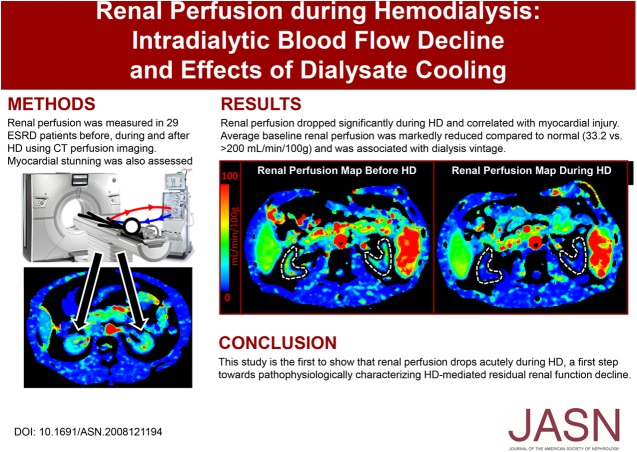
Significance Statement
Residual renal function (RRF) characteristically declines after patients with ESRD initiate dialysis. Although RRF preservation correlates with improved outcomes, poor understanding of the pathophysiology underlying RRF decline limits protection strategies. Previous research found that dialysate cooling reduces hemodialysis-induced circulatory stress and protects the brain and heart from ischemic injury. To examine renal perfusion decline during hemodialysis and the effects of cooling, the authors used computed tomography perfusion imaging to scan patients undergoing continuous dialysis with or without dialysate cooling. They found an acute decrease in renal perfusion during hemodialysis, a first step toward characterizing hemodialysis-mediated RRF loss. Dialysate cooling ameliorated this decline but this effect did not reach statistical significance. Further study is needed to explore the potential of dialysis-based interventions to slow RRF decline.
Keywords: hemodialysis, renal hemodynamics, renal ischemia, renal function decline
Visual Abstract
Abstract
Background
Residual renal function (RRF) confers survival in patients with ESRD but declines after initiating hemodialysis. Previous research shows that dialysate cooling reduces hemodialysis-induced circulatory stress and protects the brain and heart from ischemic injury. Whether hemodialysis-induced circulatory stress affects renal perfusion, and if it can be ameliorated with dialysate cooling to potentially reduce RRF loss, is unknown.
Methods
We used renal computed tomography perfusion imaging to scan 29 patients undergoing continuous dialysis under standard (36.5°C dialysate temperature) conditions; we also scanned another 15 patients under both standard and cooled (35.0°C) conditions. Imaging was performed immediately before, 3 hours into, and 15 minutes after hemodialysis sessions. We used perfusion maps to quantify renal perfusion. To provide a reference to another organ vulnerable to hemodialysis-induced ischemic injury, we also used echocardiography to assess intradialytic myocardial stunning.
Results
During standard hemodialysis, renal perfusion decreased 18.4% (P<0.005) and correlated with myocardial injury (r=−0.33; P<0.05). During sessions with dialysis cooling, patients experienced a 10.6% decrease in perfusion (not significantly different from the decline with standard hemodialysis), and ten of the 15 patients showed improved or no effect on myocardial stunning.
Conclusions
This study shows an acute decrease in renal perfusion during hemodialysis, a first step toward pathophysiologic characterization of hemodialysis-mediated RRF decline. Dialysate cooling ameliorated this decline but this effect did not reach statistical significance. Further study is needed to explore the potential of dialysate cooling as a therapeutic approach to slow RRF decline.
Most patients on incident hemodialysis (HD) are not completely anuric.1,2 The presence and preservation of even minimal amounts3–6 of residual renal function (RRF) after HD initiation is associated with better control of serum phosphate, hypervolemia, and hypertension, improved nutrition, less anemia, higher middle molecule clearance, and improved survival.7–12 Although the importance of long-term RRF maintenance is recognized,13 RRF characteristically declines after HD initiation, necessitating more aggressive fluid removal in subsequent HD sessions.14,15 This decline is linked to poorer outcomes and increased mortality.12,16 Observational studies have found that age, CKD cause, bioincompatible dialysis membranes, and elevated BP are associated with RRF decline.9,17,18 Larger epidemiologic studies have confirmed these findings and shown that hemodynamic instability during HD (i.e., intradialytic hypotension [IDH]) is independently associated with RRF loss.14,19
Intradialytic circulatory stress is associated with reduced perfusion in multiple vulnerable organs.20 Recurring subclinical ischemic injury over many HD sessions is linked to increased morbidity and mortality. One strategy that reduces IDH frequency (an independent predictor of mortality14) and ameliorates HD-induced circulatory stress is dialysate cooling (DC).21,22 This intervention does not adversely affect HD efficiency, is generally well tolerated, and can be widely implemented at no additional cost.23 Studies have found that myocardial and cerebral perfusion can be preserved using DC, providing protection against injury and longer-term organ dysfunction.22,24,25
Several authors have speculated that HD causes recurrent renal ischemic insults, which may cause irreversible injury leading to RRF loss.10,11,26 However, trials to date have not focused on measuring HD-induced renal ischemia or describing its relationship with RRF loss, preventing the evaluation of potential preservation interventions. We therefore conducted a pilot study of whole organ kidney perfusion, measured serially during HD using computed tomography (CT) perfusion imaging, to measure intradialytic renal perfusion and test two hypotheses: first, that HD is associated with acute renal perfusion decline, and second, that DC ameliorates HD-induced changes in renal hemodynamics. Confirming quantitatively that HD does in fact result in decreased renal perfusion (DRP) will represent the first crucial step toward the pathophysiologic characterization of HD-mediated RRF loss in patients with ESRD.
Methods
Patients
Thirty patients (19 men) in total from the London Health Sciences Centre Regional Renal Program were enrolled in two experiments (see Study Design below), after giving their written informed consent. Adult patients established on HD for at least 3 months and who had low RRF (<250 ml/d) were eligible. This group of patients with already low RRF was selected to limit any potential effects of contrast-induced nephropathy. Exclusion criteria included active infection/malignancy, pregnancy, breast feeding, planned pregnancy, diabetic with hypoglycemia during HD within the past 2 months, and known allergy to iodinated contrast agent. These experiments were approved by the University of Western Ontario Health Sciences Research Ethics Board and were conducted in compliance with the approved protocols, Good Clinical Practice Guidelines, and all applicable regulatory requirements.
Study Design
Two back-to-back pilot experiments were conducted and then the results were combined. In the first experiment, 14 patients were recruited to undergo a single session of standard dialysate temperature (36.5°C) HD. In the second experiment, 16 patients were recruited to undergo two sessions of HD: one session of standard dialysate temperature HD and another of cooled dialysate temperature (35.0°C) HD. Patients involved in the second experiment were randomly assigned to receive either standard or cooled HD first in a two-visit crossover study design, thereby acting as their own controls.
Combined findings from the two experiments were divided into “standard HD” (14+16=30 standard HD patients) and “standard versus cooled HD” (16 standard and cooled HD patients) and analyzed accordingly. All patients underwent uninterrupted HD in the CT scanner room. Analysis of the imaging data from the crossover experiment was performed with the operator blinded to allocation. Patients, dialysis unit staff, and the investigator at the experiment visit were not blinded to the intervention, but were not involved in the imaging data analysis.
Dynamic CT Image Acquisition and Analysis
CT perfusion imaging was performed on a GE Healthcare Revolution 256-slice CT scanner at three times during each HD session: immediately before, 3 hours into (i.e., peak stress, defined from previous studies of HD-induced myocardial injury), and 15 minutes after dialysis. For the intradialytic scan, patients were transferred to the CT bed without interrupting their HD treatment. After iodinated contrast agent injection, dynamic contrast–enhanced CT scanning of a 16 cm section of the abdomen was performed without breath hold. The type of contrast agent, iopamidol (Isovue 370; Bracco Imaging), was identical in all patients for both standard and cooled HD sessions, and was administered at a dose of 1 ml/kg of pre-HD patient weight (up to a maximum dose of 70 ml).
Scan ranges were optimized to encompass as much of both kidneys as possible. The section was divided into 32 slices of 5 mm thickness each, and was scanned 42 times at 2.8 second intervals using 120 kV and 22.4 mAs, for a duration of approximately 2 minutes. Images were reconstructed using 100% Adaptive Statistical Iterative Reconstruction (GE Healthcare) to reduce image noise, and then registered using nonrigid registration (GE Healthcare) to minimize breathing motion among images of the dynamic scan. Registered images were analyzed using CT Perfusion 4D software (GE Healthcare). An aortic region of interest (ROI) was selected for generation of perfusion maps. Next, ROIs were manually drawn over the kidneys in the blood flow maps to encompass medullar and cortical areas. Kidney ROIs were reviewed and verified by three experienced (>10 years) radiologists. Then, perfusion values were averaged over the selected slices to determine mean whole kidney blood flow values.
DRP was defined as either (1) a drop in blood flow at peak stress ≥2 SEM (SEM of the patient group), or (2) a drop in blood flow at both peak stress ≥1 SEM and after HD ≥1 SEM.
Echocardiography Analysis of Myocardial Stunning
Myocardial response to HD was assessed to provide a reference to another critical organ known to be vulnerable to HD-induced circulatory stress. Echocardiography was performed by trained investigators before commencing and 15 minutes before the end of HD, using commercially available equipment (1.5–3.6 MHz M4S probe, Vivid-iq; GE Healthcare). Standard apical two- and four-chamber views were recorded for offline digital analysis with a semi-automated computer program (EchoPac; GE Healthcare) using two-dimensional speckle tracking software.
Images were anonymized and analyzed in random order by the same trained investigators (E.Q. and C.J.G.). Three cardiac cycles at each time point were analyzed to derive segmental (12 left ventricular segments) and global longitudinal strain. Myocardial stunning (MS) was defined as a reduction in longitudinal strain of >20% in two or more segments of the left ventricle caused by regional wall motion abnormalities (RWMA). The number of left ventricular segments exhibiting a reduction in strain of >20% was also recorded.
Statistical Analyses
Kidney perfusion has never been assessed previously in the context of HD and inadequate data exist to perform a meaningful sample size calculation. As the initial proof-of-principle study for hypothesis generation, a sample size of approximately 15 patients per pilot experiment is not powered for analysis of the data with inferential statistics. However, the proposed sample size has been selected on a pragmatic basis and is comparable with published norms22,27,28 and recommendations.29,30
Statistical analysis was performed using SPSS, version 25.0 (IBM, Chicago, IL). Data were analyzed using repeated measures ANOVA with post hoc t tests (with Bonferroni correction) and baseline-adjusted analysis of covariance (ANCOVA) to detect differences between groups. Associations between variables were assessed using the Pearson product-moment correlation coefficient, and the McNemar test was used to detect differences between proportions. Two-tailed P values <0.05 were considered statistically significant.
Results
Clinical Characteristics of Study Population
Thirty patients (19 men) aged 40–84 years were enrolled in two back-to-back experiments. However, one patient could not return for the second visit of the crossover experiment and was excluded from the analysis, resulting in 29 standard HD patients, 15 of which also underwent cooled HD. The median dialysis vintage was 5.3 years (range, 0.8–46 years). Ten patients had known coronary artery disease, seven had congestive heart failure, four had peripheral vascular disease, 15 had diabetes, and 25 had hypertension. Dialysis session length ranged from 2 to 4 hours (median, 3.5 hours) and ultrafiltration (UF) ranged from 0 to 41 ml/kg (median 23 ml/kg). Table 1 presents the summary of patient baseline characteristics.
Table 1.
Baseline characteristics of study population
| Characteristics | Median (Range)a |
|---|---|
| n (standard HD, cooled HD) | 29 (29, 15) |
| Age | 64 (40–84) |
| Men, n (%) | 19 (66) |
| Dialysis vintage, yr | 5.3 (0.8–46) |
| Coronary artery disease, n (%) | 10 (34) |
| Congestive heart failure, n (%) | 7 (24) |
| Peripheral vascular disease, n (%) | 4 (14) |
| Diabetes, n (%) | 15 (52) |
| Hypertension, n (%) | 25 (86) |
| Length of HD session, h | 3.5 (2.0–4.0) |
| UF, ml/kg | 23.3 (0.0–40.9) |
Unless otherwise specified.
Renal Perfusion
Baseline renal perfusion and renal hemodynamic response to HD differed between both kidneys to a measurable extent for many patients. Therefore, perfusion data analysis was on the basis of individual kidneys among all patients.
Standard HD
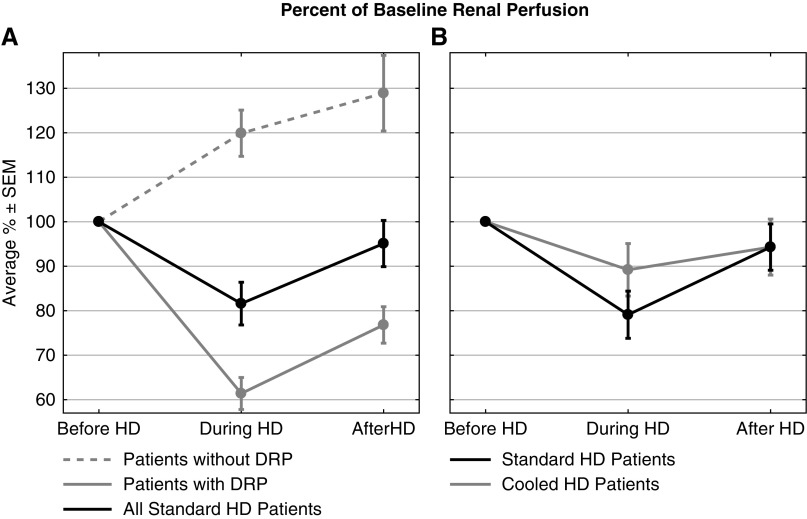
Perfusion was measured in all 29 patients, resulting in computed values for 57 kidneys (one patient had a solitary kidney). Average baseline per kidney perfusion was 33.2±2.9 ml/min per 100 g (mean±SEM) and correlated with dialysis vintage (r=−0.35; P<0.01). At peak stress, average per kidney perfusion dropped to 81.6%±4.8% of baseline (Figures 1 and 2A). After HD, average per kidney perfusion recovered to 95.1%±5.2% of baseline (Figure 2A). After repeated measures ANOVA, post hoc analysis revealed that the intradialytic renal perfusion drop was statistically significant compared with pre- and post-HD (P<0.005). Acute DRP during HD was observed in 37 out of 57 kidneys (65%), where average per kidney perfusion dropped to 61.4%±3.6% of baseline during peak stress. For the remaining 20 kidneys (35%), average per kidney perfusion increased to 119.9%±5.2% of baseline at peak stress (Figure 2A).
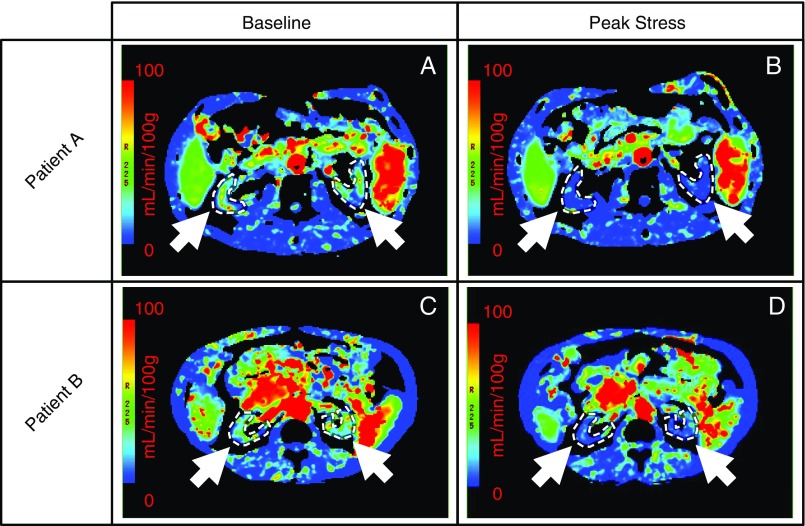
Figure 1.
Hemodialysis-induced decrease in kidney blood flow visualized with parametric renal perfusion maps. Renal blood flow at baseline (A and C) and 3 hours into dialysis (B and D) for two patients (top and bottom rows). Kidneys have been identified (white arrows and dotted contours) for both patients.
Figure 2.
Renal perfusion significantly declined during standard HD but not during cooled HD. Percent of baseline per kidney perfusion before, 3 hours into, and after dialysis, where results are given as average±SEM. (A) In 29 standard HD patients (57 kidneys), the drop in renal perfusion during HD was statistically significant compared with pre- and post-HD blood flow values (P<0.005). (B) In 15 standard and cooled HD patients (30 kidneys each), there was a smaller decline in renal perfusion during cooled HD (not statistically significant) compared with standard HD.
Standard versus Cooled HD
Perfusion was measured in all 15 crossover patients, resulting in 30 paired values under standard and cooled HD conditions. Average per kidney perfusion dropped to 79.1%±5.3% and 89.2%±5.9% of baseline at peak stress during standard and cooled HD, respectively (Figure 2B). Session-specific, baseline-adjusted ANCOVA revealed that the decline in intradialytic renal perfusion between dialysis treatments was not different [F(1,57)=1.814; P=0.18]. Average per kidney perfusion recovered to 94.3% of baseline after both standard and cooled HD. DRP was observed in 20 out of 30 kidneys (67%) during standard HD and 15 out of 30 kidneys (50%) during cooled HD (not significantly different). In those kidneys, however, the perfusion decline (37% below baseline) was the same for both dialysate temperatures.
Relationship to Cardiac Injury
Standard HD
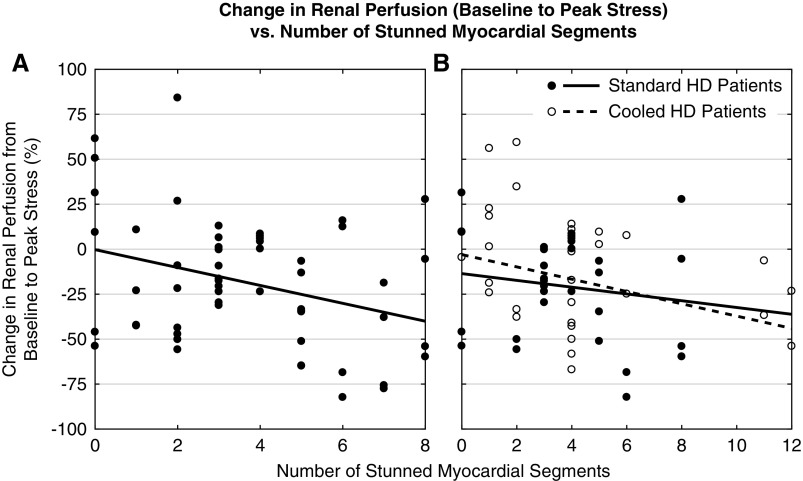
A total of 24 out of 29 patients (83%) exhibited MS. The degree of stunning correlated with DRP (r=−0.33; P<0.05) (Figure 3A). The McNemar test showed that during HD, MS incidence (83%) was significantly higher (P<0.05) than DRP incidence (65%). Patients without MS were also protected from HD-induced DRP, where peak stress perfusion declined by 4.4%±13.5% relative to baseline (not statistically significant). This change in perfusion, however, was not significantly different from the 21%±5.0% drop in the MS patients according to baseline-adjusted ANCOVA [F(1,52)=1.575; P=0.22].
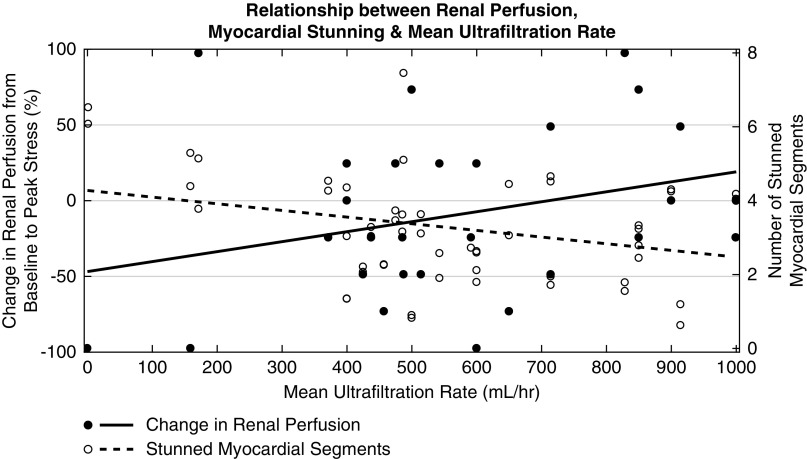
Figure 3.
Decreased renal perfusion was associated with an increased number of stunned myocardial segments during standard and cooled HD. Change in per kidney perfusion from baseline to peak stress (i.e., 3 hours into dialysis) versus the number of stunned myocardial segments measured with echocardiography. The dotted lines represent data trendlines. (A) In 29 standard HD patients (57 kidneys), there was a correlation between the degree of cardiac injury and severity of renal insult (r=-0.33; P<0.05). (B) In 15 standard and cooled HD patients (30 kidneys each), there was a correlation between the degree of cardiac injury and severity of renal insult during cooled HD (r=-0.36; P<0.05) but not during standard HD.
Standard versus Cooled HD
MS was observed in 13 out of 15 (87%) and 11 out of 15 (73%) patients during standard and cooled HD, respectively (not significantly different), where DC reduced, increased, and had no effect on the number of stunned myocardial segments in eight, five, and two patients, respectively. Although the degree of cardiac injury and severity of renal insult were not associated during standard HD, they were negatively correlated during cooled HD (r=−0.36; P<0.05) (Figure 3B). For both HD subgroups, patients without MS experienced milder DRP.
Relationship to Dialysis Stress Factors
Standard HD
Seven out of 29 patients (24%) experienced IDH (symptomatic and drop in systolic BP [SBP] >20 mm Hg). SBP and mean arterial pressure (MAP) dropped significantly during HD to 88.5% (P<0.005) and 91.2% (P<0.05) of baseline, respectively, before both recovering to 99% of baseline after HD. Although diastolic BP (DBP) behaved similarly, the intradialytic change to 95.6% of baseline was not significant. However, no correlations were found between BP changes and MS development and/or changes in renal perfusion.
Mean and total UF were associated with DRP (r=−0.31; P<0.05 and r=−0.26; P=0.05, respectively) and more stunned myocardial segments (r=0.30; P<0.05 and r=0.27; P<0.05, respectively) (Figure 4). DRP (in at least one kidney) and MS occurred in most patients. Comparing those patients who did experience DRP and stunning with those who did not, there was an association between patients who were taking β-blockers and DRP and stunning (r=0.48; P<0.01 for DRP and r=0.54; P<0.005 for MS). Also, there was a discrepancy in patient sex, where the male-to-female ratio was 12:9/7:1 for those who did/did not experience DRP and 15:9/4:1 for those who did/did not experience MS.
Figure 4.
Decreased renal perfusion and an increased number of stunned myocardial segments were both associated with higher mean ultrafiltration rates during standard HD. Change in per kidney perfusion from baseline to peak stress (open circles) and number of stunned myocardial segments (solid circles) versus mean UF rate for 29 standard HD patients (57 kidneys). The dotted and solid lines represent data trendlines for the renal perfusion and MS data, respectively. The mean UF rate was associated with a larger drop in renal perfusion from baseline to peak stress (r=-0.31; P<0.05) and a greater number of stunned myocardial segments (r=0.30; P<0.05).
Standard versus Cooled HD
Three out of 15 patients experienced IDH during both HD sessions, and ten out of 15 patients experienced an SBP drop (>20 mm Hg) during standard HD compared with eight out of 15 during cooled HD (not significantly different). Session-specific, baseline-adjusted ANCOVA revealed that BP changes during HD between dialysis treatments was not statistically significant [F(1,26)=2.814 and P=0.11; F(1,26)=0.582 and P=0.45; F(1,26)=0.382 and P=0.54 for SBP, DBP, and MAP, respectively]. During standard HD, changes in SBP and MAP correlated with MS (r=−0.36; P=0.05 and r=−0.38; P<0.05, respectively). During cooled HD, these associations persisted (r=−0.45; P<0.05 and r=−0.43; P<0.05 for SBP and MAP, respectively), and new associations emerged between BP changes and the number of stunned myocardial segments (r=−0.44; P<0.05 and r=−0.40; P<0.05 for DBP and MAP, respectively) and absolute changes in renal perfusion (r=0.47; P<0.05 for DBP).
In terms of thermal symptoms, two out of 15 patients reported feeling cold and/or were shivering during standard HD compared with six out of 15 (two same, four new) during cooled HD (not significantly different).
During standard HD, UF metrics were not associated with DRP or severity of myocardial injury. During cooled HD, UF metrics remained uncorrelated to DRP but were negatively associated with the number of stunned myocardial segments (r=−0.41; P<0.05 for mean and total UF).
Discussion
This study demonstrated that renal perfusion decreased during dialysis, even in the absence of significant hypotension, contemporaneously with MS. In addition, DRP and MS were minimized with DC (although not to a statistically significant extent). These important findings may provide a pathophysiologic explanation and potentially preventative intervention for the characteristic rapid decline of RRF in patients on HD.
Renal Perfusion
Although absolute renal perfusion does not directly represent kidney function, it is a major factor in determining GFR and urine output. As such, perfusion values measured for this study act as surrogate measures of renal function.
Standard HD
Average per kidney perfusion dropped to 81.6% of baseline during HD and 21 out of 29 patients (72%) experienced intradialytic DRP in at least one kidney. This reduction in perfusion represents a potential ischemic insult, which is repeated during recurring dialysis sessions and could result in cumulative renal tissue damage and a subsequent RRF reduction. This mechanism is reinforced by the inverse correlation between baseline renal perfusion (RRF surrogate) and dialysis vintage. Perfusion values for patients in our study (<105 ml/min per 100 g, average baseline of 33.2±2.9 ml/min per 100 g) were markedly reduced compared with normal control (typical range, 200–500 ml/min per 100 g) and earlier stage CKD values (approximately 140–300 ml/min per 100 g) measured in other studies.31–35
Recovery of perfusion to 95% of baseline after HD suggests that HD-induced DRP resolves after UF ends and hypovolemia is relieved. The role of HD-mediated hemodynamic instability and transient renal ischemia in progressive RRF decline in patients on HD has been alluded to previously.10,11,26,36,37 However, this is the first study to directly measure intradialytic renal perfusion and confirm that DRP represents the first key step toward characterizing RRF loss in patients on HD.
Patients with ESRD who are undergoing HD three to four times weekly are subjected to recurrent circulatory stress, suggesting repeated episodes of DRP. Interestingly, Ronco et al.38 list the combination of hypoperfusion, prolonged hypovolemia, and presence of comorbidities as key factors resulting in subclinical, prerenal AKI. Together with our perfusion results, these aforementioned factors are present during HD sessions of patients with ESRD. Thus, renal tubular damage due to repetitive, intradialytic ischemic AKI may contribute to kidney injury resulting in long-term RRF reduction.
Standard versus Cooled HD
DC helped ameliorate HD-induced DRP, where the cooled subgroup experienced a smaller decline in renal perfusion at peak stress and had fewer kidneys with DRP compared with the standard subgroup, although neither findings were statistically significant. Along with other studies that illustrated the protective effects of DC on the brain25 and heart,24 this study’s findings demonstrate the global hemodynamic effect of HD and the protective potential of DC.
When considering only kidneys with DRP, the change in perfusion from baseline to peak stress was the same for both dialysate temperatures. This suggests that although DC reduces DRP incidence, it does not reduce the magnitude of renal ischemia. However, because of the difference in the overall number of kidneys with DRP, the decline in average per kidney perfusion at peak stress was larger (not statistically significant) for standard HD. These results are consistent with those of a similarly designed study by Selby et al.,22 which assessed myocardial function. They found that although patients undergoing standard HD developed more RWMAs compared with cooled HD, the RWMA magnitude (i.e., percentage shortening fraction) was equal between both HD treatments.
Relationship to Cardiac Injury
Standard HD
HD causes transient ischemia in multiple vulnerable vascular beds.20,39,40 In the heart, demonstrable injury manifests as MS,41 which was measured with echocardiography and observed in 83% of patients. In non-stunning patients, intradialytic perfusion changes were lessened compared with all patients collectively. This reinforces the notion that MS is a hallmark of HD-induced systemic circulatory stress28,42 and suggests that its presence potentiates DRP. The magnitude of MS, characterized by the number of stunned segments, was associated with DRP severity, as well as with higher mean and total UF. These results suggest that HD-specific factors contributing to DRP (e.g., aggressive UF, circulating endotoxins, IDH, etc.) are the same as those responsible for intradialytic myocardial injury.42–44
Standard versus Cooled HD
Additional intradialytic cardiac dysfunction may potentiate renal injury. Therefore, for patients with minimal urine output, RRF preservation could be achieved by lessening the circulatory stress of HD via hemodynamic-protective strategies. Techniques such as DC23 and ischemic preconditioning,45 which have shown potential for attenuating the burden of dialysis upon the heart24,27 and brain,25 may also protect renal parenchyma from recurring intradialytic ischemic insults.
In this study, DC seemed to help ameliorate intradialytic myocardial injury. Fewer patients experienced MS during cooled HD and more patients received benefit from the intervention than harm in terms of severity of myocardial injury (i.e., lowering the number of stunned segments with cooling). However, neither of these outcomes were statistically significant. These findings are consistent with results of similarly designed studies that characterized and compared myocardial injury during standard versus cooled HD.22,24,27 However, certain shortcomings in the results of those studies (e.g., no difference in left ventricular ejection fraction between standard and cooled HD groups24,27) and our work suggests that although DC is a favorable intervention in terms of feasibility and effectiveness, it may be worthwhile to combine it with other interventions (e.g., biofeedback dialysis46–48) to ameliorate HD-induced circulatory stress and myocardial injury.
Relationship to Dialysis Stress Factors
Standard HD
The continual drop in RRF over many HD sessions necessitates more fluid removal (i.e., higher UF) to account for increased interdialytic hypervolemia. However, higher UF causes greater hemodynamic stress49 and increases IDH incidence, an independent predictor of RRF decline.36,50 This coincides with our findings, where DRP severity was associated with mean and total UF (r=0.31; P<0.05 and r=0.26; P=0.05, respectively), suggesting that UF-induced ischemic injury may be a key factor in progressive RRF loss in patients on HD. This establishes a vicious cycle of HD-induced RRF decline, followed by a necessitated increase in UF, followed again by RRF decline, in keeping with the observed rapid decline shortly after HD initiation.
RRF declines faster with HD compared with peritoneal dialysis.19,26 Although factors such as more gradual shifts in volume and higher biocompatibility play a role in greater RRF preservation in peritoneal dialysis relative to HD,51 another key element is dialysis-induced MS as a marker of global circulatory stress. MS incidence is much lower in peritoneal dialysis52 compared with HD, suggesting renal perfusion could be better maintained during treatment and long-term RRF loss may be slowed as a result.
The observed renal hemodynamic response of patients to HD-induced circulatory stress was heterogenous, similar to other studies of the heart43 and brain.53 Most patients exhibited DRP, but some instead demonstrated increased perfusion. Although the cause of this heterogeneity in response is unknown, it is well recognized54 and likely due to the status of patients’ circulatory compensatory mechanisms. Most patients on HD have impaired compensatory mechanisms (chronotropic incompetence,55,56 β-blocker use,57 reduced baroreflex sensitivity54), increasing their vulnerability to UF-induced hypovolemia and IDH, whereas patients with more intact mechanisms are better able to compensate for HD-induced circulatory stress. In addition, CKD-related factors, such as increased fluid volume retention, altered sympathetic nervous system activity, endothelial dysfunction, oxidative stress, inflammation, and increased arterial stiffness, may contribute to varying BP response to circulatory stress.58 This may be why no association was observed between BP changes and MS development and/or changes in renal perfusion, despite significant declines in intradialytic SBP and MAP.
There was an association between MS and DRP, and patients taking β-blockers (but not other antihypertensives). The blockade of β-adrenergic receptors in the heart may weaken compensatory mechanisms to offset HD-induced circulatory stress. This mechanism is unique to β-blockers57 and likely the cause of the observed correlation.
The discrepancy in sex between patients who did versus did not experience MS and DRP was apparent in another study that examined HD-induced myocardial injury,42 where the male-to-female ratio was 28:17 for patients who exhibited RWMAs and 19:6 for those who did not. In a study assessing stress cardiomyopathy (i.e., acute emotional stress leading to MS), 95% of patients were female.59 The authors cite several studies on myocardial injury with similar sex-related trends, but the biologic mechanisms behind this discrepancy are unknown.
Standard versus Cooled HD
DC did not demonstrate any statistically significant benefit in terms of controlling intradialytic BP compared with standard HD. However, patients were not subjected to continuous BP monitoring but only episodic checks (e.g., imaging timepoints). It is therefore entirely possible that there were BP differences between standard and cooled HD that could not be assessed. Also, DC has other effects to improve response to ischemic injury that go beyond just increasing peripheral vasoconstriction to limit hypotension60 (e.g., increasing ischemic tolerance with moderate hypothermia and effects on the splanchnic circulation helping to support circulatory volume61,62). In addition, changes in SBP, DBP, and MAP were variably associated with MS and renal perfusion changes for standard and cooled HD. The combination of impaired compensatory mechanisms and increased BP variability may be the cause of these inconsistent findings.
During cooled HD, correlations between renal perfusion and UF were abolished, whereas correlations between myocardial injury and UF were reversed. Although these findings may have been because of a lower relative sample size, they support the idea that DC better maintains hemodynamic stability during UF.63,64 Therefore, HD effectiveness could be improved by using DC to more easily achieve patient-specific UF requirements.
Four out of 15 patients (27%) were shivering and/or reported feeling cold only during cooled HD. Other studies found similar22,65 and higher63 temperature-related symptom incidence. Jefferies et al.27 used patient-individualized body temperature dialysate (i.e., 0.5°C below core temperature) to improve DC tolerability, where only one out of 11 patients reported cold-related symptoms. This individualized intervention was subsequently applied to a larger cohort of 73 patients, with no cooling-related adverse events reported.24,25
Limitations
This early phase study has several limitations. Only patients with urine output <250 ml/24 h were examined to limit contrast-induced nephropathy. As a result, the study focused on patients with low baseline RRF, and this group may be predisposed to ischemic injury. However, this was proof-of-principle work and further studies are needed in patients with higher RRF, including patients on incident HD.
There appeared to be no significant artifactual effect of contrast media on renal perfusion. First, using further exposure to contrast, we demonstrated almost complete recovery in average perfusion after HD. Second, contrast agent administration was low risk (low intravenous dose). Third, crossover experiment patients underwent the same HD treatments but exhibited improved renal hemodynamics with DC (despite identical contrast exposure). In addition, we have previously demonstrated testosterone-dependent reduction in renal perfusion, with recovery after discontinuation (using the same small contrast load).35
These were pilot experiments with a modest total sample size of 29 patients. Thus, generalizing the findings to the general population should be withheld until a larger, randomized, controlled trial is conducted. However, these experiments included detailed, multimodal imaging measurements, where both inter- and intra-patient variations were assessed. Further studies are required to examine the direct effects of standard and cooled HD upon renal perfusion in individuals with higher RRF, and to longitudinally follow patients on incident HD with respect to declining RRF.
In conclusion, recurrent HD-induced renal ischemia lays the groundwork toward pathophysiologically explaining the previously observed relationship between time spent on dialysis and declining RRF. In addition, although amelioration of the decline in renal perfusion by DC did not reach statistical significance, this intervention, which has already been applied in the protection of the brain and heart from HD-induced injury, may provide protection from recurrent kidney injury.
Disclosures
Dr. Lee reports other from GE Healthcare, outside the submitted work; and in this manuscript, renal perfusion was calculated with the CT Perfusion software developed in my lab which is licensed to GE Healthcare.
Dr. McIntyre reports grants and personal fees from INTELLOMED outside the submitted work.
Supplementary Material
Acknowledgments
The authors would like to thank Jarrin Penny, Tanya Tamasi, Justin Dorie, Sal Treesh, Luke Zordrager, Errol Stewart, Anna MacDonald, Tony Wales, and Jennifer Hadway with their invaluable assistance with dialysis and scan acquisition. Radiologists I-Lun Huang, Nanchuan Jiang, and Qingguo Wang assisted with identification and delineation of renal parenchyma on computed tomography images.
E.Q., C.J.G., T.-Y.L., and C.W.M. designed the studies. R.M., E.Q., and C.J.G. carried out experiments. R.M., E.Q., C.J.G., and T.-Y.L. analyzed the data. R.M. made the figures. R.M., E.Q., C.J.G., T.-Y.L., and C.W.M. drafted and revised the paper. All authors approved the final version of the manuscript.
This study was funded by the Kidney Foundation of Canada.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018121194/-/DCSupplemental.
Supplemental Figure 1: Plots of relative change in renal perfusion for (A) 29 standard HD patients and (B) 15 standard and cooled HD patients.
Supplemental Table 1: Patient-specific BP and renal perfusion data, including identification of patients with renal artery stenosis.
References
- 1.Kjaergaard KD, Jensen JD, Peters CD, Jespersen B: Preserving residual renal function in dialysis patients: An update on evidence to assist clinical decision making. NDT Plus 4: 225–230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Wal WM, Noordzij M, Dekker FW, Boeschoten EW, Krediet RT, Korevaar JC, et al.: Netherlands Cooperative Study on the Adequacy of Dialysis Study Group (NECOSAD) : Full loss of residual renal function causes higher mortality in dialysis patients; findings from a marginal structural model. Nephrol Dial Transplant 26: 2978–2983, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Churchill DN, Taylor DW, Keshaviah PR; Canada-USA (CANUSA) Peritoneal Dialysis Study Group : Adequacy of dialysis and nutrition in continuous peritoneal dialysis: Association with clinical outcomes. J Am Soc Nephrol 7: 198–207, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Shemin D, Bostom AG, Laliberty P, Dworkin LD: Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis 38: 85–90, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bonomini V, Albertazzi A, Vangelista A, Bortolotti GC, Stefoni S, Scolari MP: Residual renal function and effective rehabilitation in chronic dialysis. Nephron 16: 89–102, 1976 [DOI] [PubMed] [Google Scholar]
- 6.Merkus MP, Jager KJ, Dekker FW, de Haan RJ, Boeschoten EW, Krediet RT; The NECOSAD Study Group : Predictors of poor outcome in chronic dialysis patients: The Netherlands cooperative study on the adequacy of dialysis. Am J Kidney Dis 35: 69–79, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Vilar E, Wellsted D, Chandna SM, Greenwood RN, Farrington K: Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant 24: 2502–2510, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Vilar E, Farrington K: Emerging importance of residual renal function in end-stage renal failure. Semin Dial 24: 487–494, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Canaud B: Residual renal function: The delicate balance between benefits and risks. Nephrol Dial Transplant 23: 1801–1805, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Wang AY, Lai KN: The importance of residual renal function in dialysis patients. Kidney Int 69: 1726–1732, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Mathew AT, Fishbane S, Obi Y, Kalantar-Zadeh K: Preservation of residual kidney function in hemodialysis patients: Reviving an old concept. Kidney Int 90: 262–271, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shafi T, Jaar BG, Plantinga LC, Fink NE, Sadler JH, Parekh RS, et al.: Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) study. Am J Kidney Dis 56: 348–358, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Kidney Foundation : KDOQI clinical practice guidelines and clinical practice recommendations: Hemodialysis adequacy, peritoneal dialysis adequacy and vascular access: Update 2006. Am J Kidney Dis 48: S1–S322, 2006. 17045862 [Google Scholar]
- 14.Jansen MAM, Hart AAM, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT; NECOSAD Study Group : Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 62: 1046–1053, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Rottembourg J, Issad B, Gallego JL, Degoulet P, Aime F, Gueffaf B, et al.: Evolution of residual renal function in patients undergoing maintenance haemodialysis or continuous ambulatory peritoneal dialysis. Proc Eur Dial Transplant Assoc 19: 397–403, 1983 [PubMed] [Google Scholar]
- 16.Obi Y, Streja E, Rhee CM, Ravel V, Amin AN, Cupisti A, et al.: Incremental hemodialysis, residual kidney function, and mortality risk in incident dialysis patients: A cohort study. Am J Kidney Dis 68: 256–265, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Stone JC: The effect of dialyzer membrane and etiology of kidney disease on the preservation of residual renal function in chronic hemodialysis patients. ASAIO J 41: M713–M716, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Menon MK, Naimark DM, Bargman JM, Vas SI, Oreopoulos DG: Long-term blood pressure control in a cohort of peritoneal dialysis patients and its association with residual renal function. Nephrol Dial Transplant 16: 2207–2213, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, et al.: Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 11: 556–564, 2000 [DOI] [PubMed] [Google Scholar]
- 20.McIntyre CW: Recurrent circulatory stress: The dark side of dialysis. Semin Dial 23: 449–451, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Toth-Manikowski SM, Sozio SM: Cooling dialysate during in-center hemodialysis: Beneficial and deleterious effects. World J Nephrol 5: 166–171, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selby NM, Burton JO, Chesterton LJ, McIntyre CW: Dialysis-induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol 1: 1216–1225, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Selby NM, McIntyre CW: A systematic review of the clinical effects of reducing dialysate fluid temperature. Nephrol Dial Transplant 21: 1883–1898, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Odudu A, Eldehni MT, McCann GP, McIntyre CW: Randomized controlled trial of individualized dialysate cooling for cardiac protection in hemodialysis patients. Clin J Am Soc Nephrol 10: 1408–1417, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eldehni MT, Odudu A, McIntyre CW: Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol 26: 957–965, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lysaght MJ, Vonesh EF, Gotch F, Ibels L, Keen M, Lindholm B, et al.: The influence of dialysis treatment modality on the decline of remaining renal function. ASAIO Trans 37: 598–604, 1991 [PubMed] [Google Scholar]
- 27.Jefferies HJ, Burton JO, McIntyre CW: Individualised dialysate temperature improves intradialytic haemodynamics and abrogates haemodialysis-induced myocardial stunning, without compromising tolerability. Blood Purif 32: 63–68, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol 4: 1925–1931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julious SA: Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 4: 287–291, 2005 [Google Scholar]
- 30.Whitehead AL, Julious SA, Cooper CL, Campbell MJ: Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res 25: 1057–1073, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Artz NS, Sadowski EA, Wentland AL, Grist TM, Seo S, Djamali A, et al.: Arterial spin labeling MRI for assessment of perfusion in native and transplanted kidneys. Magn Reson Imaging 29: 74–82, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khatir DS, Pedersen M, Jespersen B, Buus NH: Evaluation of renal blood flow and oxygenation in CKD using magnetic resonance imaging. Am J Kidney Dis 66: 402–411, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Rossi C, Artunc F, Martirosian P, Schlemmer HP, Schick F, Boss A: Histogram analysis of renal arterial spin labeling perfusion data reveals differences between volunteers and patients with mild chronic kidney disease. Invest Radiol 47: 490–496, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Gillis KA, McComb C, Patel RK, Stevens KK, Schneider MP, Radjenovic A, et al.: Non-contrast renal magnetic resonance imaging to assess perfusion and corticomedullary differentiation in health and chronic kidney disease. Nephron 133: 183–192, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Filler G, Ramsaroop A, Stein R, Grant C, Marants R, So A, et al.: Is testosterone detrimental to renal function? Kidney Int Rep 1: 306–310, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sjolund J, Garcia Anton D, Bayes LY, Hoekstra T, Dekker FW, Munoz Mendoza J: Diuretics, limited ultrafiltration, and residual renal function in incident hemodialysis patients: A case series. Semin Dial 29: 410–415, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Daugirdas JT: Pathophysiology of dialysis hypotension: An update. Am J Kidney Dis 38[Suppl 4]: S11–S17, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Ronco C, Kellum JA, Haase M: Subclinical AKI is still AKI. Crit Care 16: 313, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, et al.: Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 3: 19–26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McIntyre CW, Goldsmith DJ: Ischemic brain injury in hemodialysis patients: Which is more dangerous, hypertension or intradialytic hypotension? Kidney Int 87: 1109–1115, 2015 [DOI] [PubMed] [Google Scholar]
- 41.McIntyre CW: Haemodialysis-induced myocardial stunning in chronic kidney disease - a new aspect of cardiovascular disease. Blood Purif 29: 105–110, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dasselaar JJ, Slart RH, Knip M, Pruim J, Tio RA, McIntyre CW, et al.: Haemodialysis is associated with a pronounced fall in myocardial perfusion. Nephrol Dial Transplant 24: 604–610, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Dorairajan S, Chockalingam A, Misra M: Myocardial stunning in hemodialysis: What is the overall message? Hemodial Int 14: 447–450, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Crowley LE, McIntyre CW: Remote ischaemic conditioning-therapeutic opportunities in renal medicine. Nat Rev Nephrol 9: 739–746, 2013 [DOI] [PubMed] [Google Scholar]
- 46.McIntyre CW, Lambie SH, Fluck RJ: Biofeedback controlled hemodialysis (BF-HD) reduces symptoms and increases both hemodynamic tolerability and dialysis adequacy in non-hypotension prone stable patients. Clin Nephrol 60: 105–112, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Ronco C, Brendolan A, Milan M, Rodeghiero MP, Zanella M, La Greca G: Impact of biofeedback-induced cardiovascular stability on hemodialysis tolerance and efficiency. Kidney Int 58: 800–808, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Selby NM, Lambie SH, Camici PG, Baker CS, McIntyre CW: Occurrence of regional left ventricular dysfunction in patients undergoing standard and biofeedback dialysis. Am J Kidney Dis 47: 830–841, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Bos WJ, Bruin S, van Olden RW, Keur I, Wesseling KH, Westerhof N, et al.: Cardiac and hemodynamic effects of hemodialysis and ultrafiltration. Am J Kidney Dis 35: 819–826, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, et al.: Longer treatment time and slower ultrafiltration in hemodialysis: Associations with reduced mortality in the DOPPS. Kidney Int 69: 1222–1228, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Marrón B, Remón C, Pérez-Fontán M, Quirós P, Ortíz A: Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int Suppl 73: S42–S51, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Selby NM, McIntyre CW: Peritoneal dialysis is not associated with myocardial stunning. Perit Dial Int 31: 27–33, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Polinder-Bos HA, García DV, Kuipers J, Elting JWJ, Aries MJH, Krijnen WP, et al.: Hemodialysis induces an Acute decline in cerebral blood flow in elderly patients. J Am Soc Nephrol 29: 1317–1325, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chesterton LJ, Selby NM, Burton JO, Fialova J, Chan C, McIntyre CW: Categorization of the hemodynamic response to hemodialysis: The importance of baroreflex sensitivity. Hemodial Int 14: 18–28, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Carreira MA, Nogueira AB, Pena FM, Kiuchi MG, Rodrigues RC, Rodrigues RR, et al.: Detection of autonomic dysfunction in hemodialysis patients using the exercise treadmill test: The role of the chronotropic index, heart rate recovery, and R-R variability. PLoS One 10: e0128123, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klein DA, Katz DH, Beussink-Nelson L, Sanchez CL, Strzelczyk TA, Shah SJ: Association of chronic kidney disease with chronotropic incompetence in heart failure with preserved ejection fraction. Am J Cardiol 116: 1093–1100, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gorre F, Vandekerckhove H: Beta-blockers: Focus on mechanism of action. Which beta-blocker, when and why? Acta Cardiol 65: 565–570, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Velasquez MT, Beddhu S, Nobakht E, Rahman M, Raj DS: Ambulatory blood pressure in chronic kidney disease: Ready for prime time? Kidney Int Rep 1: 94–104, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al.: Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 352: 539–548, 2005 [DOI] [PubMed] [Google Scholar]
- 60.McGuire S, Horton EJ, Renshaw D, Jimenez A, Krishnan N, McGregor G: Hemodynamic instability during dialysis: The potential role of intradialytic exercise. BioMed Res Int 2018: 8276912, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu AW, Ing TS, Zabaneh RI, Daugirdas JT: Effect of dialysate temperature on central hemodynamics and urea kinetics. Kidney Int 48: 237–243, 1995 [DOI] [PubMed] [Google Scholar]
- 62.Yu AW, Lai KN: Dialysis hypotension and splanchnic circulation. Int J Artif Organs 21: 774–777, 1998 [PubMed] [Google Scholar]
- 63.Dheenan S, Henrich WL: Preventing dialysis hypotension: A comparison of usual protective maneuvers. Kidney Int 59: 1175–1181, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Azar AT: Effect of dialysate temperature on hemodynamic stability among hemodialysis patients. Saudi J Kidney Dis Transpl 20: 596–603, 2009 [PubMed] [Google Scholar]
- 65.Rezki H, Salam N, Addou K, Medkouri G, Benghanem MG, Ramdani B: Comparison of prevention methods of intradialytic hypotension. Saudi J Kidney Dis Transpl 18: 361–364, 2007 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.