Significance Statement
In approximately 70%–80% of cases of primary membranous nephropathy (MN), phospholipase A2 receptor (PLA2R)/Thrombospondin Type-1 Domain–Containing 7A (THSD7A) and anti-PLA2R/THSD7A antibodies form immune complexes along the glomerular basement membrane (GBM) that characterize the condition. In other cases of primary MN and all secondary MN, the target antigen is unknown. Using proteomics and immunohistochemistry, the authors detected two proteins, exostosin 1 (EXT1) and exostosin 2 (EXT2), in the GBM of PLA2R-negative MN. EXT1 and EXT2 were absent in all cases of PLA2R-associated MN and controls. Clinical and biopsy findings showed features of autoimmune disease, including membranous lupus nephritis, in 81% of the 26 EXT1/EXT2-associated MN cases the authors identified. These findings suggest that EXT1/EXT2-associated MN represents a distinct subtype of MN, most commonly associated with autoimmune diseases (secondary MN).
Keywords: Immunology and pathology, membranous nephropathy, nephrotic syndrome
Abstract
Background
In membranous nephropathy (MN), which is characterized by deposition of immune complexes along the glomerular basement membrane (GBM), phospholipase A2 receptor (PLA2R) and thrombospondin type 1 domain–containing 7A are target antigens in approximately 70% and 1%–5% of cases of primary MN, respectively. In other cases of primary MN and in secondary MN, the target antigens are unknown.
Methods
We studied 224 cases of biopsy-proven PLA2R-negative MN and 102 controls (including 47 cases of PLA2R-associated MN) in pilot and discovery cohorts. We also evaluated 48 cases of PLA2R-negative presumed primary MN and lupus MN in a validation cohort. We used laser microdissection and mass spectrometry to identify new antigens, which were localized by immunohistochemistry.
Results
Mass spectrometry detected exostosin 1 (EXT1) and exostosin 2 (EXT2) in 21 cases of PLA2R-negative MN, but not in PLA2R-associated MN and control cases. Immunohistochemistry staining revealed bright granular GBM staining for EXT1 and EXT2. Clinical and biopsy findings showed features of autoimmune disease, including lupus, in 80.7% of the 26 EXT1/EXT2-associated MN cases we identified. In the validation cohort, we confirmed that EXT1/EXT2 staining was detected in pure class 5 lupus nephritis (eight of 18 patients) and in presumed primary MN associated with signs of autoimmunity (three of 16 patients); only one of the 14 cases of mixed class 5 and 3/4 lupus nephritis was positive for EXT1/EXT2. Tests in seven patients with EXT1/EXT2-associated MN found no circulating anti-exostosin antibodies.
Conclusions
A subset of MN is associated with accumulation of EXT1 and EXT2 in the GBM. Autoimmune disease is common in this group of patients.
Membranous nephropathy (MN) is the commonest cause of nephrotic syndrome in white adults and results because of formation of antigen-antibody immune complexes in the subepithelial region of the glomerular basement membrane (GBM).1 It is characterized by thickening of the GBM on light microscopy, bright granular staining for IgG and C3 along the glomerular capillary walls on immunofluorescence microscopy, and subepithelial GBM electron dense deposits on electron microscopy. Depending on the cause, MN is classified as primary or secondary MN, which account for 75%–80% and 20%–25% of MN, respectively.2 M-type phospholipase A2 receptor (PLA2R) and Thrombospondin Type-1 Domain–Containing 7A (THSD7A) were identified as target antigens in primary MN,3–5 and account for approximately 70%–80% and 1%–5% of primary MN, respectively.6,7 Secondary MN is associated with autoimmune diseases, malignancies, infections, and drugs.2 The target antigen in PLA2R- and THSD7A-negative MN and in secondary MN has remained elusive. The aim of this study was to determine the antigen in these cases of MN. Building on our expertise in mass spectrometry characterization and discovery of new types of amyloid, complement, and other glomerular deposits, we reasoned that we could identify new MN antigens using the same approach.
Methods
Patients
We initially selected a pilot cohort of seven cases of PLA2R-associated MN and 15 cases of PLA2R-negative MN on kidney biopsy for mass spectrometry studies. We detected unique proteins exostosin 1 (EXT1) and exostosin 2 (EXT2) in five cases of PLA2R-negative MN by mass spectrometry that we confirmed by immunohistochemistry (IHC) (Figure 1). Subsequently, we analyzed an additional 209 PLA2R-negative MN cases including eight cases of PLA2R-negative membranous lupus nephritis by IHC for EXT1 and EXT2 staining. For controls, we used 95 cases that included: Ten cases of day zero protocol transplant kidney biopsy samples that were normal on kidney biopsy sample examination, 40 cases of PLA2R-associated MN, eight cases of minimal change disease, 12 cases of FSGS (six primary and six secondary), five cases of IgA nephropathy, seven cases of diabetic nephropathy, and 13 cases of proliferative lupus nephritis without a membranous component which included two, two, and nine cases of class 2, 3, and 4 lupus nephritis, respectively. Subsequently, we confirmed the cases that were positive for EXT1/EXT2 on IHC by mass spectrometry studies (Figure 1).
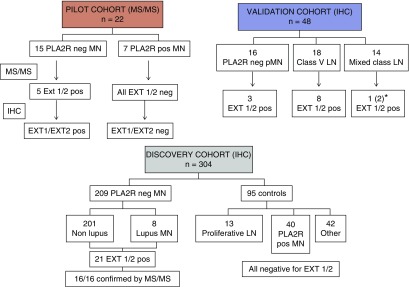
Figure 1.
Flowchart of the pilot, discovery, and validation cohorts. Initial pilot studies were done by mass spectrometry studies using 15 PLA2R-negative MN and seven PLA2R-positive MN cases. After detection of EXT1/EXT2 in five cases confirmed by IHC, we studied a large number (n=209) of PLA2R-negative MN cases and controls (n=95) for expression of EXT1/EXT2 by IHC. Controls included 13 cases of proliferative lupus nephritis (LN) without a membranous component and 40 cases of PLA2R-positive MN. Finally, we studied 48 cases of MN in a validation cohort that included PLA2R-negative primary MN (pMN), membranous (class 5) LN, and mixed class LN with a membranous component. *One case (patient #16) started with pure MN with signs of autoimmunity and then shifted to mixed class. Neg, negative; pos, positive.
These biopsy specimens were received in the Renal Pathology Laboratory, Department of Laboratory Medicine and Pathology, Mayo Clinic, for diagnosis and interpretation between January of 2015 and May of 2018. Light microscopy, immunofluorescence microscopy including PLA2R studies, and electron microscopy were performed in each case of MN. The clinical information was obtained from the accompanying charts. The study was approved by the Mayo Clinic Institutional Review Board.
Protein Identification by Laser Capture Microdissection, Trypsin Digestion, and Nano LC-Orbitrap Tandem Mass Spectrometry
For each case, 10-μm-thick formalin-fixed paraffin-embedded (FFPE) sections were obtained and mounted on a special PEN membrane laser microdissection slide and, using a Zeiss Palm Microbeam microscope, the glomeruli were microdissected to reach approximately 250–500,000 μM2 per case. Resulting FFPE fragments were digested with trypsin and collected for tandem mass spectrometry (MS/MS) analysis. The trypsin-digested peptides were identified by nano-flow liquid chromatography electrospray MS/MS using a Thermo Scientific Q-Exactive Mass Spectrometer (Thermo Fisher Scientific, Bremen, Germany) coupled to a Thermo Ultimate 3000 RSLCnano HPLC system. All MS/MS samples were analyzed using Mascot and X! Tandem set up to search a Swissprot human database. Scaffold (version 4.8.3; Proteome Software Inc., Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted at >95.0% probability by the Scaffold Local FDR algorithm, with protein identifications requiring a two-peptide minimum and a 95% probability using Protein Prophet.8 Details of laser capture and MS/MS are given in the Supplemental Material.
Immunohistochemical Staining for EXT1, EXT2, and Exostosin like 2
FFPE tissues were sectioned at 5 μm and IHC staining was performed online using EXT1, EXT2, and Exostosin like 2 (EXTL2) primary antibodies and a Polymer Refine Detection System (Leica) that included hydrogen peroxidase block, post primary and polymer reagent, DAB, and hematoxylin staining steps. Details of IHC staining are given in the Supplemental Material. In addition, a single case (#16 from the validation cohort, see below) of EXT1/EXT2-associated MN was also confirmed by immunofluorescence microscopy on paraffin sections and analyzed by confocal microscopy.
Western and Native Blotting
Patient serum antibodies for EXT1, the heterodimer EXT1/EXT2, and PLA2R were detected by western and native blotting using standard procedures (see the Supplemental Material).
Validation Cohort
Forty-eight unstained kidney biopsy specimen slides of FFPE tissue were provided by Inserm UMR-S1155 (H.D. and P.R., Tenon Hospital, Paris) and analyzed in the Renal Pathology Laboratory, Mayo Clinic, where IHC for EXT1 and EXT2 was performed. The diagnosis of the biopsy samples was not known at the time of receiving the slides. Subsequently, after the staining, the breakdown of the MN was as follows: 18 cases belonged to class 5 membranous lupus nephritis, 14 cases were class 3/4 lupus nephritis with a component of lupus class 5 MN, and 16 were primary (nonlupus) cases of MN that were negative for both PLA2R and THS7DA (Figure 1).
Results
Mass Spectrometry Results
Detection of EXT1 and EXT2 in PLA2R-Negative Biopsy Specimens
We performed MS/MS studies in the five cases of the pilot cohort and in 16 of 21 cases of the discovery cohort that stained positive for EXT1/EXT2 by IHC (Figure 2). We identified high total spectral counts of both EXT1 and EXT2 in five cases of the pilot cohort and in all 16 cases of the discovery cohort. The average total spectral count for EXT1 was 65.3 (SD±34.6, median 71, range 11–155) and the average total spectral count for EXT2 was 83.4 (SD±38.4, median 83, range 19–160). MS/MS did not detect EXT-like (EXTL) proteins in any of the EXT1/EXT2-associated MN. Also, MS/MS showed only baseline spectral counts of PLA2R in EXT1/EXT2-associated MN. Both the average EXT1 and EXT2 total spectral numbers in EXT1/EXT2-associated MN were comparable to total spectral counts of PLA2R in PLA2R-associated cases. All control and PLA2R-associated MN cases were negative for EXT1 or EXT2 spectra (Supplemental Figure 2). MS/MS spectral matches to sequences from EXT1 and EXT2 are shown in Supplemental Figure 3.
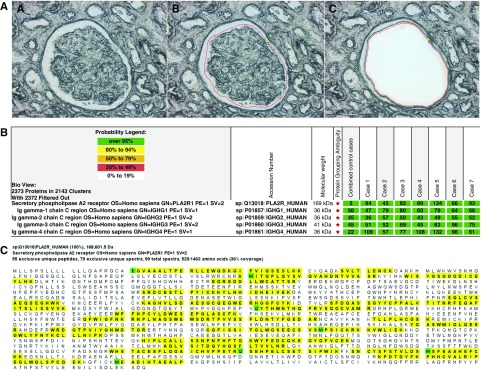
Figure 2.
Proteomic identification of PLA2R in PLA2R-associated MN and EXT1 and EXT2 in EXT1/EXT2-associated MN. Glomeruli were microdissected and analyzed using mass spectrometry as described in the Methods section. (A) Laser microdissection of glomeruli. One case of MN showing (A) unmarked glomerulus, (B) glomerulus marked for dissection, and (C) vacant space on the slide after microdissection. (B) PLA2R-associated MN: Protein identification report from seven cases is shown. Numbers in green boxes represent spectral counts of MS/MS matches to a respective protein. All seven cases show large total spectral counts for PLA2R and Ig. For comparison, the average total spectral counts from six control cases (day 0 protocol transplant biopsy specimens) are also shown. (C) Representative sequence coverage map of PLA2R from one case. Amino acids highlighted in bold letters over yellow background are the amino acids detected. Note the extensive coverage. Green highlighted boxes indicate amino acids with artifactual chemical modification induced by mass spectrometry such as oxidation of methionine. (D) EXT1/EXT2-associated MN: Protein identification from all 21 cases showing total spectral counts for both EXT1 and EXT2. For comparison, the average total spectral counts in six control cases (day 0 protocol transplant biopsy specimens) are also shown. IgG1 was the dominant IgG present. Higher spectral IgG counts in the control cases in the bottom panel compared with the top panel reflect normalization, with fewer EXT1/EXT2 cases in the lower panel compared with the top panel. (E and F) Sequence coverage maps: Representative EXT1 and EXT2 sequence coverage map from a case of EXT1/EXT2-associated MN showing the extensive amino coverage of both (E) EXT1 and (F) EXT2 by MS/MS. Amino acids highlighted in bold letters over yellow background are the amino acids detected. Note the extensive coverage. Green highlighted boxes indicate amino acids with artifactual chemical modification induced by mass spectrometry such as oxidation of methionine.
All four classes of Ig were detected in EXT1/EXT2-associated MN: IgG1 was the most abundant Ig (average 97.5, SD±35.9, median 106, range 32–173), followed by IgG2 (average 75, SD±29.5, median 77, range 23–124), IgG3 (average 74.4, SD±30.3, median 69, range 33–146), and IgG4 (average 70.8, SD±35.2, median 8, range 12–129). The average spectral count of IgG1 was higher than IgG4 in EXT1/EXT2-associated MN (P<0.01), and also when compared with the total spectral counts of IgG1 in the PLA2R-associated MN (P=0.04).
Detection of PLA2R in PLA2R-Associated MN
All seven cases showed large total spectral counts for PLA2R (Figure 2). The average PLA2R total spectra count was 86.1 (SD±27.5, median 89, range 45–134). In comparison, the average PLA2R spectral count in control cases and EXT1/EXT2-associated MN was only 7.1 (SD±5.2, median 8, range 0–19). IgG4 was the most abundant Ig (average spectral count 91.4, SD±27.6, median 96, range 47–132), followed by IgG1 (average 67.9, SD±12.3, median 66, range 47–80), IgG3 (average 64.4, SD±17.5, range 45–96), and IgG2 (average 48.9, SD±7.2, range 36–57).
Immunohistochemical Staining for EXT1 and EXT2
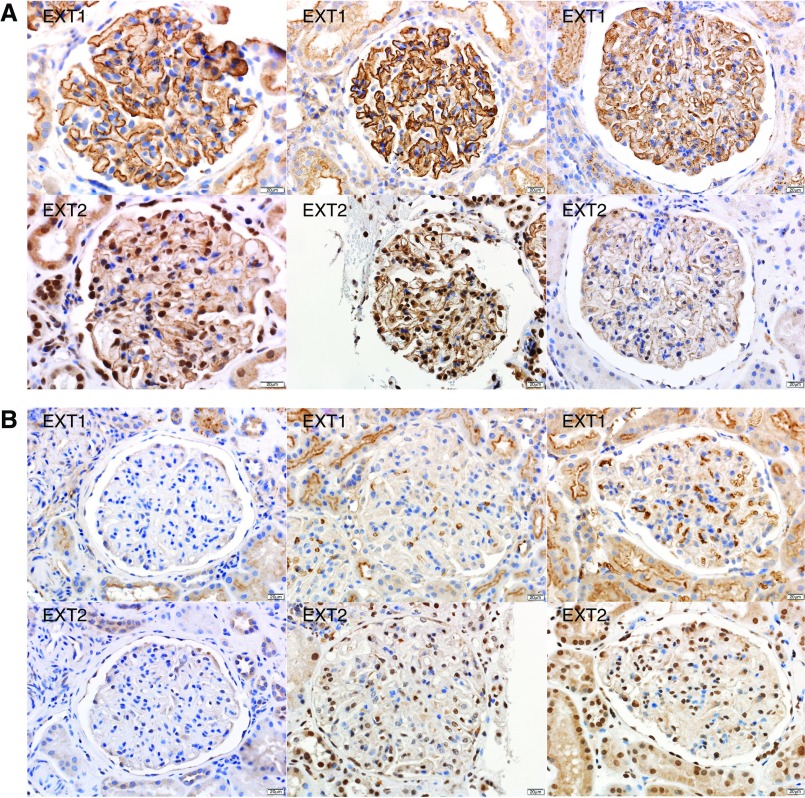
We performed IHC staining for EXT1 and EXT2 in 224 cases of PLA2R-negative MN (15 in the pilot and 209 in the discovery cohorts). Twenty-six (11.6%) cases were positive for EXT1 and EXT2 (five in the pilot and 21 in the discovery cohorts), whereas the remaining 198 (88.4%) were negative. All 26 positive cases showed bright (2–3+/3) granular staining for EXT1 and EXT2 along the GBM. Importantly, the staining was along the GBM with no significant mesangial staining. There was no staining along the Bowman’s capsule, tubular basement membranes, or in vessel walls. EXT1 and EXT2 staining in three cases is shown in Figure 3A. The positive granular staining mirrored the granular IgG along the GBM seen in each case. All 95 control cases were negative for both EXT1 and EXT2. Representative negative staining for EXT1 and EXT2 in three cases of PLA2R-associated MN is shown in Figure 3B. Figure 3C shows positive staining for EXT1 and EXT2 in a case of membranous lupus nephritis and negative staining in two cases of proliferative lupus nephritis. Representative EXT1 and EXT2 staining in the remaining EXT1/EXT2-associated MN and control cases is shown in Supplemental Figure 1.
Figure 3.
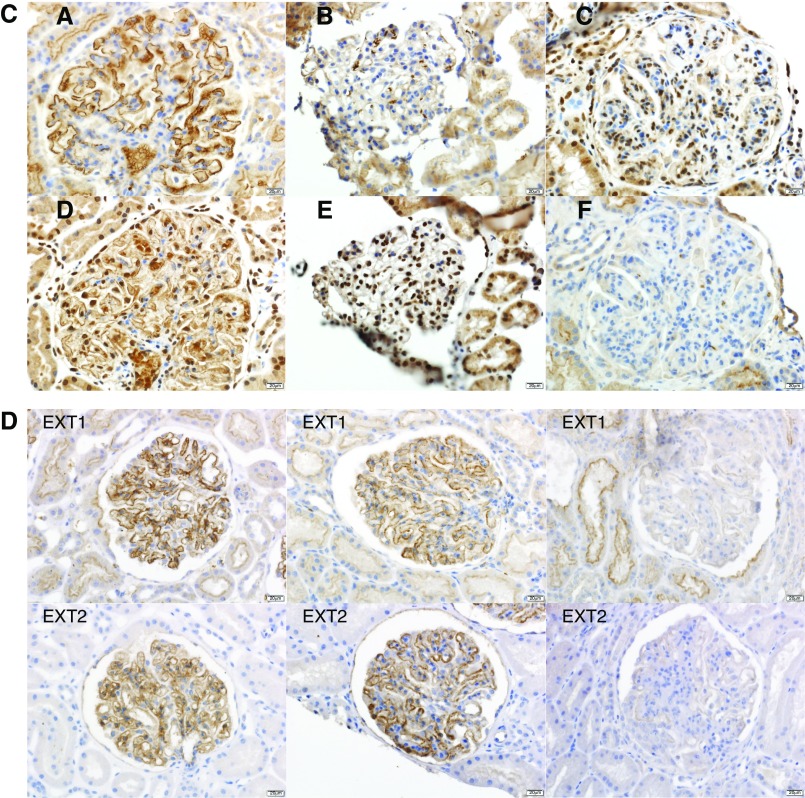
Immunohistochemical stain for EXT1 and EXT2 in EXT1/EXT2-associated MN, PLA2R-associated MN, and lupus nephritis. Each column is one case with top panel showing EXT1 and bottom panel showing EXT2 staining. (A) EXT1/EXT2-associated MN: Bright granular staining for EXT1 and EXT2 along the GBMs in three cases of EXT1/EXT2-associated MN. The first column is case 4, second column is case 7, and third column is case 25. (B) PLA2R-associated MN: Negative staining for EXT1 and EXT2 in three cases of PLA2R-associated MN. (C) Lupus nephritis: Bright positive staining for EXT1 and EXT2 in a case (case 26) of membranous lupus nephritis (column 1) and negative staining for EXT1 and EXT2 in a case of class 2 lupus nephritis (column 2) and class 4 lupus nephritis (column 3). (D) Validation cohort: Bright positive staining for EXT1 and EXT2 in two cases of membranous class 5 lupus nephritis and negative staining in one case of membranous class 5 lupus nephritis (note the focal proliferative features in this case).
Clinical and Kidney Biopsy Findings of EXT1/EXT2-Associated MN
We identified 26 cases of EXT1/EXT2-associated MN on the basis of IHC. There were 21 (80.8%) female and five (19.2%) male patients with a ratio of 4.2:1. The mean age at presentation was 35.7 (SD±13.4). The mean serum creatinine and proteinuria at presentation were 1.0 mg/dl (SD±0.9) and 5.9 g/24 h (SD±4.8), respectively. Seventeen (70.8%, n=24) had abnormal laboratory values for either anti-nuclear antibodies, anti–double-stranded DNA antibodies, anti-Smith antibodies, anti–Sjögren syndrome–related antigen A or B (SSA or SSB), or anti-ribonucleoprotein antibodies. Nine (34.6%) patients had a clinical diagnosis of SLE. None of the patients had hepatitis. One patient had a history of breast cancer and one had lung cancer.
The kidney biopsy specimens of all cases of EXT1/EXT2-associated MN showed the characteristic findings of thickened GBM on light microscopy, bright IgG and C3 staining along the capillary wall on immunofluorescence microscopy, and subepithelial deposits on electron microscopy. Overall, an average of 22 (SD±14.4) glomeruli were present, of which 2.3 (SD±3.9) were globally sclerosed. Immunofluorescence microscopy showed bright staining for IgG (2–3+/3) and C3 (2–3+/3) in all cases. Twenty-two (84.6%) of 26 cases also showed staining for IgA (1–3+/3) or IgM (1–3+/3) or both. Nineteen (73.0%) of 26 cases showed staining for C1q that ranged from 1+/3 to 3+/3. All cases showed staining for κ (2–3+/3) and λ (2–3+/3) light chains. Immunofluorescence study for PLA2R was negative in all cases. Electron microscopy showed subepithelial deposits in all cases, subendothelial deposits in nine (34.6%), and mesangial deposits in 25 (96.1%) of 26 cases. Tubuloreticular inclusions were present in 21 (80.7%) of the 26 cases. The clinical and pathology findings are shown in Tables 1 and 2.
Table 1.
Clinical and laboratory data of EXT1/EXT2-associated MN
| Case | Age/Sex/Ethnicity | Rash/Arthritis/Other | Serum Cr, mg/dl | Proteinuria, g/24 h | C3/C4 | ANA/dsDNA Other | Lupus | Hepatitis/Malignancy |
|---|---|---|---|---|---|---|---|---|
| 1 | 41/M/white | −/−/− | 1.1 | 12.0 | N/N | −/− | No | −/− |
| 2 | 32/F | −/− | 0.7 | 5.0 | N/N | +/+ | Yes | −/− |
| 3 | 60/M/Hispanic | −/−/− | 1.9 | 20.0 | N/N | −/− | No | −/− |
| 4 | 20/F/native American | −/−/− | 0.5 | 8.0 | N/N | −/− | No | −/− |
| 5 | 59/F/white | −/+/sicca | 0.6 | 5.7 | N/L | +/− SSA+, SSB+ | No | −/Breast cancer |
| 6 | 29/F/white | −/−/− | 0.8 | 6.0 | N/N | +/− | No | −/− |
| 7 | 19/F/Indian-Hispanic | −/+/− | 0.6 | 2.0 | N/N | +/− | No | −/− |
| 8 | 30/M/black | −/−/− | 0.7 | 13.0 | N/N | −/− | No | −/− |
| 9 | 55/F | −/+/− | 0.7 | 6.0 | N/N | −/− | No | −/SCC lung |
| 10 | 39/F/Indian | −/−/− | 0.5 | 3.0 | ND | +/− SSA+, SSB+ | No | −/− |
| 11 | 30/F/white | −/−/− | 0.5 | 8.4 | N/N | +/− | No | −/− |
| 12 | 32/F/black | −/−/− | 3.2 | 15.9 | L/N | +/− | No | −/− |
| 13 | 51/F/white | −/+/− | 0.7 | 3.0 | N/N | +/+ SSA+ | Noa | −/− |
| 14 | 21/F/Hispanic | −/−/lymphadenopathy | 1.7 | 11 | N/N | −/− | No | −/− |
| 15 | 34/F/black | −/+/− | 0.7 | 5.1 | N/N | +/− SSA+, SSB+ | Noa | −/− |
| 16 | 31/F/black | −/+/allergies | 0.7 | 2.2 | N/N | −/− | No | −/− |
| 17 | 17/M/white | +/+/myositis | 0.9 | NR | N/N | +/+/+anti-Smith, + RNPb | SLE/MCTD overlap | −/− |
| 18 | 25/F | +/+/ | 0.8 | NA | N/L | +/+/+SSA+ anti-Smith | Yes | −/− |
| 19 | 32/F/white | −/+/fibromyalgia | 0.6 | 11 | ND | +/+ | Yes | −/− |
| 20 | 67/F/black | — | 1.1 | 7.8 | ND | −/− | No | −/− |
| 21 | 32/F/Hispanic | −/−/pericardial effusion | 0.4 | 3 | L/L | −/− | No (treated as lupus) | −/− |
| 22 | 38/M | −/+ | 4.6 | 5 | N/N | +/+ | Yes | −/− |
| 23 | 20/F | −/+/− | 0.7 | 3 | ND | +/+ | Yes | −/− |
| 24 | 36/F/Asian | +/−/− | 0.9 | 6 | N/L | +/+ | Yes | −/− |
| 25 | 43/F/black | −/+/− | 0.7 | 2 | L/L | +/+ | Yes | −/− |
| 26 | 34/F | +/+/pleurisy | 0.9 | 2.2 | N/N | +/+/+ anti-Smith | Yes | −/− |
ANA, anti-nuclear antibody; dsDNA, anti–double-stranded DNA antibody; M, male; N, normal; F, female; L, low; SCC, squamous cell carcinoma; NR, nephrotic range, five males 20 females; NA, not available; ND, no data/data not available.
Mixed connective tissue disorder.
Anti-Smith/ribonucleoprotein antibody.
Table 2.
Kidney biopsy findings of EXT1/EXT2-associated MN
| Case | Glomeruli/Sclerosed | Mesangial or Endocapillary Hypercellularity | Interstitial Inflammation/IFTA | Arteries | Immunofluorescence Microscopy | Electron Microscopy Deposits SE/SU/ME | Tubuloreticular Inclusion |
|---|---|---|---|---|---|---|---|
| 1 | 10/0 | Not present | 0/0 | Moderate sclerosis | IgG (3+) C1q (1+) C3 (3+) | +/−/+ | + |
| 2 | 22/0 | Not present, two small crescents | 0/0 | Normal | IgG (3+) IgA (3+) IgM (1+) C1q (2+) C3 (3+) | +/−/+ | + |
| 3 | 8/2 | Not present | 0/0 | Normal | IgG (2+) IgM (1+) C3 (1+) | +/−/− | – |
| 4 | 38/0 | Not present | 0/0 | Normal | IgG (3+) IgM (1+) C3 (2+) | +/−/+ | + |
| 5 | 19/1 | Not present | 0/0 | Normal | IgG (3+) IgA (1+) IgM (1+) C1q (1+) C3 (3+) | +/+/+ | − |
| 6 | 7/0 | Not present | 0/0 | Normal | IgG (3+) IgA (2+), C1q (1+) C3 (2+) | +/+/+ | + |
| 7 | 64/0 | Not present | 0/0 | Normal | IgG (3+) C3 (1+) | +/+/+ | + |
| 8 | 23/1 | Not present | 0/10 | Normal | IgG (3+) IgA (2+) IgM (1+) C1q (2+) C3 (3+) | +/+/+ | + |
| 9 | 26/6 | Not present | 0/0 | Normal | IgG (3+) IgM (1+) C3 (2+) | +/−/+ | − |
| 10 | 48/4 | Not present | 0/10 | Normal | IgG (3+) IgA (2+) IgM (1+) C1q (1+) C3 (2+) | +/−/+ | + |
| 11 | 29/5 | Not present | 0/0 | Normal | IgG (2+) IgM (1+) C3 (3+) | +/−/+ | − |
| 12 | 54/18 | Not present | 10/30 | Mild sclerosis | IgG (3+) IgM (1+) C1q (1+) C3 (3+) | +/−/a | + |
| 13 | 18/1 | Not present | 0/0 | Normal | IgG (3+) IgA (2+) IgM (1+) C1q (2+) C3 (3+) | +/−/+ | + |
| 14 | 12/0 | Not present, three small crescents | 0/20 | Normal | IgG (3+) IgM (1+) C1q (2+) C3 (3+) | +/−/+a | + |
| 15 | 12/2 | Not present | 25/25 | Normal | IgG (3+) IgM (1+) C1q (1+) C3 (3+) | +/−/+a | + |
| 16 | 23/1 | Not present | 0/0 | Normal | IgG (3+) C3 (3+) | +/−/+ | + |
| 17 | 17/0 | Not present | 0/0 | Normal | IgG (3+) IgA (2+) C1q (1+) C3 (3+) | +/−/+ | + |
| 18 | 4/0 | Not present | 0/0 | Normal | IgG (3+) IgM (2+) C1q (1+) C3 (3+) | +/+/+ | + |
| 19 | 14/0 | Not present | 0/0 | Normal | IgG (2+) C3 (2+) | +/−/- | − |
| 20 | 18/2 | Not present | 0/0 | Normal | IgG (3+) IgA (2+) IgM (1+) C1q (2+) C3 (3+) | +/−/+ | + |
| 21 | 20/1 | Not present | 0/0 | Normal | IgG (3+) IgA (1+) IgM (1+) C1q (2+) C3 (3+) | +/−/+ | + |
| 22 | 17/9 | Not present | 0/80 | Severe sclerosis | IgG (3+) IgM (2+) C1q (1+) C3 (3+) | +/+/+ | + |
| 23 | 14/0 | Present | 10/30 | Normal | IgG (3+) IgA (3+) IgM (3+) C1q (2+) C3 (3+) | +/+/+ | + |
| 24 | 19/2 | Not present | 0/0 | Normal | IgG (3+) IgA (3+) IgM (3+) C1q (2+) C3 (3+) | +/+/+a | + |
| 25 | 27/4 | Present | 0/10 | Moderate | IgG (3+) IgM (1+) C1q (2+) C3 (3+) | +/+/+ | + |
| 26 | 16/1 | Not present | 0/25 | Normal | IgG (3+) IgM (1+) C1q (2+) C3 (2+) | +/−/+ | + |
IFTA, interstitial fibrosis and tubular atrophy; SE, subepithelial; SU, subendothelial; ME, mesangial.
Tubular basement membrane deposits.
Validation Cohort
IHC
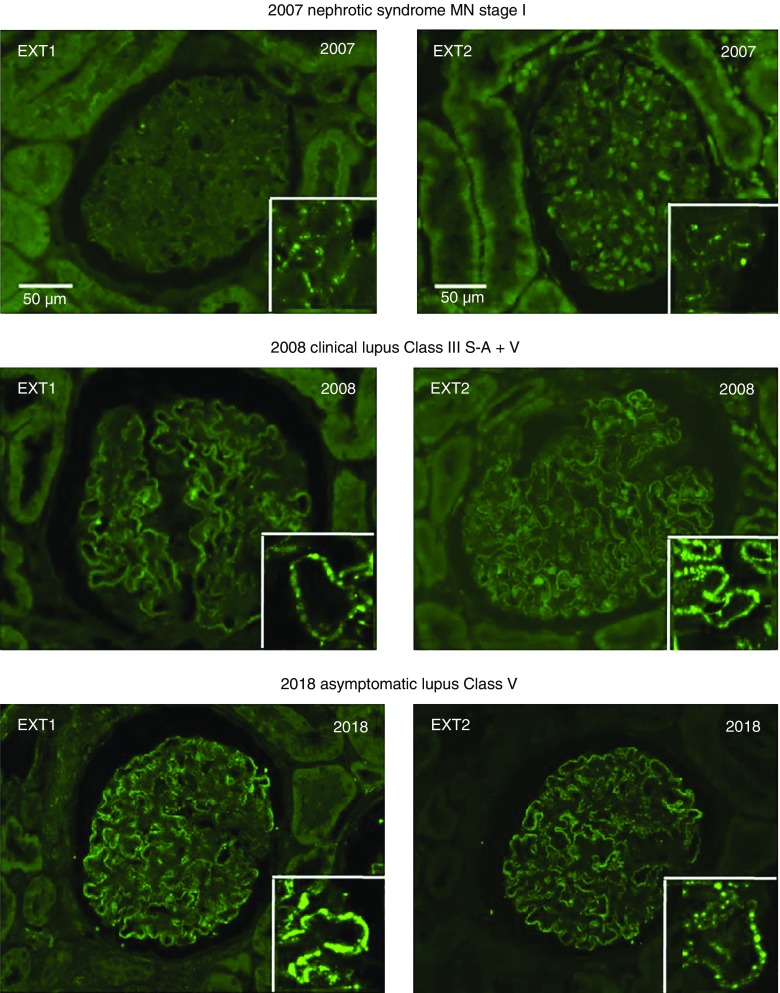
Eight of 18 (44%) cases of lupus class 5 membranous nephritis were positive for EXT1 and EXT2 staining along the GBM, whereas only one of the 14 cases of mixed class 5 and class 3/4 lupus nephritis was positive for EXT1 and EXT2 along the GBM. Three (19%) of 16 cases of PLA2R- and THS7DA-negative and nonlupus MN cases were positive for EXT1 and EXT2 staining along the GBM. These three cases had features of autoimmune disease on chart review. Patients #14 and #16 are of particular interest because they both later developed a full-blown lupus. In patient #14, the initial diagnosis was MN stage 1–2 with an immunofluorescence pattern of primary MN; 6 years later, she developed full-blown clinical lupus but the lesions and immunofluorescence pattern were unchanged. Patient #16 also had a presentation and an immunofluorescence pattern of primary MN, but she developed a year later very active clinical lupus disease with a mixed pattern of class 3 plus 5. She was referred 10 years later for nephrotic syndrome with asymptomatic lupus and class 5 MN on biopsy (Figure 4). In the two patients mentioned above, EXT1/EXT2 staining remained positive throughout evolution and histologic classes. The clinical and laboratory findings of the EXT1/EXT2-associated MN of the validation cohort are given in Supplemental Tables 1 and 2. Representative EXT1 and EXT2 staining of positive cases is shown in Figure 3D.
Figure 4.
EXT1 and EXT2 staining by immunofluorescence microscopy of a case of MN that was initially weakly positive for EXT1/EXT2 and subsequently turned strongly positive at the time of development of clinical lupus. The inserts show the EXT1 and EXT2 deposits by confocal microscopy. The year of the biopsy and the clinical and histologic diagnoses are indicated above each picture.
Western and Native Blotting
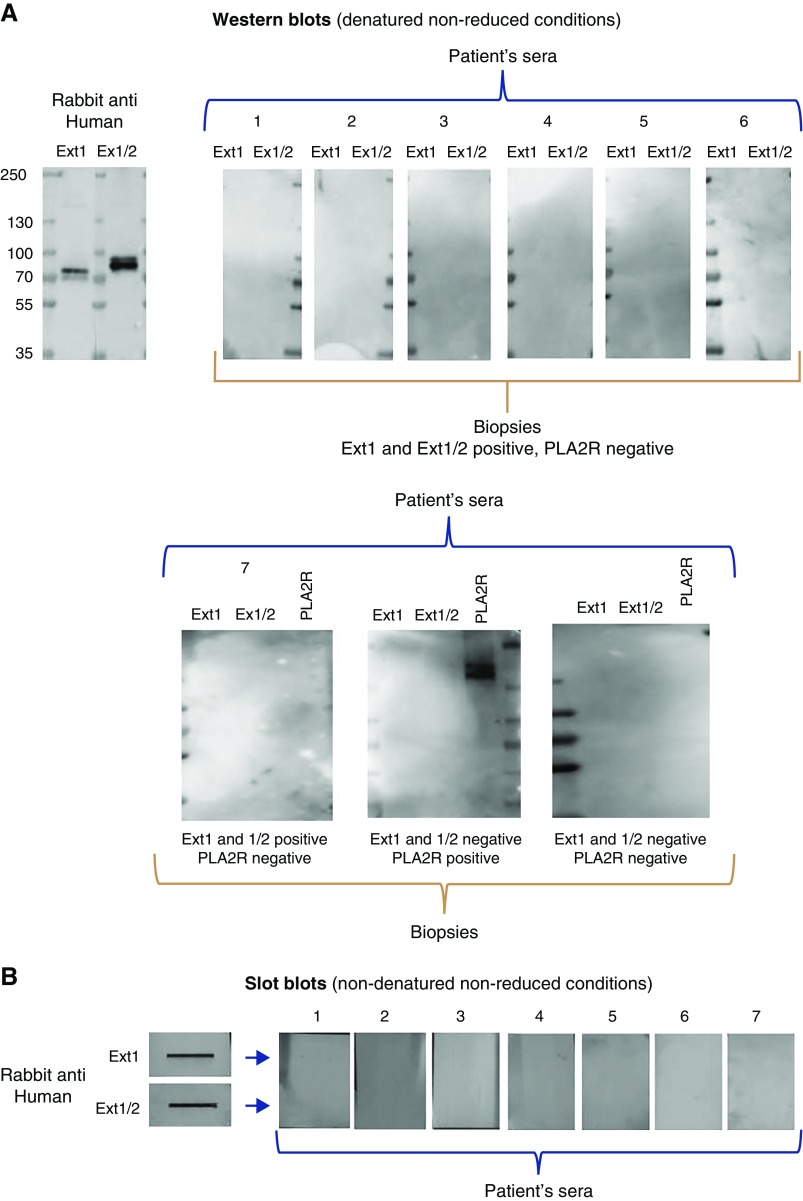
We then searched for circulating antibodies to EXT1 and the heterodimer EXT1/EXT2 in seven EXT1/EXT2-positive patients with available sera (one from the discovery and six from the validation cohorts), and in one control patient with glomerular PLA2R deposits. Western blotting was performed in SDS gels under nonreducing (Figure 5A) and reducing (not shown) conditions. Despite strong reactivity of the recombinant protein with a control rabbit anti-human EXT1 and EXT2 serum, we failed to detect any reactivity with the seven tested sera, as well as with one serum from a patient with PLA2R-related MN. In contrast, the serum from this patient strongly reacted with a 185-kd band when it was incubated with the PLA2R antigen.
Figure 5.
Western and native blotting analyses of sera from patients with EXT1/EXT2 glomerular deposits and a patient with PLA2R-related MN. (A) Western blots (denatured, nonreduced conditions). The upper panel shows lack of reactivity with recombinant EXT1 and heterodimer EXT1/EXT2 of six sera from patients with EXT1- and EXT2-positive biopsies, contrasting with strong reactivity of the rabbit anti-human EXT1 and EXT2. Here are shown five of six available sera from the validation cohort; the last serum on the right is the only one available from the discovery cohort. The sixth available serum from the validation cohort is shown in the lower panel (left). The lower panel shows reactivity of sera from three patients with or without EXT1 and EXT1/2 deposits or PLA2R deposits in the kidney biopsy specimens. For this western blot, EXT1, EXT1/2, and PLA2R recombinant antigens were run in three different, consecutive lanes before transfer, and the blots were then incubated with each of the patient sera. Note the strong reactivity with PLA2R of the serum from PLA2R-related MN and lack of reactivity with EXT1 and EXT1/2 of the three sera. (B) Slot blots (nondenaturing, nonreduced conditions). Slot blotting analysis confirms lack of reactivity in nondenaturing conditions with recombinant EXT1 and EXT1/2 protein of seven sera from patients with EXT1- and EXT2-positive biopsy specimens.
To investigate the presence of epitopes sensitive to denaturation by SDS, native blotting method was used. However, none of the seven tested sera showed reactivity with recombinant EXT1 and EXT1/2 by this method (Figure 5B). We concluded that, under our experimental conditions, we were unable to detect circulating anti-EXT1/EXT2 antibodies.
Discussion
MN is the most common cause of nephrotic syndrome in white adults. It results from accumulation of target antigens in the GBM and subsequent formation of antigen-antibody complexes. On the basis of identification of the target antigen, MN is also classified as PLA2R-positive, THSD7A-positive, or PLA2R/THSD7A double-negative, which represent approximately 20% of MN. Although PLA2R- and THSD7A-associated MN usually present as primary disease, the double-negative MN is often associated with autoimmune manifestations including SLE. The target antigen in the double-negative MN has remained elusive. In this study, we have used an original combination of laser microdissection, MS/MS, and IHC techniques to identify two novel proteins, EXT1 and EXT2, that accumulate along the GBM in a subset of PLA2R-negative MN. The staining pattern mirrors the granular IgG staining along the GBM, indicating that EXT1 and EXT2 are the likely antigens in the immune complexes. Most importantly, the clinical features and kidney biopsy findings show that EXT1/EXT2-associated MN is mostly present in patients with autoimmune manifestations or diseases including lupus. Taken together, our findings show that EXT1 and EXT2 represent novel biomarker proteins and possibly the target antigens in secondary (autoimmune) MN.
Laser microdissection with mass spectrometry is an important new methodology that allows for screening of a large number of proteins.9 Mass spectrometry also allows for semiquantitative measurement of the proteins and compares the relative abundance of the proteins. In this study, we were able to identify 1500–2000 glomerular proteins per case; most were house-keeping proteins and many were present in low total spectral counts. We found that PLA2R was among the most abundant proteins in PLA2R-associated MN compared with the house-keeping proteins. Next, we found that exostosin proteins EXT1 and EXT2 were among the most abundant proteins in 21 cases of PLA2R-negative MN. MS/MS of 21 of 26 cases of EXT1/EXT2-associated MN showed high average spectral counts of both EXT1 (65.3) and EXT2 (83.4); in the remaining five cases there was inadequate tissue to perform mass spectrometry studies. The total spectral counts for EXT1 and EXT2 were the highest among all glomerular proteins except for some complement and Ig proteins and basement membrane proteins. The high PLA2R (in PLA2R-associated MN) and EXT1/EXT2 (in EXT1/EXT2-associated MN) spectral counts were also validated by the extensive sequence coverage found for PLA2R and the EXT1 and EXT2 proteins. The EXT1 and EXT2 spectral counts in EXT1/EXT2-associated MN were comparable to the PLA2R (86.1) spectral counts in PLA2R-associated MN cases. Most importantly, EXT1 and EXT2 were absent in all PLA2R-associated MN and control cases. IHC confirmed bright granular staining for EXT1 and EXT2 along the GBM with no significant mesangial staining. It is important to note that the staining was evenly spread throughout the thickened GBM and mirrored the Ig staining, as would be expected in MN. The granular capillary wall staining for EXT1 and EXT2 was similar to the staining pattern of PLA2R in PLA2R-associated MN cases. The uniformity of EXT1/EXT2 staining along the GBM and the subepithelial deposits suggests that these proteins are shed from the podocytes rather than representing circulating antigens or immune complexes. It is unlikely that they are shed from mesangial cells or endothelial cells because there is no mesangial or subendothelial staining. It is also important to point out that we did not detect spectra for THSD7A in any of the cases of EXT1/EXT2-associated MN or in any of the PLA2R-associated MN cases. Although we found that some basement membrane proteins were also present with high spectral counts, including laminin, basement membrane–specific heparan sulfate core proteins, nidogen, type IV collagen, and structural proteins such as vinculin, these proteins had large spectral counts in both PLA2R-associated MN and in EXT1/EXT2-associated MN (data not shown).
The clinical and kidney biopsy findings revealed an interesting pattern in the EXT1/EXT2-associated MN. A total of 80.8% of the patients were women and the average age was 35.7 years. Furthermore, 70.8% of the patients had abnormal autoimmune laboratory findings, such as positive anti-nuclear, anti–double-stranded DNA, anti-SSA/SSB, or anti-ribonucleoprotein antibodies. Nine (35%) of the patients had a clinical diagnosis of lupus and three (12%) had mixed connective tissue disorder. Kidney biopsy findings revealed features suggestive of a secondary MN related to autoimmune disease in 84.6% of the patients. These included staining for C1q and/or staining for IgA/IgM on immunofluorescence studies, subendothelial and mesangial deposits, and the finding of tubuloreticular inclusions in endothelial cells on electron microscopy. Furthermore, IgG1 was the dominant IgG with spectral counts significantly greater than for IgG4 within EXT1/EXT2-associated MN and also when compared with IgG1 in PLA2R-associated MN. Taken together, these findings suggest that EXT1/EXT2 represent potential biomarkers or target antigens in secondary autoimmune MN.
We also studied other cases of primary glomerular disease with nephrotic syndrome, including minimal change disease, primary FSGS and immune complex GN including lupus nephritis (without a membranous component), and IgA nephropathy by IHC and MS/MS. All cases were negative for EXT1 and EXT2 (Supplemental Figure 1F). Most importantly, cases of lupus nephritis that did not have a membranous component were negative for EXT1 and EXT2. On the other hand, nine of the 26 positive cases of EXT1/EXT2-associated MN were consistent with membranous (class 5) lupus nephritis on the basis of clinical and kidney biopsy findings. Thus, cases with pure proliferative lupus nephritis (class 2, 3, or 4) were negative for EXT1/EXT2 GBM staining by IHC. We found two cases (cases #23 and #25) that had a proliferative component (class 3/4) along the membranous (class 5) component. Larger studies are needed to determine the percentage of positive EXT1/EXT2-associated MN in this group of patients.
These findings were confirmed by EXT1/EXT2 IHC staining of the validation cohort. For the validation studies, on the basis of our findings, we investigated cases of pure lupus class 5 nephritis, proliferative class 3/4 lupus nephritis with a membranous class 5 component, and nonlupus MN being negative for both PLA2R and THS7DA. IHC staining showed that 44% of cases with class 5 lupus nephritis were positive for EXT1 and EXT2, whereas only two cases (#16 and #35) with a mixed class (3/4 plus 5, n=14) of lupus nephritis were positive for EXT1/EXT2. However, in the case of patient #16, EXT1/EXT2 staining appeared at the first biopsy when the diagnosis of “primary” MN with signs of autoimmunity was made. Furthermore, evaluation of 16 cases of double-negative (PLA2R- and THS7DA-negative) nonlupus MN detected three positive cases of EXT1/EXT2-positive MN, all of them with biologic features of an underlying autoimmune disease. It is noticeable that two (#14 and #16) of those three patients developed full-blown lupus. Further studies are needed to determine whether EXT1/EXT2 staining is a predictor of such evolution in patients with apparently “primary” MN with biologic signs of autoimmunity and to identify the “missing” antigen in the EXT1/EXT2-negative patients with lupus MN.
The GBM is made up of mostly type IV collagen, laminin, nidogen, and heparan sulfate proteoglycans.10–17 Exostosins are glycosyltransferases that are responsible for the synthesis of the heparin sulfate backbone that add glycosaminoglycan residues to the core protein resulting in the generation of complex polysaccharides.18,19 There are five genes that encode the EXT proteins—EXT1, EXT2, EXTL1, EXTL2, and EXTL3. EXT1 and EXT2 show structural similarities, and EXT1 and EXT2 can exist as heterodimers and act as copolymerases in the elongation of the heparin sulfate chain.20 The heterodimer of EXT1/EXT2 also has increased stability and activity.21 This is the likely reason that EXT1/EXT2 (in the heterodimer form) are found together in our studies. The EXTL proteins show amino acid sequence homology with EXT1 and EXT2, and are also likely involved in heparan sulfate synthesis although their function is less well known. MS/MS did not detect EXTL proteins in any of the EXT1/EXT2-associated MN cases, although there was mild staining in one case that showed very bright EXT1/EXT2 staining, raising the possibility of crossreactivity of the EXTL2 antibody to EXT1/EXT2 (Supplemental Figure 1E). The EXT proteins are well conserved, especially in their C-terminal regions. Except for EXTL1, the EXT proteins are ubiquitously expressed in various mammalian tissues. EXT proteins are also expressed in podocytes, and a homozygous knockout of EXT1 specifically in podocytes did not lead to significant defects in glomerular filtration, although changes in podocyte architecture and focal thickening of GBM were noted.22,23 EXT proteins are transmembrane proteins in endoplasmic reticulum, and whether the EXT1 and EXT2 detected in EXT1/EXT2-associated MN are full-length proteins or represent shed partial or truncated proteins or are proteins with post-transitional modifications needs to be further studied.24 Finally, mutations in EXT1 and EXT2 are associated with an autosomal dominant disorder, hereditary multiple exostoses, which is one of the most common inherited skeletal disorders.19,25 To the best of our knowledge, we are not aware of any disorder associated with accumulation of EXT1 and EXT2.
By western and native blotting analysis in nonreducing conditions, we could not detect circulating anti-EXT1/EXT2 antibodies. This does not exclude a role for anti-EXT1/EXT2 antibodies for the following reasons. First, we used recombinant EXT1/EXT2 proteins which might not appropriately present the epitopes reactive with the patients’ antibodies. Second, the antibodies may circulate at very low concentrations and/or be cleared from the blood due to a sink effect of the deposited antigen. It is unlikely that EXT1 and EXT2 are shed from the podocyte in response to the formation of immune complexes involving other antigens, because EXT1/EXT2 appear to be specific to a well defined subset of MN with autoimmune disease. The absence of detectable antibodies to EXT1/EXT2 raises the possibility that EXT1/EXT2 proteins may represent biomarker proteins for MN associated with autoimmune disease rather than target antigens. Further studies are required to answer these questions.
In conclusion, we have identified a set of novel proteins, EXT1 and EXT2, associated with PLA2R and THSD7A double-negative MN in a subset of adult patients with clinical and biopsy findings of autoimmune disease including lupus. EXT1 and EXT2 may represent the target antigens or biomarker proteins of secondary (autoimmune) MN. This important finding has clinical importance for the molecular classification of MN and possibly for the prediction of lupus occurrence in patients with so-called primary MN but with features of autoimmunity.
Disclosures
Dr. Sethi, Dr. Fervenza, Dr. Madden, and Dr. Charlesworth have patent pending for identifying, testing and treatment of Membranous nephropathy associated with EXT1/EXT2. Dr. Ronco reports research fees from Alexion, outside of the submitted work.
Supplementary Material
Acknowledgments
We would like to thank the Mayo Clinic Genome Facility Proteomics Core (a shared resource of the Mayo Clinic Cancer Center [National Cancer Institute P30 CA15083]), the Department of Laboratory Medicine and Pathology and the Pathology Research Core, and the Fulk Family Foundation. Dr. Ronco is a recipient of European Research Council ERC-2012-ADG_20120314 grant 322947, the Seventh Framework Programme of the European Community contract 2012-305608 (European Consortium for High-Throughput Research in Rare Kidney Diseases), and the National Research Agency grant Membranous Nephropathy aims (ANR-17- CE17-0012-01). We are greatly indebted to the clinicians who took care of the patients in the Department of Nephrology and Dialysis, and to the pathologists David Buob and Isabelle Brocheriou from the Department of Pathology, all at Tenon Hospital, Paris, France.
Dr. Sethi and Dr. Fervenza designed the study. Dr. Sethi wrote the manuscript and interpreted the kidney biopsy, immunohistochemistry, and mass spectrometry data. Dr. Madden and Dr. Charlesworth performed the laser microdissection and mass spectrometry. Dr. Gross performed the immunohistochemistry. Dr. Ravindran helped in gathering the clinical information. Dr. Hummel and Dr. Specks performed the western blotting studies. Dr. Ronco and Dr. Debiec provided tissue for the validation cohort and also performed the western blot analysis and the immunofluorescence studies. Dr. Fervenza and Dr. Ronco also critically read the manuscript. All authors read the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018080852/-/DCSupplemental.
Supplemental Table. Details of laser microdissection of EXT1/EXT2-associated MN cases.
Supplemental Figure 1. Immunohistochemical staining for EXT1 and EXT2 in EXT1/EXT2-associated MN cases and control cases. EXTL2 staining is also shown in three EXT1/EXT2-associated MN cases.
Supplemental Figure 2. Representative mass spectrometry findings of control cases.
Supplemental Figure 3. Example of MS/MS spectra match to sequence from EXT1 and EXT2.
Details of methods.
Clinical, laboratory and kidney biopsy findings of validation cohort.
References
- 1.De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC: A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol 28: 421–430, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couser WG: Primary membranous nephropathy. Clin J Am Soc Nephrol 12: 983–997, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al.: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomas NM, Beck LH Jr., Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al.: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277–2287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomas NM, Hoxha E, Reinicke AT, Fester L, Helmchen U, Gerth J, et al.: Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J Clin Invest 126: 2519–2532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronco P, Debiec H: Pathophysiological advances in membranous nephropathy: Time for a shift in patient’s care. Lancet 385: 1983–1992, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Ronco P, Debiec H: Pathogenesis of membranous nephropathy: Recent advances and future challenges. Nat Rev Nephrol 8: 203–213, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Nesvizhskii AI, Keller A, Kolker E, Aebersold R: A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Sethi S, Vrana JA, Theis JD, Dogan A: Mass spectrometry based proteomics in the diagnosis of kidney disease. Curr Opin Nephrol Hypertens 22: 273–280, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Miner JH: The glomerular basement membrane. Exp Cell Res 318: 973–978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YM, Miner JH: Glomerular basement membrane and related glomerular disease. Transl Res 160: 291–297, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miner JH: Glomerular basement membrane composition and the filtration barrier. Pediatr Nephrol 26: 1413–1417, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raats CJ, Van Den Born J, Berden JH: Glomerular heparan sulfate alterations: Mechanisms and relevance for proteinuria. Kidney Int 57: 385–400, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Sugar T, Wassenhove-McCarthy DJ, Orr AW, Green J, van Kuppevelt TH, McCarthy KJ: N-sulfation of heparan sulfate is critical for syndecan-4-mediated podocyte cell-matrix interactions. Am J Physiol Renal Physiol 310: F1123–F1135, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy KJ, Wassenhove-McCarthy DJ: The glomerular basement membrane as a model system to study the bioactivity of heparan sulfate glycosaminoglycans. Microsc Microanal 18: 3–21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugar T, Wassenhove-McCarthy DJ, Esko JD, van Kuppevelt TH, Holzman L, McCarthy KJ: Podocyte-specific deletion of NDST1, a key enzyme in the sulfation of heparan sulfate glycosaminoglycans, leads to abnormalities in podocyte organization in vivo. Kidney Int 85: 307–318, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Wassenhove-McCarthy D, Yamaguchi Y, Holzman L, van Kuppevelt TH, Orr AW, et al.: Podocytes require the engagement of cell surface heparan sulfate proteoglycans for adhesion to extracellular matrices. Kidney Int 78: 1088–1099, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Busse-Wicher M, Wicher KB, Kusche-Gullberg M: The exostosin family: Proteins with many functions. Matrix Biol 35: 25–33, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Ahn J, Lüdecke HJ, Lindow S, Horton WA, Lee B, Wagner MJ, et al.: Cloning of the putative tumour suppressor gene for hereditary multiple exostoses (EXT1). Nat Genet 11: 137–143, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Busse M, Kusche-Gullberg M: In vitro polymerization of heparan sulfate backbone by the EXT proteins. J Biol Chem 278: 41333–41337, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Busse M, Feta A, Presto J, Wilén M, Grønning M, Kjellén L, et al.: Contribution of EXT1, EXT2, and EXTL3 to heparan sulfate chain elongation. J Biol Chem 282: 32802–32810, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Miner JH: Glomerular filtration: The charge debate charges ahead. Kidney Int 74: 259–261, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Wassenhove-McCarthy DJ, Yamaguchi Y, Holzman LB, van Kuppevelt TH, Jenniskens GJ, et al.: Loss of heparan sulfate glycosaminoglycan assembly in podocytes does not lead to proteinuria. Kidney Int 74: 289–299, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajjala PR, Fliser D, Speer T, Jankowski V, Jankowski J: Emerging role of post-translational modifications in chronic kidney disease and cardiovascular disease. Nephrol Dial Transplant 30: 1814–1824, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Cook A, Raskind W, Blanton SH, Pauli RM, Gregg RG, Francomano CA, et al.: Genetic heterogeneity in families with hereditary multiple exostoses. Am J Hum Genet 53: 71–79, 1993 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.