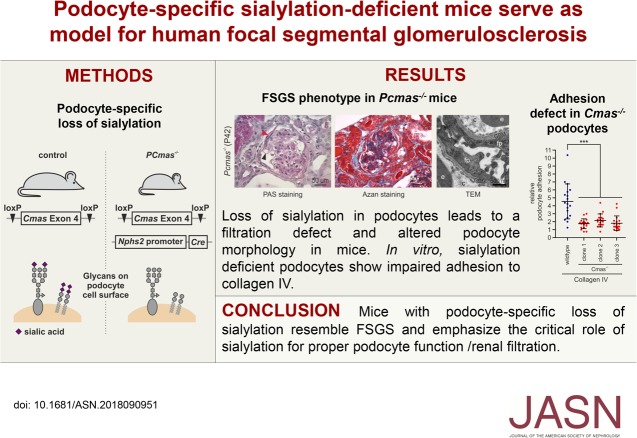
Significance Statement
Although glycosylation of the endothelial surface layer is known to be crucial for kidney function, the role of podocyte glycosylation is not well understood. The authors demonstrated that podocyte-specific ablation of sialylation in mice caused a phenotype resembling human FSGS. Loss of sialylation resulted in disturbance of podocyte homeostasis and podocyte loss in vivo. In vitro, sialylation-deficient podocytes were able to proliferate and differentiate, but did not grow out of isolated glomeruli; they also showed impaired adhesion to collagen, suggesting a crucial role of sialylation for podocyte interaction with the glomerular basement membrane. These findings strongly implicate sialylation as a factor in FSGS pathogenesis, and it therefore might serve as both a diagnostic marker and a therapeutic target to delay disease progression.
Keywords: focal segmental glomerulosclerosis, glomerular disease, podocyte, chronic kidney disease, cell adhesion, glycosylation
Visual Abstract
Abstract
Background
The etiology of steroid-resistant nephrotic syndrome, which manifests as FSGS, is not completely understood. Aberrant glycosylation is an often underestimated factor for pathologic processes, and structural changes in the glomerular endothelial glycocalyx have been correlated with models of nephrotic syndrome. Glycans are frequently capped by sialic acid (Sia), and sialylation’s crucial role for kidney function is well known. Human podocytes are highly sialylated; however, sialylation’s role in podocyte homeostasis remains unclear.
Methods
We generated a podocyte-specific sialylation-deficient mouse model (PCmas−/−) by targeting CMP-Sia synthetase, and used histologic and ultrastructural analysis to decipher the phenotype. We applied CRISPR/Cas9 technology to generate immortalized sialylation-deficient podocytes (asialo-podocytes) for functional studies.
Results
Progressive loss of sialylation in PCmas−/− mice resulted in onset of proteinuria around postnatal day 28, accompanied by foot process effacement and loss of slit diaphragms. Podocyte injury led to severe glomerular defects, including expanded capillary lumen, mesangial hypercellularity, synechiae formation, and podocyte loss. In vivo, loss of sialylation resulted in mislocalization of slit diaphragm components, whereas podocalyxin localization was preserved. In vitro, asialo-podocytes were viable, able to proliferate and differentiate, but showed impaired adhesion to collagen IV.
Conclusions
Loss of cell-surface sialylation in mice resulted in disturbance of podocyte homeostasis and FSGS development. Impaired podocyte adhesion to the glomerular basement membrane most likely contributed to disease development. Our data support the notion that loss of sialylation might be part of the complex process causing FSGS. Sialylation, such as through a Sia supplementation therapy, might provide a new therapeutic strategy to cure or delay FSGS and potentially other glomerulopathies.
FSGS is a common type of glomerular injury leading to ESRD with rising incidence around the world.1–3 Rather than being a disease itself, FSGS refers to a histopathologic pattern with sclerotic changes affecting only parts of a glomerulus and only a subset of glomeruli simultaneously.4 The etiology of FSGS is still not fully resolved, even though a variety of pathogenic factors such as genetic alterations, endogenous circulating molecules, and medication have been identified to cause or promote the disease.5,6 However, FSGS is unified by a common theme of podocyte injury. Podocytes are highly specialized cells characterized by the formation of foot processes (FP), which are interconnected by the slit diaphragm (SD), thus forming the outermost layer of the glomerular filtration barrier. Like cells of the fenestrated endothelium, podocytes are covered by a dense glycocalyx, a meshwork of glycoconjugates, proteoglycans, and glucosaminoglycans, which are also major components of the glomerular basement membrane (GBM).7,8 Alterations in the composition of the endothelial glycocalyx contribute to the development of albuminuria in a number of important diseases, such as diabetes and cardio-vascular diseases. Furthermore, structural changes in the endothelial glycocalyx have been correlated with renal failure of patients with CKD and experimental models of nephrotic syndrome.9–12 In contrast, so far there is limited knowledge on the role of the glycocalyx in podocytes. An important function for glomerular filtration is allocated to the negatively charged sugar sialic acid (Sia), which is found at the outermost position of glycoconjugates at cell surfaces. Sialylation of glycoproteins and glycolipids takes place in the Golgi apparatus before secretory vesicles are transported to the cell surface, and strictly depends on the availability of the activated sugar donor substrate CMP-Sia, generated by CMP-Sia synthetase (CMAS) (Supplemental Figure 1). Mouse models with ubiquitously reduced sialylation capacity in all cells because of mutations in enzymes involved in Sia de novo biosynthesis (N-acetylglucosamine epimerase/N-acetylmannosamine kinase [GNE], Supplemental Figure 1, GneM712T/M712T)13 or metabolic activation (Cmasnls/nls)14 suffer from proteinuria and die within the first 3 days after birth due to kidney failure. In addition, the hypomorphic Cmasnls/nls mice revealed podocytes as the cells most susceptible to restrictions in the sialylation pathway, manifesting in a maturation defect. Because the reduced sialylation capacity in the mentioned mouse models leads to premature death, and pharmacologic ways of desialylation of glycans (e.g., by neuraminidase injection) caused damage to all glomerular cell types,15,16 the effect of cell-type–specific sialylation could not be deciphered in these model systems. To address the contribution of Sia to podocyte homeostasis in vivo, we generated a podocyte-specific knockout mouse by targeting CMAS, the enzyme essential for the biosynthesis of all sialoglycoconjugates. In different model systems, we have recently confirmed that prevention of CMAS expression causes loss of sialylation on glycoproteins and glycolipids (see Supplemental Figure 1).17,18
Methods
Details on antibodies and lectins are given in Supplemental Table 1. Primers are listed in Supplemental Table 2 and further methods are described in the Supplemental Material section.
Mice
Podocyte-specific Cmas-knockout mice (PCmas−/−) mice were generated by crossing Cmasfloxed mice,17 carrying the floxed Cmas allele, with (NPHS2)-Cre mice.19 ImPCmas−/− mice were generated by crossing PCmas−/− with the H-2Kb-tsA58 transgenic mouse strain harboring the simian virus 40 large tumor antigen.20 Animals were hosted in the animal facility of the Hannover Medical School under specific-pathogen–free conditions. All animal experiments were carried out in compliance with German law for protection of animals and were approved by the local authorities (TV33.9_42502_04_16/2163 and 33.14_42502_04_13/1312; 33.19_42502_05_18A266).
Genotyping
Genotypes of mice, tissue, or isolated cells were determined by PCR analysis with isolated genomic DNA and Phusion High Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA). PCR products were analyzed on a 1%–1.5% agarose gel with GeneRuler 100 bp Plus DNA Ladder (ThermoFisher Scientific, Waltham, MA).
Urine Analysis
Creatinine and total protein of urine samples were determined in the chemical analyzer Olympus AU 400 (Olympus Deutschland GmbH, Hamburg, Germany) as described.14
GFR
The GFR of control and PCmas−/− mice was determined by transcutaneous measurement of FITC-sinistrin as described previously.21 Briefly, fluorescent signals were measured with a Transdermal GFR Monitor (MediBeacon GmbH, Mannheim, Germany) before and after retrobulbar injection of FITC-sinistrin (7.5 mg/100 g body wt) in anesthetized mice. The GFR was calculated from the t1/2 of FITC-sinistrin in the blood.
Electron Microscopy
Sample preparation and analysis were performed as described.14
Preparation of Kidney and Cell Lysates
Kidney homogenates were prepared in Triton buffer (1% Triton-X-100, 20 mM TrisHCl pH 8.0, 50 mM NaCl, 0.1 mM EDTA, 20 mM Chaps, 3 mM ATP, 200 U ml−1 aprotinin, 10 μg ml−1 leupeptin, 1 mM PMSF, 50 mM NaF, 2 mM Na3VO3). Podocytes were lysed in RIPA buffer. For sialidase treatment, an aliquot of the sample was incubated with 0.05 U Arthrobacter ureafaciens sialidase (EY Laboratories, San Mateo, CA) per mg protein at 37°C for 30 minutes before western blot analysis.
Western Blot Analysis
SDS-PAGE and western blotting was performed as described.14 Adjusted protein amounts were separated, blotted, and incubated with primary antibodies (O/N, 4°C) and appropriate secondary antibodies (1 hour, RT). Peroxidase-coupled antibodies were visualized by enhanced chemiluminescence; alkaline phosphatase-coupled antibodies were visualized by BCIP/NBT color development.
Cell Culture
Conditionally immortalized podocyte cell lines were obtained from sterile glomeruli isolated by differential sieving. Cultivation on collagen type I (Corning, Corning, NY)–coated cell culture plates was performed in RPMI 1640 medium (Biochrom, Berlin, Germany) containing 10% FCS (Life Technologies, Carlsbad, CA), 1% Penicillin/Streptomycin (Biochrom), and 20 U ml−1 IFNγ (Peprotech, Hamburg, Germany) at 33°C and 5% CO2. Podocytes were differentiated under IFNγ depletion at 37°C and 5% CO2 for 10–14 days.22 Podocyte cell clones were tested for mycoplasma contamination with a negative result.
Immunostaining of Podocytes
Podocytes were seeded on collagen type 1 (BD Biosciences, Franklin Lakes, NJ)–coated coverslips, cultured for 24 hours, fixed in 4% PFA, and permeabilized with 0.2% Triton-X 100. Blocking and antibody incubation were performed in 1% BSA for 1 hour at RT. Coverslips were mounted in DAPI containing Vectashield mounting medium (Vectorlabs, Burlingame, CA).
Generation of Cmas−/− Podocytes
Cmas−/− podocytes were generated by CRISPR/Cas9-mediated genome editing using a guide RNA targeting residues 11,154–11,173 of Cmas exon 4 (5′-TGTCGACGAGGCCGTTTCGC-3′). The target sequence was cloned into the plasmid pX330-U6-Chimeric_BB-CBh-hSpCas9 (Addgene plasmid #42230 from Feng Zhang23). Wild-type podocytes were cotransfected (ratio 10:1) with the generated plasmid and an empty vector containing a neomycin resistance gene (pcDNA3; Invitrogen, Carlsbad, CA). Transfection was carried out with FuGene (Promega, Madison, WI) according to the manufacturer’s protocol. Twenty-four hours after transfection the cells were selected with 100 µg/ml G418 (Merck) for 5 days and single-cell clones were obtained by limiting dilution. Clones were screened by PCR and products were sequenced using the primers KMB 77 and KMB 78.
In Vitro Proliferation Assay
For the analysis of cell proliferation, podocyte cell lines were seeded on collagen I–coated 96-well plates at a density of 3000 cells/well in triplicates for each time point. After 24, 48, 72, and 96 hours, 10 µl WST-1 reagent (Roche, Basel, Switzerland) was added to a triplicate of wells and incubated for 4 hours at 33°C. The reaction was measured at 450 nm (and 690 nm as reference wavelength). Wells without cells served as blank control. Three independent experiments were carried out at different days.
In Vitro Adhesion Assay
Adhesion assays were carried out with immortalized wild-type and Cmas−/− podocyte cell clones. Ninety-six–well plates were coated O/N at 4°C with 1% BSA, 10 µg/ml collagen IV (from human placenta; Sigma Aldrich, St. Louis, MO), and 10 µg/ml laminin 521 (Biolamina, Sundbyberg, Sweden), respectively. Plates were washed twice with PBS and blocked with 1% BSA for 30 minutes at 37°C. Podocytes were seeded at a density of 1×104 cells in the coated wells in RPMI 1640 medium without supplements and allowed to adhere to the matrices at 37°C for 1 hour. Wells were washed twice with PBS, fixed with 4% PFA, and nuclei of the cells were stained with Hoechst 33258. Representative images of each well were taken and cell numbers from six wells were averaged. Three individual experiments were performed and cell counts were normalized to the BSA control.
Statistical Analyses
Two groups of data sets were compared by unpaired t tests (* P<0.5, ** P<0.01, ***P<0.001, ****P<0.0001). For analysis of urinary protein-to-creatinine ratio, a nonparametric Kruskal–Wallis test and Dunn’s multiple comparison were performed (** P<0.01, ***P<0.001). Statistical analysis was performed using GraphPad Prism 5.
Results
Generation of Podocyte-Specific CMAS-Knockout (PCmas−/−) Mice
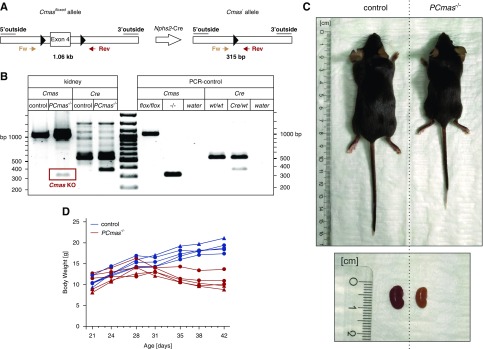
Mice with podocyte-specific ablation of CMAS (PCmas−/−) were generated by crossing a mouse line expressing Cre recombinase under the podocin (Nphs2) promotor19 with Cmasfloxed mice, carrying floxed alleles of Cmas exon 4, encoding the active site of the protein17,18,24,25 (Figure 1A). Mutant and wild-type alleles were detected by PCR analysis (Figure 1B).
Figure 1.
Podocyte-specific CMAS-knockout (PCmas−/−) mice show altered physiognomy and body weight loss. (A) Targeting strategy. Exon 4 of Cmas was flanked by loxP sites (Cmasfloxed). Podocyte-specific deletion of Cmas was achieved by expressing Cre recombinase under control of the Podocin (Nphs2) promoter. Forward (Fw) and reverse (Rev) primers for Cmas PCR analysis and product sizes are indicated. (B) Genotypic characterization of control and PCmas−/− mice. Genotypes were analyzed by PCR using genomic DNA isolated from kidney biopsy specimens sampled at P43 by the Fw/Rev primer pair (see [A]), enabling amplification of the floxed (1.06 kb) as well as the deleted (315 bp) Cmas allele (left-hand side of the ruler; Cmas− allele is highlighted in a red box). The PCR to amplify the Cre allele (372 bp) was supplemented with a control primer pair (PST Fw/PST Rev) to confirm DNA quality, giving rise to a 530 bp fragment. Specificity of PCR reactions (“PCR control”) was confirmed by using genomic DNA from mouse tail biopsy samples as template. Murine Cmas−/− ES cells (−/−) were used as template to demonstrate the size of the Cmas− allele (“PCR control”). (C) Physiognomy of PCmas−/− and littermate control mice (top) and kidneys (bottom) at P46. (D) Time course of body weight of control (blue, n=5) and PCmas−/− (red, n=5) mice. Circle, female; triangle, male.
PCmas−/− Mice Develop Progressive Proteinuria and Die Due to Renal Dysfunction
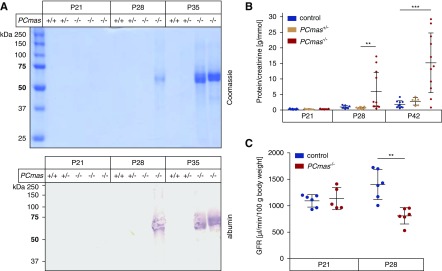
Mice of all genotypes were born in the expected Mendelian ratio. From birth until postnatal week 3 PCmas−/− mice were indistinguishable from control littermates, but showed growth retardation and body weight loss starting from postnatal day (P) 28 (Figure 1, C and D). Mutant mice died between postnatal weeks 6 and 8. Proteinuria started around P28 in PCmas−/− mice (Figure 2A) with temporally increasing severity. The urinary protein-to-creatinine ratio was at wild-type level in PCmas−/− samples until P21, but increased up to 30-fold at P42 (Figure 2B). The GFR was normal at P21 but significantly reduced at P28 in PCmas−/− mice, indicating damage of the filtering units (Figure 2C). Heterozygous mice always appeared unaffected (Figure 2, A and B).
Figure 2.
PCmas−/− mice develop proteinuria around P28. (A) SDS-PAGE followed by Coomassie Blue staining (top) and western blot analysis (bottom) of urine samples from control (+/+), heterozygous (+/−), and PCmas−/− (−/−) mice at P21, P28, and P35. Antigen-antibody binding was detected by BCIP/NBT color reaction. (B) Protein-to-creatinine ratio of urine samples from control, heterozygous, and PCmas−/− mice at P21, P28, and P42. Per genotype and time point, 6–11 individual samples were measured and tested for significance with a Kruskal–Wallis test and Dunn’s multiple comparison test (** P<0.01, ***P<0.001). (C) GFR of control and PCmas−/− mice was measured at P21 and P28 and calculated from the t1/2 of FITC-sinistrin. Per group, 5–6 animals were tested and significance was determined by unpaired t test (** P<0.01).
PCmas−/− Mice Develop FSGS
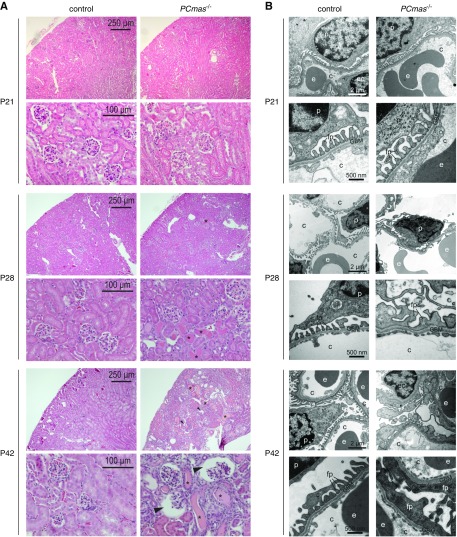
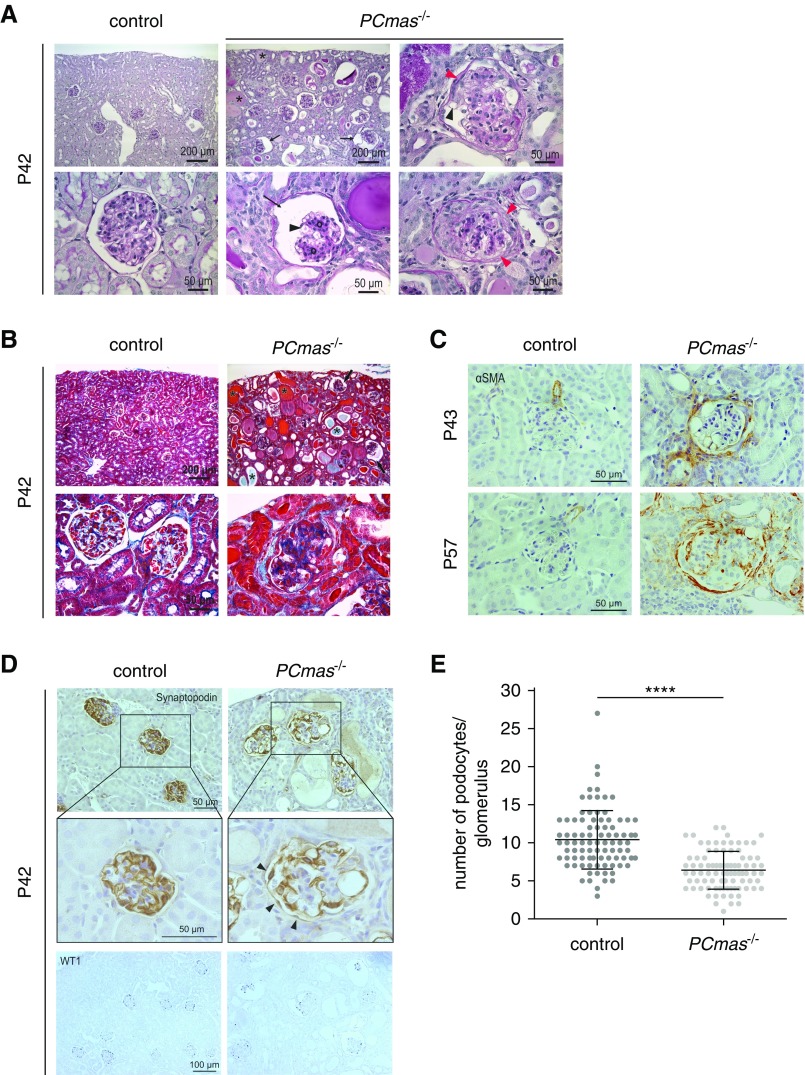
Light microscopic analysis of hematoxylin and eosin–stained kidney sections revealed no significant histologic and ultrastructural differences between different genotypes at P21 (Figure 3, A and B). At P28, glomeruli still showed wild-type morphology in PCmas−/− kidney sections, but lumina of some proximal and distal tubules were filled with eosinophilic material (Figure 3A). At ultrastructural level, podocyte FP effacement was accompanied by a reduction in mature SDs at P28, resulting in podocyte FP fusion, complete loss of SDs, and microvillous transformation at P42 (Figure 3B). Interestingly, neither endothelial fenestration nor GBM morphology was affected at any time point (Figure 3B). At light microscopic level, PAS and azan staining revealed mesangial matrix expansion (Figure 4, A and B) with increased collagen deposition (Figure 4B) and increased mesangial cell content (Figure 4A) in PCmas−/− kidney sections compared with control mice at P42. Mesangial cell activation in mutant mice was confirmed by staining of α–smooth muscle actin, which was not only detected in smooth muscle cells of the blood vessels, as observed in control mice, but also in the center of the glomerular tuft (P57) and in Bowman’s capsule (P42 and P57) (Figure 4C). Multifocal adhesions as well as complete fusion of the glomerular tuft to Bowman’s capsule (Figure 4B), and both dilatation (Figure 4B) and narrowing of glomerular capillary lumen (Supplemental Figure 2), were seen in PCmas−/− kidney sections at P42. Morphologic alterations were most prominent at P42 (Figures 3 and 4). However, although some glomeruli appeared severely affected, others showed no pathologic signs. Taken together, the histologic kidney phenotype of PCmas−/− mice strongly resembles that of human FSGS.
Figure 3.
Onset of proteinuria is associated with podocyte injury in PCmas−/− mice. (A) Hematoxylin and eosin staining of sections from paraffin-embedded renal tissue of control and PCmas−/− mice sampled at P21, P28, and P42. Asterisks, protein casts; arrowheads, enlarged Bowman’s space. (B) Ultrastructural comparison of the glomerular filtration barrier of control and PCmas−/− mice at P21, P28, and P42 assessed by transmission electron microscopy. c, capillary lumen; e, erythrocyte; ec, endothelial cell; fp, foot process; p, podocyte.
Figure 4.
PCmas−/− mice develop FSGS with podocyte loss. (A) Periodic acid–Schiff and (B) Azan staining of sections of paraffin-embedded renal tissue of control and PCmas−/− mice sampled at P42. Arrows, enlarged Bowman’s space; asterisks, dilated tubules with protein casts; black arrowheads, expanded capillaries; circles, increased mesangial content; red arrowhead, synechiae of the glomerular tuft and parietal cells of Bowman’s capsule. Immunohistochemical staining of (C) α–smooth muscle actin (αSMA) as a marker for activated mesangial cells as well as (D) the podocyte markers synaptopodin (top) and WT1 (bottom) in paraffin-embedded renal sections from control and PCmas−/− mice at P42, P43, or P57, respectively, and visualized by DAB color reaction. In PCmas−/− glomeruli, not only visceral but also parietal epithelial cells express synaptopodin (arrowheads). (E) Number of podocytes per glomerulus in control and PCmas−/− mice. WT1-positive cells were counted in renal sections from control and PCmas−/− at P42. Per genotype, three individuals, and per individual, 30 glomeruli were evaluated (n=90). Significance was determined by unpaired t test (**** P<0.0001).
To investigate whether loss of Sia on the podocyte cell surface led to podocyte depletion, we analyzed the expression of the podocyte marker proteins synaptopodin and Wilms tumor protein (WT1) in P42 kidney sections and quantified WT1-positive cells (Figure 4D). In wild-type glomeruli synaptopodin was exclusively detected in visceral epithelial cells, but in PCmas−/− sections it was additionally expressed in parietal epithelial cells lining the inside of Bowman’s capsule. However, counting of WT1-positive cells in the glomerular tuft revealed a significantly reduced number of podocytes per glomerulus in PCmas−/− mice compared with control mice (Figure 4E).
Loss of Sialylation Precedes Onset of Proteinuria in PCmas−/− Mice
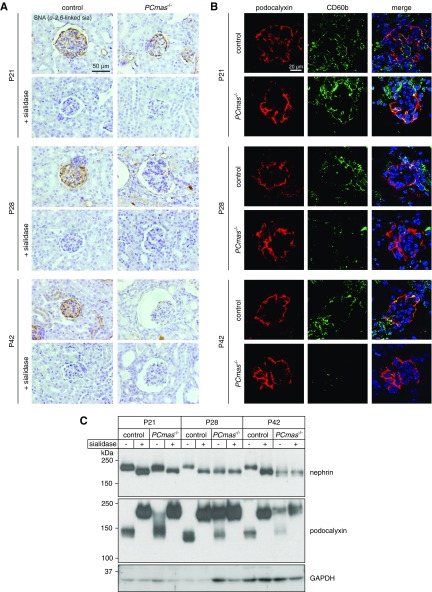
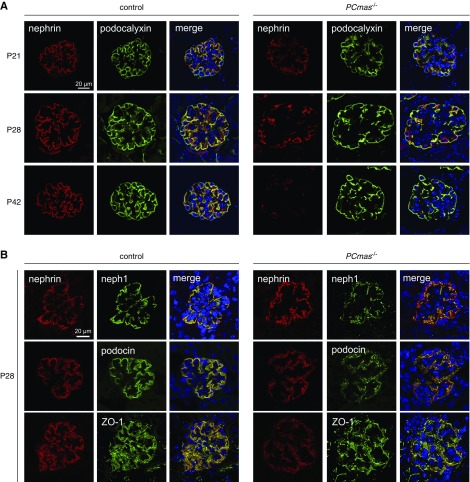
We monitored loss of CMAS at functional level by staining cell-surface sialylation with the Sambucus nigra lectin (SNA), specific for α-2,6–linked Sia (Figure 5A). Specificity of the lectin staining was confirmed by neuraminidase treatment resulting in removal of Sia. Glomeruli were highly sialylated in both genotypes at P21, whereas loss of Sia on podocytes became apparent at P28 and was prominent at P42 in PCmas−/− mice. Interestingly, at P28, juxtamedullary glomeruli of the cortex in mutant mice already showed defective podocyte sialylation whereas those in subcapsular areas were still sialylated (Supplemental Figure 3). Because glycolipids frequently get lost during processing of paraffin-embedded sections, SNA staining (Figure 5A) mainly illustrates sialylated glycoproteins. Glycolipid sialylation was investigated by monitoring the expression of the disialo-ganglioside CD60b26,27 in lipid-preserving frozen renal sections (Figure 5B). Costaining with podocalyxin identified the presence of disialo gangliosides in control glomeruli at all time points, whereas loss of these epitopes became visible around P28 in PCmas−/− glomeruli, which is in line with the SNA staining (Figure 5A). We next analyzed the sialylation status of the two sialoglycoproteins that were shown to be important determinants in the development of a renal phenotype in Cmasnls mice,14 the SD component nephrin and the mucin podocalyxin (Figure 5C). Corresponding with immunohistochemical results, time-lapse analysis of nephrin sialylation in PCmas−/− kidney lysates revealed a loss of sialylation from P28 on, whereas incipient hyposialylation of podocalyxin was already visible at P21 (Figure 5C, lower panel). In line with the observed podocyte loss, the protein amounts of nephrin and podocalyxin were reduced in mutant mice at P42 (Figures 5C and 6A). Whereas podocalyxin—although heavily hyposialylated—was regularly located at the cell surface even in severe disease state at P42, an altered localization pattern was observed for nephrin at P28 in PCmas−/− glomeruli with severely reduced expression level at P42 (Figure 6A).
Figure 5.
Loss of sialylation on glycoproteins and glycolipids precedes development of proteinuria in PCmas−/− mice. (A) Histochemical staining of paraffin-embedded kidney sections sampled at P21, P28, and P42 labeled with Sia-specific biotinylated lectin SNA. (B) Expression pattern of the disialo-ganglioside CD60b analyzed by immunofluorescence staining of frozen kidney sections sampled at P21, P28, and P42. (C) Analysis of the sialylation of nephrin and podocalyxin at P21, P28, and P42 by immunoblot. Adjusted protein amounts of whole-kidney lysates were separated by SDS-PAGE before (−) and after (+) sialidase treatment. Of note, after sialidase treatment of control kidney homogenates, asialo podocalyxin showed a slower migration behavior in SDS-PAGE (approximately 200 kDa) compared with the sialylated form (approximately 140 kDa), a phenomenon also known from other mucin-type proteins due to loss of negative charge (Takeda et al.76). For both depicted immunoblots, the same kidney lysates were applied. GAPDH staining served as loading control.
Figure 6.
Localization of asialo-podocalyxin is preserved in vivo, whereas nephrin appears mislocalized along with other SD components at P28 and dramatically reduced at P42. (A) Analysis of the localization of nephrin (red) and podocalyxin (green) in control and PCmas−/− mice by indirect immunofluorescent staining on sections of paraffin-embedded renal tissue sampled at P21, P28, and P42. Sections were costained with specific antibodies against nephrin and podocalyxin and visualized with fluorophore-conjugated secondary antibody. Nuclei were stained with DAPI. (B) Indirect immunofluorescent costaining of nephrin (red) with SD proteins neph1 and ZO-1 (green) on paraffin-embedded and podocin (green) on frozen kidney sections of control and PCmas−/− mice sampled at P28.
Asialo-Nephrin Is Mislocalized along with Other SD Proteins in PCmas−/− Mice
Because mislocalization of nephrin has also been observed in human biopsy specimens28–31 and animal models of nephrotic syndrome,32–35 and defects in trafficking have been associated with kidney disease development,36,37 we further investigated nephrin’s intracellular fate in vivo by using indirect immunofluorescence costaining with marker proteins of different cellular compartments (Supplemental Figure 4). Compared with control mice, slightly enhanced colocalization was observed with markers of the secretory pathway (giantin and VEGF) as well as the endocytic marker clathrin (Supplemental Figure 4, B–D), but neither with caveolin nor with markers for early endosomes and lysosomes (Supplemental Figure 4, E–G). Because immunohistologic analyses do not allow illustration of nephrin dynamics, nephrin turnover was investigated in sialylation-competent wild-type and asialo CMAS−/− HEK 293 cells (Supplemental Figure 5). However, in these cellular models no differences in nephrin cell-surface presentation and endocytosis could be identified, thus underpining the importance of studying these processes in complex tissues, because trafficking might depend on the cell type and multiple other factors, most importantly the crosstalk of glomerular cells with their environment. Next, we interrogated the localization of other SD components. NEPH1, podocin, and zonula occludens-1 (ZO-1) colocalized with and thereby followed mislocalization of asialo-nephrin at P28 in PCmas−/− mice, indicating that the localization of other SD proteins is also affected in mutant mice (Figure 6B).
Sialylation Appears Crucial for Podocyte Outgrowth and Adhesion In Vitro
To investigate the role of sialylation for podocyte homeostasis in vitro, we aimed to generate primary Cmas-knockout podocytes from glomeruli of immortalized PCmas−/− (imPCmas−/−) mice. As expected, imPCmas−/− mice showed a renal phenotype identical to PCmas−/− mice (Supplemental Figure 6). Cell islets grown from PCmas−/− and control glomeruli both exhibited cobblestone-like morphology and expressed the podocyte markers WT1 and synaptopodin, respectively (Supplemental Figure 7A). However, irrespective of the mouse genotype, only the Cmasfloxed allele was identified in all isolated podocyte clones, whereas the Cmas-knockout allele was never found (Supplemental Figure 7B). Although we could confirm the presence of the Cmas-knockout allele in PCmas−/− glomeruli immediately after isolation (Supplemental Figure 7C), a Cmas-negative podocyte cell line could not be obtained from PCmas−/− glomerular outgrowth in a series of independent experiments using transgenic mice of different ages (P24–P34) and analyzing a total of about 100 cell clones.
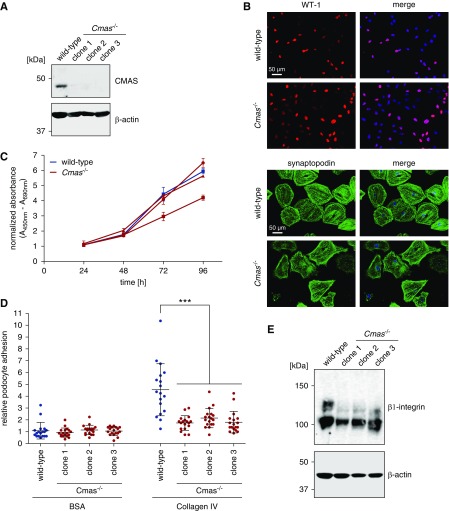
In a second attempt, we applied the CRISPR/Cas9 technology on immortalized wild-type podocytes and obtained three individual CMAS-knockout clones. Successful deletion was confirmed by sequence analysis of the targeted genomic locus (Supplemental Figure 8) and lack of CMAS protein in podocyte lysates (Figure 7A). Independent of the genotype, all cell lines expressed the podocyte marker WT1 and their capacity to differentiate in vitro was confirmed by cell morphology and synaptopodin expression (Figure 7B). Analysis of cell proliferation over a time period of 3 days did not reveal significant differences between asialo-podocytes and wild-type cells (Figure 7C). Because Sia is located at the outermost part of the cell surface and podocyte cell matrix adhesion is crucial for maintaining the filtration barrier in vivo, we also analyzed whether loss of cell-surface sialylation influences cell adhesion to the major GBM components collagen IV and laminin 521 in vitro. Importantly, CMAS-knockout podocytes showed significantly decreased adhesion to collagen when compared with wild-type (Figure 7D). In contrast, adhesion to laminin remained unchanged (Supplemental Figure 9). As expected, we found β1-integrin, which is part of the heterodimeric principal integrin receptors mediating podocyte-GBM interactions, to be hyposialylated in PCmas−/− podocytes (Figure 7E; indicated by the mass shift in western blot). Moreover, the expression level of the mature β1-integrin glycoform38 (Figure 7E, upper band) seemed to be reduced, indicating that sialylation is crucial for stability, turnover, and function of this protein in podocytes.
Figure 7.
Cmas−/− podocytes generated by use of CRISPR/Cas9 technology are able to differentiate but show impaired adhesion to collagen IV in vitro. (A) Western blot analysis of CMAS expression in wild-type and Cmas−/− podocyte cell lines. Actin staining served as loading control. (B) Indirect immunofluorescence staining of podocyte lines grown under permissive (WT1) and nonpermissive (synaptopodin) conditions. Exemplarily, one CMAS-knockout cell line is shown. (C) Cell proliferation of wild-type and Cmas−/− podocytes was assessed in a WST-1 assay over a time period of 3 days by colorimetric quantification of formazan dye produced by metabolically active cells. One experiment representative of three independent experiments is shown. (D) Cell adhesion assay of wild-type and Cmas−/− podocytes to GBM component collagen IV and BSA as control. Three individual experiments were conducted with each six technical replicates per cell line. Significance was determined by unpaired t test (*** P<0.001). (E) Western blot analysis of β1-integrin expression in wild-type and Cmas−/− podocyte cell lines. The upper band represents the mature, and the lower band the immature glycoform of the protein. Actin staining served as loading control.
Discussion
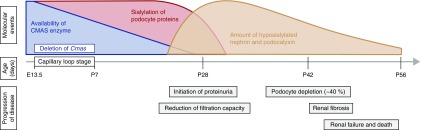
To unequivocally decipher the role of sialoglycans in podocytes in vivo, we generated a mouse model with podocyte-specific depletion of sialylation. Although podocin-promotor–driven Cre expression is first observed in immature podocytes of early capillary loop stage glomeruli (embryonic day 13.5 to P7),19 loss of sialylation was not observed until P21 in PCmas−/− mice, followed by onset of proteinuria within 1 week and renal failure before P60 (Figure 8). The unexpected late morbidity and mortality of PCmas−/− mice is likely on the basis of the long t1/2 of the CMAS protein in connection with the t1/2 of podocyte cell-surface proteins. This explication is supported by a recent study reporting a nephrin t1/2 of about 3 weeks in vivo, estimated by use of inducible podocyte-specific deletion of nephrin in a mature glomerulus.39 A time-lapse study carried out with tissue sections isolated at P21–P42 demonstrated that loss of Sia on the podocyte cell surface in PCmas−/− mice triggered a cascade of consecutive events: First, we observed podocyte FP effacement and stress, similar to the situation described in minimal change disease, followed by detachment and successive loss of injured podocytes. Podocyturia has also been reported in a number of human glomerulopathies and experimental models of kidney disease and was found to correlate with disease progression.40–42 These primary podocyte defects in PCmas−/− mice provoked secondary injuries, most prominent activation of mesangial cells43 and injury of glomerular capillaries,44,45 giving rise to phenotypic traits highly reminiscent of human FSGS. We also noticed synechiae formation by activated parietal epithelial cells, a process that can be considered an attempt to restore podocyte numbers.46,47 Our data indicate that minimal change disease and FSGS might represent different stages of the same disease, as suggested by Maas and colleagues.48 In PCmas−/− mice, the distribution of nephrin changed over time: from a capillary loop pattern of sialylated nephrin to a granular pattern of asialo-nephrin, followed by a reduction of overall nephrin expression level. Because sialoglycans are known to regulate glycoprotein trafficking,49 we made use of the PCmas−/− mouse model to analyze the fate of nephrin under pathologic conditions. Enhanced (but still fragmentary) distribution of asialo-nephrin in vesicles of the secretory and endocytic pathway portend altered turnover of incomplete glycosylated protein. However, we also still observed colocalization with other SD components, indicating that mislocalization can likewise be attributed to altered overall FP morphology. Whether nephrin mislocalization in human biopsy specimens also reflects altered turnover and FP morphology needs further investigation. In contrast to nephrin, podocalyxin was not mislocalized in PCmas−/− podocytes, confirming the reports of unaltered surface presentation of podocalyxin at the apical podocyte membrane after sialidase or PAN treatment of rats.50 However, uncoupling of rat podocalyxin from the actin cytoskeleton after sialidase treatment, as reported by Farquhar and colleagues,51 might also occur and contribute to FP effacement in PCmas−/− mice. Moreover, although related to steroid-sensitive rather than steroid-resistant nephrotic syndrome, hyposialylation of the soluble podocyte glycoprotein angiopoietin-like-4 has also been found to induce proteinuria and thereby might contribute to disease development in our mouse model.52,53 Because all cell-surface glycoconjugates in PCmas−/− mice lack sialylation, it seems allowable to speculate that the PCmas−/− phenotype results from a combination of different causes. Successful generation and biochemical analysis of immortalized asialo podocyte cell lines illustrated that sialylation per se is neither essential for viability nor for proliferation or differentiation in vitro. Supporting these results, we have recently demonstrated that other sialylation-deficient cell lines—including pluripotent mESC—are viable, proliferate, and even differentiate into the three germ layers and beating cardiomyocytes.17 Reduced binding of asialo-podocytes to collagen IV demonstrated the importance of sialylation for podocyte adhesion to the GBM and might also contribute to lack of sialo-podocytes in outgrowth of PCmas−/− glomeruli. Sialylation-dependent adhesion to collagen IV but not laminin has similarly been observed with human colon carcinoma but not with breast cancer cells,54,55 indicating a cell-type–specific characteristic. Although podocytes express a variety of cell matrix adhesion receptors, including α-dystroglycan and heparansulfate-proteoglycans, adhesion to collagen IV and laminin is mainly mediated by the integrin family, with the most abundant α3,β1-integrin (preferentially binding laminin) and α1,β1-integrin (preferentially binding collagen IV).56 The reduced β1-integrin expression level in asialo-podocytes, together with reported conformational changes of β1-integrin upon desialylation in silico,57 suggests that sialylation is crucial for integrin heterodimer assembly, clustering, activation, or association with other transmembrane proteins, e.g. tetraspanins. A contribution of impaired podocyte GBM adhesion to FSGS development is further underlined by the finding that patients with mutations in integrin genes,58,59 as well as mouse models with disrupted integrin assembly or signaling, develop FSGS.60–62 In addition, more recent work correlated defects in genes encoding enzymes of the glycosylation pathway with the development of nephrotic syndrome.63–65 These latter studies dissect specific roles of single compounds of the multifarious glycosylation machinery for glomerular filtration. However, only 30% of hereditary and 10%–20% of sporadic cases of steroid-resistant nephrotic syndrome can be attributed to gene mutations, whereas for the majority of cases the cause is still unknown.66,67
Figure 8.
PCmas−/− mice develop a progressive renal phenotype. During the capillary loop stage of glomerulogenesis (embryonic day [E] 13.5 to P7) the Cmas gene is deleted exclusively in podocytes, which leads to a reduced availability and subsequently to a complete lack of the enzyme. Presumably due to a long t1/2 of the CMAS protein in combination with long t1/2 of cell-surface glycoconjugates, loss of sialylation started around P21 and precedes development of proteinuria and FP effacement. At first, PCmas−/− mice develop a phenotype similar to patients suffering from minimal change disease (around P28), proceeding to the FSGS phenotype (around P42).
Taking into account that human podocytes are highly sialylated68 and reduced glomerular sialylation has been reported in patients with proteinuric nephrotic syndrome,69 our data support the notion that loss of sialylation might be part of the complex process causing FSGS. Thus, it is tempting to speculate that next-generation sequencing used in the diagnosis of steroid-resistant nephrotic syndrome will identify mutations in genes involved in sialylation or other glycosylation pathways. Furthermore, Sia presentation might be disturbed by genetic aberrations resulting in increased endogenous glomerular sialidase expression, as observed in membranous glomerulopathy,70 infection (by germs utilizing sialidases)72 and medication.71 Therefore, analysis of the sialylation pattern in biopsy specimens of patients with unexplained idiopathic nephrotic syndrome by staining with Sia-specific lectins may expand the diagnostic tool box. To exclude species-specific differences in the biologic role of sialylation for human glomerular function, detailed analyses of human biopsy specimens under physiologic and pathologic conditions are currently under investigation. Importantly, kidney failure and early postnatal death of GneM712T mice could be rescued by administration of Sia or its metabolic precursor, N-Acetylmannosamine,13,73,74 and a dietary Sia supplementation therapy has successfully improved muscle function in patients suffering from hereditary inclusion body myopathy.75 Our data indicate that a Sia supplementation therapy might also contribute to delayed CKD progression and a decrease in the prevalence of ESRD.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank Rita Gerardy-Schahn for continuous support and helpful discussions and Prof. Lawrence Holzman, University of Pennsylvania, Philadelphia for kindly providing the podocin-Cre mouse. Kerstin Flächsig-Schulz and Ulrike Bernard are acknowledged for expert technical assistance.
Mr. Wedekind received a scholarship from the German Academic Scholarship Foundation and Ms. Schmitz and Dr. Bräsen received funding from the Dr. Werner Jackstädt Foundation. This work was supported by funds from the German Research Foundation to Dr. Weinhold and Dr. Münster-Kühnel (WE 5585/1-1 and MU 1849/2-1).
Dr. Weinhold and Dr. Münster-Kühnel designed the study; Ms. Niculovic, Dr. Blume, Mr. Wedekind, Ms. Beuke, Ms. Albers, Ms. Kats, and Dr. Groos carried out experiments; Ms. Niculovic, Dr. Blume, Mr. Wedekind, Ms. Beuke, Dr. Melk, Dr. Groos, Dr. Abeln, Ms. Schmitz, Dr. Bräsen, Dr. Schiffer, and Dr. Münster-Kühnel analyzed the data; Ms. Niculovic, Dr. Blume, Mr. Wedekind, and Dr. Weinhold made the figures; Ms. Niculovic, Mr. Wedekind, Dr. Weinhold, and Dr. Münster-Kühnel drafted and revised the paper; all authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018090951/-/DCSupplemental.
Supplemental Methods 1. Histology.
Supplemental Methods 2. Cell-surface biotinylation and EndoH treatment in HEK 293 cells.
Supplemental Table 1. Antibodies and lectins.
Supplemental Table 2. Primers.
Supplemental Figure 1. Biosynthesis of sialylated glycoconjugates.
Supplemental Figure 2. Obliteration of glomerular capillary lumen in PCmas−/− kidney sections.
Supplemental Figure 3. Loss of sialylation in PCmas−/− kidney sections.
Supplemental Figure 4. Mislocalized nephrin is partially associated to compartments of the secretory and endocytic pathway.
Supplemental Figure 5. Biochemical characterization of wild-type and CMAS−/− HEK 293 cells stably expressing nephrin-Flag and analysis of nephrin trafficking and t1/2 in these cell lines.
Supplemental Figure 6. imPCmas−/− mice develop a phenotype identical to PCmas−/− mice.
Supplemental Figure 7. Outgrowth of imPCmas−/− glomeruli proves to be wild-type podocytes.
Supplemental Figure 8. Generation of immortalized murine Cmas−/− podocyte cell lines by use of CRISR/Cas9 technology.
Supplemental Figure 9. In vitro adhesion assay (laminin).
References
- 1.Kitiyakara C, Eggers P, Kopp JB: Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis 44: 815–825, 2004 [PubMed] [Google Scholar]
- 2.Kitiyakara C, Kopp JB, Eggers P: Trends in the epidemiology of focal segmental glomerulosclerosis. Semin Nephrol 23: 172–182, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Haas M, Spargo BH, Coventry S: Increasing incidence of focal-segmental glomerulosclerosis among adult nephropathies: A 20-year renal biopsy study. Am J Kidney Dis 26: 740–750, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Han MH, Kim YJ: Practical application of Columbia classification for focal segmental glomerulosclerosis. BioMed Res Int 2016: 9375753, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Campbell KN, Tumlin JA: Protecting podocytes: A key target for therapy of focal segmental glomerulosclerosis. Am J Nephrol 47[Suppl 1]: 14–29, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satchell S: The role of the glomerular endothelium in albumin handling. Nat Rev Nephrol 9: 717–725, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Haraldsson B, Nyström J, Deen WM: Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88: 451–487, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Padberg JS, Wiesinger A, di Marco GS, Reuter S, Grabner A, Kentrup D, et al.: Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis 234: 335–343, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Salmon AH, Satchell SC: Endothelial glycocalyx dysfunction in disease: Albuminuria and increased microvascular permeability. J Pathol 226: 562–574, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Jeansson M, Björck K, Tenstad O, Haraldsson B: Adriamycin alters glomerular endothelium to induce proteinuria. J Am Soc Nephrol 20: 114–122, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW: Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol 18: 2885–2893, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Galeano B, Klootwijk R, Manoli I, Sun M, Ciccone C, Darvish D, et al.: Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Invest 117: 1585–1594, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinhold B, Sellmeier M, Schaper W, Blume L, Philippens B, Kats E, et al.: Deficits in sialylation impair podocyte maturation. J Am Soc Nephrol 23: 1319–1328, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelberg H, Healy L, Whiteley H, Miller LA, Vimr E: In vivo enzymatic removal of α2→6-linked sialic acid from the glomerular filtration barrier results in podocyte charge alteration and glomerular injury. Lab Invest 74: 907–920, 1996 [PubMed] [Google Scholar]
- 16.Kanwar YS, Farquhar MG: Detachment of endothelium and epithelium from the glomerular basement membrane produced by kidney perfusion with neuraminidase. Lab Invest 42: 375–384, 1980 [PubMed] [Google Scholar]
- 17.Abeln M, Borst KM, Cajic S, Thiesler H, Kats E, Albers I, et al.: Sialylation is dispensable for early murine embryonic development in vitro. ChemBioChem 18: 1305–1316, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abeln M, Albers I, Peters-Bernard U, Flächsig-Schulz K, Kats E, Kispert A, et al.: Sialic acid is a critical fetal defense against maternal complement attack. J Clin Invest 129: 422–436, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, et al.: Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci U S A 88: 5096–5100, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schock-Kusch D, Geraci S, Ermeling E, Shulhevich Y, Sticht C, Hesser J, et al.: Reliability of transcutaneous measurement of renal function in various strains of conscious mice. PLoS One 8: e71519, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, et al.: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al.: Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–823, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Münster AK, Weinhold B, Gotza B, Mühlenhoff M, Frosch M, Gerardy-Schahn R: Nuclear localization signal of murine CMP-Neu5Ac synthetase includes residues required for both nuclear targeting and enzymatic activity. J Biol Chem 277: 19688–19696, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Lewandoski M, Wassarman KM, Martin GR: Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol 7: 148–151, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Baumann AM, Bakkers MJ, Buettner FF, Hartmann M, Grove M, Langereis MA, et al.: 9-O-Acetylation of sialic acids is catalysed by CASD1 via a covalent acetyl-enzyme intermediate. Nat Commun 6: 7673, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kniep B, Flegel WA, Northoff H, Rieber EP: CDw60 glycolipid antigens of human leukocytes: Structural characterization and cellular distribution. Blood 82: 1776–1786, 1993 [PubMed] [Google Scholar]
- 28.Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, et al.: Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol 158: 1723–1731, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper ME, Mundel P, Boner G: Role of nephrin in renal disease including diabetic nephropathy. Semin Nephrol 22: 393–398, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Hingorani SR, Finn LS, Kowalewska J, McDonald RA, Eddy AA: Expression of nephrin in acquired forms of nephrotic syndrome in childhood. Pediatr Nephrol 19: 300–305, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Wernerson A, Dunér F, Pettersson E, Widholm SM, Berg U, Ruotsalainen V, et al.: Altered ultrastructural distribution of nephrin in minimal change nephrotic syndrome. Nephrol Dial Transplant 18: 70–76, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Saran AM, Yuan H, Takeuchi E, McLaughlin M, Salant DJ: Complement mediates nephrin redistribution and actin dissociation in experimental membranous nephropathy. Kidney Int 64: 2072–2078, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Dijkman HB, Gerlofs-Nijland ME, van der Laak JA, Wetzels JF, Groenen PJ, Assmann KJ: Podocyte changes after induction of acute albuminuria in mice by anti-aminopeptidase A mAb. Nephron, Exp Nephrol 94: e85–e93, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Luimula P, Ahola H, Wang SX, Solin ML, Aaltonen P, Tikkanen I, et al.: Nephrin in experimental glomerular disease. Kidney Int 58: 1461–1468, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Yuan H, Takeuchi E, Taylor GA, McLaughlin M, Brown D, Salant DJ: Nephrin dissociates from actin, and its expression is reduced in early experimental membranous nephropathy. J Am Soc Nephrol 13: 946–956, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Nishibori Y, Liu L, Hosoyamada M, Endou H, Kudo A, Takenaka H, et al.: Disease-causing missense mutations in NPHS2 gene alter normal nephrin trafficking to the plasma membrane. Kidney Int 66: 1755–1765, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Doné SC, Khoshnoodi J, Bertorello A, Wartiovaara J, Berggren PO, et al.: Defective nephrin trafficking caused by missense mutations in the NPHS1 gene: Insight into the mechanisms of congenital nephrotic syndrome. Hum Mol Genet 10: 2637–2644, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Shen X, Hong MS, Moss J, Vaughan M: BIG1, a brefeldin A-inhibited guanine nucleotide-exchange protein, is required for correct glycosylation and function of integrin β1. Proc Natl Acad Sci U S A 104: 1230–1235, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verma R, Venkatareddy M, Kalinowski A, Li T, Kukla J, Mollin A, et al.: Nephrin is necessary for podocyte recovery following injury in an adult mature glomerulus. PLoS One 13: e0198013, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, et al.: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Meyer TW, Bennett PH, Nelson RG: Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42: 1341–1344, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, et al.: Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Jefferson JA, Shankland SJ: The pathogenesis of focal segmental glomerulosclerosis. Adv Chronic Kidney Dis 21: 408–416, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reidy K, Kaskel FJ: Pathophysiology of focal segmental glomerulosclerosis. Pediatr Nephrol 22: 350–354, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim BJ, Yang JW, Do WS, Fogo AB: Pathogenesis of focal segmental glomerulosclerosis. J Pathol Transl Med 50: 405–410, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Wingert RA: Regenerative medicine for the kidney: Stem cell prospects & challenges. Clin Transl Med 2: 11, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kietzmann L, Guhr SS, Meyer TN, Ni L, Sachs M, Panzer U, et al.: MicroRNA-193a regulates the transdifferentiation of human parietal epithelial cells toward a podocyte phenotype. J Am Soc Nephrol 26: 1389–1401, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF: Minimal change disease and idiopathic FSGS: Manifestations of the same disease. Nat Rev Nephrol 12: 768–776, 2016 [DOI] [PubMed] [Google Scholar]
- 49.Kitazume S, Imamaki R, Ogawa K, Komi Y, Futakawa S, Kojima S, et al.: α2,6-sialic acid on platelet endothelial cell adhesion molecule (PECAM) regulates its homophilic interactions and downstream antiapoptotic signaling. J Biol Chem 285: 6515–6521, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerjaschki D, Vernillo AT, Farquhar MG: Reduced sialylation of podocalyxin—the major Sialoprotein of the rat kidney glomerulus—in aminonucleoside nephrosis. Am J Pathol 118: 343–349, 1985 [PMC free article] [PubMed] [Google Scholar]
- 51.Takeda T, McQuistan T, Orlando RA, Farquhar MG: Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 108: 289–301, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clement LC, Macé C, Avila-Casado C, Joles JA, Kersten S, Chugh SS: Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med 20: 37–46, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clement LC, Avila-Casado C, Macé C, Soria E, Bakker WW, Kersten S, et al.: Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med 17: 117–122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgenthaler J, Kemmner W, Brossmer R: Sialic acid dependent cell adhesion to collagen IV correlates with in vivo tumorigenicity of the human colon carcinoma sublines HCT116, HCT116a and HCT116b. Biochem Biophys Res Commun 171: 860–866, 1990 [DOI] [PubMed] [Google Scholar]
- 55.Kemmner W, Morgenthaler J, Brossmer R: Alterations in cell surface carbohydrate composition of a human colon carcinoma cell line affect adhesion to extracellular matrix components. Biochimie 74: 117–122, 1992 [DOI] [PubMed] [Google Scholar]
- 56.Pozzi A, Zent R: Integrins in kidney disease. J Am Soc Nephrol 24: 1034–1039, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan D, Song Y: Role of altered sialylation of the I-like domain of β1 integrin in the binding of fibronectin to β1 integrin: Thermodynamics and conformational analyses. Biophys J 99: 208–217, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Has C, Spartà G, Kiritsi D, Weibel L, Moeller A, Vega-Warner V, et al.: Integrin α3 mutations with kidney, lung, and skin disease. N Engl J Med 366: 1508–1514, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nicolaou N, Margadant C, Kevelam SH, Lilien MR, Oosterveld MJ, Kreft M, et al.: Gain of glycosylation in integrin α3 causes lung disease and nephrotic syndrome. J Clin Invest 122: 4375–4387, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, et al.: β1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol 316: 288–301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanasaki K, Kanda Y, Palmsten K, Tanjore H, Lee SB, Lebleu VS, et al.: Integrin β1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol 313: 584–593, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, et al.: Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol 175: 33–39, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Altassan R, Witters P, Saifudeen Z, Quelhas D, Jaeken J, Levtchenko E, et al.: Renal involvement in PMM2-CDG, a mini-review. Mol Genet Metab 123: 292–296, 2018 [DOI] [PubMed] [Google Scholar]
- 64.Sinha MD, Horsfield C, Komaromy D, Booth CJ, Champion MP: Congenital disorders of glycosylation: A rare cause of nephrotic syndrome. Nephrol Dial Transplant 24: 2591–2594, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Harshman LA, Ng BG, Freeze HH, Trapane P, Dolezal A, Brophy PD, et al.: Congenital nephrotic syndrome in an infant with ALG1-congenital disorder of glycosylation. Pediatr Int (Roma) 58: 785–788, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bierzynska A, Soderquest K, Koziell A: Genes and podocytes - new insights into mechanisms of podocytopathy. Front Endocrinol (Lausanne) 5: 226, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trautmann A, Lipska-Ziętkiewicz BS, Schaefer F: Exploring the clinical and genetic spectrum of steroid resistant nephrotic syndrome: The PodoNet registry. Front Pediatr 6: 200, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faraggiana T, Malchiodi F, Prado A, Churg J: Lectin-peroxidase conjugate reactivity in normal human kidney. J Histochem Cytochem 30: 451–458, 1982 [DOI] [PubMed] [Google Scholar]
- 69.Blau EB, Haas JE: Glomerular sialic acid and proteinuria in human renal disease. Lab Invest 28: 477–481, 1973 [PubMed] [Google Scholar]
- 70.Vogtländer NP, van der Vlag J, Bakker MA, Dijkman HB, Wevers RA, Campbell KP, et al.: Expression of sialidase and dystroglycan in human glomerular diseases. Nephrol Dial Transplant 25: 478–484, 2010 [DOI] [PubMed] [Google Scholar]
- 71.Nicol BM, Prasad SB: Sialic acid changes in Dalton’s lymphoma-bearing mice after cyclophosphamide and cisplatin treatment. Braz J Med Biol Res 35: 549–553, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Corfield T: Bacterial sialidases—roles in pathogenicity and nutrition. Glycobiology 2: 509–521, 1992 [DOI] [PubMed] [Google Scholar]
- 73.Ito M, Sugihara K, Asaka T, Toyama T, Yoshihara T, Furuichi K, et al.: Glycoprotein hyposialylation gives rise to a nephrotic-like syndrome that is prevented by sialic acid administration in GNE V572L point-mutant mice. PLoS One 7: e29873, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kakani S, Yardeni T, Poling J, Ciccone C, Niethamer T, Klootwijk ED, et al.: The Gne M712T mouse as a model for human glomerulopathy. Am J Pathol 180: 1431–1440, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu X, Wang AQ, Latham LL, Celeste F, Ciccone C, Malicdan MC, et al.: Safety, pharmacokinetics and sialic acid production after oral administration of N-acetylmannosamine (ManNAc) to subjects with GNE myopathy. Mol Genet Metab 122: 126–134, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takeda T, Go WY, Orlando RA, Farquhar MG: Expression of podocalyxin inhibits cell-cell adhesion and modifies junctional properties in Madin–Darby canine kidney cells. Mol Biol Cell 11: 3219–3232, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.