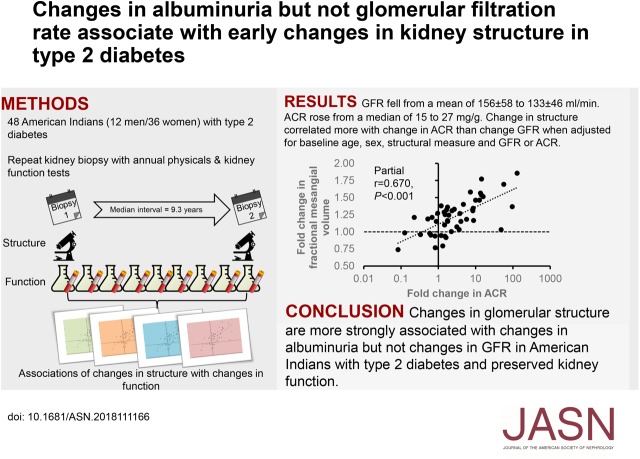
Significance Statement
Although diabetic nephropathy is assessed clinically by measuring eGFR and albuminuria, structural kidney damage typically precedes these clinical manifestations. Previous studies have assessed early structural change in type 1 diabetes but not in type 2. In a study of American Indian patients with type 2 diabetes and generally preserved kidney function, the authors found changes in kidney structure over an average of 9 years, as determined by quantitative morphometric analysis of kidney biopsy specimens. They also found that these kidney structure changes were more consistently associated with changes in albuminuria than with changes in measured GFR. This finding suggests that for people with normal or elevated GFR, increasing albuminuria may better than early GFR decline in reflecting the risk of progressive type 2 diabetic nephropathy.
Keywords: diabetic nephropathy, renal morphology., type 2 diabetes
Visual Abstract
Abstract
Background
In type 1 diabetes, changes in the GFR and urine albumin-to-creatinine ratio (ACR) are related to changes in kidney structure that reflect disease progression. However, such changes have not been studied in type 2 diabetes.
Methods
Participants were American Indians with type 2 diabetes enrolled in a clinical trial of losartan versus placebo. We followed a subset who underwent kidney biopsy at the end of the 6-year trial, with annual measurements of GFR (by urinary clearance of iothalamate) and ACR. Participants had a second kidney biopsy after a mean follow-up of 9.3 years. We used quantitative morphometric analyses to evaluate both biopsy specimens.
Results
Baseline measures for 48 participants (12 men and 36 women, mean age 45.6 years) who completed the study included diabetes duration (14.6 years), GFR (156 ml/min), and ACR (15 mg/g). During follow-up, glomerular basement membrane (GBM) width, mesangial fractional volume, and ACR increased, and surface density of peripheral GBM and GFR decreased. After adjustment for sex, age, ACR, and each morphometric variable at baseline, an increase in ACR during follow-up was significantly associated with increases in GBM width, mesangial fractional volume, and mean glomerular volume, and a decrease in surface density of peripheral GBM. Decline in GFR was not associated with changes in these morphometric variables after additionally adjusting for baseline GFR.
Conclusions
In American Indians with type 2 diabetes and preserved GFR at baseline, increasing ACR reflects the progression of earlier structural glomerular lesions, whereas early GFR decline may not accurately reflect such lesions.
Kidney function is primarily assessed in clinical practice by measurement of serum creatinine, which is used to estimate GFR, and urine albumin, measured either by albumin-to-creatinine ratio (ACR) or albumin excretion rate (AER). Persistent microalbuminuria is an important risk factor for diabetic nephropathy (DN) and its progression.1 However, microalbuminuria is not a precise predictor of DN risk,2 and is not always present when early GFR decline is first detected.3,4 Early decline in GFR in the absence of albuminuria is also a risk factor for DN progression.5,6 Abnormalities in kidney structure are present early in the course of DN, typically before detectable clinical kidney disease.7–10 However, the early progression of structural injury in DN on the basis of serial kidney biopsies has only been studied in type 1 diabetes.11,12
We reported previously that higher mesangial fractional volume [Vv(Mes/glom)], lower surface density of the peripheral glomerular basement membrane [Sv(PGBM/glom)], higher mean glomerular volume, and lower total glomerular filtration area were each associated cross-sectionally, with lower GFR and prospectively, with GFR decline in research kidney biopsy specimens in an American Indian population with type 2 diabetes.10 A subset of the persons from that study completed a second research kidney biopsy and here we examine the changes in kidney structure in the 6–12 year interval between biopsies. As changes in kidney structure are the unequivocal manifestations of DN progression, we aimed to examine how reliably changes in functional measures can reflect these early structural changes.
Methods
Study Population
Participants were American Indians with type 2 diabetes enrolled in the Renoprotection in Early Diabetic Nephropathy in Pima Indians trial, which randomized individuals to either standard of care or standard of care plus losartan (Clinicaltrials.gov identifier NCT00340678).13 At the end of the 6-year trial period, 111 out of the 169 study participants underwent a protocol kidney biopsy to determine the effects of losartan on DN lesions.10 Thereafter, participants were followed annually for measurement of albuminuria and GFR and were invited for a second biopsy after a minimum of 6 years. Of the original 111 biopsy participants, 11 had died, 27 had developed advanced CKD (stages 3–5),14 including 16 who developed ESRD, making them ineligible for a second biopsy because of safety concerns, 19 were ineligible on other clinical grounds, and two were lost to follow-up. Accordingly, 52 participants underwent a second protocol biopsy. Four participants had mostly missing data for our primary measures of interest [glomerular basement membrane [GBM] width, and Vv(Mes/glom)] because of insufficient tissue at either biopsy. Thus, 48 participants (12 men and 36 women) with glomerular morphometric measures from both baseline and follow-up kidney biopsies are included here.
Clinical Measures
GFR was measured annually by the urinary clearance of iothalamate (iGFR).15 Because of the level of obesity in these participants, absolute GFR data are presented, uncorrected for body surface area.16 At the same visits, BP was measured while the participant was resting in the seated position, and weight was obtained with the participant dressed in light clothing without shoes. Body mass index was defined as weight in kilograms divided by the square of height in meters. The iGFR measurements were performed a median of 24 days after the baseline biopsy (interquartile range [IQR], −23 to +47 days) and 1 day before the follow-up biopsy (IQR, −41 to −1 days).
Laboratory Measurements
Urine albumin was measured by nephelometry, and urine creatinine by a modified Jaffe reaction for baseline studies17,18 and by an enzymatic method during follow-up. Values measured by the modified Jaffe reaction were adjusted on the basis of a correction factor derived from samples measured by both methods (see Supplemental Appendix 1). ACR at baseline and follow-up was on the basis of a spot urine collected at the time of the GFR measurement nearest to the biopsy. Urine ACR was considered normal if <30 mg/g, microalbuminuria if ≥30 to 299 mg/g, and macroalbuminuria if ≥300 mg/g. HPLC was used to measure iothalamate15 and hemoglobin A1c (HbA1c).19
Kidney Morphometric Measurements
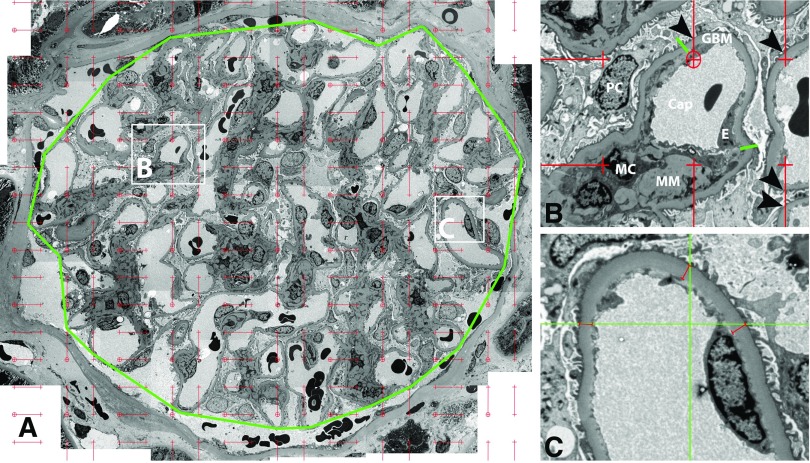
Details of the morphometric studies are provided in Supplemental Appendix 1, including measures not reported in the main text. In brief, kidney biopsies were prepared for light and electron microscopy studies according to standard procedures.12,20–23 GBM width, Sv(PGBM/glom), Vv(Mes/glom), average podocyte cell volume, fraction of the glomerulus occupied by podocytes [Vv(PC/glom)], and the podocytes number density per glomerulus were measured by unbiased morphometry on electron microscopy images as described elsewhere.24–26 Figure 1 shows electron microscopy images used for measurement of GBM width, Vv(Mes/glom) and Sv(PGBM/glom). These measures were on the basis of three randomly selected glomeruli, as we have found this a sufficient number to make reproducible measures with robust structure and function relationships, thus substantiating the diffuse distribution of diabetic glomerulopathy lesions such that careful measurements in only three glomeruli reflect functional parameters in all glomeruli in both kidneys.7,10,11,27,28 Mean glomerular volume was estimated using light microscopy by the Weibel–Gomez method29 for biopsy samples embedded in Epon, and the Cavalieri method30 for biopsy samples embedded in paraffin. An equation derived from 30 biopsy samples where both methods were used, was applied to allow for assessment of change in mean glomerular volume (see Supplemental Appendix 1). Total glomerular filtration area was calculated as the product of mean glomerular volume and Sv(PGBM/glom). We also included the percentage of glomeruli that were sclerosed as measured on light microscopy images.
Figure 1.
Morphometric measurements using electron microscopy images. (A) Montage image of a glomerular profile (approximately ×8700 magnification). The outline of the glomerular tuft is marked by a polygon (green) around outer capillary loops. A counting grid composed of short lines (red) connecting fine (crosshairs) and coarse points (circled crosshairs), which are systematically and uniformly distributed is superimposed on the image. The fine points are used to measure the area of mesangium and the coarse points are used to measure the area of the polygon and the ratio of these is Vv(Mes/glom).23,24 Magnified views of B and C squares are shown on the right for details. (B) Two green lines separate peripheral GBM of a capillary loop from mesangial region, comprising mesangial cells (MC) and matrix (MM) (approximately ×32000 magnification). The intercepts between short red lines and peripheral GBM (arrowheads) are counted to estimate Sv(PGBM/glom).23,24 (C) Higher magnification image (approximately ×46000 magnification) taken according to a systematic uniform random sampling protocol used for measurement of GBM width.23,24 Systematically and uniformly distributed grid lines (green) are superimposed on the image. Perpendicular distance from the base of foot processes to the endothelial lining (red lines) are measured wherever grid lines cross the base of foot processes. Cap, capillary; E, endothelial cell; PC, podocyte.
Embedding material for light microscopy had a significant effect on measures of tubulointerstitial structural parameters, including cortical interstitial fractional volume and tubular fractional volumes, making it impossible to assess change in these measures (see Supplemental Material for details and Supplemental Figures 1 and 2).
Thirty three participants had adequate tissue for measures of mean glomerular volume (three or more glomeruli), and hence total glomerular filtration area, at both biopsies. Measurements of percentage of sclerosed and atrophic glomeruli at both biopsies were available for 32 participants.
Statistical Methods
To simplify model fitting, most structural measures were log-transformed before analysis and changes in morphometric variables were computed as the logarithm of the follow-up minus the logarithm of the baseline measure (equal to the logarithm of the ratio of follow-up biopsy measurement to the baseline measurement). For the percentages of sclerosed and ischemic glomeruli, where many values were zero, we reported the absolute differences without logarithmic transformation. ACR was log transformed before analysis and change in ACR and GFR were examined as for the structural measurements (i.e., difference in follow-up and baseline logged values). Rate of change in iGFR (iGFR slope) was calculated by linear regression of all available iGFR measures (median, 11; IQR, 10–12). Baseline and follow-up parameters were compared by paired t test or the Kruskal–Wallis test. Associations between change in kidney structural parameters and baseline clinical measures and change in kidney function were assessed using Pearson correlations (univariate analyses) and linear regression models, adjusted for age, sex, and baseline kidney structural measurement. Data are shown as mean±SD, median (IQR), or frequencies. For data on additional structural measurements, see Supplemental Material. All analyses were undertaken using SAS v9.4 (SAS Institute, Inc., Carey, NC). P<0.05 was considered statistically significant.
Results
Most participants had normal kidney function (iGFR>60 ml/min and ACR<30 mg/g) at the time of both their baseline (n=31, 65%) and follow-up biopsy (n=25, 52%). Demographics and clinical measures at baseline and follow-up for study participants (n=48) are shown in Table 1. Glycemic control was suboptimal with mean HbA1c of 9.1%±2.0% at baseline and 9.7%±2.0% at follow-up (P=0.10), despite increased insulin use. BP was generally well controlled: 96% of participants at baseline and 92% at follow-up had BPs below 140/90 mm Hg. There was no change in systolic BP but a fall in diastolic BP and a fall in the use of antihypertensive drugs and in the use of renin-angiotensin system blockers. Decline in diastolic BP was greater in individuals receiving antihypertensives at follow-up (median change, −7 mm Hg; IQR, −14 to −2 mm Hg) than individuals not receiving antihypertensives (median change, −3 mm Hg; IQR, −12 to +4 mm Hg). At baseline, age correlated directly with diabetes duration (r=0.41; P=0.004) and systolic BP (r= 0.32; P=0.03); HbA1c did not correlate with any of the baseline clinical measures (data not shown).
Table 1.
Demographics and clinical characteristics for participants
| Characteristic | Baseline | Follow-Up | P Value |
|---|---|---|---|
| Men (%) | 12 (25%) | — | |
| Randomized to losartan in clinical trial, % | 31 (64.6%) | — | |
| Age, yr | 45.6±8.8 | 55.0±9.2 | — |
| Diabetes duration, yr | 14.6±5.0 | 23.9±5.2 | — |
| GFR, ml/min | 156±58 | 133±46 | 0.006 |
| ACR, mg/g | 15 (6–45) | 27 (17–124) | 0.002 |
| Normoalbuminuria | 32 (66.7%) | 25 (52.1%) | 0.01a |
| Microalbuminuria | 15 (31.3%) | 15 (31.3%) | |
| Macroalbuminuria | 1 (2.1%) | 8 (16.7%) | |
| Systolic BP, mm Hg | 120±13 | 119±14 | 0.55 |
| Diastolic BP, mm Hg | 77±7 | 71±9 | <0.001 |
| Body mass index, kg/m2 | 35.7±6.3 | 34.7±7.3 | 0.07 |
| HbA1c, % | 9.1±2.0 | 9.7±2.0 | 0.10 |
| Use of insulin, % | 18 (37.5%) | 28 (58.3%) | 0.03a |
| Use of antihypertensives, % | 39 (81.3%) | 32 (66.7%) | >0.99a |
| Use of renin-angiotensin system blockers, % | 37 (77.1%) | 29 (60.4%) | 0.80a |
| Use of lipid-lowering agents, % | 14 (29.2%) | 27 (56.3%) | 0.47a |
| Time between biopsies, yr | 9.4±1.3 | — | |
| Time between GFR measurements, yr | 9.3±1.3 | — | |
Data are mean±SD for continuous measures and numbers with percentages for categorical variables. Normoalbuminuria defined as ACR<30 mg/g; microalbuminuria defined as ACR 30–300 mg/g; macroalbuminuria defined as ACR>300 mg/g.
P values for paired t tests except for chi-squared test.
Participants included in this study (n=48) differed from the participants in the original biopsy study who did not have a second biopsy (n=63). The study participants had lower ACR (median, 18 mg/g; IQR, 8–54 versus median, 69 mg/g; IQR, 24–523; P<0.001), shorter diabetes duration (14.6±5.0 versus 16.8±6.3 years; P=0.05), and lower systolic BP (120±13 versus 128±16 mm Hg; P=0.006) at baseline, but there was no statistically significant difference in GFR between the two groups (156±58 versus 140±54 ml/min; P=0.13) (Supplemental Table 1). These differences were primarily because events among the original participants, including death or ESRD, precluded a second biopsy.
Changes in Kidney Function
At baseline, kidney function correlated with age (correlation coefficient for iGFR, −0.41; P=0.004; and correlation coefficient for ACR, −0.40; P=0.005) and similarly with diabetes duration (correlation coefficient for iGFR, −0.44; P=0.002; and correlation coefficient for ACR, −0.34; P=0.02), but not with HbA1c. Systolic BP was correlated with iGFR (correlation coefficient, 0.32; P=0.03) but not with ACR. Of note, the associations between ACR and age and diabetes duration were not observed in the original cohort (n=111) (age and ACR: r=−0.17; P=0.07; duration and ACR: r=0.16; P=0.09).
The median time between baseline and follow-up iGFRs was 9.3 years (range, 6.8–11.8 years). Four participants had a baseline iGFR <90 ml/min, with the lowest being 53 ml/min. During the study, iGFR declined from 156±58 to 133±46 ml/min (P=0.006; Table 1). The median rate of change of iGFR was −2.8 ml/min per year (IQR, −5.6 to 0.1) on the basis of a median of 11 (IQR, 10–12) iGFR measurements. The median ACR increased from 15 mg/g (IQR, 6–45) to 27 mg/g (IQR, 17–124) (P=0.002). The number of individuals with macroalbuminuria increased from one at baseline to eight at follow-up. Overall, 28 participants (58.3%) did not change albuminuria category (21 with normoalbuminuria, six with microalbuminuria, and one with macroalbuminuria), whereas 16 (33.3%) progressed and four (8.3%) regressed.
Changes in Kidney Morphometry
Structural parameters at baseline and follow-up are shown in Table 2. GBM width, Vv(Mes/glom), and the percentage of sclerosed glomeruli increased, whereas Sv(PGBM/glom), total glomerular filtration area, podocyte volume, and Vv(PC/glom) decreased between the baseline and follow-up biopsies. There were no significant changes in mean glomerular volume and the podocyte number density per glomerulus (Table 2). In univariate analyses, changes in GBM width, Vv(Mes/glom), Sv(PGBM/glom), and total glomerular filtration area correlated strongly with each other (Supplemental Table 2). Changes in morphometric measures were most consistently associated with baseline HbA1c and iGFR (Table 3). In univariate analyses, baseline HbA1c and iGFR were associated directly with changes in GBM width and Vv(Mes/glom), and inversely with changes in Sv(PGBM/glom) and total glomerular filtration area. In multivariate analyses the associations with HbA1c were still found, but baseline iGFR was only directly associated with GBM width and inversely with total glomerular filtration area. Baseline diastolic BP was directly associated with change in Vv(Mes/glom) and inversely associated with change in Sv(PGBM/glom) in both univariate and multivariate analyses, and directly associated with change in GBM width and mean glomerular volume in multivariate analyses. Baseline ACR was not a significant predictor of change in any of the structural variables (Table 3).
Table 2.
Glomerular morphometric measures at baseline and follow-up biopsies
| Structural Measurement | Baseline | Follow-Up | P Value |
|---|---|---|---|
| GBM width, nm | 440±78 | 528±130 | <0.001 |
| Vv(Mes/glom) | 0.25±0.05 | 0.30±0.08 | <0.001 |
| Sv(PGBM/glom), µm2/µm3 | 0.10±0.02 | 0.09±0.02 | <0.001 |
| Mean glomerular volume, 106 µm3a | 2.47±0.64 | 2.76±1.00 | 0.063 |
| Total glomerular filtration area, 105 µm2a | 0.25±0.09 | 0.22±0.07 | 0.05 |
| Podocyte volume, µm3 | 1275±627 | 814±618 | <0.001 |
| Vv(PC/glom) | 0.16±0.04 | 0.08±0.03 | <0.001 |
| Podocyte number density per glomerulus, 106 µm3 | 151±82 | 171±162 | 0.44 |
| Percentage of sclerosed glomeruli, %b | 0 (0–3) | 10 (3–19) | <0.001c |
Data are mean±SD for continuous measures and numbers with percentages for categorical variables. Missing measures because of insufficient tissue for measurement at first and/or second biopsy. n=48.
n=33.
n=32.
P value from paired t test except Kruskal–Wallis test.
Table 3.
Association of change in kidney morphometry with baseline clinical measures
| Change in Structural Measurement | Univariate, Pearson Correlation | Multivariate, Linear Regression Models | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, yr | Diabetes duration, yr | SBP, mm Hg | DBP, mm Hg | BMI, kg/m2 | Hba1c, % | iGFR, ml/min | ACR, mg/g | Age, yr | Diabetes duration, yr | SBP, mm Hg | DBP, mm Hg | BMI, kg/m2 | Hba1c, % | iGFR, ml/min | ACR, mg/g | |||
| ΔGBM width | r | −0.17 | −0.081 | 0.114 | 0.223 | 0.021 | 0.488 | 0.339 | 0.158 | β | −0.17 | −0.043 | 0.234 | 0.424 | 0.003 | −0.581 | 0.423 | 0.157 |
| P value | 0.25 | 0.584 | 0.441 | 0.128 | 0.886 | <0.001 | 0.018 | 0.282 | P value | 0.28 | 0.802 | 0.167 | 0.013 | 0.987 | <0.001 | 0.022 | 0.345 | |
| ΔVv(Mes/glom) | r | −0.22 | −0.291 | 0.087 | 0.305 | 0.091 | 0.475 | 0.398 | 0.189 | β | −0.24 | −0.157 | 0.115 | 0.416 | 0.052 | 0.561 | 0.311 | 0.130 |
| P value | 0.14 | 0.045 | 0.559 | 0.035 | 0.539 | <0.001 | 0.005 | 0.198 | P value | 0.10 | 0.338 | 0.477 | 0.010 | 0.718 | <0.001 | 0.085 | 0.396 | |
| ΔSv(PGBM/glom) | r | 0.14 | 0.126 | −0.203 | −0.414 | −0.027 | −0.446 | −0.395 | −0.223 | β | 0.18 | −0.018 | −0.118 | −0.414 | 0.149 | −0.485 | −0.230 | −0.225 |
| P value | 0.36 | 0.393 | 0.167 | 0.004 | 0.853 | 0.002 | 0.005 | 0.128 | P value | 0.2 | 0.906 | 0.453 | 0.008 | 0.294 | <0.001 | 0.194 | 0.117 | |
| ΔΜean glomerular volumea | r | −0.21 | −0.157 | −0.018 | 0.281 | 0.056 | 0.004 | 0.084 | 0.088 | β | −0.27 | −0.045 | 0.028 | 0.452 | −0.011 | 0.203 | −0.076 | 0.049 |
| P value | 0.23 | 0.383 | 0.920 | 0.113 | 0.758 | 0.981 | 0.641 | 0.628 | P value | 0.15 | 0.795 | 0.884 | 0.016 | 0.949 | 0.268 | 0.709 | 0.786 | |
| ΔTotal glomerular filtration areaa | r | 0.01 | −0.011 | −0.242 | −0.164 | 0.057 | −0.457 | −0.351 | −0.274 | β | −0.06 | −0.035 | −0.189 | −0.055 | 0.168 | −0.424 | −0.397 | −0.209 |
| P value | 0.94 | 0.954 | 0.175 | 0.363 | 0.751 | 0.008 | 0.046 | 0.123 | P value | 0.71 | 0.826 | 0.273 | 0.762 | 0.303 | 0.003 | 0.040 | 0.186 | |
| ΔMean podocyte volume | r | 0.05 | 0.092 | 0.097 | 0.144 | 0.005 | 0.138 | 0.166 | −0.071 | β | 0.006 | 0.078 | 0.098 | 0.197 | 0.067 | 0.063 | 0.233 | 0.119 |
| P value | 0.74 | 0.534 | 0.512 | 0.328 | 0.975 | 0.350 | 0.259 | 0.633 | P value | 0.97 | 0.627 | 0.542 | 0.239 | 0.643 | 0.655 | 0.177 | 0.463 | |
| ΔVv(PC/glom) | r | 0.04 | 0.115 | −0.004 | −0.127 | −0.064 | −0.027 | −0.215 | −0.023 | β | 0.003 | 0.048 | −0.039 | −0.051 | 0.044 | −0.228 | −0.215 | −0.161 |
| P value | 0.78 | 0.436 | 0.978 | 0.391 | 0.665 | 0.856 | 0.142 | 0.875 | P value | 0.98 | 0.711 | 0.764 | 0.706 | 0.706 | 0.051 | 0.122 | 0.196 | |
| ΔPodocyte number density per glomerulus | r | −0.01 | −0.004 | −0.072 | −0.173 | −0.038 | −0.115 | −0.237 | 0.039 | β | 0.01 | −0.009 | −0.091 | −0.197 | −0.057 | −0.108 | −0.295 | −0.200 |
| P value | 0.93 | 0.977 | 0.625 | 0.238 | 0.797 | 0.438 | 0.105 | 0.795 | P value | 0.93 | 0.953 | 0.572 | 0.228 | 0.687 | 0.434 | 0.082 | 0.228 | |
| ΔPercentage of sclerosed glomerulib | r | 0.05 | 0.009 | −0.255 | −0.007 | −0.291 | 0.177 | 0.043 | 0.125 | β | −0.03 | −0.120 | −0.094 | 0.220 | −0.234 | 0.153 | 0.170 | 0.308 |
| P value | 0.80 | 0.960 | 0.159 | 0.972 | 0.106 | 0.332 | 0.815 | 0.494 | P value | 0.87 | 0.539 | 0.657 | 0.263 | 0.199 | 0.375 | 0.409 | 0.162 | |
Missing morphometric measures because of insufficient tissue for measurement at first and/or second biopsy. Univariate models are Pearson correlations with the correlation coefficient and P value reported. Multivariate analyses are linear regression models with the β coefficient for the association of the clinical measure adjusted for age, sex, and baseline morphometry measure. Models for glomerular volume are also adjusted for glomerular volume method at baseline and the number of glomeruli assessed at first and second biopsy. Bold indicates P<0.05. SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index. n=48.
n=33.
n=35.
Changes in Kidney Structure and Function
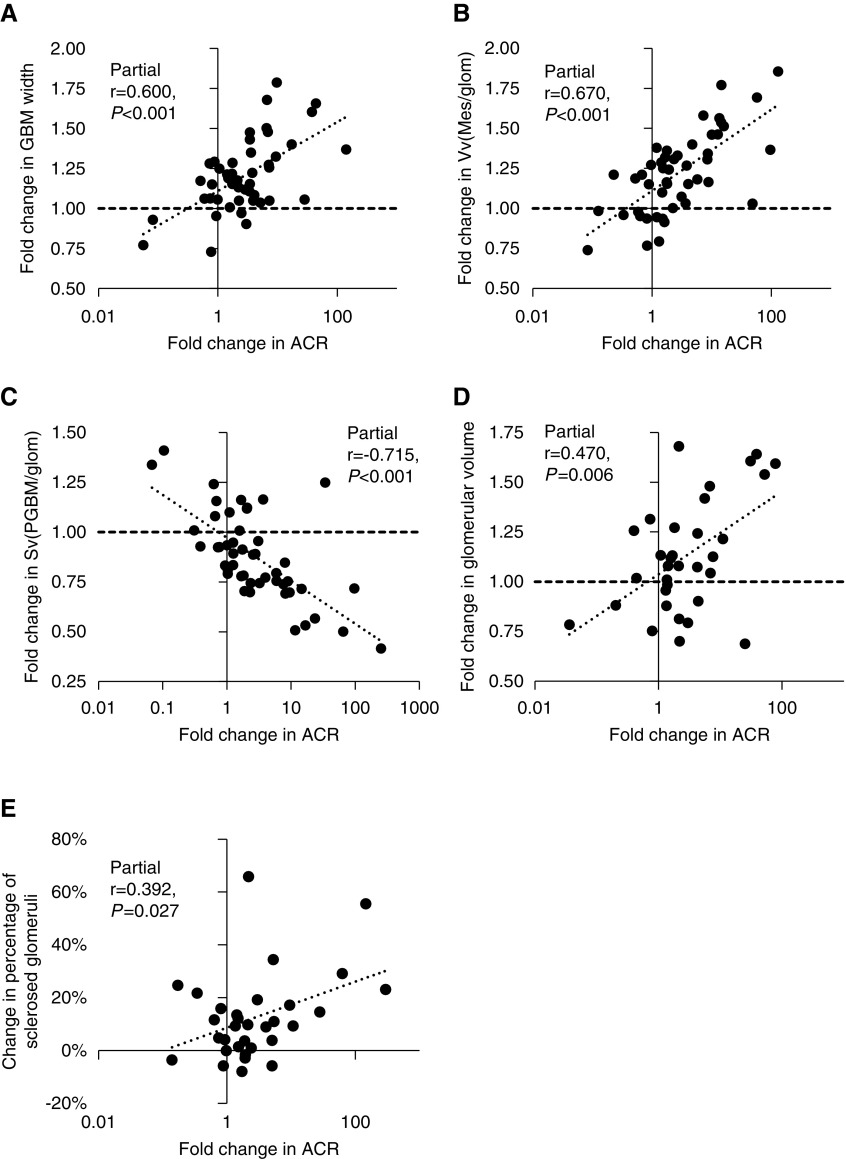
In models adjusted for age, sex, baseline structural parameters, and baseline ACR, change in ACR was associated directly with change in GBM width, Vv(Mes/glom), and mean glomerular volume, and inversely with Sv(PGBM/glom) (Figure 2). By contrast, in models adjusted for age, sex, baseline structural parameters, and baseline iGFR, change in iGFR was not associated with changes in any of the structural measures (Supplemental Figures 3 and 4). Change in percentage of sclerosed glomeruli was correlated with change in ACR (partial r=0.39; P=0.03) but not with change in iGFR (partial r=−0.23; P=0.24) in models adjusted for age, sex, baseline percentage of sclerosed glomeruli, and baseline ACR and iGFR, respectively. Neither baseline structure nor change in structural measurements was associated with treatment allocation in the clinical trial (Supplemental Table 3).
Figure 2.
Fold change in structural measures were statistically significantly associated with fold change in ACR. (A) GBM width, (B) Vv(Mes/glom), (C) Sv(PGBM/glom), (D) glomerular volume, and (E) percentage sclerosed glomeruli. For each figure, residuals were computed by regressing each of the variables on sex and baseline age, ACR, and the structural measure.
Associations between iGFR slope and change in kidney structure are shown in Table 4. Individuals with a greater rate of loss of iGFR had significantly greater increases in GBM width, Vv(Mes/glom), and podocyte volume, plus a greater decline in Sv(PGBM/glom) and podocyte number density per glomerulus. These associations were attenuated when baseline iGFR was included in the models.
Table 4.
Association of iGFR slope with change in morphometry adjusted for age, sex, and baseline structural measure
| Structural Measurement | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Partial r | P Value | Partial r | P Value | |
| GBM width, nm | −0.34 | 0.02 | −0.20 | 0.17 |
| Vv(Mes/glom) | −0.31 | 0.03 | −0.21 | 0.16 |
| Sv(PGBM/glom), µm2/µm3 | 0.30 | 0.04 | 0.24 | 0.10 |
| Mean glomerular volume, 106 µm3a | −0.04 | 0.82 | −0.09 | 0.61 |
| Total glomerular filtration area, 105 µm2/µm3a | 0.25 | 0.17 | 0.09 | 0.64 |
| Podocyte cell volume, µm3 | −0.31 | 0.04 | −0.24 | 0.10 |
| Vv(PC/glom) | 0.15 | 0.30 | 0.04 | 0.79 |
| Podocyte number density per glomerulus, 106 µm3 | 0.29 | 0.05 | 0.19 | 0.21 |
| Percentage of sclerosed glomeruli, %b | −0.11 | 0.53 | −0.03 | 0.89 |
Missing morphometric measures because of insufficient tissue for measurement at first and/or second biopsy. Model 1: correlation coefficient adjusted for age, sex, and baseline structural measure. Model 2: correlation coefficient adjusted for model 1 plus baseline measured GFR. All models of glomerular volume also include glomerular volume method and number of glomeruli assessed.
n=33.
n=32.
Discussion
In American Indians with type 2 diabetes and generally preserved kidney function, changes in kidney structure over 9.3 years reflected progression of DN.7–10 The principal structural changes included increases in GBM width, Vv(Mes/glom), and the prevalence of glomerular sclerosis along with a decline in Sv(PGBM/glom), and total glomerular filtration area. We also saw declines in podocyte volume and Vv(PC/glom). These changes were accompanied by a decline in iGFR and increase in ACR, although most participants still had normal ACR and an iGFR above the threshold for CKD at the second biopsy.31 Nevertheless, reductions of iGFR within the normal range may be important indicators of disease progression. In type 1 diabetes, the GFR breakpoint in piece-wise regression analysis, below which further decline in GFR becomes more strongly associated with GBM thickening, increased Vv(Mes/glom), and reduced Sv(PGBM/glom), was at 100 ml/min per 1.73 m2.27 However, this has not been studied in type 2 diabetes.
Changes in kidney structure were associated with baseline iGFR and HbA1c, but not with change in iGFR. However, in models of iGFR slope unadjusted for baseline iGFR, greater rate of iGFR decline was associated with increases in GBM width, Vv(Mes/glom), and mean podocyte volume along with preservation of Sv(PGBM/glom), and podocyte number density per glomerulus. By contrast, change in ACR, in models adjusted for baseline ACR, was strongly associated with change in GBM width, Vv(Mes/glom), Sv(PGBM/glom), mean glomerular volume, and percentage of sclerosed glomeruli. This suggests that, at this relatively early clinical stage of DN, change in ACR is a better indicator of early progressive structural damage than change in iGFR, particularly in individuals with preserved or elevated iGFR at baseline. In a cross-sectional study in type 1 diabetes, structural-functional relationships were weak when measured GFR values were >99 ml/min or AER<42 µg/min.27 Because iGFR values for most of the participants in this study were >99 ml/min at both biopsies, our findings are consistent with this earlier study in indicating that structural relationships with iGFR are relatively weak in those with preserved kidney function, whereas they are much stronger in associations with ACR.27 Although the majority of participants had normal ACR at their first biopsy, 48% of the participants had micro- or macroalbuminuria by their second biopsy, concordant with progression in the same structural abnormalities that explained >80% of albuminuria variance in type 1 diabetes research participants.27 Nonetheless, as 73% of the participants at baseline and 58% at follow-up had an ACR<42 µg/min, i.e., in the albuminuria zone where relationships with structure in type 1 diabetes were relatively weak,27 it may be that at higher levels, changes in albuminuria may become an even more precise predictor of structural changes. We lack data for individuals with reduced GFR, as only six participants had a baseline GFR <100 ml/min. The relative stability in iGFR over the study compared with ACR may mean that this study lacks the power to detect the more modest associations between changes in structure and iGFR compared with the greater changes in ACR at these earlier stages of DN.
Although on average there was a decrease in podocyte volume at follow-up, the iGFR slope, expressed as a negative value, was inversely associated with podocyte volume and directly with podocyte number density per glomerulus and showed a trend with number of podocytes per glomerulus. These data indicate that greater rates of iGFR loss were associated with greater podocyte hypertrophy and podocyte loss. However, it is unclear why, in many cases, podocyte volume decreased during the study. One possible explanation is that the fall in podocyte volume represents podocyte atrophy in the absence of significant changes in podocyte number or number density. Reduction in podocyte number and number density would be expected to lead to compensatory hypertrophy. In addition, those who had rapidly progressive decline in GFR and did not undergo a follow-up biopsy were likely to have more severe podocyte loss and podocyte hypertrophy. We previously showed that people with an iGFR≥154 ml/min had a higher podocyte number per glomerulus than people with lower iGFRs.10 This was not the case in the subset of participants who were included in this study because most participants with low iGFR at the time of first biopsy were not eligible for a second research kidney biopsy for safety reasons. We posit that reduced podocyte volume is rather a novel finding of potential significance. If this decrease in podocyte volume is predictive of subsequent podocyte loss and/or GFR loss, then it may be an important predictive parameter or even outcome variable. It will be important to test for this possibility in other paired biopsy studies in other cohorts with diabetes.
We previously published two biopsy studies looking at cross-sectional associations between kidney structure and function in Pima Indians, the first on the basis of 51 biopsies9 and the second on the basis of 111 biopsies,10 from which this study is a subset. The first biopsy study found that higher mean glomerular volume, GBM width, Vv(Mes/glom), and lower podocyte number density per glomerulus were each associated with macroalbuminuria.9 The second biopsy study confirmed that higher mean glomerular volume, GBM width, and Vv(Mes/glom) were also directly associated with ACR and Sv(PGBM/glom) and total glomerular filtration area were inversely associated with ACR. In addition, iGFR was directly associated with mean glomerular volume, Sv(PGBM/glom), and total glomerular filtration area and inversely associated with percentage of sclerosed glomeruli.
In a study of 71 people with type 1 diabetes and normoalbuminuria, followed for 11.0±7.2 years, higher GBM width was a risk factor for a composite outcome of ESRD, death, or macroalbuminuria.28 In a nested substudy of 20 participants where ten had progressed to albuminuria or ESRD and ten remained normoalbuminuric, baseline GBM width was higher in the progressors but there were no differences in baseline podocyte number density per glomerulus.32 In American Indians with type 2 diabetes, higher GBM width, Vv(Mes/glom), mean glomerular volume and total glomerular filtration area and lower Sv(PGBM/glom) were risk factors for a ≥40% decline in GFR over a median follow-up of 6.6 years but baseline podocyte number density per glomerulus was not.10 In a Japanese study of 29 individuals with type 2 diabetes and normo- or microalbuminuria at kidney biopsy followed for 8.0±3.5 years, higher baseline Vv(Mes/glom) was associated with lower GFR at follow-up, but no structural measures were associated with GFR slope.33 Because of the differing levels of kidney function at biopsy, variable lengths of follow-up, and the differing outcome definitions, direct comparisons between studies are difficult. However, these studies generally support associations of kidney function decline with GBM width and Vv(Mes/glom), as reported in this study.
There are few published data on serial research kidney biopsies in DN. In a study of 11 patients with type 1 diabetes with baseline and follow-up biopsies 5 years apart, and with no change in measured GFR but an increase in AER, AER change correlated with the increase in Vv(Mes/glom) but not GBM width.11 Serial kidney biopsies were also used in clinical trials in type 1 diabetes,34,35 but issues relating to selection criteria and variation by treatment make them less relevant to this study.
Change in four of the five structural measures we identified as associated with change in ACR are strongly correlated with each other. We make no claim as to whether one of these measures is the key structural change underlying the worsening of albuminuria but rather suggest that the measures are all evidence of the parallel progression of classic diabetic glomerulopathy lesions,7 which is most clearly functionally reflected at earlier DN clinical stages by increasing albuminuria.
One key strength of this study is the availability of data from serial research kidney biopsies from individuals with type 2 diabetes. Serial kidney biopsy studies of DN have only been reported previously in individuals with type 1 diabetes.11,12,27,34–37 Also, all participants in this study had extensive clinical data available at baseline and during follow-up.
Limitations of this study include changes in the methodology for assessing mean glomerular volume and the lack of tissue at baseline for assessing it by the “gold standard” serial section Cavalieri method in all samples. Nevertheless, we were able to adjust values for mean glomerular volume derived from the Weibel–Gomez method to reflect those obtained from the Cavalieri method.38 In addition, the study does not include all participants who underwent a baseline kidney biopsy. Participants who developed rapidly progressive kidney disease were excluded because the risk of a second kidney biopsy was too great to justify for research purposes. Thus, participants in this study had more stable kidney function than in the original cohort: of the 63 people not included, 25% developed ESRD. Moreover, in those with advanced kidney disease, quantitative assessment of structure is difficult because much of the normal kidney architecture is obliterated. As a result, the participants in this study had early DN and relatively stable kidney function. We used single measures of iGFR at around the time of each biopsy as our primary measures of iGFR change, but we also include data using all available iGFRs over the course of the study. Although there was no significant difference in baseline iGFR between the people included and not included in this study, the participants had lower median ACR and BP at the baseline biopsy, in keeping with their lower risk of progression. Some structural parameters could not be appropriately compared in baseline and follow-up biopsies because of either change in embedding or transport media. The change in embedding media used for the baseline and follow-up biopsies particularly affected tubulointerstitial measures. Thus, we were unable to report data for change in cortical interstitial fractional volume or for any of the tubular fractional volumes. However, on the basis of work in type 1 diabetes, before the development of renal insufficiency the association between tubulointerstitial measures and function are much weaker than for glomerular measures in the GFR ranges of this study.39 Thus, in our study, where kidney function is mostly preserved (and in some cases elevated), it is reasonable to expect that the strongest associations with function would be with the glomerular measures. The study only includes American Indians with type 2 diabetes, which raises the issue of generalizability. Although glomeruli are larger in Pima Indians than in other ethnic groups,40 findings relating structure and function are consistent with reports from other type 2 diabetes populations, including Italian and Japanese41,42 and type 1 diabetes populations.7,27 The younger age and relative lack of preexisting hypertension in the Pima Indians may allow for clearer assessment of the effect of diabetes on the kidneys than in other type 2 diabetes cohorts, and may be particularly relevant to those with youth onset type 2 diabetes.43 We include many statistical tests and report only nominal P values. This reflects the exploratory nature of the work.
Kidney biopsies are essential to our understanding of DN.44 By determining which clinical measures best reflect changes in underlying structure, we can identify individuals with progressive disease even when GFR is still preserved. Although the rate of GFR decline is considered an important determinant of progressive DN,5,6 our findings suggest that albuminuria is the more informative measure for predicting structural changes in the kidneys in the earlier stages of DN when GFR is still predominantly >60 ml/min.
Disclosures
Dr. Boustany-Kari, Mr. Hill and Dr. Guarnieri are employees of Boehringer-Ingelheim. Prof. Kretzler reports grants from National Institutes of Health and Boehringer Ingelheim during the conduct of the study, and grants from AstraZeneca, Gilead, NovoNordisc, Eli Lilly, Janssen, Merck, Eliderat, Certa, and Goldfinch Bio outside the submitted work. Prof. Kretzler has a patent PCT/EP2014/073413 “Biomarkers and methods for progression prediction for chronic kidney disease” issued. All remaining authors have nothing to disclose.
Acknowledgments
We thank Ms. Lois Jones, RN, Mr. Enrique Diaz, RN, Ms. Bernadine Waseta, and Ms. Camille Waseta for conducting the examinations, processing the samples, and entering the data, and Ms. Frida Maiers, Ms. Ann Palmer, Ms. Karen Feller, Ms. Zour Yang and Dr. Alexey Sokolovskiy for processing the tissue and performing the morphometry measurements that were done at the University of Minnesota and University of Washington.
Prof. Mauer, Dr. Nelson, and Prof. Najafian designed the study. Dr. Esplin carried out the kidney biopsies. Prof. Mauer and Prof. Najafian carried out the morphometric measurements. Dr. Looker analyzed the data and drafted the manuscript. Dr. Looker, Prof. Mauer, Prof. Saulnier, Dr. Nelson, Dr. Harder, Dr. Nair, Dr. Boustany-Kari, Dr. Guarnieri, Mr. Hill, Prof. Kretzler, and Prof. Najafian contributed to the interpretation of data and critically revised the manuscript. All authors approved the final version of the manuscript.
This study was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, a grant from the National Institutes of Health (1R24082841 to Dr. Harder, Dr. Nair, and Prof. Ketzler), and funds from Boehringer Ingelheim.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018111166/-/DCSupplemental.
Supplemental Appendix 1. Supplemental methods.
Supplemental Table 1. Demographics and clinical characteristics for participants in dual biopsy study versus participants not in dual biopsy study.
Supplemental Table 2. Pearson correlation coefficients for change in kidney morphometry measures and percentage of sclerosed glomeruli.
Supplemental Table 3. Regression coefficients for association of allocation to losartan versus placebo to baseline and change in kidney structure.
Supplemental Table 4: Additional glomerular morphometric measures at baseline and follow-up biopsies.
Supplemental Table 5: Association of change in additional kidney morphometry with baseline clinical measures.
Supplemental Table 6: Association of iGFR slope with change in additional morphometry adjusted for age, sex and baseline structural measure.
Supplemental Figure 1. Effects of embedding media on measures of cortical interstitial fractional volume. (A) Bland–Altman plot of samples at baseline and (B) estimating change over time.
Supplemental Figure 2. Bland–Altman plots of effect of embedding media at baseline on measures of (A) cortical proximal tubule fractional volume, (B) cortical distal tubule fractional volume, (C) cortical total tubular fractional volume, and (D) index of arteriolar hyalinosis.
Supplemental Figure 3. Fold change in morphometry and fold change in ACR. (A) Mesangial cell fractional volume and (B) mesangial matrix fractional volume. For each figure, residuals were computed by regressing each of the variables on sex and baseline age, ACR, and the structural measure.
Supplemental Figure 4. Fold change in morphometry and fold change in measured GFR. (A) GBM width, (B) Vv(Mes/glom), (C) mesangial cell fractional volume, (D) mesangial matrix fractional volume, (E) Sv(PGBM/glom), (F) glomerular volume, and (G) percentage glomerular sclerosis. For each figure, residuals were computed by regressing each of the variables on sex and baseline age, measured GFR, and the structural measure.
References
- 1.National Kidney Foundation : KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 60: 850–886, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Caramori ML, Fioretto P, Mauer M: Enhancing the predictive value of urinary albumin for diabetic nephropathy. J Am Soc Nephrol 17: 339–352, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group : Risk factors for renal dysfunction in type 2 diabetes: U.K. prospective diabetes study 74. Diabetes 55: 1832–1839, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Molitch ME, Steffes M, Sun W, Rutledge B, Cleary P, de Boer IH, et al.: Epidemiology of Diabetes Interventions and Complications Study Group : Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 33: 1536–1543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, et al.: Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 18: 1353–1361, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Krolewski AS: Progressive renal decline: The new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes Care 38: 954–962, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC: Structural-functional relationships in diabetic nephropathy. J Clin Invest 74: 1143–1155, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond K, Mauer M; International Diabetic Nephropathy Study Group : The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes 51: 1580–1587, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, et al.: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fufaa GD, Weil EJ, Lemley KV, Knowler WC, Brosius FC 3rd, Yee B, et al.: Structural predictors of loss of renal function in American Indians with type 2 diabetes. Clin J Am Soc Nephrol 11: 254–261, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fioretto P, Steffes MW, Sutherland DE, Mauer M: Sequential renal biopsies in insulin-dependent diabetic patients: Structural factors associated with clinical progression. Kidney Int 48: 1929–1935, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, et al.: Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 361: 40–51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weil EJ, Fufaa G, Jones LI, Lovato T, Lemley KV, Hanson RL, et al.: Effect of losartan on prevention and progression of early diabetic nephropathy in American Indians with type 2 diabetes. Diabetes 62: 3224–3231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 15.Myers BD, Nelson RG, Tan M, Beck GJ, Bennett PH, Knowler WC, et al.: Progression of overt nephropathy in non-insulin-dependent diabetes. Kidney Int 47: 1781–1789, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Delanaye P, Radermecker RP, Rorive M, Depas G, Krzesinski JM: Indexing glomerular filtration rate for body surface area in obese patients is misleading: Concept and example. Nephrol Dial Transplant 20: 2024–2028, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Vasquez B, Flock EV, Savage PJ, Nagulesparan M, Bennion LJ, Baird HR, et al.: Sustained reduction of proteinuria in type 2 (non-insulin-dependent) diabetes following diet-induced reduction of hyperglycaemia. Diabetologia 26: 127–133, 1984 [DOI] [PubMed] [Google Scholar]
- 18.Chasson AL, Grady HJ, Stanley MA: Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Technol 30: 207–212, 1960 [PubMed] [Google Scholar]
- 19.Tosoh Bioscience : HPLC technology. 2018. Available at: https://www.diagnostics.us.tosohbioscience.com/solutions/hplc-technology. Accessed May 3, 2019
- 20.Fioretto P, Steffes MW, Mauer M: Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes 43: 1358–1364, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Najafian B, Mauer M: Quantitating glomerular endothelial fenestration: An unbiased stereological approach. Am J Nephrol 33[Suppl 1]: 34–39, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M: Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339: 69–75, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim HN, Jackson S, Connaire J, Matas A, Ney A, Najafian B, et al.: Angiotensin II blockade in kidney transplant recipients. J Am Soc Nephrol 24: 320–327, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caramori ML, Kim Y, Huang C, Fish AJ, Rich SS, Miller ME, et al.: Cellular basis of diabetic nephropathy: 1. Study design and renal structural-functional relationships in patients with long-standing type 1 diabetes. Diabetes 51: 506–513, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Klein R, Zinman B, Gardiner R, Suissa S, Donnelly SM, Sinaiko AR, et al.: Renin-Angiotensin System Study : The relationship of diabetic retinopathy to preclinical diabetic glomerulopathy lesions in type 1 diabetic patients: The renin-angiotensin system study. Diabetes 54: 527–533, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Najafian B, Tøndel C, Svarstad E, Sokolovkiy A, Smith K, Mauer M: One year of enzyme replacement therapy reduces globotriaosylceramide inclusions in podocytes in male adult patients with fabry disease. PLoS One 11: e0152812, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauer M, Caramori ML, Fioretto P, Najafian B: Glomerular structural-functional relationship models of diabetic nephropathy are robust in type 1 diabetic patients. Nephrol Dial Transplant 30: 918–923, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caramori ML, Parks A, Mauer M: Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol 24: 1175–1181, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weibel E: Stereological Methods. Practical Methods for Biological Morphometry, London, Academic Press, 1979 [Google Scholar]
- 30.Lane PH, Steffes MW, Mauer SM: Estimation of glomerular volume: A comparison of four methods. Kidney Int 41: 1085–1089, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Kidney Disease Improving Global Outcomes: Chapter 2: Definition, identification, and prediction of CKD progression. Kidney Int Suppl (2011) 3: 63–72, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harindhanavudhi T, Parks A, Mauer M, Caramori ML: Podocyte structural parameters do not predict progression to diabetic nephropathy in normoalbuminuric type 1 diabetic patients. Am J Nephrol 41: 277–283, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriya T, Omura K, Matsubara M, Yoshida Y, Hayama K, Ouchi M: Arteriolar hyalinosis predicts increase in albuminuria and GFR decline in normo- and microalbuminuric Japanese patients with type 2 diabetes. Diabetes Care 40: 1373–1378, 2017 [DOI] [PubMed] [Google Scholar]
- 34.White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, et al.: Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes 51: 3083–3089, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Mauer M, Zinman B, Gardiner R, Drummond KN, Suissa S, Donnelly SM, et al.: ACE-I and ARBs in early diabetic nephropathy. J Renin Angiotensin Aldosterone Syst 3: 262–269, 2002 [DOI] [PubMed] [Google Scholar]
- 36.The European Study for the Prevention of Renal Disease in Type 1 Diabetes ESPRIT Study Group: Effect of 3 years of antihypertensive therapy on renal structure in type 1 diabetic patients with albuminuria: The European Study for the Prevention of Renal Disease in Type 1 Diabetes (ESPRIT). Diabetes 50: 843–850, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Fufaa GD, Weil EJ, Nelson RG, Hanson RL, Knowler WC, Rovin BH, et al.: CKD Biomarkers Consortium and the RASS Investigators : Urinary monocyte chemoattractant protein-1 and hepcidin and early diabetic nephropathy lesions in type 1 diabetes mellitus. Nephrol Dial Transplant 30: 599–606, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dumitrescu S, Coca I: [Clinical and technical considerations on terminal edentia. I. Anatomo-clinical, etiological and pathogenetic aspects]. Rev Chir Oncol Radiol O R L Oftalmol Stomatol Ser Stomatol 25: 205–214, 1978 [PubMed] [Google Scholar]
- 39.Najafian B, Mauer M: Morphologic features of declining renal function in type 1 diabetes. Semin Nephrol 32: 415–422, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt K, Pesce C, Liu Q, Nelson RG, Bennett PH, Karnitschnig H, et al.: Large glomerular size in Pima Indians: Lack of change with diabetic nephropathy. J Am Soc Nephrol 3: 229–235, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Nosadini R, Velussi M, Brocco E, Bruseghin M, Abaterusso C, Saller A, et al.: Course of renal function in type 2 diabetic patients with abnormalities of albumin excretion rate. Diabetes 49: 476–484, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Moriya T, Suzuki Y, Inomata S, Iwano M, Kanauchi M, Haneda M: Renal histological heterogeneity and functional progress in normoalbuminuric and microalbuminuric Japanese patients with type 2 diabetes. BMJ Open Diabetes Res Care 2: e000029, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu H, Verre MC: Type 2 diabetes mellitus in children. Am Fam Physician 98: 590–594, 2018 [PubMed] [Google Scholar]
- 44.Looker HC, Mauer M, Nelson RG: Role of kidney biopsies for biomarker discovery in diabetic kidney disease. Adv Chronic Kidney Dis 25: 192–201, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]