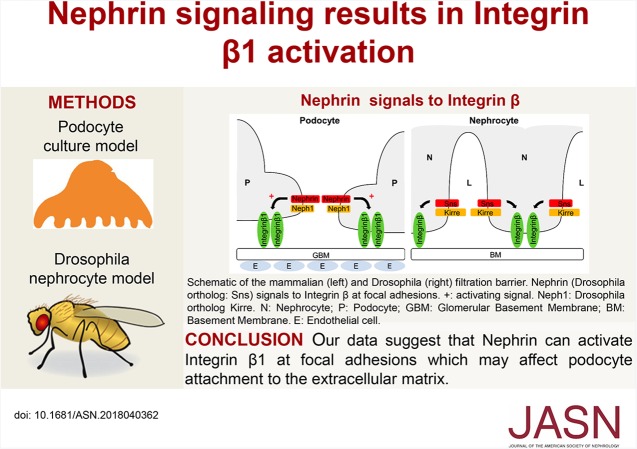
Significance Statement
The slit diaphragm protein Nephrin, which is essential for an intact glomerular filter, signals from the podocyte slit diaphragm to the Actin cytoskeleton and induces lamellipodia formation. The authors present evidence that Nephrin activation results in activation of Integrin β1 in a cultured human podocyte model, and that C3G, a guanine nucleotide exchange factor of the small GTPase Rap1, is involved in Nephrin signaling to Integrin β1. In vivo, in Drosophila nephrocytes, the Nephrin ortholog Sticks and stones is necessary for correct targeting of Integrin β1. These findings indicate that Nephrin can mediate a signaling pathway that results in activation of Integrin β1 at focal adhesions, which may affect podocyte attachment to the extracellular matrix.
Keywords: cytoskeleton, nephrin, renal cell biology, signaling
Visual Abstract
Abstract
Background
Patients with certain mutations in the gene encoding the slit diaphragm protein Nephrin fail to develop functional slit diaphragms and display severe proteinuria. Many adult-onset glomerulopathies also feature alterations in Nephrin expression and function. Nephrin signals from the podocyte slit diaphragm to the Actin cytoskeleton by recruiting proteins that can interact with C3G, a guanine nucleotide exchange factor of the small GTPase Rap1. Because Rap activity affects formation of focal adhesions, we hypothesized that Nephrin transmits signals to the Integrin receptor complex, which mediates podocyte adhesion to the extracellular matrix.
Methods
To investigate Nephrin’s role in transmitting signals to the Integrin receptor complex, we conducted genetic studies in Drosophila nephrocytes and validated findings from Drosophila in a cultured human podocyte model.
Results
Drosophila nephrocytes form a slit diaphragm–like filtration barrier and express the Nephrin ortholog Sticks and stones (Sns). A genetic screen identified c3g as necessary for nephrocyte function. In vivo, nephrocyte-specific gene silencing of sns or c3g compromised nephrocyte filtration and caused nephrocyte diaphragm defects. Nephrocytes with impaired Sns or C3G expression displayed an altered localization of Integrin and the Integrin-associated protein Talin. Furthermore, gene silencing of c3g partly rescued nephrocyte diaphragm defects of an sns overexpression phenotype, pointing to genetic interaction of sns and c3g in nephrocytes. We also found that activated Nephrin recruited phosphorylated C3G and resulted in activation of Integrin β1 in cultured podocytes.
Conclusions
Our findings suggest that Nephrin can mediate a signaling pathway that results in activation of Integrin β1 at focal adhesions, which may affect podocyte attachment to the extracellular matrix.
Podocytes build with their intercellular junctions called slit diaphragms an essential part of the kidney filter.1 Podocyte dysfunction often results in glomerular disease which can subsequently progress to ESKD.2 For most glomerular diseases, tailored molecular therapies are not available. Podocytopathies result in morphologic alteration of podocyte foot processes, which is called effacement and results in breakdown of the filtration barrier leading to proteinuria.2 Podocyte foot processes are the smallest cellular projections that are built by the Actin cytoskeleton. The slit diaphragm is located between neighboring foot processes.1 During foot process effacement, profound cytoskeletal rearrangements take place.3 Thus, it is not surprising that the Actin cytoskeleton and Actin-associated proteins appear to be promising potential therapeutic targets for glomerular disease. Two major protein complexes, the Integrin-associated focal adhesion complex, which mediates podocyte adhesion to the extracellular matrix (ECM), and the slit diaphragm junctional complex, which builds the podocyte intercellular junction, transduce signals to the Actin cytoskeleton.4–6 These protein complexes are necessary for the filtration barrier, because knockout of their key components in mice or mutations in humans result in foot process effacement and early death of the experimental mice.7–16
The slit diaphragm–associated Nephrin complex is essential for the podocyte.7 Mutations in the human NPHS1 gene encoding Nephrin cause the congenital nephrotic syndrome of the Finnish type with proteinuria in utero.8 The cell adhesion molecule Nephrin forms a receptor complex with another cell adhesion molecule, Neph1, and the Stomatin-family protein Podocin.17,18 The Src-family kinase Fyn phosphorylates Nephrin and Neph1 on intracellular tyrosine residues.19 Upon phosphorylation, multiple proteins can be recruited to Nephrin such as the cytoskeletal adapter protein Nck, the Actin-severing protein Cofilin-1, or a complex consisting of the cytoskeletal adapter proteins Cas and Crk and Focal Adhesion Kinase (FAK).20–24 Thereby, Nephrin regulates the podocyte Actin cytoskeleton. In podocyte culture, this can result in Actin polymerization directly at the Nephrin receptor complex or spatially distinct Actin polymerization by initiating lamellipodia formation.21,23 Lamellipodia are usually located at the leading edge of migrating cells and high turnover of focal adhesions (FA) underneath is necessary for orchestrated cell migration.25
At the basal side, the Integrin-associated FA complex connects podocytes with the ECM.26–28 Knockout in mice of key FA components such as Integrin or Talin results in foot process effacement, slit diaphragm breakdown, and early death of experimental mice.13,16 In cultured podocytes, Integrin activation results in Nephrin phosphorylation,29 suggesting that the FA complex transduces signals not only to the Actin cytoskeleton but also to the spatially distinct slit diaphragm.5,29
Vice versa, it is known that Nephrin activation results in lamellipodia formation23; however, it remains unclear whether Nephrin transduces signals to the Integrin complex or modulates its activity. To address this question, we used Drosophila nephrocytes, which resemble their mammalian counterparts in many ways, as a genetically tractable system and a podocyte culture model.30–32 We showed that in Drosophila the Nephrin ortholog Sticks and stones (Sns) is necessary for targeting of Integrin β. In podocyte culture, Nephrin activation leads to Integrin β1 activation.
Methods
Fly Husbandry and Genetics
Overexpression and transgenic RNAi studies were performed using the UAS-Gal4 system. All flies were grown at 25°C or 29°C on standard fly food. RNAi stocks were obtained from the Vienna Drosophila Resource Center: ControldsRNA targeting or83b (100825), which is an olfactory receptor that is not expressed in nephrocytes; snsdsRNA (109442); c3gdsRNA1 (21306); c3gdsRNA2 (29828); c3gdsRNA3 (105664); rap1dsRNA1 (20761); rap1dsRNA2 (33437); and rap1dsRNA3 (110757). UAS sns and sns-Gal4 flies were a gift from Tobias Huber (University Hospital Hamburg-Eppendorf, Hamburg, Germany).
Generation of Rabbit Anti-Sns Antibody
Rabbits were immunized (Eurogentech, Belgium) with both of the following peptides within the Sns cytoplasmic domain: amino acids 1373–1387 (QPHGILKDPNRNKQQ) and 1175–1189 (EGSDMPPPRYQKDGT). Sera from one rabbit showed specificity for Sns in immunoblots as well as in immunofluorescence analysis of garland cell nephrocytes.
Immunohistochemistry
Garland cell nephrocytes were dissected, fixed in 4% paraformaldehyde (PFA), and stained using a standard protocol.33 The following primary antibodies were used: anti-Sns (custom generated), anti-Pyd (PYD2), anti-Integrin β (CF.6G11), and anti-Talin (Talin E16B) (all from Developmental Studies Hybridoma Bank); anti-C3G (gift from Ruth Palmer, Umeå University, Sweden)34; goat–anti-mouse Alexa488, goat–anti-rabbit Alexa555, goat–anti-guinea pig Alexa488, and DAPI (all from Invitrogen).
Coimmunoprecipitation
HEK293T cells were transfected either with pQXCIP-GFP-Crb3-NephrinCT (containing the cytoplasmic term of Nephrin) or with Triple-FLAG-C3G using the calcium phosphate method.35 C3G was cloned from a human kidney library using the Gateway system (Invitrogen). Coimmunoprecipitation and immunoblot were performed using GFP-Trap (ChromoTek), antibody specific for C3G (H-300; Santa Cruz Biotechnology), Nephrin (acris), or β-Tubulin (eBiosciences).
Green Fluorescent Protein Uptake Assay
Flies of the genotype UAS::Dcr; sns-Gal4, ubi::ANF-GFP-GFP/CyO were crossed with UAS::dsRNA strains at 25°C and transferred to 29°C 48 hours after egg laying.33 Garland cell nephrocytes of L3 larvae were dissected and stained with wheat germ agglutinin (WGA) coupled to Alexa555 and DAPI. Images of nephrocytes were taken with a Zeiss LSM700 confocal microscope. The mean value of Atrial Natriuretic Factor (ANF)-GFP-GFP fluorescence per nephrocyte area was measured with ImageJ. Unpaired, two-tailed Mann–Whitney test was applied. Results are presented as mean fluorescence/µm2 cell area normalized to control condition. At least five animals and five nephrocytes per animal were evaluated for each of four separate experiments.
Red Fluorescent Protein Uptake Assay
Flies of the genotype MHC::ANF-RFP; Hand::GFP; dot-Gal4 were crossed with UAS::dsRNA strains at 25°C and transferred to 29°C 4 hours after egg laying.36 After 48 hours, uptake of ANF–red fluorescent protein (RFP) into pericardial nephrocytes was evaluated in whole larvae with an Olympus BX50 fluorescence microscope. Efficiency of protein uptake was assessed by eye and divided into two categories: no effect (−) or decreased uptake (+) of ANF-RFP compared to control.
Cell Culture, Transient Transfection, and Generation of Stable Cell Lines
Human immortalized podocytes and HEK293T cells were cultivated as previously described.23,24 Podocytes were cultured at the permissive temperature of 33°C and transfected using Lipofectamin 2000 (Thermo Fisher Scientific) according to the manufacturer’s description. Stable podocyte cell lines allowing doxycycline-dependent expression of CD16-CD7-Nephrin Cytoplasmic Domain (NCD) or CD16-CD7-HA (pInducer-21-CD16-CD7-NCD or pInducer-21-CD16-CD7-HA) were generated by lentiviral gene transfer as previously described.37
Nephrin Activation Assay
Activation of Nephrin was performed as previously described.23 In short, glass cover slips were coated with Collagen 0.1 mg/ml (Thermo Fisher Scientific) for immunofluorescence analysis of phospho-C3G in podocytes or Fibronectin 20 μg/ml (Sigma-Alldrich) for immunofluorescence analysis of Integrin activation. Podocytes transiently transfected with pEBB-CD16-CD7-NCD (CD16-NCD), pEBB-CD16-CD7-HA (CD16-HA), pEBB-CD16-CD7-NCD-Y1,2,3F (CD16-NCD-Y1,2,3F), pEBB-CD16-CD7-NCD-Y5,7,10F (CD16-NCD-Y5,7,10F), or pEBB-CD16-CD7-NCD-Y6,9F (CD16-NCDY6,9F) using Lipofectamin 2000 were serum-starved for 24 hours and CD16-Alexa647 (2 μg/ml; BD Biosciences) was added for indicated times to the medium of podocytes. Podocytes were fixed using PFA 4% (Morphisto) for 10 minutes, permeabilized in PBS containing 0.2% TritonX-100 for 5 minutes, and blocked with 3% BSA/10% FCS solution for 30 minutes at room temperature (RT). Primary antibodies p-C3G (Assay Biotech) and active Integrin-β1-Alexa488 (abcam) were diluted in 10% BSA/FCS solution and incubated for 1 hour at RT. Specificity for active Integrin β1 was previously shown.38–41 Samples were imaged using a FEI confocal microscope. Mean fluorescence intensity per cell area in square micrometers was measured using 10–30 cells per condition and separate experiments, using ImageJ and normalized to a control condition. For recruitment analysis of p-C3G to Nephrin, three independent experiments were performed and statistically analyzed separately. Altogether, for the analysis of Integrin β1 activation, three independent experiments were performed as described above. To assess the time of maximum co-localization of CD16-NCD and p-C3G, the pInducer21-puro-CD16-CD7-NCD stable podocyte line was used and Nephrin clustering was induced by adding CD16-Alexa647 to the culture medium as described above. As a control, mouse IgG was added to the medium (2 μg/ml).
CRISPR/Cas9 Knockout in Podocytes
Stable CRISPR/Cas9 knockout of the gene C3G was achieved as recently described.42 C3G gRNA sequences were cloned into pLentiCRISPRV2 using standard cloning techniques (Supplemental Table 1). HEK-293T cells were transfected with pLentiCRISPRV2, pVSV-G, and psPAX2 using calcium phosphate and podocytes were incubated with HEK supernatant followed by selection with puromycin (2 µg/ml).
Flow Cytometry Analysis
For FACS experiments, human cultured podocytes inducibly expressing CD16-CD7-NCD or CD16-CD7-HA were used. Podocytes were cultured on plastic dishes coated with Fibronectin 20 μg/ml. Before Nephrin activation, podocyte lines were treated with doxycycline 125 ng/ml for 20 hours to induce CD16-CD7-NCD or CD16-CD7-HA expression and serum-starved for 24 hours. After Nephrin activation, cells were immediately chilled on ice, scratched manually from the dish, washed with ice-cold PBS, stained with antibody specific for active Integrin β1 in 3% BSA/PBS for 30 minutes at 4°C, and fixed with 4% PFA for 10 minutes at RT. Pellets were resuspended in 500 µl cold PBS with 10% FCS and 1% sodium azide. Fluorescence was analyzed by flow cytometry using a BD FACSCalibur flow cytometer. For all three independent experiments, 20000 cells were measured.
Transmission Electron Microscopy
Garland cell nephrocytes of L3 larvae were dissected and fixed in 2.5% glutaraldehyde in Sørensen buffer for 3 days at 4°C. Samples were treated with osmium tetroxide for 1 hour, dehydrated in an ascending alcohol series, and infiltrated with epon using a series of mixtures of epon and the intermedium propylene oxide and pure epon. After embedding in epon and polymerization at 60°C for 36 hours, ultrathin slices of 60 nm were cut, and contrasted with uranyl acetate and lead citrate. Images were obtained with a Phillips CM10 transmission electron microscope.
Statistical Analysis
Unpaired, two-tailed Mann–Whitney test or unpaired, two-tailed t test was applied as indicated.
Results
c3g Is Necessary for Drosophila Nephrocyte Filter Function
The Drosophila nephrocyte forms a slit diaphragm–like structure—the nephrocyte diaphragm.30,43 It spans membrane invaginations which form a labyrinth of lacunae.30,43 There are two populations of nephrocytes in Drosophila, the pericardial nephrocytes, which are located next to the heart, and the garland nephrocytes, which surround the esophagus of Drosophila.30 Nephrocytes take up proteins of defined size. This process relies on the integrity of the nephrocyte diaphragm and functional protein uptake mechanisms.31,32,36,44 Orthologs of major slit diaphragm proteins such as Nephrin (Sns in Drosophila melanogaster [Dm]), Neph1 (Kirre in Dm), Podocin (Mec2 in Dm), and CD2AP (Cindr in Dm) are expressed and localize to nephrocyte diaphragms.30,43 To identify Actin-associated genes that are essential for nephrocyte function, we used an in vivo filtration assay where RFP-tagged ANF is secreted into the hemolymph.36 This strain also expresses the yeast expression factor Gal4 under the control of a nephrocyte-specific enhancer and the transgene hand::GFP, marking nephrocytes.36 This strain was crossed with flies that carry UAS::dsRNA.45 Altogether, a knockdown of 52 genes related to regulation of the Actin cytoskeleton, focal adhesions, and the nephrocyte diaphragm was accomplished. RFP accumulation was evaluated in L3 larvae as a surrogate of nephrocyte filter and protein uptake function (Supplemental Table 2).31,36,46 We identified the rap guanine nucleotide exchange factor c3g (RAPGEF1) as well as the small GTPase rap1 to be necessary for nephrocyte function. In other cells, the small GTPase Rap1 and its guanine nucleotide exchange factor C3G mediate crosstalk from intercellular junctions to Integrin at focal adhesions.47,48
Knockdown of c3g in Nephrocytes Reduces Protein Uptake
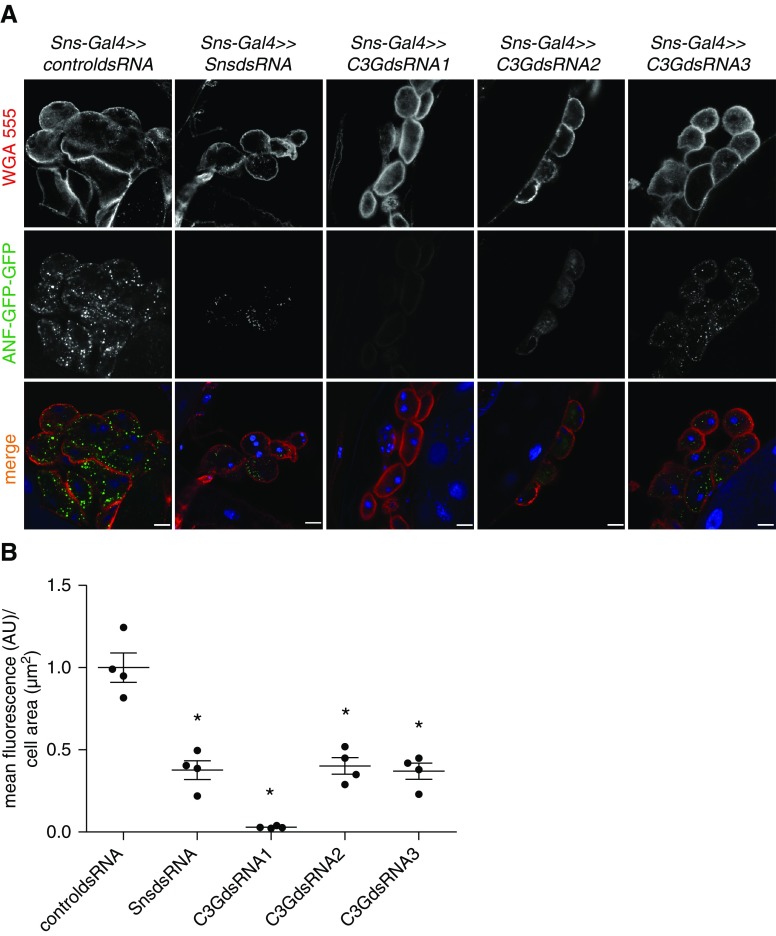
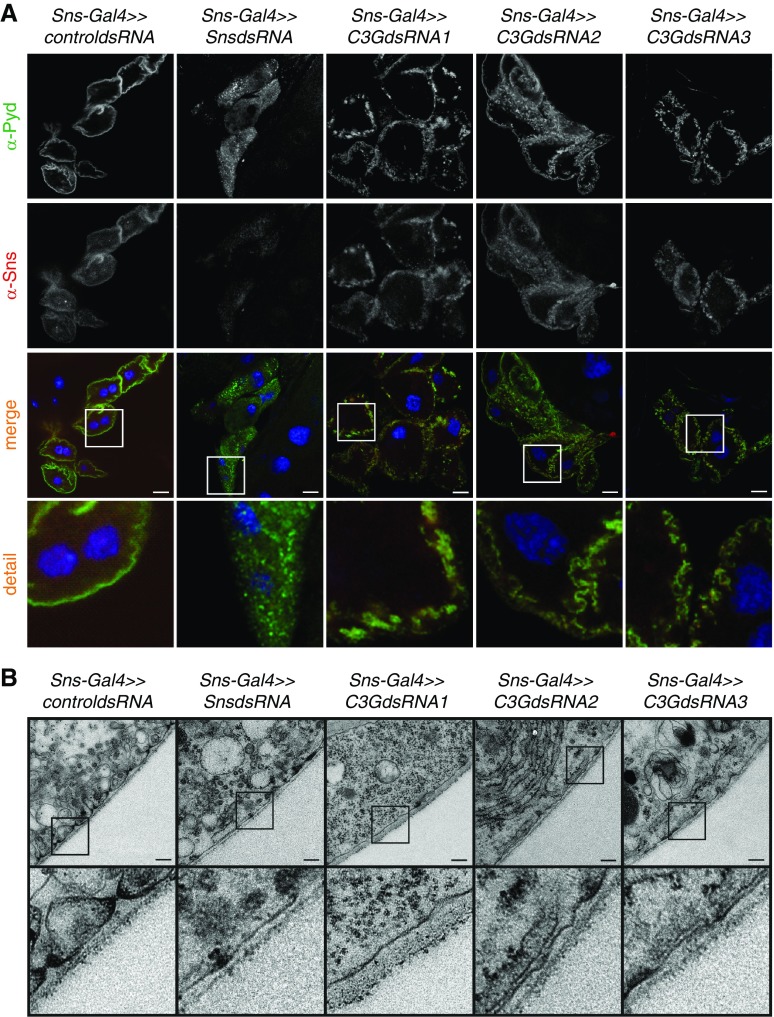
We previously showed that Nephrin transduces signals via the cytoskeletal adapter protein Crk in podocytes, which directly interacts with C3G in other cellular models.23,24,49 Thus, we hypothesized that Nephrin also signals via C3G. To explore the relationship of Nephrin (Sns in Dm) and C3G, we used the Drosophila model. First, we showed that endogenous C3G was expressed in nephrocytes (Supplemental Figure 1). To show that c3g was essential for nephrocyte function, we used another in vivo uptake assay with ubiquitously expressed and secreted ANF-GFP-GFP in larval nephrocytes.33 Nephrocytes with a knockdown of either sns or c3g exhibited significantly reduced uptake of ANF-GFP-GFP (Figure 1, A and B). Because C3G is a guanine nucleotide exchange factor for the small GTPase Rap1,48 we tested whether Rap1 is necessary for nephrocyte function. Indeed, cell type–specific rap1 knockdown in nephrocytes also resulted in a reduced ANF-GFP-GFP uptake (Supplemental Figure 2). Control nephrocytes and nephrocytes with knockdown of either sns or c3g were stained for the nephrocyte diaphragm protein Sns or the Drosophila ortholog to mammalian ZO-1/2 (Pyd). Control nephrocytes showed a distinct membrane staining pattern for Sns and Pyd, whereas nephrocytes with sns knockdown exhibited almost complete absence of Sns from the membrane and an irregular, dotty staining pattern for Pyd (Figure 2A). Nephrocytes with c3g knockdown exhibited a diffuse, dotty staining pattern for both Sns and Pyd (Figure 2A). To verify binding specificity of the newly generated Sns antibody, we performed immunofluorescence analysis of nephrocytes with nephrocyte-specific silencing of sns or control nephrocytes (Supplemental Figure 3). We then investigated whether knockdown of either sns or c3g disturbed nephrocyte diaphragm formation or maintenance. Although nephrocytes from control Drosophila showed a regular pattern of lacunae and nephrocyte diaphragms by transmission electron microscopy, nephrocytes with knockdown of either sns or c3g showed a significantly reduced number of nephrocyte diaphragms (Figure 2B).
Figure 1.
Knockdown of sns or c3g impairs nephrocyte function in vivo. (A) Uptake of secreted ANF-GFP-GFP into nephrocytes is shown in prepared control (RNAi for or83b), c3g, or sns knockdown nephrocytes. Knockdown in nephrocytes was accomplished using sns-Gal4. Samples were stained with wheat germ agglutinin-Alexa555 (WGA) to visualize membranes. Merged images are shown in the lower panel. Scale bar, 10 µm. (B) Accumulation of ANF-GFP-GFP in nephrocytes was quantified. Shown are means and SEM in arbitrary units (AU) per µm2 cell area normalized to control condition. *P<0.05 by unpaired two-tailed Mann–Whitney test. n=4.
Figure 2.
Knockdown of sns or c3g leads to loss of nephrocyte diaphragms and lacunae. (A) Nephrocytes were prepared and immunofluorescence analysis was performed with antibody specific for Sns (red) and Pyd (green) (mammalian ortholog: ZO-1/2). Merged images and higher magnifications (×4) are shown in the bottom panels. Scale bar, 10 µm. (B) TEM analysis of control (RNAi for or83b), sns, or c3g knockdown nephrocytes is shown. Note that three different RNAi lines were used for c3g knockdown. Knockdown in nephrocytes was accomplished using sns-Gal4. Upper panel scale bar, 200 nm. Lower panel, zoom ×4 compared with the upper panel. (A and B) n=3.
Knockdown of sns or c3g Alters Integrin and Talin Localization In Vivo
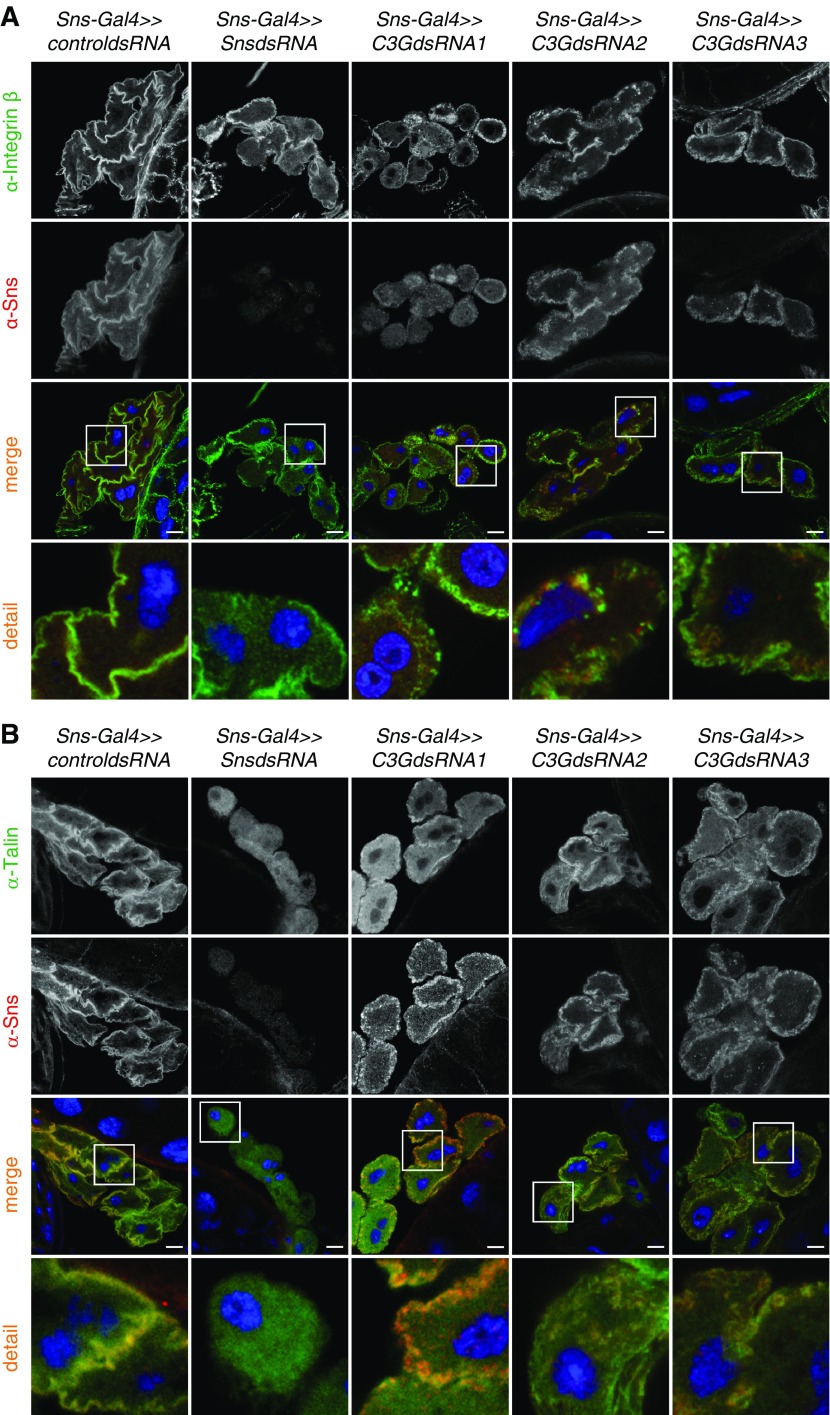
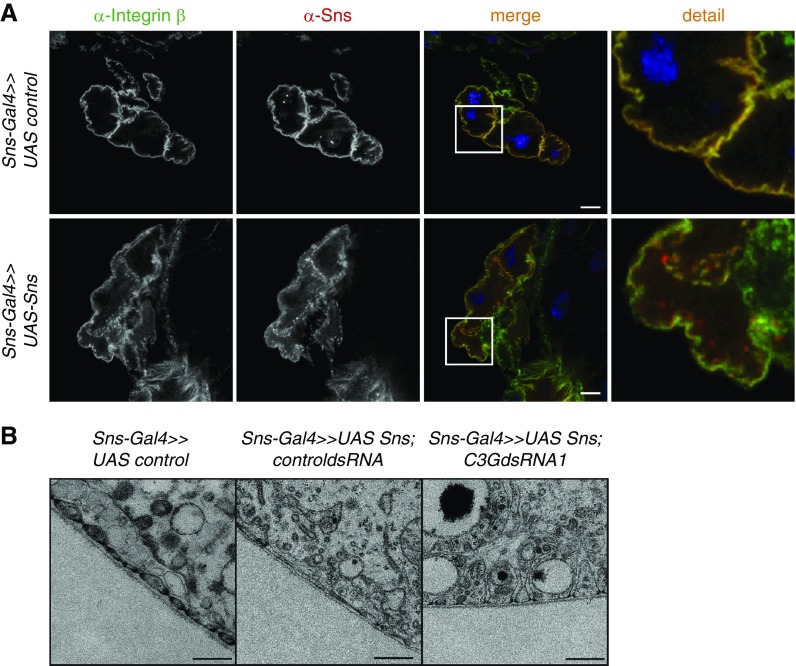
To test whether knockdown of sns resulted in alterations of FA in nephrocytes, nephrocytes with either sns or c3g knockdown were stained with an antibody specific for the Drosophila ortholog of Integrin β or the FA protein Talin. In contrast to control nephrocytes showing Integrin expression distinctly at the plasma membrane, sns or c3g knockdown led to a dotty expression pattern of Integrin with less membrane association (Figure 3A). Similarly, in sns or c3g knockdown nephrocytes, Talin expression showed less plasma membrane accumulation than in control nephrocytes (Figure 3B). Compared with the expression pattern of Integrin in sns or c3g knockdown nephrocytes, Talin expression was less dotty and largely cytoplasmic (Figure 3B).
Figure 3.
Knockdown of sns or c3g results in altered targeting of the focal adhesion proteins Integrin and Talin in nephrocytes. Immunofluorescence analysis of control (RNAi for or83b), sns, or c3g knockdown nephrocytes is shown. Knockdown in nephrocytes was accomplished using sns-Gal4. Nephrocytes were prepared and immunofluorescence analysis was performed with antibody specific for Integrin (A), Talin (B), and Sns. Merged images and higher magnifications (×4) of the marked area are shown in the bottom panels. Third panel scale bar, 10 µm. (A and B) n=3.
sns and c3g Genetically Interact in Nephrocytes
Drosophila has long been known to allow genetic dissection of complex signaling cascades.50–53 In general, the ability to suppress a phenotype caused by overexpression of a given gene (gain-of-function phenotype) by suppression of another gene is taken as evidence that the two genes interact.50–53 Because Nephrin interacts with Crk in podocytes, which in turn directly interacts with C3G in other cell models,23,54 we used the Drosophila system to test whether sns and c3g genetically interact in nephrocytes. Flies overexpressing sns (sns-Gal4; UAS sns) caused an sns gain-of-function phenotype. These flies were then either crossed with UAS c3g dsRNA- or UAS control dsRNA-containing flies and the offspring were analyzed for modulation of nephrocyte phenotypes.
sns overexpression resulted in moderate mislocalization of Sns, as shown by immunofluorescence analysis (Figure 4A). Furthermore, overexpression of sns led to mild mislocalization of Integrin β (Figure 4A). Although nephrocytes with sns overexpression exhibited a reduced number of nephrocyte diaphragms, as evaluated by transmission electron microscopy, nephrocytes with sns overexpression and concomitant c3g knockdown showed mostly intact nephrocyte diaphragms (Figure 4B). Thus, c3g knockdown partly rescued the sns gain-of-function phenotype in nephrocytes.
Figure 4.
sns and c3g genetically interact in nephrocytes. (A) Immunofluorescence analysis of nephrocytes overexpressing sns or control plasmid by sns-Gal4. Nephrocytes were stained with antibodies specific for Integrin β (green) and Sns (red). Merged images and higher magnifications (×4) of the marked area are shown in the columns to the right. Scale bar, 10 µm. (B) Shown are TEM images from Drosophila with overexpression of sns and knockdown of the control gene or83b, or flies with sns overexpression and c3g knockdown in nephrocytes. Knockdown in nephrocytes was accomplished using sns-Gal4. Scale bar, 200 nm. n=3.
Phosphorylated C3G Is Recruited to Activated Nephrin in Podocytes
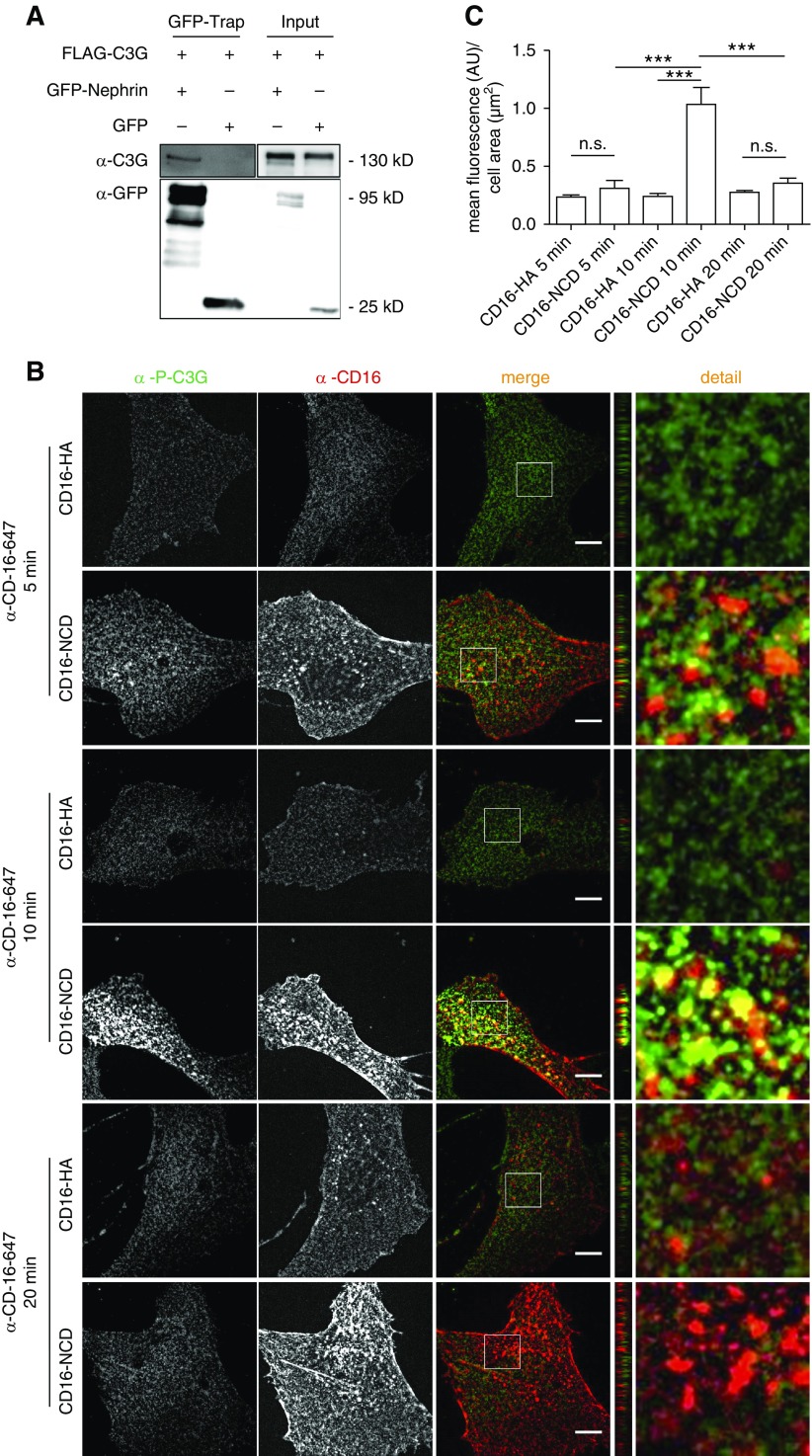
Because sns or c3g knockdown results in Integrin β mislocalization in Drosophila nephrocytes, we tested whether Nephrin signals via C3G in mammalian podocytes. To test whether Nephrin and C3G interact, we performed coimmunoprecipitation experiments with lysates from HEK293T cells expressing the GFP-tagged C terminus of Nephrin and FLAG-tagged C3G. GFP-trap pull-down experiments demonstrated that GFP-Nephrin coprecipitated with C3G, suggesting that Nephrin and C3G were part of one protein complex (Figure 5A). To test whether activation of Nephrin was necessary for recruiting C3G to Nephrin, we performed immunofluorescence analyses in cultured podocytes using a previously published assay for Nephrin activation.21,23 Addition of anti-CD16 antibody to the medium of cultured podocytes expressing recombinant chimeric Nephrin (CD16-CD7-NCD) induces “clustering” of Nephrin and subsequent phosphorylation of Nephrin on distinct tyrosine residues within its cytoplasmic domain—a process we refer to as “Nephrin activation.”21,23 After Nephrin activation in cultured podocytes, we performed costainings with an antibody specific for phospho-C3G. The phospho-C3G antibody recognizes Y504, which is a major activating regulatory phosphorylation site of C3G.54 There was significant colocalization of Nephrin and p-C3G, with a peak in colocalization and C3G phosphorylation 10 minutes after Nephrin activation (Figure 5, B and C, Supplemental Figure 4A). Activated Nephrin partly colocalized with endogenous C3G compared with controls (Supplemental Figure 4B).
Figure 5.
phospho-C3G is recruited to activated Nephrin. (A) Flag-C3G and either GFP-Nephrin or GFP were transiently expressed in HEK293T cells. GFP-trap analysis revealed that Nephrin and C3G are part of one protein complex. (B) Podocytes were transiently transfected with CD16-NCD or CD16-HA and anti-CD16-Alexa647 antibody was added to the medium for the indicated time to induce Nephrin clustering. Cells were fixed, and immunofluorescence analysis was performed with antibody specific for p-C3G. (C) Statistical analysis of mean fluorescence of p-C3G in arbitrary units (AU) per µm2 cell area. Shown are means and SEM. ***P<0.001 by unpaired two-tailed t test; NS, not significant. One representative experiment of three is shown. Ten cells per condition were evaluated. Scale bar, 10 µm.
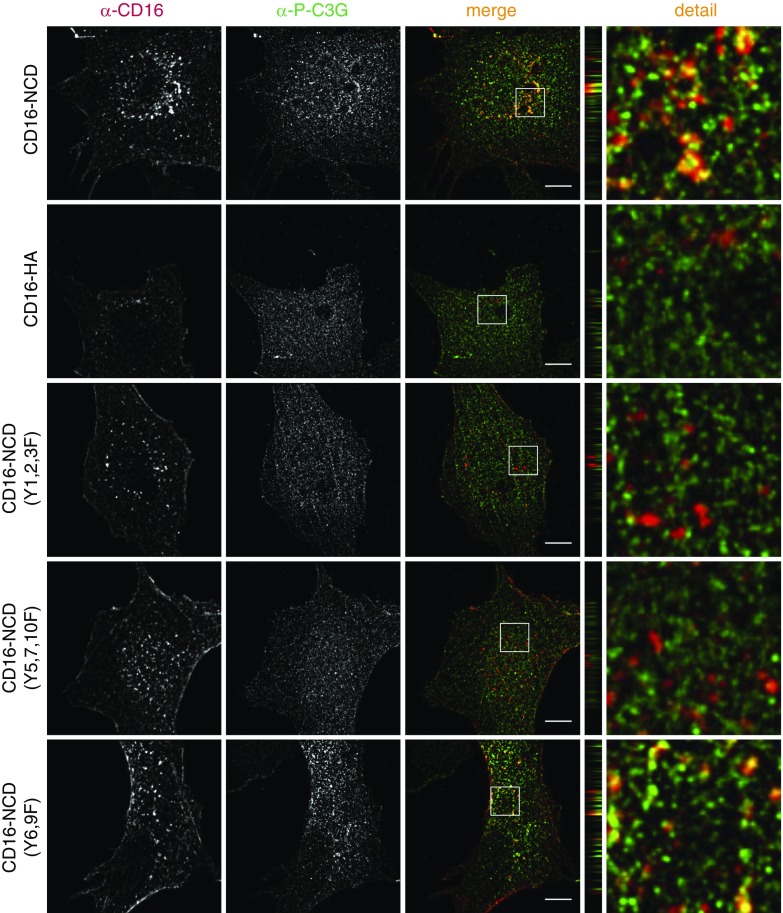
Next, we tested which of ten tyrosine residues within the cytoplasmic domain of Nephrin were necessary for recruitment of p-C3G to Nephrin by transiently expressing three Nephrin mutants in cultured podocytes where subsets of tyrosine (Y) residues were mutated to phenylalanine (F), followed by Nephrin activation (Supplemental Table 3). Immunofluorescence analysis of p-C3G staining revealed that both tyrosine clusters within Nephrin that are necessary for recruitment of the FAK-Cas-Crk complex to Nephrin were also essential for recruitment of p-C3G to Nephrin (Figure 6).23,24
Figure 6.
Recruitment of phospho-C3G to Nephrin depends on tyrosine residues also necessary for recruitment of the FAK-Cas-Crk complex to Nephrin. Human podocytes that transiently express CD16-NCD or Nephrin Y-to-F mutants as indicated were incubated with anti–CD16-Alexa647 antibody to induce Nephrin clustering. Podocytes were fixed and immunofluorescence analysis was performed with antibody specific for p-C3G. z-panel to the right. Zoom in to the far right. Scale bar, 10 µm; n=3.
Nephrin Activation Results in Integrin β1 Activation
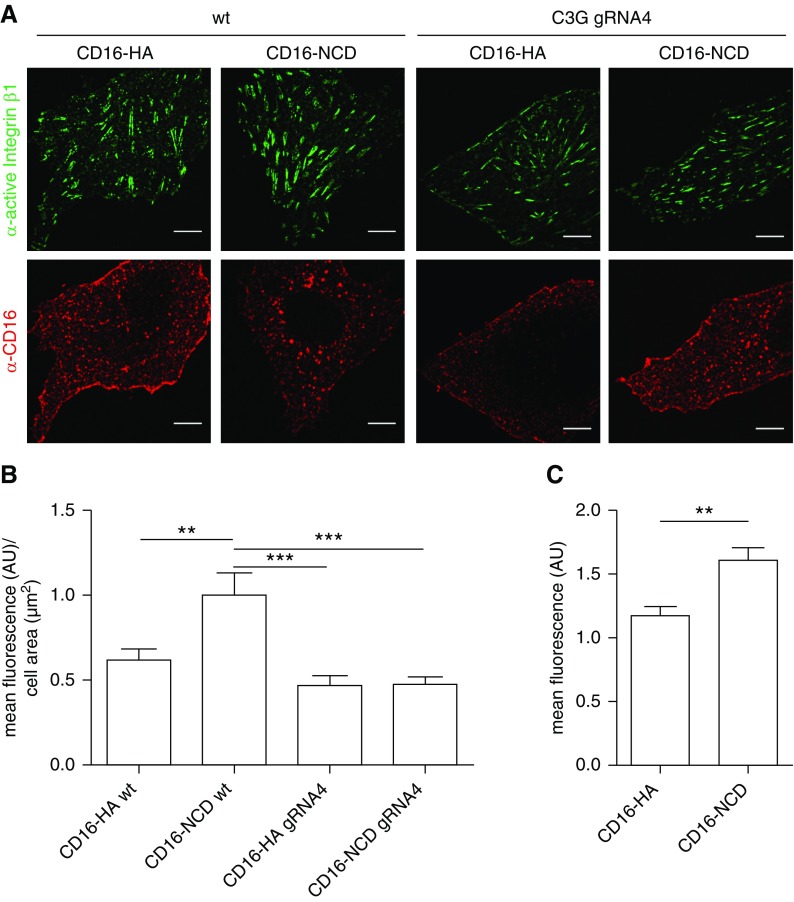
Previously, we showed that Nephrin activation results in lamellipodia formation.23 Newly formed lamellipodia are stabilized by adhesion to the ECM, which is mediated by Integrins.55 On the basis of our findings mentioned above, we hypothesized that Nephrin can modulate the Integrin activation status. To test this, transfected Nephrin was activated in cultured podocytes and podocytes were tested for endogenous active Integrin β1.38–41 This showed that Nephrin activation resulted in increased activation of Integrin β1 (Figure 7, A and B, Supplemental Figure 5, A and B). Activation of Integrin β1 after Nephrin clustering in podocyte culture was confirmed by flow cytometry analysis as an independent method (Figure 7C). We then generated podocytes with a knockout of C3G by CRISPR/Cas9 technology. To verify C3G knockout, we performed immunoblot experiments with wild-type podocytes or podocytes expressing C3G-specific gRNA (Supplemental Figure 6). We analyzed whether C3G was essential for Nephrin-mediated activation of Integrin β1 by transiently expressing recombinant Nephrin in cultured C3G knockout podocytes followed by Nephrin activation. Immunofluorescence analysis of active Integrin β1 showed that although Integrin β1 activation was increased in control podocytes after Nephrin activation, Integrin β1 activation was not substantially altered in C3G knockout podocytes after Nephrin clustering (Figure 7, A and B, Supplemental Figure 5, A and B).
Figure 7.
Nephrin activation results in Integrin β1 activation. (A) Control podocytes or C3G KO podocytes (gRNA 4) transiently expressing CD16-NCD or CD16-HA were incubated with anti-CD16-Alexa647 antibody to initiate Nephrin clustering. Podocytes were fixed, and immunofluorescence analysis was performed using antibody specific for active Integrin β1. Scale bar, 10 µm. (B) Statistical analysis of mean fluorescence intensity of active Integrin β1 in arbitrary units (AU) per µm2 cell area. **P<0.01, ***P<0.001 by unpaired two-tailed t test. n=3; 20 cells per condition were evaluated. (C) Flow cytometry analysis of cultured podocytes inducibly expressing CD16-NCD or CD16-HA that were incubated with anti–CD16-Alexa647 antibody to initiate Nephrin clustering is shown. Podocytes were scraped from the dish, stained with antibody specific for active Integrin β1, and fixed. The statistical analysis by unpaired two-tailed t test shows the mean fluorescence intensity of active Integrin β1 in AU normalized to the control condition. n=3, **P<0.01. KO, knockout; wt, wild type.
Discussion
In this study, we used the Drosophila model to identify the RAPGEF1 C3G and its small GTPase Rap1 to be necessary for nephrocyte function. We showed that changes in the sns or c3g gene dosage resulted in impaired nephrocyte function, loss of nephrocyte diaphragms, and mislocalization of Integrin β1. These findings were tested in a human podocyte culture model. We found that Nephrin transduced signals that activated Integrin β1 (Figure 8).
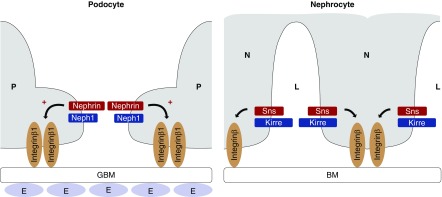
Figure 8.
Nephrin signals to Integrin β in podocytes and Drosophila nephrocytes. To the left, a schematic of the mammalian filtration barrier is shown, with the fenestrated endothelium (E), the glomerular basement membrane (GBM), and two mammalian podocyte (P) foot processes with the slit diaphragm in between. Slit diaphragm proteins Nephrin and Neph1 are depicted as well as Integrin β1, which connects the basal podocyte with the GBM. Nephrin activation in podocyte culture results in activation of Integrin β1 (arrow marked with +). To the right, a schematic of the Dm nephrocyte (N) filtration barrier is shown, which consists of the basement membrane (BM) and the intracellular junction that spans the gaps between invaginations in the nephrocyte plasma membrane. Nephrocyte slit diaphragm proteins that are orthologs of Nephrin (Sns in Dm) and Neph1 (Kirre in Dm) are shown. Drosophila Integrin β connects the nephrocyte with the basement membrane. Sns gain of function results in mislocalization of Integrin β (depicted by arrow). L, nephrocyte lacunae.
In past years, Drosophila genetics have often served as a potent discovery tool to disclose components of complex signaling pathways. On the one hand, modulation of a weak loss-of-function phenotype by reduction of the gene dose of an interacting gene provided information on genetic networks. On the other hand, the suppression of gain-of-function phenotypes, for example caused by the constitutive activation of a receptor tyrosine kinase, provided important insights into signaling cascades.50–53 Drosophila nephrocytes form intracellular slit diaphragm–like structures that require expression of many proteins known from the mammalian slit diaphragm such as Nephrin (Sns in Dm), Neph1 (Kirre in Dm), and many orthologous adapter proteins.30,43 The similarities of the slit diaphragms and the feasibility of performing high-throughput in vivo screening experiments make Drosophila an attractive model for identifying functionally relevant slit diaphragm constituents.31,36
Endocytosis of E-Cadherin results in activation of Rap1, which may in turn lead to redistribution of Integrin and Integrin-regulating proteins to novel adhesion sites.47 Rap1 and Rap1-regulating proteins play a central role in inside-out activation of Integrin.49 Furthermore, in T lymphocytes signal transduction from the immunologic synapse leads to C3G-mediated activation of Rap1 and to Integrin affinity maturation.56 In Drosophila, c3g mutants display abnormal muscle morphology and attachment as well as defective targeting of Integrin to muscle attachment sites.34 These data are in line with our finding that Nephrin transmits signals from the podocyte intercellular junction to regulate Integrin-dependent cell matrix adhesion and that this process may be C3G-dependent. This provides further evidence that signals are transduced from intercellular junctions to FA to regulate cell adhesion with the ECM. In mammalian cells, Crk binds C3G.54 Because Nephrin signals via Crk proteins, C3G could be recruited to the Nephrin signaling cluster by Crk.23
Both Integrin-associated signaling proteins at the basal side of the podocyte as well as proteins of the slit diaphragm signaling hub are essential for functional podocytes.7–16 Furthermore, Rap1 as a central mediator of Integrin activation is activated by C3G and is necessary for podocyte function.57–59 In mice, loss of function of Rap1a and Rap1b results in massive glomerulosclerosis and premature death.58 Overexpression of the Rap inactivating protein Rap1GAP mediates reduced activation of Integrin β1.58 Rap1GAP is upregulated in human glomerular disease.58 The hypothesis that Rap1 activation is dysregulated in glomerular disease is supported by the finding that RAPGEF1 C3G is upregulated in a rat model of anti-glomerular basement membrane (GBM) antibody–mediated GN.59 A potential Nephrin-C3G-Rap1 pathway could be an important regulator of podocyte detachment, which is a considerable pathomechanism that contributes to the progression of glomerular disease.60–62 Up to now, it was unclear which upstream stimuli converge on this pathway. Our finding that Nephrin activation can initiate Integrin β1 activation sheds light on upstream regulating mechanisms that connect slit diaphragm signaling to FA sites in podocytes. Because Nephrin is activated in subsets of human glomerular disease, these diseases may be susceptible to molecular targeting of components of this pathway.
Gene silencing of c3g in vivo in nephrocytes results in almost complete loss of lacunae and nephrocyte diaphragms in larvae. C3G knockout in mice is lethal on embryonic day 7.5 before the pronephros arises.63 Because Nephrin is necessary for foot process development in mice and C3G may act downstream of Nephrin, it would be interesting to test whether C3G is essential for foot process formation in mice by using a conditional knockout strategy.64
In summary, our data suggest that Nephrin can activate a signaling pathway that results in activation of Integrin β1 at FA, which may affect podocyte attachment to the ECM. This pathway may be instrumental in podocyte loss and GBM denudation—processes that ultimately result in CKD.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Lawrence B. Holzman (University of Pennsylvania) for providing phospho-Nephrin antibody and numerous plasmids, and Zhe Han (Washington University) and Tobias Huber (University Hospital Hamburg-Eppendorf, Hamburg, Germany) for providing Drosophila.
This work was funded by grants from the German Research Foundation (GE 2158/3-1) to Dr. George, the German Research Foundation (SFB1009-B10) to Dr. Klämbt, Dr. Wedlich-Söldner and Dr. Pavenstädt, the German Research Foundation (WE 2550/2-2) to Dr. Weide, the German Research Foundation (SFB1348-A05) to Dr. Krahn, “Innovative Medizinische Forschung” (GE111303) to Dr. George, “Interdisziplinäres Zentrum für Klinische Forschung” (SEED06/15) to Dr. Dlugos, a Cells in Motion Bridging grant (German Research Foundation) to Dr. Picciotto, and a MedK scholarship to M. Krakow (Medical Faculty Münster). This work was also supported by the Medical Faculty Münster (Technology Platform Drosophila).
The work contains parts of the thesis of Dr. Picciotto, the thesis of C. Lepa, and the thesis of M. Krakow.
Dr. Dlugos, Dr. Pavenstädt, Dr. Weide, and Dr. George designed the study. Dr. Dlugos, Dr. Picciotto, M. Krakow, A. Stöber, Dr. Van Marck, C. Lepa, and Dr. Eddy carried out the experiments. Dr. Dlugos, Dr. Picciotto, A. Stöber, Dr. Klingauf, Dr. Krahn, Dr. Jeibmann, Dr. Wedlich-Söldner, Dr. Klämbt, and Dr. George analyzed the data. Dr. Dlugos, Dr. Picciotto, and Dr. George made the figures. Dr. Dlugos, Dr. Picciotto, Dr. Weide, Dr. Pavenstädt, Dr. Klämbt, Dr. Wedlich-Söldner, Dr. Jeibmann, Dr. Klingauf, and Dr. George drafted and revised the paper. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018040362/-/DCSupplemental.
Supplemental Figure 1. Drosophila nephrocytes express C3G.
Supplemental Figure 2. Rap1 is necessary for nephrocyte function.
Supplemental Figure 3. Anti-Sns antibody specifically recognizes Sns.
Supplemental Figure 4. Activated Nephrin recruits C3G.
Supplemental Figure 5. Nephrin activation results in Integrin β1 activation (additional data).
Supplemental Figure 6. C3G knockout in human podocytes.
Supplemental Table 1. Guide RNA (gRNA) sequences used for generation of C3G knockout podocytes by CRISPR/Cas9 technology.
Supplemental Table 2. Genes identified to be necessary for nephrocyte function.
Supplemental Table 3. Nomenclature of mouse Nephrin tyrosine (Y) residues.
References
- 1.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Scott RP, Quaggin SE: Review series: The cell biology of renal filtration. J Cell Biol 209: 199–210, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harburger DS, Calderwood DA: Integrin signalling at a glance. J Cell Sci 122:159–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George B, Holzman LB: Signaling from the podocyte intercellular junction to the actin cytoskeleton. Semin Nephrol 32: 307–318, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, et al.: Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol 21: 4829–4836, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santín S, García-Maset R, Ruíz P, Giménez I, Zamora I, Peña A, et al.: FSGS Spanish Study Group : Nephrin mutations cause childhood- and adult-onset focal segmental glomerulosclerosis. Kidney Int 76: 1268–1276, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, et al.; NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Roselli S, Heidet L, Sich M, Henger A, Kretzler M, Gubler M, et al. : Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol 24: 550–560, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman L, Potla U, Coleman S, Dikiy S, Hata Y, Kurihara H, et al.: Up-regulation of the homophilic adhesion molecule sidekick-1 in podocytes contributes to glomerulosclerosis. J Biol Chem 285: 25677–25685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lausecker F, Tian X, Inoue K, Wang Z, Pedigo CE, Hassan H, et al.: Vinculin is required to maintain glomerular barrier integrity. Kidney Int 93: 643–655, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian X, Kim JJ, Monkley SM, Gotoh N, Nandez R, Soda K, et al.: Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J Clin Invest 124: 1098–1113, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartleben B, Schweizer H, Lübben P, Bartram MP, Möller CC, Herr R, et al.: Neph-Nephrin proteins bind the Par3-Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J Biol Chem 283: 23033–23038, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borza CM, Su Y, Chen X, Yu L, Mont S, Chetyrkin S, et al.: Inhibition of integrin α2β1 ameliorates glomerular injury. J Am Soc Nephrol 23: 1027–1038, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, et al.: Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol 316: 288–301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barletta G, Kovari IA, Verma RK, Kerjaschki D, Holzman LB. Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem 278(21):19266–19271, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Garg P, Verma R, Holzman LB: Slit diaphragm junctional complex and regulation of the cytoskeleton. Nephron, Exp Nephrol 106: e67–e72, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, et al.: Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem 278: 20716–20723, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, et al.: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB: Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg P, Verma R, Cook L, Soofi A, Venkatareddy M, George B, et al.: Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J Biol Chem 285: 22676–22688, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George B, Verma R, Soofi AA, Garg P, Zhang J, Park TJ, et al.: Crk1/2-dependent signaling is necessary for podocyte foot process spreading in mouse models of glomerular disease. J Clin Invest 122: 674–692, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George B, Fan Q, Dlugos CP, Soofi AA, Zhang J, Verma R, et al.: Crk1/2 and CrkL form a hetero-oligomer and functionally complement each other during podocyte morphogenesis. Kidney Int 85: 1382–1394, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krause M, Gautreau A: Steering cell migration: Lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol 15: 577–590, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Geiger B, Spatz JP, Bershadsky AD: Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10: 21–33, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Margadant C, Monsuur HN, Norman JC, Sonnenberg A: Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol 23: 607–614, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Ginsberg MH: Integrin activation. BMB Rep 47: 655–659, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma R, Venkatareddy M, Kalinowski A, Patel SR, Garg P: Integrin ligation results in nephrin tyrosine phosphorylation in vitro. PLoS One 11: e0148906, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weavers H, Prieto-Sánchez S, Grawe F, Garcia-López A, Artero R, Wilsch-Bräuninger M, et al.: The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457: 322–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermle T, Braun DA, Helmstädter M, Huber TB, Hildebrandt F: Modeling monogenic human nephrotic syndrome in the Drosophila garland cell nephrocyte. J Am Soc Nephrol 28: 1521–1533, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cagan RL: The Drosophila nephrocyte. Curr Opin Nephrol Hypertens 20: 409–415, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Hochapfel F, Denk L, Mendl G, Schulze U, Maaßen C, Zaytseva Y, et al.: Distinct functions of Crumbs regulating slit diaphragms and endocytosis in Drosophila nephrocytes. Cell Mol Life Sci 74: 4573–4586, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirinian M, Popovic M, Grabbe C, Varshney G, Hugosson F, Bos H, et al.: The Rap1 guanine nucleotide exchange factor C3G is required for preservation of larval muscle integrity in Drosophila melanogaster. PLoS One 5: e9403, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colwill K, Wells CD, Elder K, Goudreault M, Hersi K, Kulkarni S, et al.: Modification of the Creator recombination system for proteomics applications--improved expression by addition of splice sites. BMC Biotechnol 6: 13, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang F, Zhao Y, Han Z: An in vivo functional analysis system for renal gene discovery in Drosophila pericardial nephrocytes. J Am Soc Nephrol 24: 191–197, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulze U, Vollenbröker B, Braun DA, Van Le T, Granado D, Kremerskothen J, et al.: The Vac14-interaction network is linked to regulators of the endolysosomal and autophagic pathway. Mol Cell Proteomics 13: 1397–1411, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandt DT, Baarlink C, Kitzing TM, Kremmer E, Ivaska J, Nollau P, et al.: SCAI acts as a suppressor of cancer cell invasion through the transcriptional control of β1-integrin. Nat Cell Biol 11: 557–568, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Tanner MR, Pennington MW, Laragione T, Gulko PS, Beeton C: KCa1.1 channels regulate β1-integrin function and cell adhesion in rheumatoid arthritis fibroblast-like synoviocytes. FASEB J 31: 3309–3320, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacquemet G, Baghirov H, Georgiadou M, Sihto H, Peuhu E, Cettour-Janet P, et al.: L-type calcium channels regulate filopodia stability and cancer cell invasion downstream of integrin signalling. Nat Commun 7: 13297, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu Z, Liu F, Tonkova EA, Lee SY, Tschumperlin DJ, Brenner MB: Soft matrix is a natural stimulator for cellular invasiveness. Mol Biol Cell 25: 457–469, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanjana NE, Shalem O, Zhang F: Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods 11: 783–784, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhuang S, Shao H, Guo F, Trimble R, Pearce E, Abmayr SM: Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 136: 2335–2344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Na J, Cagan R: The Drosophila nephrocyte: Back on stage. J Am Soc Nephrol 24: 161–163, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Duffy JB: GAL4 system in Drosophila: A fly geneticist’s Swiss army knife. Genesis 34: 1–15, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Fu Y, Zhu JY, Zhang F, Richman A, Zhao Z, Han Z: Comprehensive functional analysis of Rab GTPases in Drosophila nephrocytes. Cell Tissue Res 368: 615–627, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balzac F, Avolio M, Degani S, Kaverina I, Torti M, Silengo L, et al.: E-cadherin endocytosis regulates the activity of Rap1: A traffic light GTPase at the crossroads between cadherin and integrin function. J Cell Sci 118: 4765–4783, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Retta SF, Balzac F, Avolio M: Rap1: A turnabout for the crosstalk between cadherins and integrins. Eur J Cell Biol 85: 283–293, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Radha V, Mitra A, Dayma K, Sasikumar K: Signalling to actin: Role of C3G, a multitasking guanine-nucleotide-exchange factor. Biosci Rep 31: 231–244, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Simon MA, Bowtell DD, Dodson GS, Laverty TR, Rubin GM: Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell 67: 701–716, 1991 [DOI] [PubMed] [Google Scholar]
- 51.Fortini ME, Simon MA, Rubin GM: Signalling by the sevenless protein tyrosine kinase is mimicked by Ras1 activation. Nature 355: 559–561, 1992 [DOI] [PubMed] [Google Scholar]
- 52.Brunner D, Oellers N, Szabad J, Biggs WH 3rd, Zipursky SL, Hafen E: A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell 76: 875–888, 1994 [DOI] [PubMed] [Google Scholar]
- 53.Gaul U, Chang H, Choi T, Karim F, Rubin GM: Identification of ras targets using a genetic approach. Ciba Found Symp 176: 85–92; discussion 92–95, 1993 [DOI] [PubMed] [Google Scholar]
- 54.Tanaka S, Morishita T, Hashimoto Y, Hattori S, Nakamura S, Shibuya M, et al.: C3G, a guanine nucleotide-releasing protein expressed ubiquitously, binds to the Src homology 3 domains of CRK and GRB2/ASH proteins. Proc Natl Acad Sci U S A 91: 3443–3447, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parsons JT, Horwitz AR, Schwartz MA: Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 11: 633–643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kloog Y, Mor A: Cytotoxic-T-lymphocyte antigen 4 receptor signaling for lymphocyte adhesion is mediated by C3G and Rap1. Mol Cell Biol 34: 978–988, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu L, Yang J, Bromberger T, Holly A, Lu F, Liu H, et al.: Structure of Rap1b bound to talin reveals a pathway for triggering integrin activation. Nat Commun 8: 1744, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Potla U, Ni J, Vadaparampil J, Yang G, Leventhal JS, Campbell KN, et al.: Podocyte-specific RAP1GAP expression contributes to focal segmental glomerulosclerosis-associated glomerular injury. J Clin Invest 124: 1757–1769, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rufanova VA, Lianos E, Alexanian A, Sorokina E, Sharma M, McGinty A, et al.: C3G overexpression in glomerular epithelial cells during anti-GBM-induced glomerulonephritis. Kidney Int 75: 31–40, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, et al.: Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, et al.: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O’Connor C, et al.: Glomerular aging and focal global glomerulosclerosis: A podometric perspective. J Am Soc Nephrol 26: 3162–3178, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohba Y, Ikuta K, Ogura A, Matsuda J, Mochizuki N, Nagashima K, et al.: Requirement for C3G-dependent Rap1 activation for cell adhesion and embryogenesis. EMBO J 20: 3333–3341, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rantanen M, Palmén T, Pätäri A, Ahola H, Lehtonen S, Aström E, et al.: Nephrin TRAP mice lack slit diaphragms and show fibrotic glomeruli and cystic tubular lesions. J Am Soc Nephrol 13: 1586–1594, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.