Significance Statement
Colorectal cancer screening using fecal immunochemical testing (FIT) is recommended for patients with CKD, whose risk of developing and dying from this malignancy is at least 1.5 times higher than that of the sex- and age-matched general population. However, FIT accuracy in this setting is unknown and is likely to be affected by occult gastrointestinal bleeding from dysfunctional platelets and increased bleeding sensitivity to aspirin. In a large, multinational study, the authors found that FIT appears to be an accurate screening test for patients with CKD, but the risk of major complications from work-up colonoscopies (1.5%) is high compared with this risk in the general population. These findings provide useful estimates of harms and test accuracies to inform colorectal cancer screening decisions across the full spectrum of CKD.
Keywords: cancer, chronic kidney disease, screening, test performance
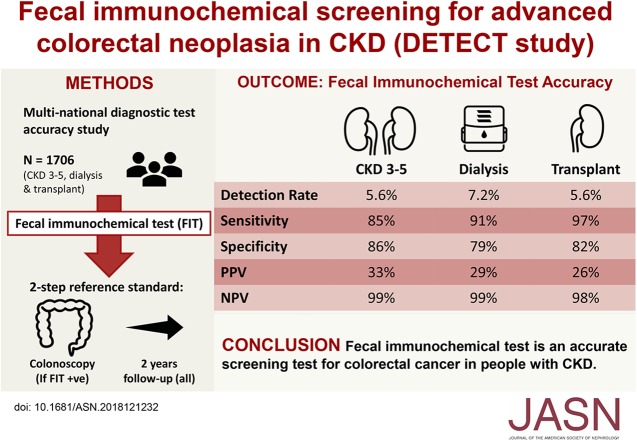
Visual Abstract
Abstract
Background
In patients with CKD, the risk of developing colorectal cancer is high and outcomes are poor. Screening using fecal immunochemical testing (FIT) is effective in reducing mortality from colorectal cancer, but performance characteristics of FIT in CKD are unknown.
Methods
To determine the detection rates and performance characteristics of FIT for advanced colorectal neoplasia (ACN) in patients with CKD, we used FIT to prospectively screen patients aged 35–74 years with CKD (stages 3–5 CKD, dialysis, and renal transplant) from 11 sites in Australia, New Zealand, Canada, and Spain. All participants received clinical follow-up at 2 years. We used a two-step reference standard approach to estimate disease status.
Results
Overall, 369 out of 1706 patients who completed FIT (21.6%) tested positive; 323 (87.5%) underwent colonoscopies. A total of 1553 (91.0%) completed follow-up; 82 (4.8%) had died and 71 (4.2%) were lost. The detection rate of ACN using FIT was 6.0% (5.6%, 7.4%, and 5.6% for stages 3–5 CKD, dialysis, and transplant). Sensitivity, specificity, and positive and negative predictive values of FIT for ACN were 0.90, 0.83, 0.30, and 0.99, respectively. Of participants who underwent colonoscopy, five (1.5%) experienced major colonoscopy-related complications, including bowel perforation and major bleeding.
Conclusions
FIT appears to be an accurate screening test for patients with CKD, such that a negative test may rule out the diagnosis of colorectal cancer within 2 years. However, the risk of major complications from work-up colonoscopy are at least ten-fold higher than in the general population.
Colorectal cancer is common in patients with CKD, with an excess risk of at least 1.5 times that of the age-matched population.1,2 Colorectal cancer also occurs at a much younger age in patients with CKD, particularly for those with a kidney transplant. The risk of colorectal cancer is increased by about 2–10 times in transplant recipients, depending upon the age at transplantation.3 The increased risk of colorectal cancer in transplant recipients is largely attributed to altered T cell immunity and loss of cancer immune editing resulting from chronic immunosuppression.4 Cancer outcomes are poor in those with CKD.5 Five-year survival after a colorectal cancer diagnosis is <50% for kidney transplant recipients and <30% for patients on dialysis.6,7
Early detection and subsequent management of treatment-responsive colorectal cancer through screening is a major cancer control strategy that is widely implemented. Fecal immunochemical testing (FIT) is an acceptable, accurate, and cost-effective strategy for screening for colorectal cancer in average-risk populations,8–10 but this may not be the case in the setting of CKD. The perceived harms associated with screening and downstream diagnostic procedures, such as colonoscopy, are expected to be higher in people with kidney disease.11 Occult gastrointestinal bleeding from dysfunctional platelets secondary to uremia, anticoagulation during dialysis, and antiplatelet agents for cardiovascular risk prevention, may also increase the false-positive rate.12 The risk of cardiovascular disease and the competing risk of death from cardiovascular causes are also very high, so the benefits of early detection and treatment of colorectal cancer may not accrue.13 Consequently, the Choosing Wisely campaign, initiated by the American Society of Nephrology in 2012, recommended against routine cancer screening among patients receiving maintenance dialysis with limited life expectancy,14 but a recent observational study in the United States indicated patients undergoing dialysis (with expected overall survival of <10 years) are receiving screening tests at a much higher rate relative to their life expectancy than their counterparts without ESKD.15
Given the uncertainty regarding the potential harms as well as the accuracy of the screening test, the aim of this study was to determine the consequences of a one-time screening for advanced colorectal neoplasia using FIT in patients with a broad spectrum of CKD. In particular, it was designed to determine the detection rates and the test performance characteristics for advanced colorectal neoplasia (i.e., colorectal cancer and advanced adenomas) in patients with CKD stages 3–5, patients on dialysis, and kidney transplant recipients.
Methods
Study Design
From June 2010 to November 2015, we enrolled consecutive participants with CKD (CKD stages 3–5, patients on dialysis, and transplant recipients), aged 35–74 years, at 11 sites throughout Australia, New Zealand, Spain, and Canada, including hospital, academic, and private practice clinics. Baseline recruitment was completed in December 2015 and the last work-up colonoscopy was carried out in April 2016. The study protocol16 was approved by the Human and Research Ethics Committee of all participating centers (Westmead, Gosford, Royal North Shore, Blacktown, Nepean, Sir Charles Gairdner, Concord, Royal Prince Alfred, Toronto General, and Christchurch Hospitals, and the Hospital Clinic of Barcelona). All participants provided written informed consent. The design, conduct, and reporting are in accordance with the Standards for Reporting of Diagnostic Accuracy Studies.17
Study Population and Screening Protocol
Asymptomatic patients with CKD (CKD stages 3–5, dialysis, and transplant) were assessed for eligibility across all sites. Patients were excluded if they had a first-degree relative with colorectal cancer, a prior history of colorectal cancer and/or inflammatory bowel disease, were medically unfit for a colonoscopy, pregnant, had FITs within 1 year, or had a colonoscopy performed within the past 2 years. Compared with the standard starting age threshold used in the general population for screening using FIT (50 years), a lower starting age threshold (35 years) was used, reflecting epidemiologic data demonstrating that prevalence of colorectal cancer in people with CKD is equivalent to those who are about 20 years older.3
Screening and Diagnostic Procedures
Participants underwent screening using standard methods,18 with FIT applied to two consecutive fecal samples per single test kit. Test positivity was defined as ≥10 μg of hemoglobin per gram of feces in either or both of the two stool samples collected using the automated semiquantitative OC-Sensor (Eiken Chemical) without dietary or mediation restrictions. Samples were sent to a central laboratory at the corresponding sites (Australia, Canada, New Zealand, and Spain). All participants with positive FITs were invited to undergo colonoscopy. A combination of sodium picosulfate and GlycoPrep-C (Macrogol 3350) were used as routine preparation for the work-up colonoscopies. Serum electrolytes were obtained from participants the day before and within 1 week after bowel preparation for monitoring of electrolytes and metabolic disturbances. Experienced colonoscopists (who performed over 400 colonoscopies per year) performed the colonoscopies at each site. It was a requirement for all colonoscopists to describe the location and record the size of the lesions. If polyps were found, polypectomies were performed where possible.
Histologic assessment of the biopsies and surgical specimens were performed by a designated and experienced histopathologist at each study site. Colonic polyps were considered as growths arising from the intestinal epithelium of the colon and/or rectum. Polyps were categorized as non-neoplastic or neoplastic. Advanced colorectal neoplasia was defined in accordance with accepted international standards as any colorectal adenoma with at least one of the following features: ≥10 mm in size, villous component (≥25%) or high-grade dysplasia; or any serrated lesion ≥10 mm in size, or with a dysplastic component; or any invasive colorectal cancer.19 All cancers identified were staged and participants were referred to the colorectal surgical, gastroenterology, or oncology team, depending upon the stage of initial diagnoses. Tumor staging was determined using the American Joint Committee on Cancer staging system.20 Patients were classified according to the most advanced lesion. Results of the FIT screens as well as the colonoscopy findings were reported to their treating nephrologists and general practitioners.
Reference Standard
To overcome the issue of differential verification bias, a two-step reference standard approach was used.21 Participants, irrespective of the test screen results, received clinical follow-up at 2 years. Participants were asked whether they had any unusual bowel symptoms, such as rectal bleeding or changes in bowel motions, and whether they had additional bowel investigations, such as colonoscopies and repeat FIT as part of the National Bowel Cancer Screening Programs in Australia and Spain,22,23 during the follow-up period.
Outcomes
The primary outcome was test specificity of FIT (number of participants without advanced colorectal neoplasia divided by the number of participants without advanced colorectal neoplasia and those with false-positive results). The other outcomes measured were positivity rate (number of individuals with positive FIT divided by the number of screened participants), detection rate (number of individuals in whom an advanced colorectal neoplasia was detected divided by the number of individuals who had received FIT), test sensitivity (number of participants with advanced colorectal neoplasia detected through initial screens divided by the total number of participants with advanced colorectal neoplasia detected through initial screens and the total number of participants with advanced colorectal neoplasia diagnosed during the 2-year follow-up period after negative FIT), and positive (proportion of all participants with positive FIT who had advanced colorectal neoplasia) and negative (proportion of all participants with negative FIT who did not have the disease within the 2 years of follow-up) predictive values for advanced colorectal neoplasia, and all major adverse events associated with colonoscopies.
Sample Size Calculation
We estimated a minimum sample size of 1700 on the basis of a positivity rate of 20%, a prevalence of advanced colorectal neoplasia between 3.1% in CKD stages 3–5 and 4.2% among transplant recipients, with test sensitivity and test specificity of FIT of 75% and 80%, and the maximally acceptable half-width of the 95% confidence interval (95% CI) for the primary outcome specificity as 2.5%.
Statistical Analyses
Baseline demographic characteristics were analyzed using standard methods, with continuous variables presented as means with SD or medians (interquartile range), if not normally distributed and binary variables reported as percentages. Differences between CKD stages were determined by chi-squared test or ANOVA where appropriate. Test sensitivity, specificity, and positive and negative predictive values were expressed as proportions and their 95% CIs were constructed using binomial distribution to reach an exact estimate. Post hoc analyses of the subgroups were conducted to account for possible differences in age, sex, and use and nonuse of antiplatelet agents or anticoagulants in the test performance characteristics of FIT. To compare the test sensitivity and specificity between subgroups (CKD stage, age group, use and nonuse of anticoagulants and antiplatelets), two-sample tests for binomial proportions were used to compute the exact P values and the confidence intervals. Sensitivity analyses (best and worst case scenarios) were conducted to determine whether the test performance characteristics would be affected by verification biases because of missing data on those that did not complete the 2-year follow-up. For the best case scenario, we assumed all nonverified cases did not have advanced colorectal neoplasia. For the worst case scenario, all nonverified cases were assumed to have advanced colorectal neoplasia. Additional analyses were also conducted for the assessment of the test performance characteristics of colorectal cancers (excluding colorectal adenomas). All analyses were undertaken using SAS (version 9.4; SAS Institute Inc., Cary, NC). P values <0.05 in two-tailed testing were considered statistically significant.
Results
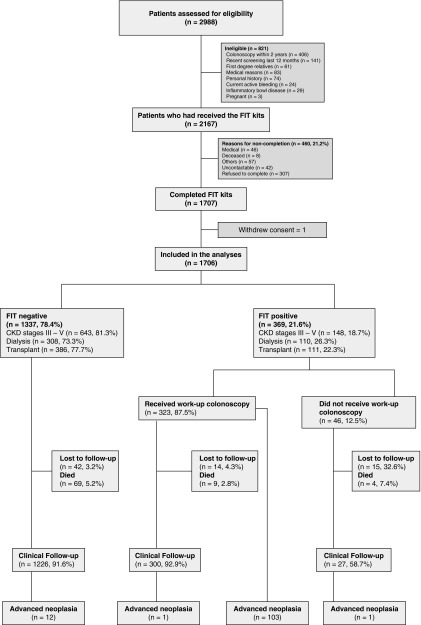
A total of 2988 patients with CKD were screened for study eligibility. Of these, 2167 (72.5%) were eligible and 1707 (78.8%) completed FIT. One withdrew consent after screening, leaving 1706 in the final analyses. A total of 460 (21.2%) eligible patients did not undergo screening because of death, refusal, or medical reasons including hospitalization after screening kits were given (Figure 1). Compared with nonparticipants, participants were older (mean age of 58.7 versus 56.2 years; P<0.001), more likely to be white (71.2% versus 43.0%; P<0.001), had received tertiary education (56.1% versus 55.8%; P=0.03), were engaged in full-time or part-time employment (12.7% versus 5.7%; P<0.001), had a prior cancer diagnosis (20.6% versus 10.7%; P<0.001), had diabetes mellitus (34.0% versus 32.2%; P<0.001), had cardiovascular disease (23.3% versus 18.7%; P<0.001), were less likely to have cerebrovascular disease (6.6% versus 7.8%; P<0.001), and were less likely to be on dialysis (24.5% versus 31.5%; P<0.001). There were no significant differences in sex, marital status, obesity or other comorbidities (including hypertension and dyslipidemia) between participants and nonparticipants.
Figure 1.
Study flow.
Baseline Characteristics
Of the 1706 participants, 791 (46.4%) were CKD stages 3–5, 418 (24.5%) were on dialysis, and 497 (29.1%) were kidney transplant recipients (Table 1). Overall, 1032 (60.5%) were men, with a median age of 59.9 years. The majority were white (71.2%); 63.7% had at least one of the comorbidities diabetes mellitus, cardiovascular disease, and cerebrovascular disease; 20.6% had a history of prior cancer (skin and nonskin cancers); and 52.5% were either ex- or current smokers. Of the 418 patients on dialysis, 69.9% were on hemodialysis, and 62.2% of all kidney transplant recipients (309 out of 497) had received deceased donor organs. The majority of kidney transplant recipients were maintained on prednisone (90.7%), tacrolimus (61.4%), and mycophenolate mofetil (77.1%).
Table 1.
Baseline characteristics of the study participants (n=1706)
| Characteristics | CKD Stages 3–5 (n=791, 46.4%) | Dialysis (n=418, 24.5%) | Transplant (n=497, 29.1%) | All (n=1706) |
|---|---|---|---|---|
| Age, yr | ||||
| 35–49 | 116 (14.7) | 85 (20.3) | 184 (37.0) | 385 (22.6) |
| 50–64 | 278 (35.1) | 228 (54.5) | 240 (48.3) | 746 (43.7) |
| ≥65 | 397 (50.2) | 105 (25.1) | 73 (14.7) | 575 (33.7) |
| Sex | ||||
| Women | 337 (42.6) | 153 (36.6) | 184 (37.0) | 674 (39.5) |
| Men | 454 (57.4) | 265 (63.4) | 313 (63.0) | 1032 (60.5) |
| Race or ethnic groups | ||||
| White | 547 (69.2) | 277 (66.3) | 390 (78.5) | 1214 (71.2) |
| Asian | 81 (10.2) | 60 (14.4) | 44 (8.9) | 185 (10.8) |
| Middle eastern | 30 (3.8) | 16 (3.8) | 18 (3.6) | 64 (3.8) |
| Aboriginal/Torres Strait Islander/Maori/Pacific Islanders | 18 (2.3) | 22 (5.3) | 13 (2.6) | 53 (3.1) |
| Other | 115 (14.5) | 43 (10.3) | 32 (6.4) | 190 (11.1) |
| Education level | ||||
| High school graduate or less | 318 (40.2) | 161 (38.5) | 240 (48.3) | 719 (42.1) |
| College/university | 458 (57.9) | 245 (58.6) | 250 (50.3) | 953 (55.9) |
| Unknown | 15 (1.9) | 12 (2.9) | 7 (1.4) | 34 (2.0) |
| Marital status | ||||
| Single | 64 (8.1) | 62 (14.8) | 74 (14.9) | 200 (11.7) |
| Married/partnered | 567 (71.7) | 279 (66.7) | 372 (74.8) | 1218 (71.4) |
| Divorced/separated | 157 (19.8) | 74 (17.7) | 48 (9.7) | 279 (16.4) |
| Unknown | 3 (0.4) | 3 (0.7) | 3 (0.6) | 9 (0.5) |
| Smoking status | ||||
| Current | 83 (10.5) | 26 (6.2) | 18 (3.6) | 127 (7.4) |
| Ex-smoker | 357 (45.1) | 198 (47.4) | 215 (43.3) | 770 (45.1) |
| Never | 341 (43.1) | 187 (44.7) | 256 (51.5) | 784 (46.0) |
| Unknown | 10 (1.3) | 7 (1.7) | 8 (1.6) | 25 (1.5) |
| Diabetes mellitus | ||||
| Yes | 320 (40.5) | 151 (36.1) | 109 (21.9) | 580 (34.0) |
| No | 471 (59.5) | 267 (63.9) | 388 (78.1) | 1126 (66.0) |
| Cardiovascular disease | ||||
| Yes | 209 (26.4) | 113 (27.0) | 73 (14.7) | 395 (23.2) |
| No | 582 (73.6) | 305 (73.0) | 424 (85.3) | 1311 (76.8) |
| Cerebrovascular disease | ||||
| Yes | 58 (7.3) | 30 (7.2) | 24 (4.8) | 112 (6.6) |
| No | 733 (92.7) | 388 (92.8) | 473 (95.2) | 1594 (93.4) |
| Body mass index, kg/m2 | ||||
| <20 | 20 (2.5) | 33 (7.9) | 18 (3.6) | 71 (4.2) |
| 20–25 | 155 (19.6) | 126 (30.1) | 157 (31.6) | 438 (25.7) |
| 25–30 | 281 (35.5) | 127 (30.4) | 181 (36.4) | 589 (34.5) |
| >30 | 302 (38.2) | 110 (26.3) | 123 (24.7) | 535 (31.4) |
| Unknown | 33 (4.2) | 22 (5.3) | 18 (3.6) | 73 (4.3) |
| Prior cancer | ||||
| Yes | 149 (18.8) | 83 (19.9) | 119 (23.9) | 351 (20.6) |
| No | 642 (81.2) | 335 (80.1) | 378 (76.1) | 1355 (79.4) |
| Prior lower gastrointestinal endoscopy | ||||
| Yes | 281 (35.5) | 112 (26.8) | 134 (27.0) | 527 (30.9) |
| No | 505 (63.8) | 306 (73.2) | 362 (72.8) | 1173 (68.8) |
| Unknown | 5 (0.6) | 0 | 1 (0.2) | 6 (0.4) |
| Daily use of antiplatelet agents | ||||
| Yes | 241 (30.5) | 148 (35.4) | 137 (25.6) | 526 (30.8) |
| No | 550 (69.5) | 270 (64.6) | 360 (72.4) | 1180 (69.2) |
| Daily use of anticoagulation | ||||
| Yes | 67 (8.5) | 65 (15.6) | 26 (5.2) | 158 (9.3) |
| No | 724 (91.5) | 353 (84.4) | 471 (94.8) | 1548 (90.7) |
| Types of dialysis | ||||
| Hemodialysis | — | 291 (69.6) | — | 291 (69.6) |
| Peritoneal dialysis | — | 127 (30.4) | — | 127 (30.4) |
| Donor types | ||||
| Deceased | — | — | 309 (62.2) | 309 (62.2) |
| Living | — | — | 188 (37.8) | 188 (37.8) |
| Daily immunosuppression use | ||||
| Prednisone | 40 (5.1) | 29 (6.9) | 451 (90.7) | 520 (30.5) |
| Azathioprine | 9 (1.1) | 4 (1.0) | 52 (10.5) | 65 (3.8) |
| Mycophenolate mofetil | 9 (1.1) | 3 (0.7) | 383 (77.1) | 395 (23.2) |
| Tacrolimus | 1 (0.1) | 3 (0.7) | 305 (61.4) | 309 (18.1) |
| Cyclosporine | 5 (0.6) | 1 (0.2) | 105 (21.1) | 111 (6.5) |
| Regular use of erythropoiesis-stimulating agents | ||||
| Yes | 38 (4.8) | 203 (48.6) | 31 (6.2) | 272 (15.9) |
| No | 753 (95.2) | 215 (51.4) | 466 (93.8) | 1434 (84.1) |
All data are displayed as n (%). —, not applicable.
Test Positivity Rate
All participants with positive FIT (369/1706, 21.6%) were invited to undergo colonoscopy. Forty-six of these participants declined for medical reasons, with 323 (87.5%) proceeding to colonoscopy. The median time between FIT results and colonoscopy was 2.7 months (interquartile range, 2.0 months). A total of 32 (9.9%) participants had inadequate/poor bowel preparation at initial work-up colonoscopy, but all underwent repeat examination.
Characteristics of Colorectal Polyps
Of the 323 participants who tested positive and received colonoscopy, 223 (69.0%) had colorectal polyps (CKD stages 3–5: n=95, 42.6%; dialysis: n=66, 29.6%; and transplant: n=62, 27.8%) (Table 2). On average, two colorectal polyps were found per patient. The average size of the largest polyp was 5.8 (SD 4.3) mm in diameter and did not differ by CKD stage (P=0.09). Approximately 70% of all participants with polyps had neoplastic lesions. The distribution of the neoplastic lesions by type was as follows: tubular adenomas (n=149, 66.8%), tubulovillous adenomas (n=62, 27.8%), sessile serrated adenomas/polyps (n=10, 4.5%), and villous adenomas (n=3, 1.3%).
Table 2.
Characteristics of colonic polyps in screen positive participants who underwent work-up colonoscopy (n=223)
| Characteristics | All | CKD Stages 3–5 | Dialysis | Transplant | P Valuea |
|---|---|---|---|---|---|
| Participants with polyps | 223 (100) | 95 (42.6) | 66 (29.6) | 62 (27.8) | 0.03 |
| Polyps per patientb | |||||
| 1 polyp | 83 (37.2) | 34 (35.8) | 17 (25.8) | 32 (51.6) | 0.01 |
| 2–3 polyps | 90 (40.4) | 38 (40.0) | 29 (43.9) | 23 (37.1) | 0.7 |
| >3 polyps | 50 (22.4) | 23 (24.2) | 20 (30.3) | 7 (11.3) | 0.03 |
| Location of the largest polyp | |||||
| Cecum | 36 (16.1) | 23 (24.2) | 4 (6.1) | 9 (14.5) | 0.01 |
| Ascending colon | 35 (15.7) | 18 (18.9) | 11 (16.7) | 6 (9.7) | 0.3 |
| Transverse colon | 33 (14.8) | 9 (9.5) | 17 (25.8) | 7 (11.3) | 0.01 |
| Descending colon | 22 (9.9) | 7 (7.4) | 9 (13.6) | 6 (9.7) | 0.4 |
| Sigmoid colon/rectum | 95 (42.6) | 37 (38.9) | 24 (36.4) | 34 (54.8) | 0.07 |
| Other | 2 (0.9) | 1 (1.1) | 1 (1.5) | 0 | 0.6 |
| Size of the largest polyp, mm | |||||
| <5 | 40 (17.9) | 17 (17.9) | 10 (15.2) | 13 (21.0) | 0.7 |
| 5–10 | 124 (55.6) | 53 (55.8) | 39 (59.1) | 32 (51.6) | 0.7 |
| >10 | 59 (26.5) | 25 (26.3) | 17 (25.8) | 17 (27.4) | 0.9 |
| Neoplastic lesionsc | |||||
| Tubulovillous adenomas | 62 (27.8) | 30 (31.6) | 16 (24.2) | 16 (25.8) | 0.5 |
| Tubular adenomas | 149 (66.8) | 62 (65.3) | 46 (69.7) | 41 (66.1) | 0.8 |
| Villous adenomas | 3 (1.3) | 2 (2.1) | 0 | 1 (1.6) | 0.5 |
| Sessile serrated adenomas/polyps | 10 (4.5) | 6 (6.3) | 1 (1.5) | 3 (4.8) | 0.6 |
| Non-neoplastic lesionsc | |||||
| Inflammatory polyps | 7 (3.1) | 1 (1.1) | 3 (4.5) | 3 (4.8) | 0.3 |
| Hyperplastic/others | 44 (19.7) | 16 (16.8) | 18 (27.3) | 10 (16.1) | 0.5 |
| Advanced neoplasiab | 103 (46.1) | 44 (46.3) | 31 (47.0) | 28 (45.2) | 0.9 |
| Colorectal cancers | 7 (3.1) | 2 (2.1) | 3 (4.5) | 2 (3.2) | 0.7 |
P values are on the basis of chi-square analyses for comparison between the three groups (CKD stages) in each row.
Advanced colorectal neoplasia was defined as any colorectal adenoma with at least one of the following features: ≥1 cm in size, villous component (≥25%) or high-grade dysplasia; or any serrated lesion ≥1 cm in diameter, or with dysplastic component; or any invasive colorectal cancer.
Participants can have more than one of the neoplastic and non-neoplastic lesions.
Detection of Advanced Colorectal Neoplasia
Seven colorectal cancers (CKD stages 3–5: n=2, 2.1%; dialysis: n=3, 4.5%; and transplant: n=2, 3.2%) and 96 advanced colorectal adenomas (CKD stages 3–5: n=42, 44.2%; dialysis: n=28, 40.9%; and transplant: n=26, 41.9%) were detected (Table 2). The overall detection rate of advanced colorectal neoplasia in CKD was 6.0% (95% CI, 5.0% to 7.2%) (CKD stages 3–5: n=44, 5.6% [95% CI, 4.2% to 7.4%]; dialysis: n=31, 7.4% [95% CI, 5.1% to 10.1%]; and transplant: n=28, 5.6% [95% CI, 3.9% to 8.1%]). These advanced colorectal neoplasia were most commonly located in the sigmoid colon (n=46, 44.7%), followed by cecum (n=27, 26.2%) and the ascending colon (n=16, 15.5%). The overall detection rates of advanced colorectal neoplasia for participants aged <50 years and ≥50 years were 2.3% (95% CI, 1.1% to 4.5%) and 7.2% (95% CI, 5.7% to 8.6%), respectively.
Adverse Effects and Harms
Major colonoscopy-related complications occurred in five (1.5%) participants: postpolypectomy bleeding requiring arterial embolization in two participants, peritonitis in two participants, and large bowel perforation in one participant (Table 3). Of these, 40% (n=2) occurred in patients on dialysis. No significant metabolic and/or electrolyte disturbances were observed, and no AKI related to the bowel preparation were documented. No participant died as a consequence of the screening program.
Table 3.
Complications associated with work-up colonoscopies
| Complications | Total, n (%), n=323 |
|---|---|
| Anesthetic complications | 6 (1.9) |
| Aspiration | 2 (0.6) |
| Minor intraprocedural bleed | 6 (1.9) |
| Minor postprocedural bleed | 26 (8.0) |
| Major postprocedural bleed requiring hospital admission | 2 (0.6) |
| Inadequate bowel preparation requiring repeat colonoscopies | 32 (9.9) |
| Perforation | 1 (0.3) |
| Peritonitis | 2 (0.6) |
Clinical Follow-Up
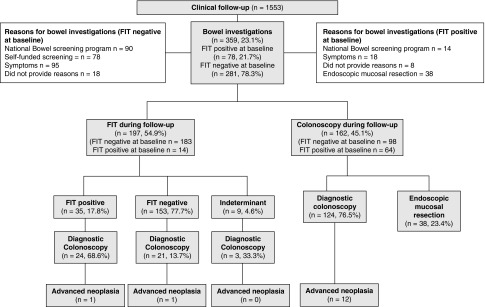
A total of 1553 (91.0%) participants completed the 2-year clinical follow-up (82 [4.8%] died and 71 [4.2%] were lost) (Figure 2). A total of 359 participants (23.1%) received bowel examinations during the follow-up period. Among those with positive FIT screens at baseline (n=78), 14 (17.9%) participants had repeat FIT as part of the bowel cancer screening programs, 18 (23.1%) participants experienced symptoms (such as change in bowel motions) and underwent diagnostic colonoscopies, 38 (48.7%) participants underwent endoscopic mucosal resection as a second procedure to remove the large superficial spreading adenomas diagnosed at baseline, and eight participants (10.2%) did not provide reasons for the bowel examinations. Among those with negative FIT screens at baseline (n=281), 90 (32.0%) received repeat FIT as part of the bowel cancer screening programs in Australia and Spain, 78 (27.8%) self-funded their annual FIT screens, and 95 (33.8%) participants had bowel symptoms and underwent colonoscopies. Eighteen participants (6.4%) did not provide reasons for the bowel examinations.
Figure 2.
Two-year clinical follow-up.
During clinical follow-up, advanced colorectal neoplasia were identified in 12 patients who had colonoscopies but did not receive FIT. Of those with positive FIT (n=35, 17.8%) at follow-up, 24 (68.6%) had work-up colonoscopies and advanced colorectal neoplasia was found in one patient. Of those with negative FIT at follow-up, 21 (13.7%) participants received colonoscopies and a single advanced neoplasia was identified.
Test Performance Characteristics of FIT
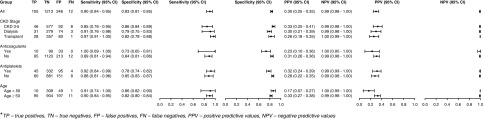
The overall test sensitivity, specificity, and positive and negative predictive values of FIT for advanced colorectal neoplasia were 0.90 (95% CI, 0.84 to 0.95), 0.83 (95% CI, 0.81 to 0.85), 0.30 (95% CI, 0.25 to 0.35), and 0.99 (95% CI, 0.98 to 1.0), respectively (Figure 3). The test sensitivity did not differ by CKD stage (CKD stages 3–5 versus dialysis P=0.69; CKD stages 3–5 versus transplant P=0.15; dialysis versus transplant P=0.76), use and nonuse of antiplatelets (P=0.76) or anticoagulants (P=0.39), or age group (<50 versus ≥50 years) (P=0.97). However, there were differences in test specificity between regular use and nonuse of anticoagulants (P<0.001) and antiplatelet agents (P=0.002), and participants on dialysis (versus those with CKD stages 3–5; P=0.003).
Figure 3.
Test performance characteristics of fecal immunochemical test for advanced colorectal neoplasia stratified by CKD stage, age, and medication use. FN, false negative; FP, false positive; NPV, negative predictive value; PPV, positive predictive value; TN, true negative; TP, true positive.
For colorectal cancer (excluding advanced colorectal adenoma), the overall test sensitivity, specificity, and positive and negative predictive values FIT were 1.0 (95% CI, 0.59 to 1.0), 0.79 (95% CI, 0.77 to 0.81), 0.02 (95% CI, 0.01 to 0.04), and 1.0 (95% CI, 0.99 to 1.0), respectively.
Sensitivity Analyses
Under the worst case scenario, test sensitivity of FIT reduced to 0.50 (95% CI, 0.44 to 0.56), but no difference in specificity was observed (0.83; 95% CI, 0.81 to 0.85). In the best case scenario, the test performance characteristics of FIT remained largely unchanged (Tables 4 and 5).
Table 4.
Best case scenario: assuming all nonverified cases did not have advanced colorectal neoplasms
| Population | Test Sensitivity | Test Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|
| CKD stages 3–5 | 0.85 (0.76–0.95) | 0.86 (0.83–0.89) | 0.31 (0.24–0.39) | 0.98 (0.97–0.99) |
| Dialysis | 0.90 (0.76–0.98) | 0.79 (0.75–0.83) | 0.27 (0.19–0.36) | 0.99 (0.97–0.99) |
| Transplant | 0.97 (0.82–0.99) | 0.82 (0.79–0.86) | 0.25 (0.17–0.33) | 0.99 (0.98–1.00) |
| All | 0.89 (0.84–0.95) | 0.83 (0.81–0.85) | 0.28 (0.24–0.33) | 0.99 (0.98–0.99) |
Data are defined as point estimate (95% CI).
Table 5.
Worst case scenario: assuming all nonverified cases had advanced colorectal neoplasms
| Population | Test Sensitivity | Test Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|
| CKD stages 3–5 | 0.46 (0.37–0.55) | 0.86 (0.84–0.88) | 0.38 (0.30–0.46) | 0.90 (0.87–0.91) |
| Dialysis | 0.57 (0.45–0.69) | 0.75 (0.79–0.84) | 0.33 (0.24–0.42) | 0.91 (0.88–0.94) |
| Transplant | 0.52 (0.39–0.64) | 0.82 (0.78–0.85) | 0.28 (0.20–0.36) | 0.92 (0.90–0.95) |
| All | 0.50 (0.44–0.56) | 0.83 (0.81–0.85) | 0.33 (0.29–0.38) | 0.90 (0.89–0.92) |
Data are defined as point estimate (95% CI).
Discussion
This multinational prospective study in patients across the spectrum of CKD demonstrated a very high screen positivity rate of 21.6%, approximately four times higher than in the general population.24,25 The participation rate was also high (79%), although patients on dialysis were less likely to participate compared with transplant recipients and those with earlier stage CKD. The detection rate of advanced colorectal neoplasia after FIT was 6.0% and was consistent across all stages of CKD. The overall test sensitivity and specificity estimates were favorable (over 90% and 80%, respectively), with a negative predictive value of 99%. Although the test sensitivity did not differ by subgroups, regular use of antiplatelet agents and anticoagulants and dialysis appeared to give higher false-positive rates compared with nonuse of antiplatelet agents and anticoagulants and CKD stages 3–5.
Patients with kidney disease are expected to receive routine colorectal cancer screening,26 but data on the screening patterns, harms, and efficiency of colorectal screening across the spectrum of CKD stages are lacking. There are concerns about the higher risk of gastrointestinal bleeding from uremic platelets and anticoagulation use, and its effect on the frequency of false-positive results, as well as potential adverse renal and metabolic effects such as dehydration, volume depletion, hypernatremia, and hyperphosphatemia associated with the bowel preparation.12 This study has provided reliable evidence suggesting FIT is an accurate screening tool for advanced colorectal neoplasia in patients with CKD, but the major complication rates associated with work-up colonoscopies performed consequent to a positive FIT were at least ten-fold higher compared with the incidence of colonic perforation and major bleeding reported in the general population.27 This is an important consideration for patients on dialysis and those on regular dosage of anticoagulants and antiplatelet agents, given the slightly higher false-positive and complication rates observed in these participants. False-positive tests may lead to unnecessary invasive tests and unintended psychologic harm, including anxiety and fear associated with the work-up colonoscopies.
Using a threshold hemoglobin concentration cut-off value of 10 μg/g of stool, the pooled test sensitivities and specificities of one-time FIT in the general population were reported to be approximately 89% and 90%, respectively, for the detection of colorectal cancer in a recent systematic review and meta-analysis.28 Our study findings are reassuring, suggesting the false-positive and false-negative rates in the overall CKD setting are comparable to the general population. Although the risk of harms associated with the work-up colonoscopies may be higher than in the general population, clinicians and patients should be mindful that a positive FIT screen should not be ignored and further investigations are required to delineate important and precancerous pathologies. On the contrary, a negative test may potentially rule out a diagnosis of colorectal cancer.
The high positive predictive values (around 30%) and detection rates for premalignant advanced colorectal neoplasia in the CKD population warrants consideration as a cancer-control strategy. The World Health Organization (WHO) stated objective of mass screening for advanced colorectal neoplasia is to detect 50 prevalent cases among 10,000 people aged >50 years.29 The present findings have exceeded this goal (estimated 310 prevalent cases per 10,000 people aged >50 years and 130 prevalent cases per 10,000 people aged between 35 and 49 years), highlighting the efficiency of FIT in CKD in comparison with the general population. Although the detection rate was lower among younger participants, our findings suggest that screening on the basis of presence of CKD in 34- to 49-year-olds may be considered, because the estimated prevalence in this study was similar to the WHO recommendations for people aged >50 years.
The decision to screen in patients with chronic illness such as those with CKD is complex. Many have advocated against routine screening because of the lack of benefits from the reduced life expectancy.2 Others have refuted and suggested early detection for cancer should be considered as part of standard clinical care because of the higher incidence of certain types of cancer in those with chronic illnesses such as kidney disease.30 Screening decisions should therefore be a shared-informed process,31 and involves bidirectional flow of information about the cost-benefits of the screening services, potential harms, and elucidation of patients’ preferences, priorities, and values. Our prior modeling analyses and qualitative work have indicated the test performance characteristics of the screening tool (i.e., FIT) is a key determinant of the cost-benefit ratio of routine screening in this population,32 and have identified the fear and anxiety of a positive test and the invasiveness of the work-up colonoscopies as key barriers to screening.33 Although our study was not designed to generate data regarding the marginal health benefits associated with screening in patients with reduced kidney function and comorbidities, it has provided precise estimates of the test performance characteristics and potential harms to guide decision making.
To our knowledge, this is the largest diagnostic test accuracy study of FIT screening for advanced colorectal neoplasm in CKD, with sufficient participation rates to provide precise estimates of the test performance of FIT screening. Our study was conducted prospectively in 11 centers across four countries in a routine screening setting. The study population is therefore representative of the general CKD population and the results are generalizable to diverse CKD groups. However, our study has a number of potential limitations. First, direct comparisons of harms and benefits between screen and clinically detected disease was not feasible within a similar timeframe, given the delays in clinical diagnosis and infrequency of outcome. Second, selection bias may occur in any screening study as a limited range of participants will consent to screening. Patients with CKD may be more likely to elect to participate than those in the general population, possibly because of their increased exposure to, and experience with, the health care system. Third, the diagnostic test accuracy of the reference standard (colonoscopy) was assumed to be the same across centers but this may vary with operator experience. We sought to limit this variability by using the same experienced and qualified colonoscopist within each participating site. It is also important to note that this study was not powered to examine the differences in test performance characteristics between subgroups. Caution should be taken when interpreting the findings of the post hoc analyses. Finally, despite the low attrition rates, partial verification bias may still occur. Given the average time of disease progression from adenoma to carcinoma takes approximately 5–10 years,34 the 2-year follow-up time may not be sufficient to rule out the presence of advanced colorectal adenoma at baseline, leading to overestimation of the test sensitivity. However, progression from adenoma to carcinoma may be hastened in the context of immunosuppression (as in the case of kidney transplantation) and persistent inflammation associated with uremia.4 Performing work-up colonoscopies in all participants regardless of the index test results may mitigate the issue of differential verification bias, but it would be unethical to conduct potentially harmful and unnecessary investigations in individuals who are at risk of serious complications. Nevertheless, to overcome this limitation, performance characteristics of FIT to detect colorectal cancer, a more robust indicator, have been reported separately.
In conclusion, a high detection rate of advanced colorectal neoplasia is observed in patients with CKD. FIT appears to be an accurate screening test, such that a negative result may ultimately rule out the diagnosis of colorectal cancer (but not advanced adenoma) during the ensuing 2 years, and a positive result should necessitate colonoscopy to confirm or exclude the diagnosis of relevant and important colonic pathologies. Patients and clinicians should also be cognizant of the potential harms associated with routine screening.
Disclosures
None.
Acknowledgments
We would like to thank all of the participants of the Detecting Bowel Cancer in CKD (DETECT) study for generously participating in this research. We would also like to thank the clinicians, nurses, and clinical research staff who have kindly assisted us by collecting information from participants for this study. We would like to extend our deepest gratitude to Dr. Hope for his unfailing support throughout the study. Without his dedication and perseverance over the many years, the DETECT study would have not been possible. He is sadly missed by all members of the DETECT team, his patients, and fellow researchers.
Dr. Wong and Dr. Craig had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. Dr. Wong and Dr. Craig conceived and designed the study. All authors acquired and interpreted the data, revised the manuscript, and gave final approval of the manuscript. Dr. Wong and Dr. Craig drafted the manuscript. Dr. Wong and Dr. Au were responsible for the statistical analyses and the figures.
The DETECT study is funded by the National Health and Medical Research Screening and Test Evaluation Program grant (APP 633003) and Better Evidence And Translation in Program grant (APP 1092579).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
aDeceased.
References
- 1.Wong G, Hayen A, Chapman JR, Webster AC, Wang JJ, Mitchell P, et al.: Association of CKD and cancer risk in older people. J Am Soc Nephrol 20: 1341–1350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al.: Cancer incidence before and after kidney transplantation. JAMA 296: 2823–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Webster AC, Craig JC, Simpson JM, Jones MP, Chapman JR: Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: A cohort study of 15,183 recipients. Am J Transplant 7: 2140–2151, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Angriman I, Furian L, Scarpa M, Fassan M, Morgan S, Porzionato A, et al.: Effects of immune suppression for transplantation on inflammatory colorectal cancer progression. Oncogenesis 7: 46, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iff S, Craig JC, Turner R, Chapman JR, Wang JJ, Mitchell P, et al.: Reduced estimated GFR and cancer mortality. Am J Kidney Dis 63: 23–30, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Au E, Wong G, Chapman JR: Cancer in kidney transplant recipients. Nat Rev Nephrol 14: 508–520, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Chien CC, Han MM, Chiu YH, Wang JJ, Chu CC, Hung CY, et al.: Epidemiology of cancer in end-stage renal disease dialysis patients: A national cohort study in Taiwan. J Cancer 8: 9–18, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Towler B, Irwig L, Glasziou P, Kewenter J, Weller D, Silagy C: A systematic review of the effects of screening for colorectal cancer using the faecal occult blood test, hemoccult. BMJ 317: 559–565, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominitz JA, Provenzale D: Patient preferences and quality of life associated with colorectal cancer screening. Am J Gastroenterol 92: 2171–2178, 1997 [PubMed] [Google Scholar]
- 10.Gyrd-Hansen D: Fecal occult blood tests. A cost-effectiveness analysis. Int J Technol Assess Health Care 14: 290–301, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Wong G, Li MW, Howard K, Hua DK, Chapman JR, Bourke M, et al.: Health benefits and costs of screening for colorectal cancer in people on dialysis or who have received a kidney transplant. Nephrol Dial Transplant 28: 917–926, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Akmal M, Sawelson S, Karubian F, Gadallah M: The prevalence and significance of occult blood loss in patients with predialysis advanced chronic renal failure (CRF), or receiving dialytic therapy. Clin Nephrol 42: 198–202, 1994 [PubMed] [Google Scholar]
- 13.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al.: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Williams AW, Dwyer AC, Eddy AA, Fink JC, Jaber BL, Linas SL, et al.: American Society of Nephrology Quality, and Patient Safety Task Force : Critical and honest conversations: The evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol 7: 1664–1672, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Carlos CA, McCulloch CE, Hsu CY, Grimes B, Pavkov ME, Burrows NR, et al.: Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : Colon cancer screening among patients receiving dialysis in the United States: Are we choosing wisely? J Am Soc Nephrol 28: 2521–2528, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong G, Howard K, Chapman JR, Tong A, Bourke MJ, Hayen A, et al.: Test performance of faecal occult blood testing for the detection of bowel cancer in people with chronic kidney disease (DETECT) protocol. BMC Public Health 11: 516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al.: STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 6: e012799, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson DJ, Lee JK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, et al.: Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: A consensus statement by the US Multi-Society task force on colorectal cancer. Gastroenterology 152: 1217–1237.e3, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Cancer Council Australia Colorectal Cancer Guidelines Working Party: Clinical practice guidelines for the prevention, early detection and management of colorectal cancer . 2018. Available at: https://wiki.cancer.org.au/australiawiki/index.php?oldid=191477. Accessed April 3, 2019 [Google Scholar]
- 20.Mace AG, Pai RK, Stocchi L, Kalady MF: American Joint Committee on Cancer and College of American Pathologists regression grade: A new prognostic factor in rectal cancer. Dis Colon Rectum 58: 32–44, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Rosman AS, Korsten MA: Effect of verification bias on the sensitivity of fecal occult blood testing: A meta-analysis. J Gen Intern Med 25: 1211–1221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Australian Government Department of Health: National Bowel Cancer Screening Program. 2016. Available at: http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/bowel-screening-1. Accessed July 20, 2016
- 23.Binefa G, Garcia M, Milà N, Fernández E, Rodríguez-Moranta F, Gonzalo N, et al.: Colorectal cancer screening programme in Spain: Results of key performance indicators after five rounds (2000-2012). Sci Rep 6: 19532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Symonds EL, Osborne JM, Cole SR, Bampton PA, Fraser RJ, Young GP: Factors affecting faecal immunochemical test positive rates: Demographic, pathological, behavioural and environmental variables. J Med Screen 22: 187–193, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, et al.: COLONPREV Study Investigators : Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 366: 697–706, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Wong G, Chapman JR, Craig JC: Cancer screening in renal transplant recipients: What is the evidence? Clin J Am Soc Nephrol 3[Suppl 2]: S87–S100, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viiala CH, Zimmerman M, Cullen DJ, Hoffman NE: Complication rates of colonoscopy in an Australian teaching hospital environment. Intern Med J 33: 355–359, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Lee JK, Liles EG, Bent S, Levin TR, Corley DA: Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann Intern Med 160: 171, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson JM, Jungner YG: [Principles and practice of mass screening for disease]. Bol Oficina Sanit Panam 65: 281–393, 1968 [PubMed] [Google Scholar]
- 30.Webster AC, Wong G, Craig JC, Chapman JR: Managing cancer risk and decision making after kidney transplantation. Am J Transplant 8: 2185–2191, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Irwig L, McCaffery K, Salkeld G, Bossuyt P: Informed choice for screening: Implications for evaluation. BMJ 332: 1148–1150, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong G, Howard K, Craig JC, Chapman JR: Cost-effectiveness of colorectal cancer screening in renal transplant recipients. Transplantation 85: 532–541, 2008 [DOI] [PubMed] [Google Scholar]
- 33.James LJ, Wong G, Craig JC, Ju A, Williams N, Lim WH, et al.: Beliefs and attitudes to bowel cancer screening in patients with CKD: A semistructured interview study. Clin J Am Soc Nephrol 12: 568–576, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al.: Colorectal cancer. Nat Rev Dis Primers 1: 15065, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]