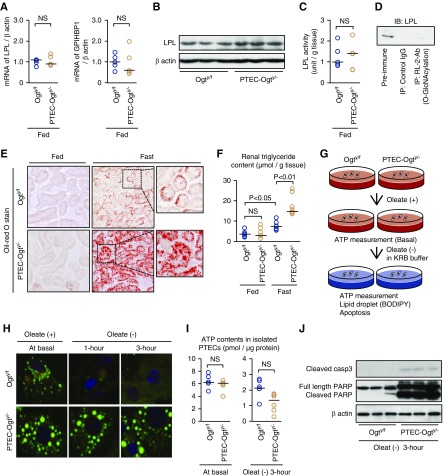
Next, FA metabolism was evaluated. LPL is essential for FA uptake into cells during the fed state.8 The expression and lipase activity of LPL were similar in Ogty/f and PTEC-Ogty/− mice during the fed state (Figure 4, A–C). Furthermore, LPL was not O-GlcNAcylated (Figure 4D), and the expression of glycosylphosphatidylinositol-anchored HDL-binding protein 1, an adaptor protein for LPL, was also similar in the two genotypes (Figure 4A). Thus, the LPL system was not affected by the Ogt deficiency. These findings are consistent with the finding of no marked renal phenotype in the fed PTEC-Ogty/− mice.
Figure 4.
Lipid droplet breakdown is impaired in proximal tubular epithelial cell–specific O-GlcNAc transferase knockout (PTEC-Ogty/-) mice. (A) There were no significant differences in the renal mRNA expression of lipoprotein lipase (LPL) and glycosylphosphatidylinositol-anchored HDL-binding protein 1 (GPIHBP1) between 20-week-old control Ogty/f mice and PTEC-Ogty/− mice under ad libitum–fed condition (n=5 per group). (B and C) There were no significant differences in renal protein expression and activity of LPL between the two genotypes under the fed condition. (D) Immunoprecipitation (IP) study using an RL2 antibody that recognizes protein O-GlcNAcylation followed by immunoblotting (IB) with an LPL antibody. LPL was not O-GlcNAcylated. (E and F) Renal neutral lipid accumulation identified using (E) Oil-red O staining and (F) triglyceride content measurement. The fasting-induced increase in renal triglyceride was significantly greater in PTEC-Ogty/− mice. Original magnification, ×200 in E. (G) Protocol for the cell culture study using isolated proximal tubular epithelial cells (PTECs). (H) Oleate treatment led to the formation of boron-dipyrromethene (BODIPY)–stained lipid droplets in the isolated PTECs of 20-week-old Ogty/f mice and PTEC-Ogty/− mice. Lipid droplet degradation after oleate removal did not occur in the PTECs of PTEC-Ogty/− mice. Original magnification, ×400. (I) After oleate removal, the ATP concentration in the PTECs of PTEC-Ogty/− mice was lower than in cells from control Ogty/f mice (n=6 per group), although the basal ATP concentration before oleate removal did not differ between the two genotypes (n=5 per group). (J) After oleate removal, cleavage of caspase 3 was higher in cultured PTECs from PTEC-Ogty/− mice. Horizontal bars in the graphs of A, C, F, and I indicate the median values. P<0.05 was considered a statistical significance. KRB, Krebs–Ringer buffer; PARP, poly(ADP-ribose) polymerase.
In contrast, 48 hours of fasting was associated with greater intracellular lipid droplet formation and higher renal triglyceride content in the PTECs of Ogty/f mice, and the difference were greater in the PTEC-Ogty/− mice (Figure 4, E and F). This finding provoked the hypothesis that Ogt deficiency impairs lipid droplet breakdown and subsequent utilization of FAs for ATP production during fasting. To investigate this possibility, a primary cell culture study was conducted (Figure 4G). When isolated PTECs were treated with oleate, an unsaturated FA, in complete medium, lipid droplets were formed in the cells, but ATP concentration did not differ between two genotypes (Figure 4, H and I). After 3 hours of culture in Krebs–Ringer buffer without oleate and glucose, the lipid droplets disappeared from the Ogty/f cells, and apoptosis did not occur (Figure 4, H–J). In contrast, the droplets remained, and there was more apoptosis in cells from PTEC-Ogty/− mice accompanied by a lower ATP concentration (Figure 4, H–J). These observations are consistent with the above hypothesis.
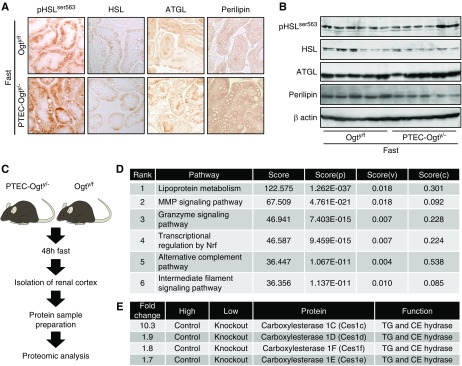
Altered Expression of Lipolytic Enzymes in Fasted PTEC-Ogty/− Mice
After 48 hours of fasting, there were no differences in the renal phosphorylation of hormone-sensitive lipase (HSL) or the renal expression of HSL, adipose triglyceride lipase, or perilipin, key regulators of intracellular lipid droplet breakdown,33−35 between the two genotypes (Figure 5, A and B). Therefore, to identify the proteins and pathways mediating the impairment in lipolysis in the fasted PTEC-Ogty/− mice, a proteomic analysis of renal cortical samples from fasted Ogty/f and PTEC-Ogty/− mice was conducted (Figure 5C). Proteomic analysis and subsequent pathway analysis revealed that PTEC-Ogty/− mice had significant differences in renal lipid metabolism from controls (Figure 5D). There were several lipolytic enzymes listed in the output of the proteomic analysis (Supplemental Table 3), including much lower protein expression of CES1 family proteins, which can act as triglyceride hydrolases as well as cholesterol esterases36−38 (Figures 5E and 6A).
Figure 5.
Carboxylesterase 1 (CES1) expression is decreased in the kidney of fasted proximal tubular epithelial cell–specific O-GlcNAc transferase knockout (PTEC-Ogty/-) mice. (A) Immunostaining and (B) Western blot analysis of phosphorylated hormone-sensitive lipase (pHSL), hormone-sensitive lipase (HSL), adipose triglyceride lipase (ATGL), and perilipin. During fasting, there were no significant differences in renal phosphorylation or the expression levels of the indicated proteins between 20-week-old control Ogty/f mice and PTEC-Ogty/− mice (n=6 per group). Original magnification, ×400. (C) Brief protocol for the proteomic analysis of renal cortical samples from 20-week-old control Ogty/f and PTEC-Ogty/− mice after 48 hours of fasting. (D) Pathway analysis of data from the proteomic analysis. The detailed meaning of each calculated Score, Score(P), Score(V), are Score(C) is given in Supplemental Appendix. (E) In the proteomic analysis, the expression levels of carboxylesterase 1 (CES1) family proteins were lower in fasted PTEC-Ogty/− mice. CE cholesterol ester; TG, triglyceride.
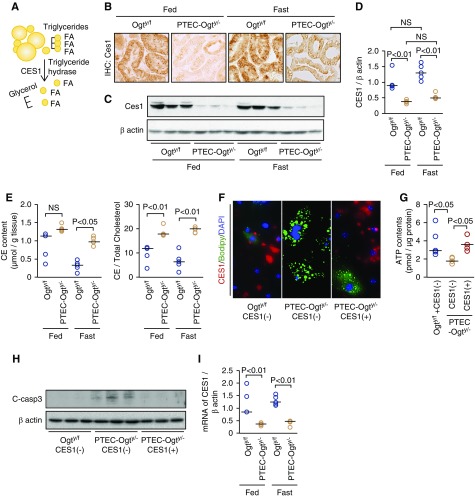
Figure 6.
Decreased Carboxylesterase 1 (CES1) expression is responsible for the impaired lipid droplet breakdown in proximal tubular epithelial cell–specific O-GlcNAc transferase knockout (PTEC-Ogty/-) mice. (A) CES1 is a triglyceride hydrolase that hydrolyzes triglyceride to liberate glycerol and fatty acids (FAs). (B–D) Renal CES1 expression in 20-week-old control Ogty/f mice and PTEC-Ogty/− mice, demonstrated using (B) immunostaining and (C and D) Western blotting, in the kidneys of fasted PTEC-Ogty/− mice was lower than in fasted Ogty/f mice, regardless of feeding status (n=5 each). Original magnification, ×400. (E) Renal cholesterol ester (CE) concentration and the ratio of cholesterol ester to total cholesterol concentrations were higher in PTEC-Ogty/− mice (n=5 per group). (F) Overexpression of CES1 restored triglyceride degradation in the isolated proximal tubular epithelial cells (PTECs) of 20-week-old PTEC-Ogty/− mice. Red, green, and blue colors indicate CES1 protein expression, boron-dipyrromethene (BODIPY)–stained lipid droplets, and 4′,6-diamidino-2-phenylindole (DAPI)–stained nuclei, respectively. Original magnification, ×400. (G and H) Overexpression of CES1 (G) restored ATP production (n=5 per group) and (H) inhibited apoptosis, which is indicated by cleavage of caspase 3 in isolated PTECs from the 20-week-old PTEC-Ogty/− mice. (I) Renal CES1 mRNA expression in the PTEC-Ogty/− mice was lower, regardless of feeding status (n=5 per group). Horizontal bars in the graphs of D, E, G, and I indicate the median values. P<0.05 was considered statistical significance.
The finding of a large difference in CES1 protein level was corroborated by immunohistochemistry and Western blot analysis (Figure 6, B–D). Furthermore, the concentration of cholesterol ester and the ratio of cholesterol ester to total cholesterol concentrations in renal cortical samples from PTEC-Ogty/− mice were significantly higher than in control mice (Figure 6E), suggesting that renal CES1 activity is lower in PTEC-Ogty/− mice. In the isolated PTECs, the impairment in lipid droplet breakdown was accompanied by lower CES1 expression in the PTEC-Ogty/− mice (Figure 6F, center panel). However, Ogt-deficient PTECs that overexpressed CES1 after transfection with a CES1-overexpression vector, which appear red in the right panel of Figure 6F, demonstrated a restored capacity for lipid droplet breakdown. Furthermore, CES1 overexpression significantly ameliorated the low ATP production and inhibited apoptosis in nutrient-deprived primary Ogt-deficient PTECs after an oleic acid load (Figure 6, G and H).
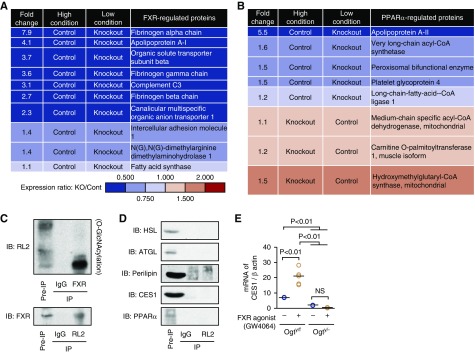
In addition to the difference in protein expression level, renal mRNA expression of CES1 was also lower in the kidneys of PTEC-Ogty/− mice (Figure 6I), suggesting that the activity of a transcription factor regulating CES1 expression might have been inhibited by Ogt deficiency. Previous studies have demonstrated that peroxisome proliferator-activated receptor-α (PPARα) and FXR regulate CES1 expression at the transcriptional level.39,40 The proteomic data showed that the expression of several proteins regulated by FXR was lower in the fasted PTEC-Ogty/− mice (Figure 7A), but the differences in the expression of proteins regulated by PPARα varied (Figure 7B). A series of immunoprecipitation studies revealed that FXR was O-GlcNAcylated but that HSL, adipose triglyceride lipase, perilipin, CES1, and PPARα were not (Figure 7, C and D). Furthermore, FXR agonist (GW4064) treatment significantly increased CES1 mRNA expression in isolated PTECs from Ogty/f mice, but it failed to increase expression in cells from PTEC-Ogty/− mice (Figure 7E). These data suggest that the low CES1 expression is dependent on an interaction between FXR and O-GlcNAcylation.
Figure 7.
O-GlcNAcylation to farnesoid X receptor (FXR) is essential for Carboxylesterase 1 (CES1) expression. (A and B) Fold differences in the renal expression of proteins regulated by (A) FXR and (B) peroxisome proliferator-activated receptor-α (PPARα) in the proteomic data. The expression of FXR-regulated proteins was lower in the kidneys of PTEC-Ogty/− mice, and the expression of PPARα-regulated proteins was inconsistent. (C) Immunoprecipitation studies using RL-2 and FXR antibodies in renal cortex samples from 20-week-old wild-type mice. FXR was O-GlcNAcylated. (D) Immunoprecipitation study using an RL-2 antibody to detect O-GlcNAcylated proteins. Hormone-sensitive lipase (HSL), adipose triglyceride lipase (ATGL), perilipin, carboxylesterase 1 (CES1), and PPARα were not O-GlcNAcylated. (E) FXR agonist (GW4064) treatment significantly increased mRNA expression of CES1 in isolated proximal tubular epithelial cells from 20-week-old Ogty/f mice, but it failed to increase it in cells from PTEC-Ogty/− mice (n=5 per group). Horizontal bars in the graphs of E indicate the median values. P<0.05 was considered statistical significance. IB, immunoblot; IP, immunoprecipitation; KO, knockout.
Low-Protein O-GlcNAcylation and CES1 Expression in an Animal Model of Atherosclerogenic Diabetes
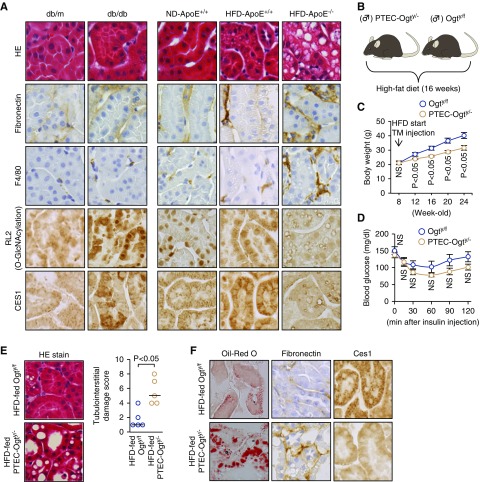
In addition to fasting, insulin resistance in adipose tissue under obese and/or diabetic conditions is associated with high plasma free FAs and a greater influx of free FAs into the kidneys, leading to renal lipotoxicity.5,23,41 Therefore, we next evaluated the role of O-GlcNAcylation in lipotoxic kidney injury. We first measured protein O-GlcNAcylation in the kidneys of diabetic db/db mice, HFD-fed obese ApoE+/+ mice, and HFD-fed ApoE−/− mice. Consistent with the results of previous studies,23,42,43 the interstitial lesions in diabetic db/db mice and HFD-induced obese mice were not severe, but those in HFD-fed ApoE−/− mice were more evident (Figure 8A). Protein O-GlcNAcylation was more extensive in the kidneys of the first two models, but it was much lower in the kidneys of HFD-fed ApoE−/− mice (Figure 8A).
Figure 8.
Deficient O-GlcNAcylation exacerbates lipotoxicity-related kidney injury in high-fat diet (HFD)-induced obese type 2 diabetic mice. (A) Renal tubulointerstitial lesions, identified by hematoxylin and eosin (HE) staining and immunostaining for fibronectin and F4/80, were not severe in 18-week-old db/db mice and 32-week-old HFD-fed ApoE+/+ mice, but they were more severe in 32-week-old HFD-fed ApoE−/− mice. Renal protein O-GlcNAcylation and carboxylesterase 1 (CES1) expressions were high in db/db mice and HFD-fed ApoE+/+ mice, but they were lower in HFD-fed ApoE−/− mice. Original magnification, ×400. (B) HFD intervention study in Ogty/f mice and PTEC-Ogty/− mice (n=5 per group). (C) Change in mean body mass. PTEC-Ogty/− mice showed lower body mass gain during the HFD-feeding period. (D) Change in mean blood glucose concentration during an intraperitoneal insulin tolerance test. There was no significant difference in insulin sensitivity between 24-week-old Ogty/f and PTEC-Ogty/− mice. (E) Renal tubular cell damage in HE-stained sections was significantly worse in PTEC-Ogty/− mice. Original magnification, ×400. (F) Oil-red O staining of positive neutral lipid and fibronectin deposition was higher in the kidneys of PTEC-Ogty/− mice, and it was accompanied by lower CES1 expression. Original magnification, ×400. Horizontal bars in E indicate the median values for each group. P<0.05 was considered statistical significance. ND, normal diet; TM, tamoxifen.
Exacerbation of HFD-Induced Kidney Pathology in PTEC-Ogty/− Mice
The above findings suggest that deficient protein O-GlcNAcylation in PTECs is involved in the exacerbation of tubulointerstitial lesions in HFD-fed ApoE−/− mice. To evaluate this possibility, PTEC-Ogty/− mice were fed an HFD for 16 weeks to induce obesity and diabetes (Figure 8B). Although the body mass of HFD-fed PTEC-Ogty/− mice was significantly lower and glucose tolerance was slightly better than in HFD-fed Ogty/f mice (Figure 8, C and D), tubular dilation, larger numbers of vesicle-like structures, and fibronectin accumulation were observed in kidney sections of PTEC-Ogty/− mice (Figure 8, E and F) accompanied by lower CES1 expression and a massive accumulation of lipid droplets (Figure 8F).
Discussion
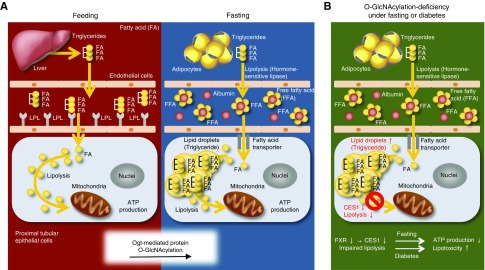
Because prolonged fasting is a life-threatening event, the process of evolution has caused organisms to develop a variety of cellular mechanisms for the maintenance of whole-body ATP production, regardless of feeding status. In mammals, the substrate used to generate ATP depends on the cell type. PTECs metabolize FAs to provide ATP during both feeding and fasting. Here, we show that protein O-GlcNAcylation is involved in the maintenance of lipolysis during prolonged fasting (Figure 9A). Therefore, this modification likely represents one of the abilities that PTECs have acquired to cope with prolonged fasting during the course of evolution.
Figure 9.
Proposed hypothesis. (A) Ogt-mediated protein O-GlcNAcylation plays a critical role in maintaining renal lipolysis–dependent ATP production in proximal tubular cells during the switch from the fed to the fasting state. (B) Deficient O-GlcNAcylation impairs intracellular lipid droplet breakdown, which leads to lower ATP production during fasting and an exacerbation of intrarenal lipotoxicity in diabetes. The farnesoid X receptor (FXR)-carboxylesterase 1 (CES1) pathway may be involved in the maintenance of renal lipid metabolism, and it is regulated by O-GlcNAcylation. FA, fatty acid; FFA, free fatty acid.
Although one previous report has suggested that O-GlcNAcylation in PTECs is important for the maintenance of sodium-glucose transport protein function during hypoxia,18 the precise role of O-GlcNAcylation in PTECs has not been fully characterized to date, in part because appropriate genetically modified mouse models have not previously been available. This study, which used genetic deletion of protein O-GlcNAcylation in PTECs, has demonstrated that this modification is essential for normal renal energy homeostasis and the function and survival of PTECs. In the light of our recent report showing that O-GlcNAcylation is also critical for podocyte function,44 this modification may be important for many types of kidney cells in addition to PTECs and podocytes, because it can be identified in most parts of the nephron.
The high levels of renal O-GlcNAcylation present in disease have been thought to be pathogenic. For instance, diabetes-associated hyper O-GlcNAcylation is associated with less phosphorylation of endothelial nitric oxide synthase and Akt16 as well as changes in the microvilli of PTECs mediated by higher expression of α-actinin 4.17 In addition, hypertension is associated with greater renal O-GlcNAcylation, which leads to the downregulation of megalin in PTECs and subsequent proteinuria.15 In contrast, we have demonstrated that deficient O-GlcNAcylation also causes Fanconi syndrome–like abnormalities in fasting and exacerbates tubulopathy in mice with diabetic atherosclerosis (Figure 9B). These apparently conflicting results may imply that both deficiency and excess of O-GlcNAcylation are harmful to PTECs, and the maintenance of O-GlcNAcylation within a narrow effective range is important for the normal function of PTECs.
The circumstances under which protein O-GlcNAcylation is suppressed or enhanced remain to be established. In general, hyperglycemia has been thought to increase cellular O-GlcNAcylation levels in various tissues.45,46 Actually, in this study, protein O-GlcNAcylation in PTECs was greater in diabetic db/db mice and HFD-induced obese mice, but no tubulointerstitial lesions were evident in these models. Surprisingly, the additional presence of atherosclerotic lesions in HFD-induced obese condition was associated with much lower renal protein O-GlcNAcylation and severe tubulopathy. These findings suggest that hypernutrition in diabetes is associated with greater renal protein O-GlcNAcylation as previously reported,46 but proatherosclerotic stimuli, such as hypoxia, may have the opposite effect. Recently, atherosclerosis and hypoxia have been shown to be key pathogenic factors in the progressive decline in renal function that occurs in patients with diabetes who do not show albuminuria.47 Thus, a deficiency in protein O-GlcNAcylation may be involved particularly in DKD without albuminuria rather than in typical progressive DKD showing albuminuria.
Our proteomic analysis has shown that renal CES1 expression is downregulated by deficient O-GlcNAcylation. CES1 is generally thought to be a cholesterol esterase.37 In fact, renal cholesterol ester accumulated in PTEC-Ogty/− mice during both the fed and fasting states, suggesting that Ogt deficiency suppressed CES1 activity. Furthermore, a recent study has demonstrated that this enzyme has a triglyceride hydrolase activity.38 Our data showing that CES1 overexpression is able to restore lipid droplet breakdown in isolated PTECs from PTEC-Ogty/− mice provides additional evidence for the role of CES1 in triglyceride metabolism. In this study, although PTEC-Ogty/− mice demonstrated low CES1 protein expression, regardless of feeding status, they developed renal abnormalities only after fasting. This suggests that the use of triglyceride, rather than cholesterol ester, as a substrate for CES1 is more important for ATP production during fasting.
Previous work has demonstrated that loss and gain of function of CES1 in mice worsens and improves hepatosteatosis, respectively.40 These results are consistent with our findings that low CES1 expression is associated with renal lipotoxicity in HFD-fed mice and that high CES1 expression ameliorates cell damage in primary Ogt-deficient PTECs, and they suggest that CES1 gain of function may represent a promising therapeutic target for lipotoxicity-associated kidney diseases. Our data also suggest that the transcription factor FXR is a candidate for regulation by O-GlcNAcylation, although a direct relationship between CES1 and FXR has not been fully shown in this study. Given that a renoprotective effect of an FXR agonist has recently been reported48−50 and that this agonist increased renal CES1 expression in our study, CES1 overexpression may be involved in the mechanism underpinning FXR-mediated renoprotection.
Our findings are consistent with the notion that a novel finding regarding the physiology of renal lipolysis can lead to clarification of the pathogenesis of kidney diseases. However, our findings have revealed only part of the physiology of renal lipolysis; additional work is required to elucidate the mechanisms in more depth. In most mammalian cell types, LPL-dependent lipolysis of circulating triglycerides at the endothelial surface and CD36-dependent uptake of albumin-bound free FAs have been thought to be the means by which cells obtain FAs from the bloodstream during feeding and fasting, respectively.8,9 However, it has still not been proven that LPL is critical for FA uptake by PTECs during feeding. Furthermore, a recent study showed that CD36 is not involved in FA uptake by PTECs during fasting.9 Thus, the regulation of FA uptake by PTECs seems to be different from that in other cell types. If the unique mechanisms involved in renal lipid metabolism can be elucidated in greater depth, this should contribute to better understanding of the pathogenesis of CKD, including DKD.
There were a couple of limitations to this study. First, NDRG1 is expressed in parts of the nephron other than PTECs. Therefore, although the Fanconi syndrome–like phenomenon and changes in renal cortical samples in PTEC-Ogty/− mice most likely reflect changes in PTEC function, not all of the changes that occurred in this mouse model may be explained by abnormalities in PTECs. Second, regulation of lipid metabolism during fasting is one of the roles of renal O-GlcNAcylation. Actually, our proteomics analysis identified some proteins with changed expression levels in the kidneys of PTEC-Ogty/− mice. For example, Renin-2 protein levels were largely increased in the kidneys of the fasted PTEC-Ogty/− mice. We still do not know the exact cause and significance of this change. However, given that active renin was found in proximal convoluted tubules,51 intrarenal protein O-GlcNAcylation may be directly involved in the regulation of RAS. Thus, unraveling the remaining cell-specific effects of protein O-GlcNAcylation should provide additional insights into renal physiology.
In conclusion, intracellular lipid metabolism is maintained in PTECs using protein O-GlcNAcylation, despite increases in FA uptake in situations, such as starvation or obesity and diabetes. The protein O-GlcNAcylation-FXR-CES1 axis may be the mechanism responsible. Our results provide novel insight into proximal tubule physiology and should contribute to better understanding of the kidney diseases associated with abnormal lipid metabolism.
Disclosures
Dr. Kume reports grants from Lilly Grant Office, grants from Bayer Academic Support, grants from Sanofi Grant, and grants from the Japan Society for the Promotion of Science during the conduct of the study. Dr. Chin-Kanasaki reports grants from MSD Grant and grants from the Japan Society for the Promotion of Science during the conduct of the study. Dr. Maegawa reports grants from Lilly Grant Office, grants from Bayer Academic Support, and grants from the Japan Society for the Promotion of Science during the conduct of the study. Dr. Sugahara, Dr. Tomita, Dr. Yasuda-Yamahara, Dr. Yamahara, Dr. Takeda, Dr. Osawa, Dr. Yanagita, and Dr. Araki had nothing to disclose.
Supplementary Material
Acknowledgments
We thank Naoko Yamanaka and Keiko Kosaka (Shiga University of Medical Science) and the Central Research Laboratory of Shiga University of Medical Science for technical assistance.
This study was supported by Grants-in-Aid for Scientific Research (KAKENHI) 25713033 (to Dr. Kume), 25461219 (to Dr. Chin-Kanasaki), and 18H02862 (to Dr. Maegawa) from the Japan Society for the Promotion of Science; grants from the Lilly Grant Office (to Dr. Kume and Dr. Maegawa); Bayer Academic Support (Dr. Kume and Dr. Maegawa); a Sanofi Grant (to Dr. Kume); and an MSD Grant (to Dr. Chin-Kanasaki).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018090950/-/DCSupplemental.
Supplemental Appendix. Spreadsheet.
Supplemental Figure 1. Fatty acid transport into cells during feeding and fasting
Supplemental Figure 2. Full gel scan images for the immune blots of the indicated figure number.
Supplemental Table 1. List of antibodies used in this study.
Supplemental Table 2. List of primer sets used in this study.
Supplemental Table 3. Fold changes in expression levels of proteins associated with fatty acid metabolism in the proteomic analysis using renal cortex samples obtained from Ogty/f mice (control) and PTEC-Ogty/− mice (knockout).
References
- 1.Schmidt U, Guder WG: Sites of enzyme activity along the nephron. Kidney Int 9: 233–242, 1976 [DOI] [PubMed] [Google Scholar]
- 2.Balaban RS, Mandel LJ: Metabolic substrate utilization by rabbit proximal tubule. An NADH fluorescence study. Am J Physiol 254: F407–F416, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Gullans SR, Brazy PC, Mandel LJ, Dennis VW: Stimulation of phosphate transport in the proximal tubule by metabolic substrates. Am J Physiol 247: F582–F587, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Uchida S, Endou H: Substrate specificity to maintain cellular ATP along the mouse nephron. Am J Physiol 255: F977–F983, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Kume S, Uzu T, Araki S, Sugimoto T, Isshiki K, Chin-Kanasaki M, et al.: Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J Am Soc Nephrol 18: 2715–2723, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al.: Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, et al.: Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem 280: 32317–32325, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Goldberg IJ, Eckel RH, Abumrad NA: Regulation of fatty acid uptake into tissues: Lipoprotein lipase- and CD36-mediated pathways. J Lipid Res 50[Suppl]: S86–S90, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scerbo D, Son NH, Sirwi A, Zeng L, Sas KM, Cifarelli V, et al.: Kidney triglyceride accumulation in the fasted mouse is dependent upon serum free fatty acids. J Lipid Res 58: 1132–1142, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardivillé S, Hart GW: Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab 20: 208–213, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanover JA, Krause MW, Love DC: Bittersweet memories: Linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol 13: 312–321, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Janetzko J, Walker S: The making of a sweet modification: Structure and function of O-GlcNAc transferase. J Biol Chem 289: 34424–34432, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misra J, Kim DK, Jung YS, Kim HB, Kim YH, Yoo EK, et al.: O-GlcNAcylation of orphan nuclear receptor estrogen-related receptor γ promotes hepatic gluconeogenesis. Diabetes 65: 2835–2848, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Ohashi N, Morino K, Ida S, Sekine O, Lemecha M, Kume S, et al.: Pivotal role of O-GlcNAc modification in cold-induced thermogenesis by brown adipose tissue through mitochondrial biogenesis. Diabetes 66: 2351–2362, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Silva-Aguiar RP, Bezerra NCF, Lucena MC, Sirtoli GM, Sudo RT, Zapata-Sudo G, et al.: O-GlcNAcylation reduces proximal tubule protein reabsorption and promotes proteinuria in spontaneously hypertensive rats. J Biol Chem 293: 12749–12758, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gellai R, Hodrea J, Lenart L, Hosszu A, Koszegi S, Balogh D, et al.: Role of O-linked N-acetylglucosamine modification in diabetic nephropathy. Am J Physiol Renal Physiol 311: F1172–F1181, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Akimoto Y, Miura Y, Toda T, Wolfert MA, Wells L, Boons GJ, et al.: Morphological changes in diabetic kidney are associated with increased O-GlcNAcylation of cytoskeletal proteins including α-actinin 4. Clin Proteomics 8: 15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh HN, Lee YJ, Kim MO, Ryu JM, Han HJ: Glucosamine-induced Sp1 O-GlcNAcylation ameliorates hypoxia-induced SGLT dysfunction in primary cultured renal proximal tubule cells. J Cell Physiol 229: 1557–1568, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Peruchetti DB, Silva-Aguiar RP, Siqueira GM, Dias WB, Caruso-Neves C: High glucose reduces megalin-mediated albumin endocytosis in renal proximal tubule cells through protein kinase B O-GlcNAcylation. J Biol Chem 293: 11388–11400, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shafi R, Iyer SP, Ellies LG, O’Donnell N, Marek KW, Chui D, et al.: The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci U S A 97: 5735–5739, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endo T, Nakamura J, Sato Y, Asada M, Yamada R, Takase M, et al.: Exploring the origin and limitations of kidney regeneration. J Pathol 236: 251–263, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Huang ZH, Reardon CA, Mazzone T: Endogenous ApoE expression modulates adipocyte triglyceride content and turnover. Diabetes 55: 3394–3402, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Tanaka Y, Kume S, Araki S, Isshiki K, Chin-Kanasaki M, Sakaguchi M, et al.: Fenofibrate, a PPARα agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney Int 79: 871–882, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Ueda S, Ozawa S, Mori K, Asanuma K, Yanagita M, Uchida S, et al.: ENOS deficiency causes podocyte injury with mitochondrial abnormality. Free Radic Biol Med 87: 181–192, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Yamahara K, Kume S, Koya D, Tanaka Y, Morita Y, Chin-Kanasaki M, et al.: Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J Am Soc Nephrol 24: 1769–1781, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura T, Takabatake Y, Takahashi A, Kaimori JY, Matsui I, Namba T, et al.: Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 22: 902–913, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soumura M, Kume S, Isshiki K, Takeda N, Araki S, Tanaka Y, et al.: Oleate and eicosapentaenoic acid attenuate palmitate-induced inflammation and apoptosis in renal proximal tubular cell. Biochem Biophys Res Commun 402: 265–271, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Iwai T, Kume S, Chin-Kanasaki M, Kuwagata S, Araki H, Takeda N, et al.: Stearoyl-CoA desaturase-1 protects cells against lipotoxicity-mediated apoptosis in proximal tubular cells. Int J Mol Sci 17: pii: E1868, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuda M, Tanaka Y, Kume S, Morita Y, Chin-Kanasaki M, Araki H, et al.: Fatty acids are novel nutrient factors to regulate mTORC1 lysosomal localization and apoptosis in podocytes. Biochim Biophys Acta 1842: 1097–1108, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Bligh EG, Dyer WJ: A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 31.Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, et al.: Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 120: 1043–1055, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, et al.: Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell 14: 193–204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egan JJ, Greenberg AS, Chang MK, Wek SA, Moos MC Jr, Londos C: Mechanism of hormone-stimulated lipolysis in adipocytes: Translocation of hormone-sensitive lipase to the lipid storage droplet. Proc Natl Acad Sci U S A 89: 8537–8541, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A: Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res 50: 3–21, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C: Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 266: 11341–11346, 1991 [PubMed] [Google Scholar]
- 36.Alam M, Ho S, Vance DE, Lehner R: Heterologous expression, purification, and characterization of human triacylglycerol hydrolase. Protein Expr Purif 24: 33–42, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Crow JA, Middleton BL, Borazjani A, Hatfield MJ, Potter PM, Ross MK: Inhibition of carboxylesterase 1 is associated with cholesteryl ester retention in human THP-1 monocyte/macrophages. Biochim Biophys Acta 1781: 643–654, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolinsky VW, Gilham D, Alam M, Vance DE, Lehner R: Triacylglycerol hydrolase: Role in intracellular lipid metabolism. Cell Mol Life Sci 61: 1633–1651, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh S, Natarajan R: Cloning of the human cholesteryl ester hydrolase promoter: Identification of functional peroxisomal proliferator-activated receptor responsive elements. Biochem Biophys Res Commun 284: 1065–1070, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Li Y, Chen WD, Xu Y, Yin L, Ge X, et al.: Hepatic carboxylesterase 1 is essential for both normal and farnesoid X receptor-controlled lipid homeostasis. Hepatology 59: 1761–1771, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U: Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res 55: 561–572, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chin M, Isono M, Isshiki K, Araki S, Sugimoto T, Guo B, et al.: Estrogen and raloxifene, a selective estrogen receptor modulator, ameliorate renal damage in db/db mice. Am J Pathol 166: 1629–1636, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shibuya K, Kanasaki K, Isono M, Sato H, Omata M, Sugimoto T, et al.: N-acetyl-seryl-aspartyl-lysyl-proline prevents renal insufficiency and mesangial matrix expansion in diabetic db/db mice. Diabetes 54: 838–845, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Ono S, Kume S, Yasuda-Yamahara M, Yamahara K, Takeda N, Chin-Kanasaki M, et al.: O-linked β-N-acetylglucosamine modification of proteins is essential for foot process maturation and survival in podocytes. Nephrol Dial Transplant 32: 1477–1487, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Copeland RJ, Bullen JW, Hart GW: Cross-talk between GlcNAcylation and phosphorylation: Roles in insulin resistance and glucose toxicity. Am J Physiol Endocrinol Metab 295: E17–E28, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Degrell P, Cseh J, Mohás M, Molnár GA, Pajor L, Chatham JC, et al.: Evidence of O-linked N-acetylglucosamine in diabetic nephropathy. Life Sci 84: 389–393, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Bolignano D, Zoccali C: Non-proteinuric rather than proteinuric renal diseases are the leading cause of end-stage kidney disease. Nephrol Dial Transplant 32[Suppl_2]: ii194–ii199, 2017 [DOI] [PubMed] [Google Scholar]
- 48.Zhao K, He J, Zhang Y, Xu Z, Xiong H, Gong R, et al.: Activation of FXR protects against renal fibrosis via suppressing Smad3 expression. Sci Rep 6: 37234, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gai Z, Gui T, Hiller C, Kullak-Ublick GA, Farnesoid X: Farnesoid X receptor protects against kidney injury in uninephrectomized obese mice. J Biol Chem 291: 2397–2411, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang XX, Wang D, Luo Y, Myakala K, Dobrinskikh E, Rosenberg AZ, et al.: FXR/TGR5 dual agonist prevents progression of nephropathy in diabetes and obesity. J Am Soc Nephrol 29: 118–137, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen M, Harris MP, Rose D, Smart A, He XR, Kretzler M, et al.: Renin and renin mRNA in proximal tubules of the rat kidney. J Clin Invest 94: 237–243, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.