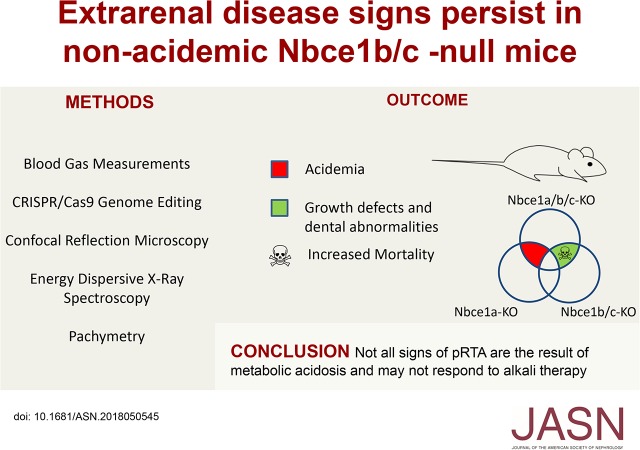
Significance Statement
Recessive SLC4A4 mutations are a cause of proximal renal tubular acidosis (pRTA), a rare but devastating disorder associated with loss of electrogenic sodium bicarbonate cotransporter 1 (NBCe1) function in kidney and other organs. Alkali therapy is the preferred treatment for pRTA, which is characterized by acidemia, developmental impairment, and vision loss, and often enamel hypomineralization. However, which nonrenal findings are secondary to acidemia is poorly understood. The authors describe the phenotype of a line of transgenic mice in which NBCe1 expression is blocked in all tissues except the proximal tubule. These mice are not acidemic but still exhibit many of the extrarenal signs associated with pRTA, revealing the potential limitations of pH correction by alkali therapy in pRTA and the need to develop novel therapies.
Keywords: chronic metabolic acidosis, genetic renal disease, Intracellular pH, renal proximal tubule cell, bicarbonate, NBCe1
Visual Abstract
Abstract
Background
The SLC4A4 gene encodes electrogenic sodium bicarbonate cotransporter 1 (NBCe1). Inheritance of recessive mutations in SLC4A4 causes proximal renal tubular acidosis (pRTA), a disease characterized by metabolic acidosis, growth retardation, ocular abnormalities, and often dental abnormalities. Mouse models of pRTA exhibit acidemia, corneal edema, weak dental enamel, impacted colons, nutritional defects, and a general failure to thrive, rarely surviving beyond weaning. Alkali therapy remains the preferred treatment for pRTA, but it is unclear which nonrenal signs are secondary to acidemia and which are a direct consequence of NBCe1 loss from nonrenal sites (such as the eye and enamel organ) and therefore require separate therapy. SLC4A4 encodes three major NBCe1 variants: NBCe1-A, NBCe1-B, and NBCe1-C. NBCe1-A is expressed in proximal tubule epithelia; its dysfunction causes the plasma bicarbonate insufficiency that underlies acidemia. NBCe1-B and NBCe1-C exhibit a broad extra-proximal-tubular distribution.
Methods
To explore the consequences of Nbce1b/c loss in the absence of acidemia, we engineered a novel strain of Nbce1b/c-null mice and assessed them for signs of pRTA.
Results
Nbce1b/c-null mice have normal blood pH, but exhibit increased mortality, growth retardation, corneal edema, and tooth enamel defects.
Conclusions
The correction of pRTA-related acidemia should not be considered a panacea for all signs of pRTA. The phenotype of Nbce1b/c-null mice highlights the physiologic importance of NBCe1 variants expressed beyond the proximal tubular epithelia and potential limitations of pH correction by alkali therapy in pRTA. It also suggests a novel genetic locus for corneal dystrophy and enamel hypomineralization without acidemia.
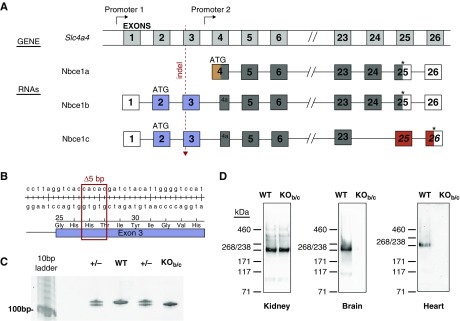
Electrogenic sodium bicarbonate cotransporter 1 (NBCe1; encoded by the SLC4A4 gene) is the archetypal and the most extensively studied of the five Na+-coupled bicarbonate transporters of the SLC4 solute carrier family.1,2 SLC4A4 expresses five NBCe1 variants, designated A, B, C, D, and E, from two distinct promoters (Figure 1A).3,4 Each is composed of an approximately 400 amino-acid cytosolic amino-terminal domain (Nt), 14 transmembrane spans, and an approximately 100 amino-acid cytosolic carboxy-terminal sequence (Ct). NBCe1-A is expressed in the basolateral membranes of renal proximal tubule epithelia and is a critical part of the mechanism that supplies blood plasma with newly generated and reabsorbed HCO3− to maintain whole-body pH.5,6 NBCe1-B and NBCe1-C (hereafter collectively referred to as NBCe1-B/C) are expressed in a wide variety of epithelial and excitable cells where they import HCO3−, either to maintain intracellular pH (e.g., in neurons) or to support transepithelial anion/fluid secretion (e.g., in gastrointestinal epithelia).7–9 NBCe1-A and NBCe1-B/C differ not only in their distribution but also in their Nt and Ct sequences; NBCe1-A includes a unique 41 amino-acid autostimulatory domain (encoded by the orange section of exon 4 in Figure 1A), whereas NBCe1-B/C include in its place an 85 amino-acid autoinhibitory domain (encoded by exons 2 and 3; blue in in Figure 1A).10 The consequence of these alternative Nt sequences is that NBCe1-A exhibits a greater conductance than NBCe1-B/C. However, under the influence of the autoinhibitory domain–binding, secretagogue-activated protein IRBIT, NBCe1-B/C can achieve a similar magnitude of conductance to NBCe1-A.10–12 The consequence of the Ct differences between NBCe1-B and NBCe1-C (46 amino-acid domain in NBCe1-B is replaced by a different 61 amino-acid domain in NBCe1-C) have yet to be fully understood. Of the two variants, NBCe1-B appears to exhibit a broader distribution than NBCe1-C, with expression of the latter being predominantly limited to neurons and glia.8,13 NBCe1-D and NBCe1-E are a subset of NBCe1-A and NBCe1-B transcripts with apparently overlapping expression profiles that, because of alternative splicing, omit a nine amino-acid sequence from the amino-terminal domain of the cognate protein.14 Little is known of their unique physiologic contribution, but it is to be noted that any disruption of NBCe1-A should also disrupt NBCe1-D and any disruption of NBCe1-B should also disrupt NBCe1-E.
Figure 1.
Nbce1b/c is specifically disrupted in Nbce1b/c-null (KOb/c) mice. (A) Cartoon depicting the structure of the Slc4a4 gene (not to scale) and its major products Nbce1a, Nbce1b, and Nbce1c (minor products Nbce1d and e are produced by choice of an alternate 5′ splice site within exon 6 of a and b).14 Numbered gray boxes are exons. Exons that are included in each gene product are reproduced below the gene cartoon: Noncoding exons are colorless, exons common to all variants are colored dark gray, exons encoding sequence unique to individual exons are colored orange, blue, or red. Exons 7–22 are included in all variants and are omitted to highlight the variable regions. (B) Genomic DNA and encoded protein sequence around the 5 bp deletion in the described strain of Nbce1b/c-null mouse. (C) Example of a genotyping gel showing the shift in molecular weight of the amplicon caused by the 5 bp deletion. (D) Western blots showing the effect of the 5 bp deletion upon the abundance of Nbce1 in lysates prepared from the kidneys, brains, and hearts of Nbce1b/c-null mice. Each blot is representative of results obtained from lysates prepared from three pairs of wild-type versus KOb/c littermates and probed with the anti-Slc4a4 antibody. Four further paired kidney lysates were probed with the anti–NBCe1-A/B antibody αrb1NBC (a gift from Walter Boron at Case Western Reserve University8) with similar results. WT, wild-type.
Recessive inheritance of mutations in SLC4A4 causes the rare but systematically devastating disease proximal renal tubular acidosis (pRTA).15,16 All of the 15 affected individuals who have been described to date exhibit acidemia (low plasma [HCO3−] due to a renal reabsorption defect), growth retardation, and one or more of the following ocular pathologies: band keratopathy, cataracts, and glaucoma.15,17–28 The other signs and sequelae of pRTA exhibit variable penetrance and include intellectual impairment, dental abnormalities, and a variety of other neurologic and neuromuscular features including migraine, muscle weakness, and bilateral calcification of the basal ganglia (reviewed in Parker and Boron1). Nbce1a/b/c-null mice model several aspects of the human disease, namely acidemia, growth retardation, and dental abnormalities, as well as several other signs that have not been observed with human pRTA, such as corneal edema, impacted intestines, and greater mortality.24,29,30 No strain of Nbce1-null mice has been described to exhibit any of the neurologic signs that are common to individuals with pRTA.
The cause of the acidemia in pRTA is logically considered to be NBCe1-A loss, but it is unclear which of the other signs or sequelae of pRTA are secondary to acidemia, specifically due to NBCe1-B/C loss, or are the combined results of acidemia and NBCe1-B/C loss. The current treatment for pRTA is the same as for any form of metabolic acidosis: alkali administration.31–33 Although this approach, if well administered, can correct acidemia, it would not be expected to ameliorate those signs that follow NBCe1-B/C loss.
We have generated a novel mouse model to discover the consequence of Nbce1b/c loss in the absence of acidemia. This mouse was designed to model pRTA with a perfectly administered alkali therapy and highlight those signs that require additional therapies.
A preliminary report of this work has appeared in abstract form.34
Methods
Ethical Statement
All procedures involving mice were approved by and performed in accordance with the rules and recommendations of the Institutional Animal Care and Use Committee of the University at Buffalo.
Generation of Nbce1b/c-Null Mice
Slc4a4-targeted founder mice were generated by the Gene Targeting and Transgenic Shared Resource at the Roswell Park Comprehensive Cancer Center (Buffalo, NY) using CRISPR/Cas9 technology with a guide RNA (5′-GGACCCCAATGTAGATCGTGNGG-3′) that targets the antisense strand of the second coding exon of Slc4a4. The guide was designed, tested, and generated by the Genome Engineering and iPSC Center at Washington University in St. Louis (St. Louis, MO). A female C57BL/6J mosaic mouse that harbored a 5-nt deletion in the targeted exon was generated via pronuclear microinjection. The female was then bred with a wild-type male C57BL/6J mouse (Jackson Laboratory, Bar Harbor, ME) to confirm germline transmission. Heterozygous pups from this union were designated as generation F1 and thenceforth backcrossed onto the C57BL/6J genetic background. All experimental animals were produced from heterozygote×heterozygote crosses using animals backcrossed to F3–F10 generation.
Genotyping
Genomic DNA was prepared from mouse tail snips using a DNeasy Blood and Tissue Kit (QIAGEN Inc., Germantown, MD) and used as a template for PCR. PCR primers (5′-CATGGGTTGAAATGACCGTTG-3′ forward, 5′- CCTTGTGCCCAGCCTTCCTC-3′ reverse) flanking the site of the deletion were used to amplify a 125-bp product for wild-type mice or a 120-bp product for Nbce1b/c-null mice. The products were resolved by electrophoresis on 6% TBE-Urea gels (Thermo Fisher Scientific, Waltham, MA) alongside a Track-It 10bp ladder (Thermo Fisher Scientific; Figure 1C). Initially we confirmed the identity of these PCR products and the presence of the 5-nt deletion by subcloning into a TOPO vector (Thermo) followed by DNA sequencing (Eurofins Genomics, Louisville, KY: data not shown).
Blood Gas Measurements
Blood drawn by cardiac puncture from isoflurane anesthetized mice (5%, inhaled) was immediately transferred into a blood gas electrolyte and metabolite test card and analyzed using an epoc reader according to the manufacturer’s instructions (both Siemens Medical Solutions USA Inc., Malvern, PA).
Western Blotting
Organs were homogenized in 1 ml of ice-cold homogenization buffer (100 mM NaCl, 25 mM HEPES, 250 mM sucrose, pH 7.4) plus cOmplete Protease Inhibitor Cocktail (Sigma-Aldrich, St. Louis, MO). Then, 5 µg/lane of organ lysate was resolved on a 3%–8% Tris-Acetate gel and transferred onto a PVDF membrane (Thermo Fisher Scientific). The PVDF was incubated overnight in TBS containing 0.1% Tween-20 and 5% milk powder and probed using an anti-SLC4A4 rabbit polyclonal antibody ESAP14635 (#E-AB-14348; Elabscience Biotechnology Inc., Houston, TX; characterized in this study by single band of appropriate molecular weight by Western blotting, as well as loss of immunoreactivity from Nbce1b/c mice) followed by an HRP-conjugated goat–anti-rabbit secondary antibody (MP Biomedicals, Solon, OH). Immunoreactive protein bands were disclosed using ECL2 reagent and the chemiluminescent signal was imaged using a myECL imager (Thermo Fisher Scientific). Densitometry was performed using FiJi software.35,36 Further details are provided in the Supplemental Material.
Bone Measurement
Mice were euthanized by isoflurane overdose followed by cervical dislocation, and then vacuum-sealed into plastic bags. The sealed bags were incubated in a 50°C water bath overnight and the limb bones were removed and defleshed using a mascara brush. The bones were air-dried overnight and their lengths measured using a dissecting microscope equipped with an OMAX digital camera and Toupview software (Amscope.com).
Immunofluorescence
Sections of paraformaldehyde-fixed eyes were mounted on slides and blocked with Rodent Block M (Biocare Medical, Pacheco, CA). NBCe1 immunoreactivity was probed using the anti-NBCe1 antibody ESAP14635 (Elabscience Biotechnology Inc.) followed by an Alex488-conjugated goat–anti-rabbit secondary antibody A-11034 (Thermo Fisher Scientific). The labeled tissue was fixed beneath a coverslip using Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Images were acquired using an Axio Imager 2 fluorescence microscope (Carl Zeiss Inc., Thornwood, NY). Further details are provided in the Supplemental Material.
Confocal Reflection Microscopy
A freshly enucleated eye was held in a putty ring containing mineral oil such that the cornea was apposed to the cover glass of a glass-bottomed microwell dish (MatTek Corps., Ashlan, MA). z-stacked images (0.24 µm spacing) were obtained using a White Light Laser Confocal Microscope (TCS SP8; Leica Microsystems, Wetzlar, Germany) with a 488 nm laser emission and a band-pass filter set at 480–496 nm to capture reflected light. From z-stack projections, the stroma was selected as an area of interest and pixel intensity profiles were generated from grayscale images using FiJi.35 Density (pixel intensity) medians and variances were calculated using Microsoft Excel 1997 using the formulae SUMPRODUCT ([“pixel intensity” array], [“number of pixels” array])/SUM([“number of pixels” array] for medians and SUMPRODUCT (([“pixel intensity” array]−“calculated median”)^2, [“number of pixels” array])/SUM([“number of pixels” array]−1) for variance.
Pachymetry
A drop of proparacaine hydrochloride anesthetic solution (Akorn, Lake Forest, IL) was applied to each eye of a scruffed mouse before the measurement of in vivo corneal thickness using a handheld Pachette-3 ultrasonic pachymeter with flap option (DGH Technology Inc., Exton, PA). The probe was applied for several seconds perpendicular to the center of the cornea to record the mean of 25 consecutive measurements.
Scanning Electron Microscopy and Energy-Dispersive X-Ray Spectroscopy
Extracted lower incisors were air dried, embedded in acrylic resin, and polished to reveal their cross section in the transverse plane. The polished surface was coated with evaporated carbon and the tissue imaged using a Hitachi SU70 field emission SEM operated at 20 KeV. The images collected were backscattered electron images in which the pixel intensities are related to atomic number, in this case showing differences in mineral density in the tissues. EDS spectra were collected with beam energy of 20 KeV and live collection time of 60 seconds. Pixel intensities from electron micrographs were analyzed in the same way as the corneal confocal images.
Statistical Analyses
Statistical analyses were performed in Microsoft Excel 1997 (paired and unpaired t tests assuming equal variances) or MiniTab version 17 (ANOVA with Tukey post hoc analysis). Paired t tests were performed in instances where data were taken from sex-matched littermates.
Results
Expression of Nbce1 Protein in Wild-Type versus Nbce1b/c-Null Mice
The genome of the Nbce1b/c-null mouse strain reported in this study (hereafter referred to as “KOb/c”) lacks 5 bp in exon 3 of Slc4a4 (Figure 1, B and C), a frameshifting deletion that is predicted to result in the expression of the prematurely truncated Nbce1b/c polypeptide p.His27Aspfs40X. Figure 1D shows Western blots of lysates produced from wild-type (WT) versus KOb/c littermate organs showing that NBCe1 immunoreactivity (the approximately 240 kD band is consistent with the predicted molecular weight of an NBCe1 dimer) persists in the (predominantly NBCe1a-expressing) kidneys of Nbce1b/c-null mice, but is lost from the other (predominantly Nbce1b/c-expressing) organs such as the brain and heart. We detected no significant difference in the intensity of Nbce1 immunoreactivity in kidney lysates from WT versus KOb/c mice; the WT normalized NBCe1-immunofluorescence signal intensity was 108±13% in KOb/c kidney preparations (n=7; P=0.96; result of a paired, two-tailed t test assuming equal variance).
Blood Gas and Chemistry Measurements from KOb/c Mice
Table 1 shows the results of measurements of blood parameters among WT, heterozygous (+/−), and KOb/c mice. None of the measured parameters was significantly different between wild-type and +/− mice (each parameter was individually assessed by ANOVA among the groups). Although KOb/c mice have normal blood pH and no deficit in plasma [HCO3−], they exhibit an increased pCO2 (+7 mm Hg) with increased [HCO3−] (+3 mmol/L) and decreased pO2 (−20 mm Hg), which are signs of a chronic (compensated) respiratory acidosis.
Table 1.
Blood analysis data
| Parameter | WT | +/− | KOb/c | Unit |
|---|---|---|---|---|
| Blood gas | ||||
| pH | 7.36±0.01 | 7.35±0.01 | 7.33±0.01 | |
| pCO2 | 36.4±2.3 | 36.5±2.2 | 44.3±2.0a | mm Hg |
| pO2 | 65.8±7.7 | 70.5±6.1 | 46.2±4.8a | mm Hg |
| HCO3− (calculated) | 20.2±1.0 | 20.1±1.0 | 23.2±0.6a | mmol/L |
| Blood chemistry | ||||
| Na+ | 143±1 | 142±1 | 142±1 | mmol/L |
| K+ | 4.85±0.27 | 4.69±0.14 | 4.54±0.22 | mmol/L |
| Ca2+ | 1.25±0.02 | 1.26±0.02 | 1.29±0.02 | mmol/L |
| Cl− | 111±1 | 111±1 | 108±1 | mmol/L |
| Blood metabolites | ||||
| Glu | 284±11 | 271±6 | 283±16 | mg/dL |
| Lac | 4.85±0.47 | 5.78±0.39 | 5.92±0.49 | mg/dL |
Blood gas, chemistry, and metabolite data from wild-type (n=11), heterozygous (n=13), and Nbce1b/c-null (n=11) littermate mice. Note that these data correspond to mixed arterial venous blood collected by cardiac puncture from anesthetized mice.
Significantly different from WT by ANOVA with Tukey post hoc analysis.
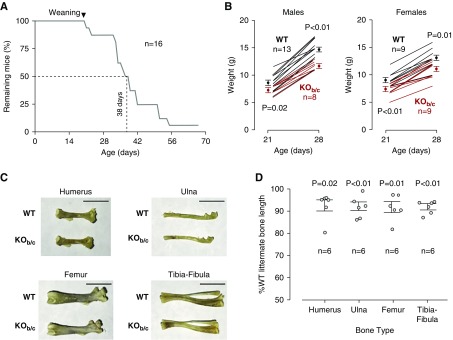
KOb/c Mice Have Increased Mortality
We examined KOb/c mice for the increased mortality that is exhibited by Nbce1a/b/c-null mice. The average size of litters born to heterozygous couples was 6±1 pups (n=38). Statistically fewer KOb/c pups were born to each pair than WT pups (P=0.01; one-tailed, paired t test). Of 232 pups, 63 were WT, 120 were +/−, and 45 were KOb/c; a Mendelian deficit that indicates that as many as 25% of Nbce1b/c-null mice could have died pre/perinatally. Figure 2A tracks the postnatal mortality of 16 KOb/c mice. A total of 50% of this cohort of KOb/c mice died within 6 weeks of birth. The cause of death is undetermined. The oldest KOb/c mouse in this cohort was euthanized at 67 days. All eight WT and ten +/− littermates included in this study survived to 70 days or longer before being euthanized. Even at 6 months, there was no mortality among a separate cohort of eight heterozygous mice (F6–F9 generation) that were kept for breeding purposes (data not shown).
Figure 2.
Nbce1-b/c-null (KOb/c) mice exhibit reduced survival and impaired growth. (A) Mortality of 16 KOb/c mice. Eight wild-type (WT) and ten +/− littermates served as controls and survived beyond the 70 day period. (B) The weight of WT and KOb/c mice at weaning (21 days) and at 28 days. Males and females are plotted on separate axes. Mean weights±SEM at each time point are shown as filled circles and the growth trajectories of individual mice are represented as lines. Statistics are results of unpaired, one-tailed t tests between WT and KOb/c data at 21 or 28 days. (C) Representative photographs of limb bones from six WT mice and sex-matched KOb/c littermates (four male wild-type versus KOb/c pairs, two female wild-type versus KOb/c pairs, 20–43 days old). Scale bar, 5 mm. (D) The length of the six KOb/c bones normalized to their WT counterparts are shown in the graph. Statistics are results of paired, one-tailed t tests between prenormalized WT and KOb/c data for each bone type.
KOb/c Mice Exhibit Lower than Normal Weight and Smaller Limb Bones
We examined KOb/c mice for signs of failure to thrive that are exhibited by Nbce1a/b/c-null mice. KOb/c mice are noticeably smaller than their WT littermates. Two pieces of data speak to a growth defect in KOb/c mice. Figure 2B shows the weight of WT and KOb/c mice at weaning (i.e., day 21) and at 1 week after weaning (i.e., day 28: 7 days on solid chow.) At both time points, male and female KOb/c mice weigh less on average than their WT counterparts. For both males and females, this difference is significant at 21 days and 28 days (one-tailed, unpaired t test with Bonferroni correction for multiple comparisons, setting significance at P=0.025=0.05/2). The difference at 28 days is exacerbated in males by a significantly smaller increase in weight for KOb/c mice over the 7 day interval (+4 g versus +6 g; P<0.01, two-tailed, unpaired t test). Interestingly, for females, the ability to gain weight during the first postweaning week is not compromised (+4 g versus +4 g; P=0.23, two-tailed unpaired t test). Figure 2C shows example photographs of the long bones isolated from age- and sex-matched WT and KOb/c mice. Figure 2D reports their relative lengths. The humeri, ulnae, femora, and tibia-fibulae of KOb/c mice are consistently approximately 10% shorter than those isolated from comparable WT mice and in our samples, the difference is significant in all cases except the humeri. (one-tailed, paired t test with Bonferroni correction for multiple comparisons, setting significance at P=0.013=0.05/4).
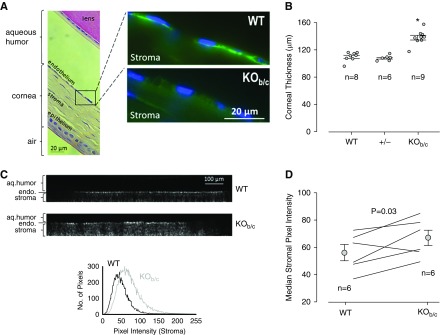
KOb/c Mice Have Thickened Corneas
We examined KOb/c mice for signs of the corneal edema that is exhibited by Nbce1a/b/c-null mice. Figure 3A shows the NBCe1 immunoreactivity in corneal sections from wild-type and KOb/c mice. Nbce1 immunoreactivity is detected in the basolateral membrane of corneal endothelial cells of WT mice, but is absent from the corneal endothelial cells of KOb/c mice. Figure 3B shows the average in vivo corneal thickness of WT, +/−, and KOb/c mice, as measured by ultrasonic pachymetry. The corneas of KOb/c mice are significantly thicker than those of WT or +/− mice. A similar degree of thickening was evident in KOb/c mice of that were 25 (the youngest examined), 40, and 47 (the oldest examined) days old (not shown). Although slit-lamp examination did not reveal obvious signs of corneal clouding in KOb/c mice (not shown), the corneal stroma of KOb/c mice reflected more light, consistent with an increase in opacity (Figure 3, C and D).
Figure 3.
Nbce1b/c-null (KOb/c) mice exhibit corneal swelling and increased corneal opacity. (A) Light micrograph of a hematoxylin and eosin stained wild-type (WT) mouse eye section showing the approximate locations highlighted in the accompanying fluorescence microscopy images of the corneas from WT and KOb/c mice. Note the absence of Nbce1 immunoreactivity (using the anti-Slc4a4 antibody) from the corneal endothelia of KOb/c mice (representative of images gathered from eye sections from three pairs of mice). (B) The in vivo (left eye) corneal thickness of WT mice (two males, six females), together with data gathered from their heterozygous (+/−; four males, two females) and KOb/c (four males, five females) littermates. *P<0.05: significantly different from WT thickness according to ANOVA with Tukey post-hoc analysis. (C) Representative z-axis projections of confocal reflection micrographs of the corneas of freshly enucleated WT and KOb/c mouse eyes (the endothelium is visible as a bright band, the epithelium could not be imaged because of its proximity to the highly reflective coverslip) together with a distribution plot of the pixel intensities of the stroma. (D) The median stromal pixel intensities for a larger number of WT and KOb/c mice.
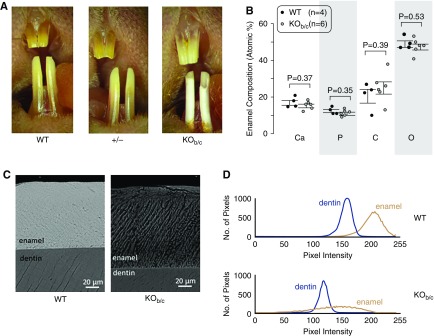
KOb/c Mice Have Defective Tooth Enamel
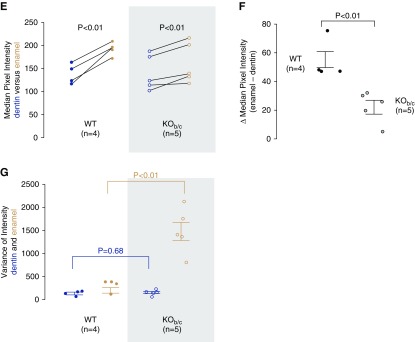
We examined KOb/c mice for signs of the weakened dental enamel that is exhibited by Nbce1a/b/c-null mice. Figure 4A shows representative photographs of the teeth of WT, +/−, and KOb/c mice (more than ten per group). As most clearly seen for the lower incisors, the enamel of KOb/c mice, unlike the normal appearance of the enamel of wild-type and heterozygous mice, is markedly chalky in appearance, chipped at the biting edge, and lacks iron pigmentation. EDS revealed no gross defects in the mineral composition of the enamel from KOb/c mice (Figure 4B), although analysis of electron micrographs (such as shown in Figure 4C) showed differences in the medians and variance of the mineral density (Figure 4D). Specifically, although both wild-type and KOb/c mice incisors have enamel that is denser than dentin in the same tooth (Figure 4E), the difference between enamel and dentin density is significantly smaller in KOb/c mice (Figure 4F) and the enamel has a substantially more heterogeneous composition (Figure 4G).
Figure 4.
Nbce1b/c-null (KOb/c) mice exhibit enamel defects. (A) Photographs of the incisors of wild-type (WT), +/−, and KOb/c littermates (representative of more than ten mice each). (B) The results of EDS analysis of the enamel of a greater number of incisors taken from preparations such as those shown in the following panel. Note that a parallel analysis of dentin also showed no significant difference in composition between WT and KOb/c mice (data not shown). (C) Representative electron micrograph of polished cross-sections of incisors from a pair of WT and KOb/c littermates. (D) Representative plots of pixel intensity for dentin and enamel taken from electron micrographs of a WT and KOb/c incisor. Note that the pixel intensities can only be compared between dentin and enamel in the same tooth and not between teeth as the intensity depends on the distance between the electron detector and the sample surface, which was not constant among samples [an exception being the images in (C)]. (E) A comparison of the median pixel intensity of dentin and enamel from a greater number of electron micrographs of WT and KOb/c incisors (one micrograph per tooth). (F) A comparison of the difference in median pixel intensities between dentin and enamel from the same images. (G) Variation of pixel intensity between dentin and enamel from the same images.
Discussion
KOb/c mice exhibit many of the signs exhibited by Nbce1a/b/c-null mice (increased mortality, growth retardation, corneal edema, and enamel defects) despite a normal plasma pH and no deficit in plasma [HCO3−]. This observation reveals, for the first time, those signs of pRTA that are not secondary to metabolic acidosis, are not amenable to alkali therapy, and which therefore require treatment beyond alkali therapy.
Increased Mortality
The effect of NBCe1 mutations on human mortality is unknown: cases of pRTA are rare and most probands are still young at the time of report. The oldest individual was 50 years of age.28 However, there is some anecdotal evidence among this small cohort of increased childhood mortality. The sister of one proband died at 1 day of age of unknown cause, but her pRTA status is unknown.37 One of two brothers with pRTA died at age 4 years after an inappropriately severe reaction to a mild head trauma, although the genetic cause of pRTA was not verified in either brother.38 What is certain is that NBCe1a/b/c-null mice exhibit a severe increase in perinatal mortality, with none surviving beyond 30 days.24,29 Studies in which alkali therapy was administered to Nbce1a/b/c-null mice led to an imperfect correction of acidosis but a substantial increase in survival: the majority of prenatally treated mice survived beyond 60 days and one mouse survived to 120 days.39 However, expectations that perfect plasma pH correction might further enhance survivability are at odds with the poor survival of KOb/c mice; although their mortality is not as great as that of Nbce1a/b/c-null mice, few KOb/c mice survive beyond 2 months. Furthermore, acidemic Nbce1a-null mice have a normal lifespan, suggesting that acidemia is not a prime cause of mortality.40,41 Taken together these data suggest that Nbce1b/c loss is a key factor in early mortality of Nbce1-null mice, but that acidemia secondary to Nbce1a loss worsens the outlook. Thus, although alkali therapy might enhance survival, it is not sufficient to normalize lifespan.
Growth Defects
Individuals with pRTA are typically several standard deviations below average height and weight, often with an inappropriately young bone age.17,37 A study of one girl with pRTA indicated that early administration of alkali therapy could improve height velocity.26 Similarly, alkali-supplemented Nbce1a/b/c-null mice exhibited an improvement in weight gain.39 Together these data indicate that acidosis is a major factor in growth retardation; indeed, acidosis would be expected to impair bone growth. However, even KOb/c mice exhibit a significant growth retardation, suggesting that alkali therapy, even if perfectly administered, would not be sufficient to completely correct the growth defects in pRTA (except perhaps in instances where the genetic defect specifically affects NBCe1-A20). We are unaware of a specific role for Nbce1b/c in bone formation, but recent studies indicate that Nbce1b/c loss from the intestinal epithelia impairs electrolyte and nutrient absorption,42 which would contribute toward a general failure to thrive. An unusual feature of Nbce1a/b/c-null mice that has not been noted in individuals with pRTA is severe impaction of the intestines. Nbce1b/c supports intestinal fluid secretion and so it might be predicted that its loss would contribute to such a phenotype.29,42 However, it has also been noted that ENaC is upregulated in the colons of Nbce1a/b/c-null mice, secondary to hypovolemia and acidemia; hyperabsorption would also contribute to the phenotype.29 We did not see evidence of the impacted intestines in postmortem studies of Nbce1b/c-null mice, further supporting a complex cause.
Corneal Edema
The ocular phenotype of pRTA has variable expressivity and penetrance. Almost all individuals with pRTA exhibit band keratopathy (or increased corneal opacity18,23), glaucoma (or at least elevated intraocular pressure22), and cataracts. The cause of these signs is not well understood, although an individual with a mutation that affects only NBCe1-A exhibited glaucoma but no other ocular pathology, whereas aged Nbce1a-null mice develop elevated intraocular pressure.20,43 Among the other signs, only corneal opacity has been reported in Nbce1a/b/c-null mice and histologic study of their corneas revealed corneal edema.24 Nbce1b has long been suggested to play a critical role in the corneal HCO3− pumping mechanism.44–46 Our data show that the in vivo baseline corneal thickness in KOb/c mice is increased to an extent that would be considered clinically significant in humans.47 The greater stromal opacity that we observed by confocal microscopy in KOb/c mice is similar to that observed by in vivo imaging of edematous human corneas from individuals with Fuchs dystrophy.48,49 These observations demonstrate a direct and critical role for Nbce1b in maintaining corneal health. These data also implicate the region that encompasses promoter 1 and exons 1–3 of SLC4A4 as a novel candidate locus for cases of recessively inherited corneal dystrophies whose genetic origin remains obscure.50,51
Enamel Defects
Dental abnormalities are frequently noted in individuals with pRTA,18,25 and easily chipped, hypomineralized enamel is characteristic of the teeth of Nbce1a/b/c-null mice.29,52 The apparently normal mineral composition of KOb/c enamel combined with its unusually heterogeneous structure are consistent with hypomineralization. The relative contribution of Nbce1a versus Nbce1b/c loss to this phenotype has been controversial. On the one hand, Nbce1b/c is expressed in the ameloblasts of the enamel organ where it is hypothesized to support enamel remodeling.53 On the other hand, wild-type and Nbce1a/b/c-null molars developed similarly when mandibles from those mice were explanted and cultured in the kidney capsules of wild-type mice, suggesting that correction of acidemia could prevent hypomineralization.54 The exhibition of defective enamel in KOb/c mice establishes the importance of Nbce1b/c for enamel formation and demonstrates that the enamel defect in pRTA cannot be prevented by normalizing blood pH. With regard to the contrary explant data, we note that neither the wild-type nor the Nbce1a/b/c-null explanted teeth mineralized their enamel as well as in situ teeth, and development of the explanted teeth may have been sufficiently slow to allow even disabled ameloblasts to perform adequately.
Chronic Respiratory Acidosis
The elevation of pCO2 in KOb/c mice is not a feature shared with Nbce1a/b/c-null mice. In fact, metabolic acidosis typically results in hyperventilation producing a compensatory reduction of pCO2, which may mask such a defect. However, inappropriately high pCO2 (≥35 mm Hg, consistent with a confounding respiratory acidosis) has been reported in one case of pRTA18 and can be calculated from the pH and [HCO3−] values provided in two other cases.22,23 The cause is unknown but we note that Nbce1b is expressed in lung epithelia, myocytes, and neurons,1 any and all of which can influence the set point of pCO2.
The Role of Renal Nbce1b
Finally, in reference to a recent report of Nbce1b expression in the renal medulla,9 the ability of the kidneys to maintain an apparently appropriately elevated plasma [HCO3−] does not reveal any gross renal acid-base handling defect in KOb/c mice.
Future Directions
Although alkali therapy is considered beneficial for treatment of many kidney diseases, the phenotype of Nbce1b/c-null mice reveals the limitations of alkali therapy as a catch-all treatment for the signs of pRTA, highlights the need to develop separate therapies for the ocular, dental, and growth defects, and indicates that mutations specific to the NBCe1-B/C–encoding region of SLC4A4 could underlie unusual cases of corneal dystrophy and enamel hypomineralization. Pending the development of conditional Nbce1-null mice, further work will need to be done to establish a cause of chronic respiratory acidosis and death in Nbce1b/c-null mice, knowledge of which may be useful to prolong their lifespan and increase their usefulness as a model for developing novel therapies.
Disclosures
Dr. Patel reports grants from NIH during the conduct of the study. Dr. Parker reports grants from NIH, grants from ASN Foundation during the conduct of the study.
Supplementary Material
Acknowledgments
Dr. Parker is grateful for the assistance of Dr. Wade Sigurdson in the Confocal Microscope and Flow Cytometry Facility at The University at Buffalo, Peter Bush in the South Campus Instrument Center at The University at Buffalo, Ms. Aimee Stablewski in the Gene Targeting and Transgenic Shared Resource at the Roswell Park Comprehensive Cancer Center (funded by National Cancer Institute grant P30CA16056), and Dr. Shondra Miller in the Genome Engineering and iPSC Center at Washington University in St. Louis. We thank Dr. Pamela DenBesten at the University of California San Francisco School of Dentistry and Dr. Michael Garrick from the University at Buffalo Department of Biochemistry for helpful discussions.
Dr. Parker and Dr. Patel designed the study. Ms. Salerno, Dr. Patel, Mrs. A. Marshall, Ms. J. Marshall, Mr. Alsufayan, Mr. Mballo, Ms. Quade, and Dr. Parker carried out experiments. Ms. Salerno, Dr. Patel, Mrs. A. Marshall, Ms. J. Marshall, Mr. Alsufayan, Mr. Mballo, Ms. Quade, and Dr. Parker analyzed the data. Ms. Salerno, Dr. J. Marshall, Mr. Alsufayan, Ms. Quade, and Dr. Parker made the figures. Dr. Patel and Dr. Parker drafted and revised the paper. All authors approved the final version of the manuscript.
This work was supported by start-up funding from the Dean of the School of Medicine and Biomedical Sciences and the Department of Physiology and Biophysics at The University at Buffalo and a Carl W. Gottschalk Research Scholar grant from the American Society of Nephrology Foundation for Kidney Research (to Dr. Parker), and an R01 grant from the National Institutes of Health National Eye Institute (EY028580) (to Dr. Parker and Dr. Patel).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Veterans Administration, or the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018050545/-/DCSupplemental.
References
- 1.Parker MD, Boron WF: The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev 93: 803–959, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero MF, Hediger MA, Boulpaep EL, Boron WF: Expression cloning and characterization of a renal electrogenic Na+/HCO3- cotransporter. Nature 387: 409–413, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, et al.: Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem 273: 17689–17695, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Abuladze N, Song M, Pushkin A, Newman D, Lee I, Nicholas S, et al.: Structural organization of the human NBC1 gene: kNBC1 is transcribed from an alternative promoter in intron 3. Gene 251: 109–122, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF: Immunolocalization of the electrogenic Na+-HCO-3 cotransporter in mammalian and amphibian kidney. Am J Physiol 276: F27–F38, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Romero MF, Fong P, Berger UV, Hediger MA, Boron WF: Cloning and functional expression of rNBC, an electrogenic Na(+)-HCO3- cotransporter from rat kidney. Am J Physiol 274: F425–F432, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Choi I, Romero MF, Khandoudi N, Bril A, Boron WF: Cloning and characterization of a human electrogenic Na+-HCO-3 cotransporter isoform (hhNBC). Am J Physiol 276: C576–C584, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF: An electrogenic Na+-HCO3- cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. Am J Physiol Cell Physiol 278: C1200–C1211, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Fang L, Lee H-W, Chen C, Harris AN, Romero MF, Verlander JW, et al.: Expression of the B splice variant of NBCe1 (SLC4A4) in the mouse kidney. Am J Physiol Renal Physiol 315: F417–F428, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO: Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: Functional comparison and roles of the amino and carboxy termini. J Gen Physiol 127: 639–658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, et al.: IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3- cotransporter 1 (pNBC1). Proc Natl Acad Sci U S A 103: 9542–9547, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S-K, Boron WF, Parker MD: Relief of autoinhibition of the electrogenic Na-HCO3 cotransporter NBCe1-B: Role of IRBIT vs.amino-terminal truncation. Am J Physiol Cell Physiol 302: C518–C526, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majumdar D, Maunsbach AB, Shacka JJ, Williams JB, Berger UV, Schultz KP, et al.: Localization of electrogenic Na/bicarbonate cotransporter NBCe1 variants in rat brain. Neuroscience 155: 818–832, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Xu JY, Wang DK, Wang L, Chen LM: Cloning and identification of two novel NBCe1 splice variants from mouse reproductive tract tissues: A comparative study of NCBT genes. Genomics 98: 112–119, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, et al.: Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet 23: 264–266, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Romero MF, Chen AP, Parker MD, Boron WF: The SLC4 family of bicarbonate (HCO3−) transporters. Mol Aspects Med 34: 159–182, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demirci FYK, Chang M-H, Mah TS, Romero MF, Gorin MB: Proximal renal tubular acidosis and ocular pathology: A novel missense mutation in the gene (SLC4A4) for sodium bicarbonate cotransporter protein (NBCe1). Mol Vis 12: 324–330, 2006 [PubMed] [Google Scholar]
- 18.Dinour D, Chang MH, Satoh J, Smith BL, Angle N, Knecht A, et al.: A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem 279: 52238–52246, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Horita S, Yamada H, Inatomi J, Moriyama N, Sekine T, Igarashi T, et al.: Functional analysis of NBC1 mutants associated with proximal renal tubular acidosis and ocular abnormalities. J Am Soc Nephrol 16: 2270–2278, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Igarashi T, Inatomi J, Sekine T, Seki G, Shimadzu M, Tozawa F, et al.: Novel nonsense mutation in the Na+/HCO3- cotransporter gene (SLC4A4) in a patient with permanent isolated proximal renal tubular acidosis and bilateral glaucoma. J Am Soc Nephrol 12: 713–718, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Igarashi T, Sekine T, Inatomi J, Seki G: Unraveling the molecular pathogenesis of isolated proximal renal tubular acidosis. J Am Soc Nephrol 13: 2171–2177, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Inatomi J, Horita S, Braverman N, Sekine T, Yamada H, Suzuki Y, et al.: Mutational and functional analysis of SLC4A4 in a patient with proximal renal tubular acidosis. Pflugers Arch 448: 438–444, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kari JA, El Desoky SM, Singh AK, Gari MA, Kleta R, Bockenhauer D: The case | Renal tubular acidosis and eye findings. Kidney Int 86: 217–218, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Lo YF, Yang SS, Seki G, Yamada H, Horita S, Yamazaki O, et al.: Severe metabolic acidosis causes early lethality in NBC1 W516X knock-in mice as a model of human isolated proximal renal tubular acidosis. Kidney Int 79: 730–741, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Myers EJ, Yuan L, Felmlee MA, Lin YY, Jiang Y, Pei Y, et al.: A novel mutant Na+/HCO3- cotransporter NBCe1 in a case of compound-heterozygous inheritance of proximal renal tubular acidosis. J Physiol 594: 6267–6286, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiohara M, Igarashi T, Mori T, Komiyama A: Genetic and long-term data on a patient with permanent isolated proximal renal tubular acidosis. Eur J Pediatr 159: 892–894, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Suzuki M, Vaisbich MH, Yamada H, Horita S, Li Y, Sekine T, et al.: Functional analysis of a novel missense NBC1 mutation and of other mutations causing proximal renal tubular acidosis. Pflugers Arch 455: 583–593, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Suzuki M, Van Paesschen W, Stalmans I, Horita S, Yamada H, Bergmans BA, et al.: Defective membrane expression of the Na+-HCO3- cotransporter NBCe1 is associated with familial migraine. Proc Natl Acad Sci U S A 107: 15963–15968, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, et al.: Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3- cotransporter. J Biol Chem 282: 9042–9052, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Parker MD: Mouse models of SLC4-linked disorders of HCO3--transporter dysfunction. Am J Physiol Cell Physiol 314: C569–C588, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adeva-Andany MM, Fernández-Fernández C, Mouriño-Bayolo D, Castro-Quintela E, Domínguez-Montero A: Sodium bicarbonate therapy in patients with metabolic acidosis. ScientificWorldJournal 2014: 627673, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris RC Jr., Sebastian A: Alkali therapy in renal tubular acidosis: Who needs it? J Am Soc Nephrol 13: 2186–2188, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Raphael KL: Approach to the treatment of chronic metabolic acidosis in CKD. Am J Kidney Dis 67: 696–702, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Salerno EE, Patel SP, Marshall A, Mballo CS, Parker MD: NBCe1-B/C knockout mice exhibit signs associated with proximal renal tubular acidosis. FASEB J 31: 1007.29–1007.29, 2017
- 35.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al.: Fiji: An open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, et al.: ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18: 529, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deda G, Ekim M, Güven A, Karagöl U, Tümer N: Hypopotassemic paralysis: A rare presentation of proximal renal tubular acidosis. J Child Neurol 16: 770–771, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Winsnes A, Monn E, Stokke O, Feyling T: Congenital persistent proximal type renal tubular acidosis in two brothers. Acta Paediatr Scand 68: 861–868, 1979 [DOI] [PubMed] [Google Scholar]
- 39.Fang YW, Yang SS, Chau T, Nakamura M, Yamazaki O, Seki G, et al.: Therapeutic effect of prenatal alkalization and PTC124 in Na(+)/HCO3(-) cotransporter 1 p.W516* knock-in mice. Gene Ther 22: 374–381, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Chen A-P, Holmes HL, Chang M, Romero MF: Targeted NBCe1A deletion causes proximal RTA: Whole nbce1 (sh_nbce1) KO vs. nbce1A KO mice. J Am Soc Nephrol 25: 71A, 2014 [Google Scholar]
- 41.Lee HW, Osis G, Harris AN, Fang L, Romero MF, Handlogten ME, et al.: NBCe1-A regulates proximal tubule ammonia metabolism under basal conditions and in response to metabolic acidosis. J Am Soc Nephrol 29: 1182–1197, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Q, Liu X, Liu Y, Riederer B, Li T, Tian DA, et al.: Defective small intestinal anion secretion, dipeptide absorption, and intestinal failure in suckling NBCe1-deficient mice. Pflugers Arch 468: 1419–1432, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hildenbrand J, Chen A-P, Landry G, Holmes H, Chang M-H, Romero M: Metabolic acidosis, ocular pressure and cysts in NBCe1 knockout. FASEB J 29: 844.23, 2015 [Google Scholar]
- 44.Bonanno JA: Molecular mechanisms underlying the corneal endothelial pump. Exp Eye Res 95: 2–7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodson S: Evidence for a bicarbonate-dependent sodium pump in corneal endothelium. Exp Eye Res 11: 20–29, 1971 [DOI] [PubMed] [Google Scholar]
- 46.Sun XC, Bonanno JA, Jelamskii S, Xie Q: Expression and localization of Na+-HCO3- cotransporter in bovine corneal endothelium. Am J Physiol Cell Physiol 279: C1648–C1655, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Paliwal P, Sharma A, Tandon R, Sharma N, Titiyal JS, Sen S, et al.: Congenital hereditary endothelial dystrophy - mutation analysis of SLC4A11 and genotype-phenotype correlation in a North Indian patient cohort. Mol Vis 16: 2955–2963, 2010 [PMC free article] [PubMed] [Google Scholar]
- 48.Wacker K, McLaren JW, Kane KM, Patel SV: Corneal optical changes associated with induced edema in Fuchs endothelial corneal dystrophy. Cornea 37: 313–317, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amin SR, Baratz KH, McLaren JW, Patel SV: Corneal abnormalities early in the course of Fuchs’ endothelial dystrophy. Ophthalmology 121: 2325–2333, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah SS, Al-Rajhi A, Brandt JD, Mannis MJ, Roos B, Sheffield VC, et al.: Mutation in the SLC4A11 gene associated with autosomal recessive congenital hereditary endothelial dystrophy in a large Saudi family. Ophthalmic Genet 29: 41–45, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Akhtar S, Bron AJ, Meek KM, Bennett K: Congenital hereditary endothelial dystrophy and band keratopathy in an infant with corpus callosum agenesis. Cornea 20: 547–552, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Lacruz RS, Nanci A, White SN, Wen X, Wang H, Zalzal SF, et al.: The sodium bicarbonate cotransporter (NBCe1) is essential for normal development of mouse dentition. J Biol Chem 285: 24432–24438, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jalali R, Guo J, Zandieh-Doulabi B, Bervoets TJ, Paine ML, Boron WF, et al.: NBCe1 (SLC4A4) a potential pH regulator in enamel organ cells during enamel development in the mouse. Cell Tissue Res 358: 433–442, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen X, Kurtz I, Paine ML: Prevention of the disrupted enamel phenotype in Slc4a4-null mice using explant organ culture maintained in a living host kidney capsule. PLoS One 9: e97318, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.