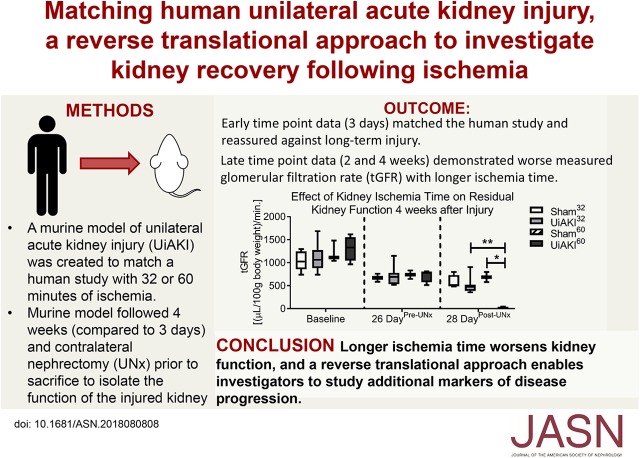
Significance Statement
Unilateral ischemia-reperfusion AKI (UiAKI) occurs during kidney-sparing surgeries, but the effect of ischemia duration on kidney injury or recovery remains unclear. Some have interpreted recent findings from a human study that described reassuring short-term outcomes after UiAKI, even with ischemia lasting up to an hour, as suggesting that the human kidney is remarkably tolerant to prolonged ischemia. Using a murine model matched to parameters of that human study, the authors described longer-term histologic and functional outcomes 14 and 28 days after UiAKI, finding increased fibrosis and reduced GFR in the injured kidney that corresponded to the duration of ischemia. These findings demonstrate that the duration of ischemia affects outcomes, including long-term kidney scarring and functional decline, and that short-term findings can be falsely reassuring.
Keywords: acute kidney injury, animal models of disease, reverse translational research approach, chronic kidney disease, unilateral ischemia reperfusion injury
Visual Abstract
Abstract
Background
The duration of renal ischemia that is associated with (or leads to) renal injury in patients is uncertain, and a reverse translational research approach has been proposed to improve animal models of AKI to facilitate clinical translatability. We developed a two murine models of unilateral renal ischemia to match a recently published human study that investigated renal injury after unilateral renal ischemia during partial nephrectomy.
Methods
Eight 10-week-old C57BL/6 male mice underwent left UiAKI or sham procedure, with or without intra-operative ice packs. Functional, histological, and biomarker outcomes were followed at 2, 6 and 24 hours, or 14 or 28 days later. The 14 and 28 day cohorts were duplicated such that contralateral nephrectomy could be performed 3 days prior to sacrifice with functional measurements obtained to isolate the glomerular filtration rate of the injured kidney.
Results
The short-term outcomes correlated with the human study findings with urine and serum biomarkers of injury peaking around 24 hours and then normalizing, and reassuring immediate histological outcomes. Functional and histological outcomes at the later time-points (14 and 28 days) demonstrate an increase in fibrosis markers, and a reduction in glomerular filtration rate in the injured kidney, corresponding to the duration of ischemia, while serum and urine biomarkers remained reassuring.
Conclusions
Our findings suggest that clinically available biomarkers of renal function are falsely reassuring against long-term injury following UiAKI, and that the duration of ischemia correlates with impaired function and increased fibrosis.
Unilateral renal ischemia (UiAKI) occurs during renal-sparing surgeries, and there has been an increase in the utilization of nephron-sparing techniques in the treatment of renal cancers, which have an incidence of >10 per 100,000 individuals in developed countries.1,2 Although numerous nonsurgical variables affect postpartial nephrectomy kidney function, the duration of ischemia that allows for complete renal recovery remains controversial. The definition of warm ischemia is still debated3,4; however, warm ischemia time generally refers to the technique of inducing ischemia-reperfusion while maintaining core body temperature is maintained intraoperatively and efforts are made to minimize intraoperative surgical heat loss. There are multiple methods of inducing cold ischemia depending on surgical approach. Two general approaches of inducing cold ischemia include (1) cooling the core body temperature to 32°C, and (2) cooling the kidney to 4°C via an ice bath.5 The latter method is commonly used clinically during renal-sparing surgeries via in situ ice packing for the first 10 minutes of ischemia. Recovery from cold ischemia has been found to be superior from that of warm ischemia.6 Shorter ischemia times are tolerated in warm ischemia than in cold ischemia, with warm ischemia times >20–25 minutes being associated with AKI and progression to significant CKD.7,8 Additionally, the parameters to define kidney function “recovery” are poorly described.9 Use of serum creatinine (SCr) is inherently flawed and insensitive because SCr is a late marker of impaired GFR, and there is inherent variability in measurements depending on the assay utilized.10,11 Direct measurements of GFR are time-consuming, costly, and infrequently performed. Longer-term histologic evidence of injury and fibrosis are typically not clinically assessed. In sum, studies investigating this important clinical question have yielded variable results, and the ideal duration of ischemia time remains unknown.12–14
Recently, the most comprehensive assessment of histologic and biomarker injury during unilateral ischemia in patients was published by Parekh et al.15 In this prospective study, patients undergoing UiAKI during partial nephrectomy were evaluated for kidney histologic, serum, and urine AKI biomarker changes pre-, during, and post-ischemia. Their findings suggested that although there was evidence of acute biomarker and immediate histologic injury associated with ischemia, the injury was short-lived and did not correlate with duration of ischemia. A limitation of this study was the lack of available outcomes beyond the immediate postoperative period, with the latest time point evaluated for histologic injury being 5 minutes postreperfusion. Ischemia times ranged from 15 to 61 minutes, and the duration of renal ischemia time did not appear to correlate with histologic injury, which was relatively mild at 5 minutes postreperfusion. The increase in SCr and renal injury biomarkers was also mild. A follow-up study published by this group evaluated renal function 1 year after surgery16 and demonstrated a 12.99% reduction in estimated GFR by SCr. Interestingly, this study suggested that duration of ischemia did not correlate with outcome, although the P value did approach significance (P=0.09) when duration of ischemia was evaluated as a continuous variable. Notably, this study did not perform long-term functional measurements nor investigate the split function of the kidneys.
In murine models of ischemic AKI, ischemia time directly correlates to renal injury and the rise in SCr and histologic injury is typically dramatic. On the basis of these observations, some have concluded that that murine models of ischemic AKI may not be relevant to humans; especially because histologic injury was minimal in the human study and widespread tubular necrosis is observed in murine models.17 It is important to note, however, that there are important differences between the human study and most animal models. Murine models are typically designed to optimize, or rather, increase the degree of renal injury to study pathways of disease recovery versus progression. For obvious reasons, human studies aim to minimize injury to diminish disease progression. For this reason, most animal models use warm ischemia rather than cold, and take care not to overhydrate the animals after injury. Conversely, standard clinical practice often uses cold ischemia in combination with brisk hydration and mannitol administration. Another key point of consideration is whether unilateral versus bilateral ischemia is performed, as unilateral versus bilateral ischemia results in very different renal and nonrenal sequelae.18 Unilateral ischemia typically does not result in an increase in either SCr or BUN, nor an increase in cytokines associated with AKI such as IL-6, and deleterious systemic effects such as lung inflammation do not occur.19 It has also been recognized that the healthy contralateral kidney affects fibrotic outcomes in unilateral ischemia, with removal of the contralateral kidney mitigating fibrosis in the injured kidney.20 In contrast, bilateral ischemia causes a dramatic rise in SCr and BUN as well as an increase in systemic inflammation, including a rise in serum IL-6 and lung inflammation. Therefore, both unilateral and bilateral ischemia-reperfusion animal models have been used to help understand human disease progression, and much work has been performed in murine models to delineate the functional and histologic outcomes of both unilateral and bilateral ischemia-reperfusion.21 Notably, disease progression depends on the model of injury used, as well as the animal species and strain. Thus, translation of murine models to humans requires careful attention to the details (e.g., unilateral versus bilateral ischemia, cold versus warm ischemia, etc.) of both the murine and human condition studied to assure translatability.22 Finally, the timing of the measurements after injury is critically important when comparing human and murine models– in murine models of ischemia-reperfusion injury, histologic injury is typically assessed at 24 hours postischemic reperfusion, whereas histologic injury in the human study was assessed at 5 minutes. Thus, there are many differences between the typical models of murine ischemia-reperfusion injury and the detailed results in the human model described.
In light of these challenges and limitations of translatability from murine to human disease, a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop was recently convened and published recommendations on overcoming translational barriers in AKI.23 A key recommendation from this report includes a reverse translational research approach, which proposes that murine models should be matched to a specific human disease process and then studied to provide insights into disease progression. This is a significant departure from the traditional translational approach, which typically starts with investigation of an animal model of disease, which then leads to human clinical trials on the basis of animal research findings. We have therefore adopted this recommendation and have herein utilized a reverse translational approach to study the effect of ischemia time on kidney recovery. We developed two models of murine unilateral ischemia-reperfusion that closely match the published human model by Parekh et al.15 We hypothesized that a matched murine model would (1) produce similar short-term insights in renal recovery after UiAKI found in the human study, and (2) provide invaluable insights into the long-term disease progression, not readily available in human studies.
Methods
Surgical Procedures
All surgical procedures and animal care were approved by the Institutional Animal Care and Use Committee at the University of Colorado. Adult (10–12 week old) male C57BL/6 mice (Jackson Labs, Bar Harbor, ME) underwent UiAKI on the left kidney. Controls included healthy mice as well as those that underwent sham procedure, which was identical to the UiAKI procedure sans clamping. In the first model, mice were subjected to cold ischemia, meaning there was no heating pad throughout the procedure. Two ischemia times were used, 32 minutes (UiAKI32) and 60 minutes (UiAKI60). In the second model, cold ice packs were utilized around the left kidney for the first 10 minutes of the sham or ischemia-reperfusion procedure with both the 32 and 60 minute times used (UiAKI32ice and UiAKI60ice).
A total of 4 ml normal saline was given within 2 hours of the procedure time. Blood was collected via retro-orbital sampling 2 hours postoperative with 1 ml of normal saline being delivered subcutaneously immediately after the blood draw. Blood was also collected at 6 hours via retro-orbital collection. Animals were euthanized at either 5 minutes, 2 hours, 6 hours, 24 hours, 14 days, or 28 days postreperfusion or sham procedure. Animals were weighed to detect any fluid overload, and no concerns of volume overload arose in any groups throughout the study, as would be expected given the presence of the uninjured contralateral kidney. Blood collection at euthanasia was performed via cardiac puncture. We duplicated the 14 and 28 day cohort study time points to perform unilateral nephrectomy (UNx) of the contralateral kidney 2 days before euthanizing via dorsal approach. For both procedures, anesthesia was provided via ketamine/xylazine and analgesia via buprenorphine as previously described.24
Transcutaneous GFR
Transcutaneous GFR (tGFR) measurements, normalized for animal weight, were obtained on the 14 and 28 day cohorts at: (1) baseline, before the sham or UiAKI procedure, (2) 2 days before the UNx if applicable, and (3) just before euthanizing. Measurements were obtained in the 28 day “ice” cohorts at (1) baseline, (2) 1 day postprocedure, (3) 2 days pre-UNx and (4) just before euthanizing. The NIC-Kidney device (MediBeacon Inc., Amtsgericht, Germany) was utilized for tGFR measurements per the manufacturer’s instructions.25
Tissue Collection and Analysis
Blood samples were processed as previously described.26 Urine, when available, was collected 1 day postoperatively and at euthanasia. Kidneys were collected at euthanasia. BUN (BioAssay Systems QuantiChrom, Hayward, CA), enzymatic creatinine (Pointe Scientific, Canton, MI), cystatin C (R&D Systems, Minneapolis, MN), neutrophil gelatinase-associated lipocalin (NGAL) (R&D Systems), liver fatty acid-binding protein (R&D Systems), kidney injury molecule-1 (KIM-1) (R&D Systems), and albumin (Excocell Albuwell, Philadelphia, PA) were processed and analyzed per the manufacturers’ instructions.
RNA was isolated from flash-frozen kidney halves and complementary DNA synthesis was performed as described previously.27 RNA extracted with RNA-Bee reagent (TEL-TEST, Inc., Friendswood, TX). RNA quantification and quality was determined using a NanoDrop 2000c instrument (Thermo Fisher Scientific, Waltham, MA). Complementary DNA was amplified and labeled using SYBR Green Supermix PCR (Bio-Rad, Hercules, CA). Gene expression is expressed as relative gene expression calculated using the 2ΔΔCT method, as described.28 Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) mRNA was used as a loading control. Primer sequences were as follows: Gapdh: forward TGGAGAAACCTGCCAAGTATGA, reverse GAAGAGTGGGAGTTGCTGTTGA; Kim-1: forward AAACCAGAGATTCCCACACG, reverse GTCGTGGGTCTTCCTGTAGC; NGAL: forward GCAGGTGGTACGTTGTGGG, reverse CTCTTGTAGCTCATAGATGGTGC; Collagen type 1: forward CCCGCCGATGTCGCTAT, reverse GCTACGCTGTTCTTGCAGTGAT; Collagen type 3: forward CTGTAACATGGAAACTGGGGAAA, reverse CCATAGCTGAACTGAAAACCACC.
Histologic Analysis
Tissue was fixed in formalin and embedded in paraffin. Then, 4 μm sections were cut and stained for Picrosirius red (PSR) (Sigma-Aldrich, St. Louis, MO). ImageJ software was used to measure the percentage of collagen-stained cortical area with consistent threshold colors set to detect positive PSR staining. Immunohistochemistry for collagen type 3 was performed on kidney tissue following standard protocols, using goat anti-type 3 collagen antibody (Southern Biotech, Birmingham, AL) (1:100) overnight at 4°C followed by rabbit anti-goat horseradish peroxidase (Dako, Carpinteria, CA) (1:200) for 45 minutes. Twenty cortical images at 200× magnification were obtained per sample and imaging software (Aperio) was used to analyze images for a positive pixel counts, measuring the percentage of positive immunohistochemistry staining per high-power field. Periodic acid–Schiff (PAS) stain was performed using standard manufacturing instructions (Merck KGaA, Darmstadt, Germany). For quantitative scoring of tubular injury, all nonoverlapping fields in cortex were semiquantitated separately by a blinded pathologist (×400 magnification) using the following scoring system: 0, no injury; 1, 1%–25% of area injured; 2, 26%–50%; 3, 51%–75%; and 4, 76%–100%. Tubular injury was defined as sloughing of tubular epithelial cells, tubular cast formation, tubular dilatation, tubular cell vacuolization, or necrosis. For electron microscopy, kidneys were dissected and immersed in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M cacodylate buffer at pH 7.4 for a minimum of 24 hours at 4°C. For processing, the tissue was trimmed into small cubes (approximately 1 mm3), rinsed in 100 mM cacodylate buffer, and then immersed in 1% osmium for 1 hour, and then rinsed again five times for 2 minutes each in cacodylate buffer and two times briefly in water. The tissue was dehydrated in standard fashion and transferred through propylene oxide at room temperature and embedded in LX112 and cured for 48 hours at 60°C in an oven. Ultrathin sections (65 nm) were cut on a Reichert Ultracut S. Sections were counterstained with uranyl acetate and lead citrate and imaged on a FEI Tecnai G2 transmission electron microscope (Hillsboro, OR) with a Gatan digital camera (Pleasanton, CA).
Hydroxyproline Assay
Frozen kidney tissues (20–30 mg tissue) were homogenized in water using a bead homogenizer, TissueLyser LT (Qiagen, Germantown, MD). An aliquot was removed for direct protein measurement using a Bradford assay (Bio-Rad). The remaining solution was mixed with 12N HCl (Fisher Scientific, Hampton, NH) and hydrolyzed at 120°C for 24 hours. An equal amount of protein per sample was loaded into 2.0 ml tubes (Eppendorf, Hamburg, Germany) and the solvent was evaporated in a fume hood. Hydroxyproline standards (Sigma) and samples were analyzed together. An acetate/citrate buffer (pH 6.0) containing 1.4% chloramine T (Sigma) was first added to the samples. Ehrlich reagent, containing 60% perchloric acid and para-dimethylbenzaldehyde (Sigma) was then added and the samples were heated to 60°C for 25 minutes. The hydroxyproline content was measured by reading the absorbance at 560 nm.
Statistical Analyses
Each group size was n=5–11. Data are reported as means±SEM. For multiple groups, ANOVA with P<0.05 was used to determine statistical significance with Dunnett post hoc test. For direct comparisons between two groups, an unpaired t test with Welch correction and P<0.05 was used. In figures, a single asterisk indicates a statistical difference between the UiAKI group and its paired sham control (e.g., UiAKI32 versus sham32); a double asterisk indicates a statistical difference between UiAKI32 and UiAKI60.
Results
Acute (within 24 Hours) Results
To demonstrate that our murine model of UiAKI appropriately matched the human study, histology, estimated kidney function (SCr, BUN), and renal injury biomarkers were determined after either 32 or 60 minutes of UiAKI reperfusion. These two ischemia times were the mean and upper limit of ischemia times, respectively, reported in the human study.
Acute Histologic Outcomes
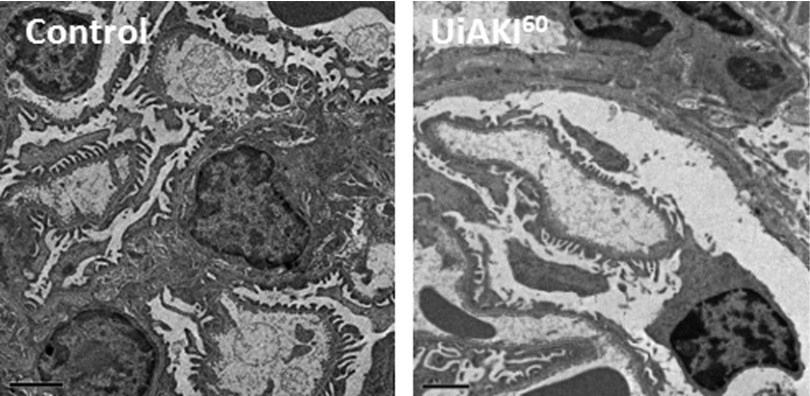
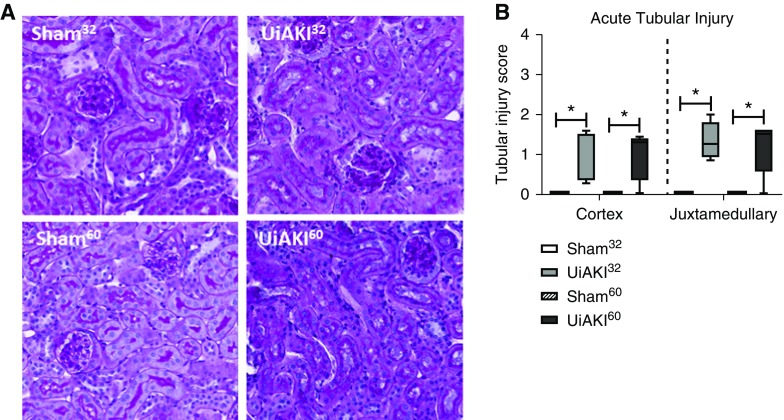
Electron microscopy obtained 10 minutes after reperfusion demonstrated no acute changes between the healthy controls and UiAKI60 cohorts (Figure 1). Tubular injury scores were determined on PAS-stained tissue obtained 5 minutes postprocedure (Figure 2) and demonstrate mild cortical and juxtamedullary tubular injury in both the UiAKI32 and UiAKI60 cohorts, similar to the results in the human study, with only minimal tubular injury noted (Figure 2B). As in the human study, there was no significant difference in tubular injury between the different ischemia times.
Figure 1.
Ischemia does not affect immediate histology. Histologic changes were assessed in the left kidneys 10 minutes after 60 minutes of left unilateral ischemia AKI (UiAKI) or sham procedure. Electron microscopy demonstrates no acute changes between the healthy control (left) and 60 minutes of ischemia (right). Scale bar, 2 μm.
Figure 2.
Ischemia leads to mild acute tubular injury. Histologic changes were assessed in the left kidneys 10 minutes after either 32 or 60 minutes of left unilateral ischemia AKI (UiAKI) or sham procedure. (A) Representative images of PAS (×200 magnification) staining and (B) acute tubular necrosis scoring demonstrate tubular injury in the ischemic kidneys was mild and without significant difference between ischemia times. n=5; P<0.05. * indicates a statistical difference between the UiAKI group and its paired sham control (e.g., UiAKI32 versus sham32); ** indicates a statistical difference between the 32-minute and 60-minute ischemia times.
Acute Postoperative Estimated Renal Function and Injury using Serum and Urine Biomarkers
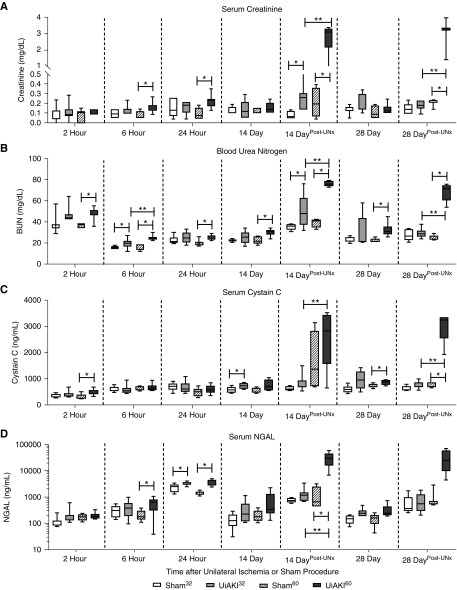
To assess kidney function and injury after UiAKI, SCr, BUN, serum cystatin C, serum and urine NGAL, urine Kim-1, and urine albumin were determined at 2, 6, and 24 hours postprocedure. SCr peaked at 24 hours, with a statistically significant increase in UiAKI60 (0.22 mg/dl) versus UiAKI32 (0.16 mg/dl) and sham (Figure 3A). BUN and serum cystatin C both peaked 2 hours after UiAKI60, and did not increase at any time after UiAKI32 or sham (Figure 3, B and C).
Figure 3.
Duration of ischemia affects serum biomarkers over time. To determine the effect of ischemia time on serum biomarkers of renal function, mice underwent unilateral left ischemia or sham procedure for either 32 or 60 minutes, and (A) SCr, (B) BUN, (C) serum cystatin C, and (D) serum NGAL were measured at 2, 6, and 24 hours, and 14 and 28 days. To isolate the residual function of the left injured kidney, additional 14 and 28 day cohorts underwent right UNx 2 days before the 14 and 28 day time points (14 DayPost-Unx and 28 DayPost-Unx). n=5–7; P<0.05. * indicates a statistical difference between the UiAKI group and its paired sham control (e.g., UiAKI32 versus sham32); ** indicates a statistical difference between the 32-minute and 60-minute ischemia times.
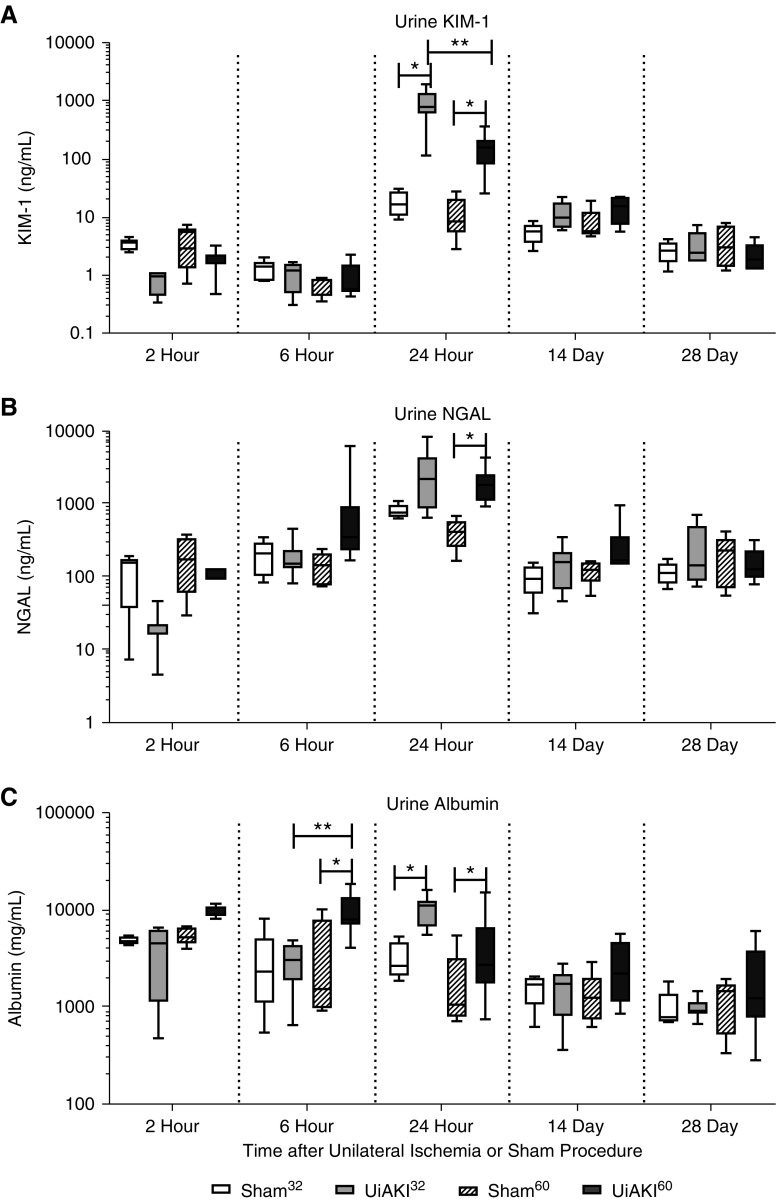
Serum NGAL peaked at the 24 hour time point and was increased in both UiAKI32 (3271 ng/ml) versus sham32 (2347 ng/ml) and UiAKI60 (3506 ng/ml) versus sham60 (1441 ng/ml) (P=0.03), but no statistical difference between the two ischemia times was observed. Urine NGAL and KIM-1 both peaked at 24 hours with increases in both UiAKI32 and UiAKI60 versus sham32 and sham60, respectively (Figure 4, A and B). Urine albumin peaked at 6 hours and (Figure 4C) was increased in the UiAKI60 (10126 ng/ml) cohort at that time compared with both the sham60 (3554 ng/ml) and UiAKI32 (2931 ng/ml) groups.
Figure 4.
Urine biomarkers normalize early, regardless of ischemia duration. To determine the effect of ischemia time on renal injury, mice underwent unilateral left ischemia or sham procedure for either 32 or 60 minutes, and urine was collected 2, 6, and 24 hours, and 14 and 28 days after the procedure and (A) KIM-1, (B) NGAL, and (C) albumin and were measured. n=3–7; P<0.05. * indicates a statistically significant difference between either the 32 or 60 minute sham and UiAKI groups, ** indicates a statistically significant difference between the 32 and 60 minute UiAKI groups.
Acute mRNA Expression
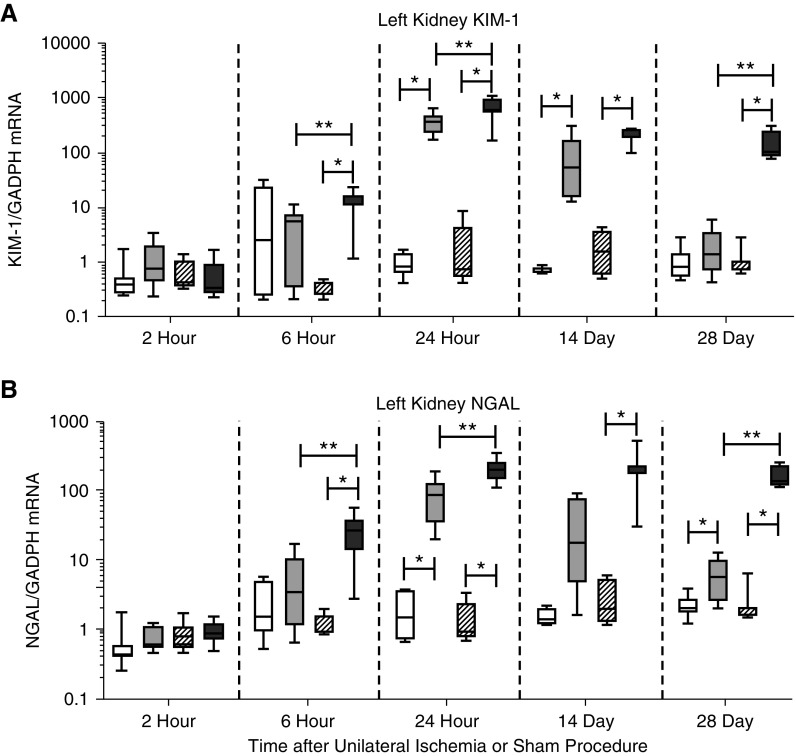
KIM-1 mRNA increased after both the UiAKI32 and UiAKI60 versus sham, and was statistically increased in UiAKI60 versus UiAKI32 (Figure 5A). NGAL mRNA also peaked at 24 hours postinjury, with a statistically significant increase in expression between the UiAKI60 and sham60 cohorts (Figure 5B).
Figure 5.
Ischemia duration correlates with early and late mRNA expression of injury biomarkers. To determine the effect of ischemia time on expression of KIM-1 and NGAL, mice underwent unilateral left ischemia or sham procedure for either 32 or 60 minutes. The left kidneys were collected after euthanasia at 2, 6, and 24 hours, and 14 and 28 days after the procedure, and (A) KIM-1 and (B) NGAL were measured via RT-PCR. n=3–10; P<0.05. * indicates a statistically significant difference between either the 32 or 60 minute sham and UiAKI groups, ** indicates a statistically significant difference between the 32 and 60 minute UiAKI groups.
Taken in sum, these early markers of renal injury correlate well with the early findings in the human study of UiAKI; specifically, commonly utilized clinical serum biomarkers and novel urine biomarkers of AKI peaked within 24 hours and then normalized.
Late Results (14 and 28 Days)
After establishing a matched murine model to the human study, we set out to evaluate the longer term sequelae of UiAKI by assessing serum, urine, histologic, and functional outcomes at 14 and 28 days after injury. These outcomes match the recommended metrics to evaluate renal recovery after AKI.23
To specifically assess the kidney function of the single kidney that experienced ischemia, we duplicated the 14 and 28 day cohorts and performed a right UNx 2 days before assessing end points at days 14 and 28. Thus, kidney function measurements (e.g., via tGFR) would reflect only the function of the kidney that had undergone UiAKI or sham and could assess whether ischemia resulted in any functional impairment that might be masked by compensatory hyperfiltration of the uninjured contralateral kidney.
Estimated Renal Function via Serum and Urine Biomarkers at 14 and 28 Days
In the mice that did not undergo UNx, the majority of serum and urine biomarkers of kidney injury had normalized by 14 and 28 days; specifically, SCr, serum and urine NGAL, urine KIM-1, and urine albumin were similar between sham and UiAKI at these time points, as well as compared with baseline, suggesting that kidney function had returned to normal in both UiAKI32 and UiAKI60. However, the cohorts that underwent UNx 2 days before either day 14 or 28 demonstrated significant increases in serum biomarkers of function, SCr and BUN. In mice without UNx on day 14, cystatin C was increased in UiAKI32 versus sham32, but levels were similar between UiAKI60 versus sham60; at 28 days, cystatin C was not different between UiAKI32 versus sham32, but was increased in UiAKI60 versus sham60 (Figure 3C). In the cohorts that underwent UNx 2 days before day 14 as well as day 28, serum cystatin C was increased in UiAKI60 compared with both the sham60 and UiAKI32 groups. These findings suggest that despite reassuring serum biomarkers without UNx, the ischemic kidney had poor residual kidney function. Furthermore, both SCr and BUN were increased to a greater extent in UiAKI60 compared with UiAKI32, indicating that the longer ischemia time correlates with the degree of long-term impairment. Indeed, upon isolation of the injured kidney, this correlation between duration of ischemia and biomarker levels was a consistent finding, with only the 28 day UiAKI60 NGAL not demonstrating a further significance between ischemia times, although it did trend toward significance (P=0.06).
Expression of KIM-1 mRNA remained elevated in both the UiAKI32 and UiAKI60 groups at the 14 and 28 day time points with an increase the UiAKI60 cohort compared with UiAKI32 at 28 days (Figure 5A). NGAL expression (Figure 5B) mirrored that of KIM-1, except that there was no statistically significant increase in the UiAKI32 compared with sham32 at 14 days.
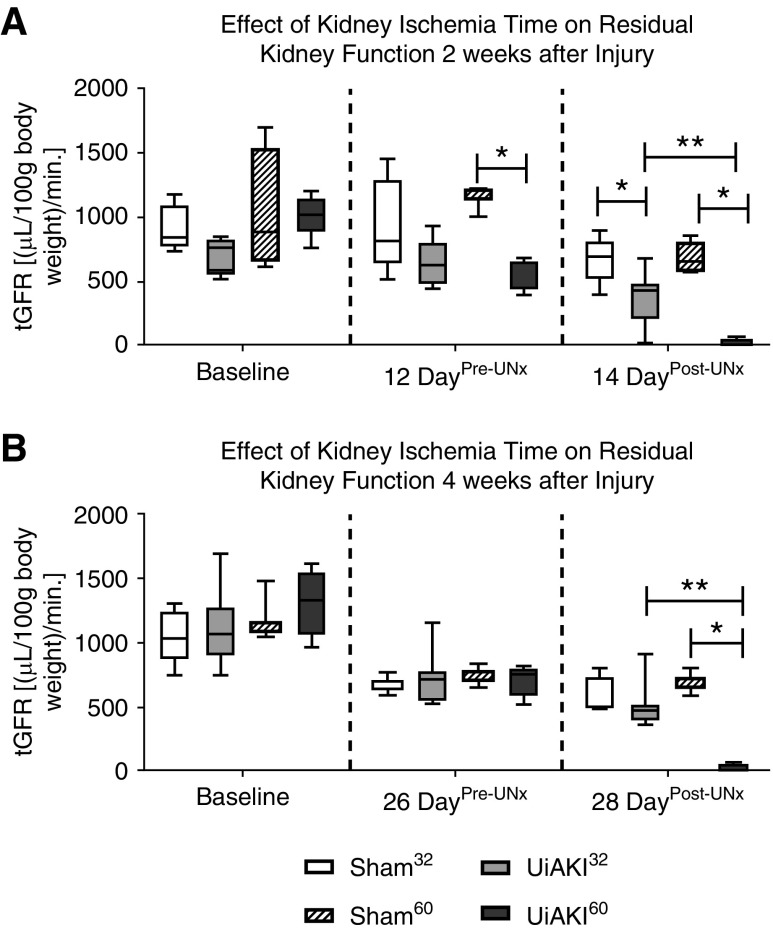
Measured Kidney Function via tGFR
Both the 14 and 28 day cohorts that underwent UNx had serial tGFR measurements pre- and post-UNx (Figure 6). Before the UNx, the tGFR reflects the combined function of both the healthy and injured kidneys. After the UNx, the tGFR reflects the residual function of only the injured kidney that had undergone UiAKI or sham.
Figure 6.
Measured kidney function post-contralateral nephrectomy unmasks the loss of function in the injured kidney. To assess the effect of ischemia time on residual renal function, serial measurements of tGFR were measured at baseline, after 32 or 60 minutes of left unilateral ischemic AKI (UiAKI) or sham procedure before contralateral nephrectomy (Pre-UNx) and again after contralateral nephrectomy (Post-UNx), in both the 14 and 28 day cohorts. Removal of the contralateral right kidney allows for a functional assessment of the left injured kidney. There was no statistical difference in baseline tGFR (A and B). Pre-UNx, tGFR was reduced in the 60 minute ischemia group at (A) the 12 day time point, but not (B) the 26 day time point. Post-UNx, both ischemia times resulted in a decrease in measured kidney function at the 14 day time point (A), whereas only 60 minutes of ischemia resulted in a significant decrease in function at the 28 day time point (B). The residual kidney function of the left kidneys submitted to 60 minutes of ischemia was nil at both the 14 and 28 day time points (A and B). n=5–7; P<0.05. * indicates a statistical difference between the UiAKI group and its paired sham control (e.g., UiAKI32 versus sham32); ** indicates a statistical difference between the 32-minute and 60-minute ischemia times.
Before UNx, tGFR at 12 days was reduced in UiAKI60 but not UiAKI32 versus their shams, respectively (Figure 6A); at 26 days, tGFR was similar among sham32, UiAKI32, sham60, and UiAKI60, and was similar to baseline values (Figure 6B).
In the 28 day cohort, after UNx, the group that had undergone UiAKI60 demonstrated a marked reduction in function of the remaining left kidney. Kidneys having undergone UiAKI32 were able to function significantly better, with no significant reduction compared with the sham32 and sham60 groups at the 28 day time point (Figure 6B). Conversely, the cohort euthanized 14 days after UiAKI or sham demonstrated a significant reduction in tGFR in both the UiAKI32 and UiAKI60 groups. However, the UiAKI32 group’s average tGFR was appropriately approximately 50% of pre-UNx tGFR, indicating fairly equal function compared with the healthy, nephrectomized kidney; this finding was similar in the sham32 cohort. Conversely, the UiAKI60 group’s average tGFR was almost nil, indicating severe reduction in residual renal function. This indicates that nearly the entirety of the pre-UNx tGFR in the UiAKI60 cohort was contributed by the healthy, uninjured kidney.
Markers of Fibrosis
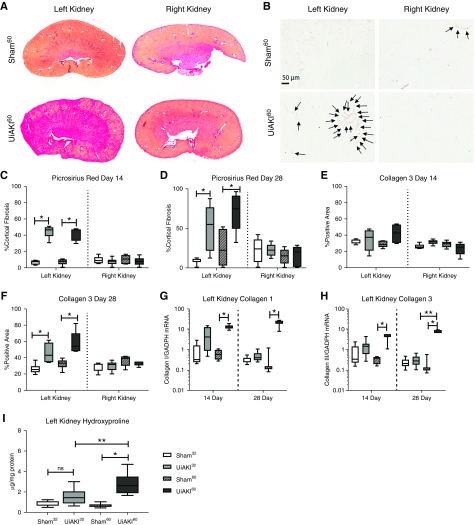
Quantification of PSR staining at 14 and 28 days (Figure 7, C and D) demonstrates an increase in fibrosis in both the UiAKI32 and UiAKI60 cohorts in the left kidneys that underwent ischemia with no difference in the right kidneys. Quantification of Collagen type 3 immunohistochemistry shows no significant difference at 14 days (Figure 7E), but an increase in both the UiAKI32 and UiAKI60 cohorts compared with sham controls in the left kidneys at 28 days (Figure 7F), with no significant difference in the right kidneys. Expression of Collagen type 1 and Collagen type 3 (Figure 7, G and H) demonstrated an increase in the UiAKI60 cohort at 14 and 28 days. Expression of Collagen type 3 at 28 days was also significantly increased in the UiAKI60 compared with UiAKI32 group. Hydroxyproline (Figure 7I) content, a biochemical assay that can detect collagen content with a high degree of sensitivity and specificity,29 measured in the left kidneys of the 28 day cohorts demonstrated an increase in the UiAKI60 cohort compared with both the sham60 and UiAKI32 cohorts.
Figure 7.
Duration of ischemia correlates with markers of renal fibrosis 14 days and 28 days after injury. Histologic changes were assessed in the left and right kidneys after either 32 or 60 minutes of left unilateral ischemia AKI (UiAKI) or sham procedure. (A) PSR staining and (B) immunohistochemistry for collagen type 3 demonstrates increased fibrosis and collagen type 3 deposition in the left (injured) kidneys. Quantification of PSR staining at (C) 14 days and (D) 28 days demonstrates increased fibrosis in the left kidneys, but no difference in the right kidneys. Quantification of immunohistochemistry for collagen type 3 demonstrates an increase in collagen type 3 staining in the left kidneys at (F) the 28 day time point for both 32 and 60 minutes of ischemia (UiAKI32 and UiAKI60), but no difference in the right kidneys at that time-point, and no statistically significant difference in either the left or right kidneys at (E) the 14 day time point. (G and H) Collagen type 1 and Collagen type 3 expression was measured via RT-PCR on left kidneys collected 14 and 28 days after injury, and demonstrate an increased expression in the injured versus sham groups, with further significance noted between ischemia times in Collagen type 3 expression at the 28 day time point. (I) Hydroxyproline content was measured in the left kidneys in the 28 day cohorts and demonstrated a significant increase in the UiAKI versus sham cohorts for both ischemia times, and a further increase in the UiAKI60 versus UiAKI32. n=5–7; P<0.05. Arrows indicate areas of Collagen 3 staining. * indicates a statistically significant difference between either the 32 or 60 minute sham and UiAKI groups, ** indicates a statistically significant difference between the 32 and 60 minute UiAKI groups.
“Ice” Model of Cold Ischemia
To further replicate the clinical study in our reverse translational approach, we developed a the “ice” model of cold ischemia-reperfusion in which the left kidney was packed with sterile ice packs for the first 10 minutes of the sham or UiAKI procedure. We performed the same ischemia/sham times of 32 or 60 minutes, and followed these cohorts for 28 days with a contralateral nephrectomy performed 2 days before euthanizing, on day 26. Serum biomarkers of renal function, histology, and tGFR were measured, and the results are summarized in Figure 8.
Figure 8.
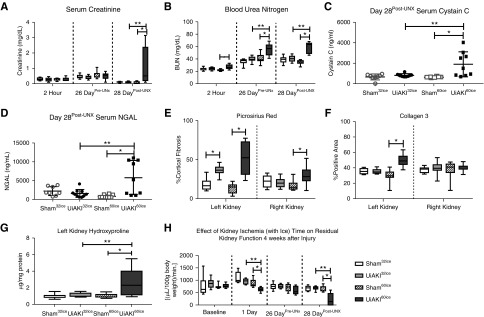
Despite addition of intra-operative ice packs, the duration of ischemia impacts functional biomarkers, markers of fibrosis and measured kidney function 28 days after injury. An additional model of cold ischemia was developed to more closely replicate the study by Parekh et al.15 In these cohorts, the 28 day study was repeated with an ice pack surrounding the left sham or UiAKI kidney for the first 10 minutes of the 32 or 60 minute procedure time. Serum biomarkers of renal function were obtained 2 hours after injury, 2 days before the contralateral nephrectomy (Pre-UNx), and at the time of euthanasia (Post-UNx). (A) SCr demonstrated no significant differences among the groups at either the 2 hour or Pre-UNx time points, but a significant increase in creatinine in the UiAKI60ice cohort Post-UNx. (B) BUN demonstrated a significant increase in the UiAKI60ice cohort at all three time points, and an additional increase between the UiAKI60ice compared with the UiAKI32ice at both the Pre-UNx and Post-UNx time points. (C) Serum cystatin C and (D) NGAL were measured Post-UNx. Both serum biomarkers of injury demonstrated a significant increase in the UiAKI60ice cohort compared with the sham and UiAKI32ice cohorts. There was no increase in the UiAKI32ice cohort compared with sham. (E) Quantification of PSR staining demonstrated an increase in fibrosis in both the UiAKI32ice and UiAKI60ice groups compared with sham in the left kidneys, and a statistically significant increase in fibrosis in the UiAKI60ice group in the right kidney. (F) Quantification of Collagen type 3 immunohistochemistry demonstrated an increase in staining in the UiAKI60ice in the left kidney. (G) Hydroxyproline quantification of the left kidneys demonstrated an increase in the UiAKI60ice group compared with the sham60ice as well as the UiAKI32ice groups. (H) tGFR was measured in all groups at baseline, 1 day after sham or UiAKI procedure, Pre-UNx, and Post-UNx at the time of euthanasia on day 28. There was no difference in function between the cohorts at baseline. One day postprocedure, there was a marked reduction in tGFR in the UiAKI60ice group, but not the UiAKI32ice cohort. 26 days later, Pre-UNx, there was no difference in measured tGFR among the cohorts. Post-UNx, the tGFR of left injured UiAKI60ice cohort was significantly reduced compared with both the sham60ice and UiAKI32ice cohorts. There was no difference in the UiAKI32ice group compared with sham32ice. n=8–11; P<0.05. * indicates a statistical difference between the UiAKI group and its paired sham control (e.g., UiAKI32 versus sham32); ** indicates a statistical difference between the 32-minute and 60-minute ischemia times.
Overall, the results of this experiment mirrored the findings in the original cohorts. There was no difference in SCr at the earliest time point of 2 hours, and it was reassuringly normal at the 26 day time point. Only after the contralateral UNx was performed was there a noticeable increase in SCr in the UiAKI60ice cohort (Figure 8A). BUN was more sensitive to injury and demonstrated a significant increase at all time points in the UiAKI60ice cohort with a significant increase compared with both sham60ice and UiAKI32ice at both the pre- and post-UNx time points (Figure 8B). Serum cystatin C (Figure 8C) and NGAL (Figure 8D) were measured on the post-UNx samples and both demonstrated a significant increase in the UiAKI60ice cohort above both the sham60ice and UiAKI32ice cohorts. Quantification of fibrosis was performed via PSR staining (Figure 8E), immunohistochemistry for Collagen type 3 (Figure 8F), and hydroxyproline content (Figure 8G). In the left kidneys, PSR demonstrated an increase in staining in both the UiAKI32ice and UiAKI60ice compared with their shams. In the right kidneys, there was a slight but statistically significant increase in the UiAKI60ice cohort compared with sham60ice. Collagen type 3 quantification of the left kidneys demonstrated an increase in only the UiAKI60ice compared with sham60ice, and no statistically significant difference was detected in the right kidneys. Hydroxyproline content in the left kidneys demonstrated a significant increase in the UiAKI60ice cohort compared with both its sham60ice control and the UiAKI32ice cohort. Throughout the ice study, tGFR was measured serially at baseline, 1 day postprocedure, and then before and after contralateral UNx (Figure 8H). There was no difference in the tGFR at baseline among the cohorts. One day postprocedure, there was a significant reduction in tGFR in the UiAKI60ice cohort compared with both the sham60ice and UiAKI32ice cohorts. There was no statistically significant reduction in tGFR in the UiAKI32ice cohort compared with sham32ice. Immediately before the contralateral nephrectomy, there was no difference in measured tGFR among the cohorts. Post-UNx, on the day of euthanizing, there was a marked reduction in the tGFR of the UiAKI60ice cohort compared with the sham60ice and UiAKI32ice cohorts. There was no reduction in tGFR in the UiAKI32ice group compared with the sham32ice. Unlike the UiAKI60 cohort, in which the 28 day post-UNx tGFR was nil (Figure 6B), the measured tGFR in the UiAKI60ice cohort was reduced, but not nil.
Table 1 summarizes our reverse translational approach, and compares the findings in the human study with our UiAKI32, UiAKI60, and UiAKIice murine models.
Table 1.
Reverse translational research approach to unilateral ischemia-reperfusion AKI
| Study Parameters | Parekh et al.15 | UiAKI32 | UiAKI60 | UiAKI32ice | UiAKI60ice |
|---|---|---|---|---|---|
| Human study | Murine model | Murine model | Murine model | Murine model | |
| UiAKI model | |||||
| Sex | Male and female | Male | Male | Male | Male |
| Ischemia time | Warm (32.3 min) | Cold (32 min) | Cold (60 min) | Ice pack in surgical site for the first 10 minutes out of 32 | Ice pack in surgical site for the first 10 minutes out of 60 |
| Cold (48 min) | |||||
| Average (37.4 min) | |||||
| BiomarkersSerum | |||||
| BUN | Not performed | Returned to baseline by 24 h | BUN persistently elevated at 28 d | Normal throughout | Elevated at 2 h, normal at 26 d and elevated post-UNx |
| SCr | SCr peaked at 24 h, returned to baseline by 72 h | SCr normalized by 24 h | SCr normalized by day 14 | Normal throughout | SCr normal until post-UNx |
| Cystatin C | No change | Mild elevation in at 14 d | Elevated at 28 d | Normal post-UNx at 28 d | Elevated post-UNx at 28 d |
| NGAL | No change | Peaked at 24 h then normalized | Peaked at 24 h then normalized | Normal post-UNx at 28 d | Elevated post-UNx at 28 d |
| BiomarkersUrine | |||||
| LFABP | Peaked at 2 h | Not performed | Not performed | Not performed | Not performed |
| NAG | Peaked at 2 h | Not performed | Not performed | Not performed | Not performed |
| IL-18 | Elevated 2–24 h | Not performed | Not performed | Not performed | Not performed |
| NGAL | Elevated 2–24 h | Peaked at 24 h | Peaked at 24 h | Not performed | Not performed |
| KIM-1 | Elevated 2–24 h | Peaked at 24 h | Peaked at 24 h | Not performed | Not performed |
| Albumin | Peaked at 2 h | Elevated at 24 h | Elevated at 6–24 h | Not performed | Not performed |
| Acute histology | |||||
| Thick section | No change | EM not performed | No change | EM not performed | EM not performed |
| EM | |||||
| IF actin | No change | IF not performed | IF not performed | IF not performed | IF not performed |
| IF | Changes in end noted Clamp and postclamp IF staining noted | Not performed | Not performed | Not performed | Not performed |
| Phosphotyrosine | Not performed | Not performed | Not performed | Not performed | |
| IF β-1 integrin | |||||
| IF ICAM-1 | Not performed | Not performed | Not performed | Not performed | |
| Tubular injury score | Mild tubular injury | Mild tubular injury | Tubular injury score not performed | Tubular injury score not performed | |
| Late histology | |||||
| PSR | Not performed | Increase in left kidney fibrosis at 14 and 28 d; increase in collagen III at 28 d | Increase in left kidney fibrosis at 14 and 28 d; increase in collagen III at 28 d | Increase in left kidney fibrosis at 28 d; no increase in collagen III at 28 d | Increase in left and right kidney fibrosis at 28 d; increase in left kidney collagen III at 28 d |
| IHC Collagen III | Not performed | ||||
| mRNA via RT-PCR | |||||
| KIM-1 | Not performed | Increase in KIM-1 at 6 h-28 d and in NGAL at 24 h and 28 d. Increase in Collagen I and III at 24 h | Increase in KIM-1 and NGAL at 6 h-28 d. Increase in Collagen I and III at 24 h through 28 d | Not performed | Not performed |
| NGAL | Not performed | Not performed | Not performed | ||
| Collagen I | Not performed | Not performed | Not performed | ||
| Collagen III | Not performed | Not performed | Not performed | ||
| Not performed | |||||
| Hydroxyproline | Normal at 28 d | Elevated at 28 d | Normal at 28 d | Elevated at 28 d | |
| Measured GFR | |||||
| Pre-UNx | Not performed | No pre-UNx change at 14 or 28 d | Reduced pre-UNx at 14 d | Normal 1 day postprocedure, 26 d postprocedure | Reduced 1 day postprocedure, normal pre-UNx on 26 d |
| Not performed | |||||
| Post-UNx | Reduced post-UNx at 14 d but not 28 d | Reduced post-UNx at 14 and 28 d with nil function | Normal post-UNx at 28 d. | Reduced post-UNx at 28 d |
LFABP, liver fatty acid-binding protein; NAG, N-acetyl-β-D-glucosamine; EM, electron microscopy; IF, immunofluorescence; ICAM, intracellular adhesion molecule-1; IHC, immunohistochemistry.
UiAKI, unilateral ischemic acute kidney injury; UiAKIice, UiAKI utilizing intraoperative ice packs; min, minute; SCr, serum creatinine; NGAL, neutrophil gelatinase-associated lipocalin; LFABP, liver fatty acid-binding protein; NAG, N-acetyl-β-D-glucosaminidase; IL-18, interleukin-18; EM, electron microscopy; IF, immunofluorescence; ICAM, intracellular adhesion molecule-1; IHC, immunohistochemistry; UNx, contralateral nephrectomy.
Discussion
Herein, we aimed to develop a murine model of UiAKI that was similar to a recently published human study, to better compare the effects of renal ischemia on kidney injury and biomarkers, and examine potential long-term effects of ischemia time on kidney function and fibrosis. Our approach comports with recent recommendations made by the NIDDK calling for reverse translational research, as well this group’s recommended metrics of evaluating kidney recovery: function, histology, injury biomarkers, and fibrosis.23 To match the human study, we first altered our routine bilateral ischemia-reperfusion model as follows: (1) rather than utilizing warm ischemia to induce severe AKI, we utilized cold ischemia to help prevent severe AKI; (2) we performed unilateral, rather than bilateral ischemia-reperfusion; (3) we utilized two separate clamp times, 32 and 60 minutes, to study the effect of longer ischemia times; (4) we increased the postoperative fluid hydration in our model to align with the preoperative and postoperative management described.15 In our UiAKIice model, we further replicated the human study by adding ice packs to the surgical site for the first 10 minutes of the procedure. Our cold and ice models depart from typical murine models of AKI that aim to optimize the degree of injury via warm ischemia. A limitation of our models’ alignment with the human study includes the exclusion of female mice. We evaluated only male mice, whereas estrogen is known to protect against ischemic AKI.30–33 Therefore, our results do not include this renal-protective effect whereas the human study did. Additionally, the murine model was limited to healthy, uniformly young mice without comorbidities, partial nephrectomy did not occur, mannitol was not given, and only two ischemia times were tested (versus a range between 15 and 61 minutes). The inclusion of sham controls is a major benefit of the animal model, as this can elucidate outcomes related to the surgery itself, apart from renal ischemia. Another benefit of the animal model was the capability to perform a contralateral nephrectomy to unmask the degree of long-term injury in the affected kidney. This is a notable addition to human studies that have relied upon estimated kidney function via SCr and have not performed functional measurements.16
By matching ischemia times and the timing of renal injury assessments to the human study, we demonstrate that UiAKI in the murine model is markedly similar to human UiAKI within 24 hours and is similarly characterized by the following in both: (1) modest rise in SCr, (2) no increase in serum cystatin C (with 32 minutes of ischemia), (3) rise in serum NGAL that peaks at 24 hours, (4) increase in urine KIM-1 that peaks at 24 hours, (5) increase in urine NGAL and albumin relative to baseline levels, and (6) no correlation of histologic injury to ischemia time when assessed within 5 minutes of reperfusion. Important differences included a higher correlation between ischemia times and biomarkers of injury in the murine model compared with the human model; this likely reflects the more controlled experimental approach that animal experiments enable, which were enumerated earlier. On the basis of our early time point results, we have demonstrated matched murine models to the human study. In the human study, as well as both of our models, injury biomarkers rose and then normalized relatively early. The ability of acute biomarkers to predict the severity of long-term sequelae remains controversial. Much of the foundational biomarker work has utilized murine models of renal disease, most often bilateral models.34–36 Human studies involving bilateral renal disease have indicated that acute biomarkers such as urine NGAL and TIMP-2*IGFBP-7 effectively predict long-term outcomes such as kidney function, fibrosis, and cardiovascular morbidity.37–40 The extent these biomarkers can predict long-term functional decline in unilateral models of disease remains unclear. The study by Parekh et al.15 of unilateral cold ischemia in humans only evaluated these biomarkers for 72 hours postinjury and did not measure kidney function. In mice, others have demonstrated that acute renal injury biomarkers can detect AKI after unilateral ischemia,41 but to our knowledge, this is the first murine study to evaluate these acute biomarkers long-term in mice in a unilateral model of disease, and we have not found corresponding experiments in human studies. Despite clear differences in both measured GFR and histologic outcomes in the UiAKI60 cohort, the day 14 and 28 serum and urine biomarkers showed no significant differences between the groups. These results highlight that serum and urine biomarkers of injury may be poor predictors of long-term histologic and functional outcomes after UiAKI, which is to be expected in unilateral, rather than bilateral renal ischemia because of compensatory kidney function in the contralateral kidney. However, this finding remains somewhat controversial in light of human studies indicating acute biomarkers can predict long-term sequalae, and suggests that further matched studies investigate separate models of kidney injury (unilateral versus bilateral ischemia, sepsis, nephrotoxicity, etc.) in a reverse translational manner may elucidate these relationships. Importantly, a robust evaluation of both acute and chronic biomarkers in each model, in both human and murine studies, would further reveal the applicability and translatability of these promising biomarkers.
Our data also suggest that the immediate histologic results do not correlate with the long-term histologic and functional outcome. In light of the Kidney Precision Medicine Project, which aims to obtain human kidney biopsy samples after AKI, great care will be needed to identify the time from AKI to biopsy such that results can be interpreted appropriately.
Importantly, the murine models facilitate evaluation of long-term outcomes not easily obtainable in humans: 14 and 28 day biomarkers, measurements of GFR with isolation of the injured kidney, and histologic outcomes. Our post-UNx tGFR data provide long-term functional insights to the residual function of the kidney that underwent ischemic injury and demonstrate that the duration of ischemia time correlates with poor functional outcomes. Such measurements of GFR were not included in long-term follow-up evaluation in the human cohort, and although the estimates of GFR via SCr did not demonstrate any statistically significant reduction in function with longer duration of ischemia, the results did trend toward significance.16 In our murine models, before removal of the contralateral kidney there was no significant difference in tGFR among the day 28 UiAKI or UiAKIice cohorts, but there was a difference in function in the day 14 UiAKI cohorts. Only with removal of the contralateral kidney was the true degree of impaired function in the injured kidney unmasked. We propose that this novel approach may be ideal for isolating the injured kidney to investigate functional outcomes of UiAKI in animal models moving forward. This approach also conforms with the recommendation to include functional along with histologic and biomarker outcomes in reverse translational studies investigating recovery after AKI.23 The difference in the 14 and 28 day results in the UiAKI study likely reflects the time needed for the contralateral kidney to compensate via hyperfiltration. Furthermore, our data imply that the duration of ischemia contributes to the long-term function of the kidney, with the UiAKI60 cohort demonstrating significantly impaired function compared with the UiAKI32 group at both the 14 and 28 day time points. Further, although adding intraoperative ice packs mitigated the deterioration in tGFR resulting from longer ischemia time, the function was still significantly reduced despite a reassuring pre-UNx SCr. This reinforces prior notions that ischemia time should ideally be minimized to preserve long-term renal function, and that serum biomarkers of renal function are an insensitive predictor of renal disease. In the 1 year follow-up study that demonstrated a marginal decrease in eGFR such histologic samples were not collected, nor was GFR measured or split kidney function assessed via nuclear renal scan.
We suggest that these results underscore the importance of matched animal models for investigating human disease, and that animal models may also serve as springboards for further human studies and may be useful to predict outcomes in additional human studies. For example, on the basis of these data, we would predict that patients who underwent longer ischemia times during partial nephrectomy would have worse residual kidney function in that kidney if a nuclear MAG3 scan were performed 28 days after the procedure, after correcting for reduced renal mass status after partial nephrectomy. A prior human study that measured kidney function before and after unilateral ischemia demonstrated reduced renal function 3 months after injury.42 In this study, function was maintained in patients who underwent warm ischemia times <44 minutes. Although the clinical significance of mildly decreased kidney function in one kidney can be debated, we would advocate that the basic tenet that ischemia time does indeed affect kidney recovery be considered when determining best practice, and that further matched models of human disease be designed to study the efficacy and translatability of novel biomarkers in predicting long-term functional outcomes.
In conclusion, we have developed a novel model of UiAKI model using a reverse translational approach as recommended by a recent NIDDK expert panel workshop. Our data lend significant insight into the utility of biomarkers in the setting of unilateral ischemia, and support the notion that prolonged ischemia time adversely affects kidney recovery. We also demonstrate that acute biomarkers of injury may be unreliable during unilateral ischemia to predict long-term outcomes.
Disclosures
None.
Acknowledgments
The authors would also like to thank Nataliya Skrypnyk for processing the mRNA data.
Dr. Soranno and Dr. Faubel contributed to the study design, data analysis, figure creation, and manuscript preparation. Dr. Gil contributed to the study design, surgeries, and data analysis. Dr. Kirkbride-Romeo, Dr. Altmann, and Dr. Montford contributed to the surgical procedures and data analysis. Dr. Yang performed the acute tubular necrosis scoring. Dr. Montford performed the hydroxyproline assays and contributed to the data analysis and manuscript. Dr. Levine contributed to the data analysis.
The authors would like to thank the following groups for their research support: Center for Fibrosis Research and Translation (Dr. Soranno), Research Institute at the Children’s Hospital Colorado (Dr. Soranno), National Institute of Diabetes and Digestive and Kidney Diseases (grant 1K08DK109226-01A1 to Dr. Soranno), the National Institute of Heart Lung and Blood Institute (grant R01 1R01HL130084-01 to Dr. Faubel) and the Veterans Affairs Administration (grant 1 I01 BX001498 VA Merit to Dr. Faubel, and 1 IK2BX003839 VA CDA-2 to Dr. Montford).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Wei JT: National utilization trends of partial nephrectomy for renal cell carcinoma: A case of underutilization? Urology 67: 254–259, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Medina-Rico M, Ramos HL, Lobo M, Romo J, Prada JG: Epidemiology of renal cancer in developing countries: Review of the literature. Can Urol Assoc J 12: E154–E162, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halazun KJ, Al-Mukhtar A, Aldouri A, Willis S, Ahmad N: Warm ischemia in transplantation: Search for a consensus definition. Transplant Proc 39: 1329–1331, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Secin FP: Importance and limits of ischemia in renal partial surgery: Experimental and clinical research. Adv Urol 2008: 102461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Clef N, Verhulst A, D’Haese PC, Vervaet BA: Unilateral renal ischemia-reperfusion as a robust model for acute to chronic kidney injury in mice. PLoS One 11: e0152153, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatsugami K, Eto M, Yokomizo A, Kuroiwa K, Inokuchi J, Tada Y, et al.: Impact of cold and warm ischemia on postoperative recovery of affected renal function after partial nephrectomy. J Endourol 25: 869–873, discussion 873–874, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al.: Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol 58: 340–345, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al.: Renal function after partial nephrectomy: Effect of warm ischemia relative to quantity and quality of preserved kidney. Urology 79: 356–360, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Simmons MN, Schreiber MJ, Gill IS: Surgical renal ischemia: A contemporary overview. J Urol 180: 19–30, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Gaspari F, Ferrari S, Stucchi N, Centemeri E, Carrara F, Pellegrino M, et al.: MY.S.S. Study Investigators : Performance of different prediction equations for estimating renal function in kidney transplantation. Am J Transplant 4: 1826–1835, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Lee E, Collier CP, White CA: Interlaboratory variability in plasma creatinine measurement and the relation with estimated glomerular filtration rate and chronic kidney disease diagnosis. Clin J Am Soc Nephrol 12: 29–37, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George AK, Herati AS, Srinivasan AK, Rais-Bahrami S, Waingankar N, Sadek MA, et al.: Perioperative outcomes of off-clamp vs complete hilar control laparoscopic partial nephrectomy. BJU Int 111[4 Pt B]: E235–E241, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Zhao J, Velet L, Ercole CE, Remer EM, Mir CM, et al.: Functional recovery from extended warm ischemia associated with partial nephrectomy. Urology 87: 106–113, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Thompson RH: Words of wisdom: Re: Tolerance of the human kidney to isolated controlled ischemia. Eur Urol 64: 684–685, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Parekh DJ, Weinberg JM, Ercole B, Torkko KC, Hilton W, Bennett M, et al.: Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol 24: 506–517, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kallingal GJ, Weinberg JM, Reis IM, Nehra A, Venkatachalam MA, Parekh DJ: Long-term response to renal ischaemia in the human kidney after partial nephrectomy: Results from a prospective clinical trial. BJU Int 117: 766–774, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mir MC, Pavan N, Parekh DJ: Current paradigm for ischemia in kidney surgery. J Urol 195: 1655–1663, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Fu Y, Tang C, Cai J, Chen G, Zhang D, Dong Z: Rodent models of AKI-CKD transition. Am J Physiol Renal Physiol 315: F1098–F1106, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoke TS, Douglas IS, Klein CL, He Z, Fang W, Thurman JM, et al.: Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol 18: 155–164, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 1p following 143, 2010 [DOI] [PMC free article] [PubMed]
- 21.Skrypnyk NI, Harris RC, de Caestecker MP. Ischemia-reperfusion model of acute kidney injury and post injury fibrosis in mice. J Vis Exp (78): 50495, 2013 [DOI] [PMC free article] [PubMed]
- 22.Lieberthal W, Nigam SK: Acute renal failure. II. Experimental models of acute renal failure: Imperfect but indispensable. Am J Physiol Renal Physiol 278: F1–F12, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Zuk A, Palevsky PM, Fried L, Harrell FE Jr., Khan S, McKay DB, et al.: Overcoming translational barriers in acute kidney injury: A report from an NIDDK workshop. Clin J Am Soc Nephrol 13: 1113–1123, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andres-Hernando A, Altmann C, Bhargava R, Okamura K, Bacalja J, Hunter B, et al.: Prolonged acute kidney injury exacerbates lung inflammation at 7 days post-acute kidney injury. Physiol Rep 2: e12084, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreiber A, Shulhevich Y, Geraci S, Hesser J, Stsepankou D, Neudecker S, et al.: Transcutaneous measurement of renal function in conscious mice. Am J Physiol Renal Physiol 303: F783–F788, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Soranno DE, Rodell CB, Altmann C, Duplantis J, Andres-Hernando A, Burdick JA, et al. : Delivery of interleukin-10 via injectable hydrogels improves renal outcomes and reduces systemic inflammation following ischemic acute kidney injury in mice. Am J Physiol Renal Physiol 311: F362–F372, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiba T, Skrypnyk NI, Skvarca LB, Penchev R, Zhang KX, Rochon ER, et al.: Retinoic acid signaling coordinates macrophage-dependent injury and repair after AKI. J Am Soc Nephrol 27: 495–508, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmittgen TD, Livak KJ: Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Hewitson TD, Smith ER, Samuel CS: Qualitative and quantitative analysis of fibrosis in the kidney. Nephrology (Carlton) 19: 721–726, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Tanaka R, Yazawa M, Morikawa Y, Tsutsui H, Ohkita M, Yukimura T, et al.: Sex differences in ischaemia/reperfusion-induced acute kidney injury depends on the degradation of noradrenaline by monoamine oxidase. Clin Exp Pharmacol Physiol 44: 371–377, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Tanaka R, Tsutsui H, Ohkita M, Takaoka M, Yukimura T, Matsumura Y: Sex differences in ischemia/reperfusion-induced acute kidney injury are dependent on the renal sympathetic nervous system. Eur J Pharmacol 714: 397–404, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Kang KP, Lee JE, Lee AS, Jung YJ, Kim D, Lee S, et al.: Effect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in mice. Mol Med Rep 9: 2061–2068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchens MP, Fujiyoshi T, Komers R, Herson PS, Anderson S: Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am J Physiol Renal Physiol 303: F377–F385, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al.: Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin: A novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol 24: 307–315, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Trof RJ, Di Maggio F, Leemreis J, Groeneveld AB: Biomarkers of acute renal injury and renal failure. Shock 26: 245–253, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa M, Ishii J, Kitagawa F, Takahashi H, Sugiyama K, Tada M, et al.: Plasma neutrophil gelatinase-associated lipocalin as a predictor of cardiovascular events in patients with chronic kidney disease. BioMed Res Int 2016: 8761475, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim IY, Kim JH, Kim MJ, Lee DW, Hwang CG, Han M, et al.: Plasma neutrophil gelatinase-associated lipocalin is independently associated with left ventricular hypertrophy and diastolic dysfunction in patients with chronic kidney disease. PLoS One 13: e0205848, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singer E, Schrezenmeier EV, Elger A, Seelow ER, Krannich A, Luft FC, et al.: Urinary NGAL-positive acute kidney injury and poor long-term outcomes in hospitalized patients. Kidney Int Rep 1: 114–124, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koyner JL, Shaw AD, Chawla LS, Hoste EA, Bihorac A, Kashani K, et al.: Sapphire Investigators : Tissue inhibitor metalloproteinase-2 (TIMP-2)⋅IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol 26: 1747–1754, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaucsár T, Godó M, Révész C, Kovács M, Mócsai A, Kiss N, et al.: Urine/plasma neutrophil gelatinase associated lipocalin ratio is a sensitive and specific marker of subclinical acute kidney injury in mice. PLoS One 11: e0148043, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shekarriz B, Shah G, Upadhyay J: Impact of temporary hilar clamping during laparoscopic partial nephrectomy on postoperative renal function: A prospective study. J Urol 172: 54–57, 2004 [DOI] [PubMed] [Google Scholar]