Abstract
Background: Typically, closed-loop control (CLC) studies excluded patients with significant hypoglycemia. We evaluated the effectiveness of hybrid CLC (HCLC) versus sensor-augmented pump (SAP) in reducing hypoglycemia in this high-risk population.
Methods: Forty-four subjects with type 1 diabetes, 25 women, 37 ± 2 years old, HbA1c 7.4% ± 0.2% (57 ± 1.5 mmol/mol), diabetes duration 19 ± 2 years, on insulin pump, were enrolled at the University of Virginia (N = 33) and Stanford University (N = 11). Eligibility: increased risk of hypoglycemia confirmed by 1 week of blinded continuous glucose monitor (CGM); randomized to 4 weeks of home use of either HCLC or SAP. Primary/secondary outcomes: risk for hypoglycemia measured by the low blood glucose index (LBGI)/CGM-based time in ranges.
Results: Values reported: mean ± standard deviation. From baseline to the final week of study: LBGI decreased more on HCLC (2.51 ± 1.17 to 1.28 ± 0.5) than on SAP (2.1 ± 1.05 to 1.79 ± 0.98), P < 0.001; percent time below 70 mg/dL (3.9 mmol/L) decreased on HCLC (7.2% ± 5.3% to 2.0% ± 1.4%) but not on SAP (5.8% ± 4.7% to 4.8% ± 4.5%), P = 0.001; percent time within the target range 70–180 mg/dL (3.9–10 mmol/L) increased on HCLC (67.8% ± 13.5% to 78.2% ± 10%) but decreased on SAP (65.6% ± 12.9% to 59.6% ± 16.5%), P < 0.001; percent time above 180 mg/dL (10 mmol/L) decreased on HCLC (25.1% ± 15.3% to 19.8% ± 10.1%) but increased on SAP (28.6% ± 14.6% to 35.6% ± 17.6%), P = 0.009. Mean glucose did not change significantly on HCLC (144.9 ± 27.9 to 143.8 ± 14.4 mg/dL [8.1 ± 1.6 to 8.0 ± 0.8 mmol/L]) or SAP (152.5 ± 24.3 to 162.4 ± 28.2 [8.5 ± 1.4 to 9.0 ± 1.6]), P = ns.
Conclusions: Compared with SAP therapy, HCLC reduced the risk and frequency of hypoglycemia, while improving time in target range and reducing hyperglycemia in people at moderate to high risk of hypoglycemia.
Keywords: Type 1 diabetes, Hypoglycemia, Artificial pancreas, Closed-loop systems
Introduction
The DCCT1,2 and EDIC3 studies demonstrated that lower hemoglobin A1c (HbA1c) levels reduce the risk of long-term complications from diabetes. However, in the EDIC population, with lowering of HbA1c levels, severe hypoglycemia rates increased and persisted at a rate of about 36–41 episodes per 100 patient-years.4 In the T1D Exchange Registry in 2015, there was a 10%–15% yearly incidence of severe hypoglycemia in adults (defined as seizure or loss of consciousness), with the lowest incidence in those with HbA1c levels between 7% and <7.5% and the highest incidence in those with HbA1c levels <6.5%.5
In the last few years, there have been several studies testing in-home use of closed-loop control (CLC) systems to improve glucose control over multiple months.6–14 Using these systems, the overall amount of time <70 mg/dL (<3.9 mmol/L) was reduced from 3%–6.4% to 1.3%–3.4%. Overnight, there were even more striking reductions in hypoglycemia with the percent time <70 mg/dL (<3.9 mmol/L) decreasing from 3.5%–6.6% to 0.5%–3.2%. In one 6-month outpatient study that used the same algorithm as the present study, the median percent time <70 mg/dL (<3.9 mmol/L) was reduced to 0.5%.12
These previous trials, however, typically excluded patients at high risk for hypoglycemia and, in particular, those with recent history of severe hypoglycemia or hypoglycemia unawareness. With the consistent reduction in hypoglycemia obtained by this closed-loop system, the purpose of the present study was to determine if 4 weeks of hybrid CLC (HCLC) would be able to reduce the risk and frequency of hypoglycemia in patients with moderate to high risk of hypoglycemia.
Research Design and Methods
Study protocol, investigational device exemption, and institutional review board approval
The protocol was approved by the review boards of the participating institutions. The study also received FDA approval (IDE #G140169) and was registered with ClinicalTrials.gov: NCT02302963 (UVA). Written informed consent was obtained from all participants.
Eligibility criteria
We recruited subjects, ages 12–70 years, with type 1 diabetes on insulin for ≥1 year, and on insulin pump therapy for ≥6 months with HbA1c <10.0% (86 mmol/mol) (if HbA1c <6.0% [42 mmol/mol] then total daily insulin had to be ≥0.5 U/kg). Subjects had a risk of hypoglycemia or hypoglycemia unawareness as defined by any of the following: (1) Clarke Hypoglycemia Perception Awareness questionnaire score of ≥415; (2) average daily risk range (ADRR) >40 as assessed from SMBG readings from the prior month16; (3) low blood glucose index (LBGI) >2.5 as assessed from SMBG from the prior month17 or LBGI >1.1 as assessed from 1 week of continuous glucose monitoring (CGM) readings18; or (4) no recognition of hypoglycemia until the glucose is <60 mg/dL (<3.3 mmol/L) and no adrenergic symptoms at glucose of 60 mg/dL (3.3 mmol/L) (shakiness, palpitations, diaphoresis). Additional eligibility criteria included the ability to speak and read English and use basic technology such as a cell phone, current use of an insulin-to-carbohydrate ratio, access to Internet or cell phone service in the subject's local environment, willingness to maintain uninterrupted availability via personal cell phone, willingness to perform SMBG testing four to six times daily (before meals, bedtime, before driving, before exercise, and as indicated), and living with a diabetes care partner ≥18 years old. Subjects were recruited at two clinical sites (University of Virginia and Stanford University) with the goal of 44 completed subjects.
Exclusion criteria included admission for diabetic ketoacidosis in the past 12 months, severe hypoglycemia in the past 3 months, hematocrit less than the lower limit of normal, cystic fibrosis, pregnancy, breastfeeding, or intention of becoming pregnant, and conditions that may be riskier in the setting of hypoglycemia such as coronary artery disease, congestive heart failure, cardiac arrhythmia, seizure disorder, cerebrovascular event or transient ischemic attack, hypoglycemia-induced migraine within the past 6 months, or neurological disease.
Additional exclusions included inpatient psychiatric treatment in the past 6 months, presence of an adrenal disorder, abnormal liver function tests (transaminase >3 times the upper limit of normal), abnormal renal function (estimated glomerular filtration rate <60 mL/min/1.73 m2), gastroparesis, lack of stability on antihypertensive, thyroid, antidepressant, or lipid-lowering medication, uncontrolled thyroid disease (TSH undetectable or >10 mIU/L), current or recent abuse of alcohol or recreational drugs, infectious process not anticipated to resolve before study procedures, any skin condition in the area of sensor or pump placement, diagnosed with celiac disease, and not following a gluten-free diet, or other significant medical disorders, injuries, or medications that in the judgment of the investigator would affect the completion of the protocol. Subjects also could not be taking any noninsulin medication to lower blood glucose, beta-blockers, glucocorticoids, or pseudoephedrine.
Study procedures
After written informed consent, a screening history and physical (±blood draw for screening laboratories) were completed and a meter ± CGM download was performed to evaluate subject eligibility (i.e., ADRR >40 or LBGI >2.5 from SMBG data, or LBGI >1.1 from CGM data). Baseline HbA1c and hypoglycemia symptom awareness (presence of adrenergic symptoms at blood glucose [BG] 60 mg/dL [3.3 mmol/L] and Clarke Hypoglycemia Perception Awareness questionnaire15) were also assessed. Qualifying subjects completed 1 week of baseline blinded CGM and SMBG at least four times daily. If LBGI >1.1 was confirmed by blinded CGM, the subject was randomized to USS Virginia AP experimental treatment or sensor-augmented pump control treatment. The experimental and control groups were matched by predefined LBGI categories (LBGI 1.1–2.4, LBGI 2.5–5.0, and LBGI >5.0, i.e., the LBGI category was used as a stratification factor) and prestudy CGM use (active CGM use [≥5 days/week] or no CGM use). All subjects were trained on the Dexcom Share AP CGM. Control subjects subsequently completed 5 weeks of study CGM and home pump use. Experimental subjects proceeded to study pump and the HCLC system training. There was an initial 1 week of home use in pump mode (functionally the same as an insulin pump without active algorithms), followed by 4 weeks of closed-loop mode.
USS Virginia CLC algorithms
The system consists of several modules with different functions adapted to different activities during the day and night. The safety supervision module (SSM) is active at all times and has “veto” power over any insulin delivery requested by the other modules. This module predicts hypoglycemic risk and computes a maximum allowable insulin rate according to the patient's current basal rate and a risk-based attenuation factor. Insulin-on-board supervisor (IOBsup) computes the insulin on board based on past injected insulin and broadcasts IOB to all other control modules. It therefore enables the avoidance of insulin stacking, regardless of the current or predicted glycemic risk.
Outputs from SSM and IOBsup are combined to determine the hypoglycemia and hyperglycemia red light system, informing the patient of the hypoglycemic risk status: green, no perceived risk, yellow: predicted risk resulting in insulin dampening, and red: predicted imminent hypoglycemia with external intervention needed; and hyperglycemic risk status: green, no perceived risk, yellow: predicted risk resulting in basal rate increase, and red: perceived hyperglycemia with external intervention needed. Basal rate modulator (BRM) is designed to elevate the basal rate when hyperglycemia is developing. During the day, as it takes meal insulin into account, it is mostly inactive unless significant and prolonged hyperglycemia develops.
The BRM control module ensures that the level of insulin-on-board is adapted to the current glucose level and the predetermined target. The target is gradually lowered overnight to obtain optimal conditions before breakfast. The hyperglycemia mitigation system provides automated correction boluses during the day (8 am–11 pm) to a target of 110 mg/dL (6.1 mmol/L), but only if glucose is predicted to be above a predetermined threshold and if the current IOB is insufficient (meal insulin included). The system also includes a meal bolus calculator that takes into account the current glucose state of the subject and available insulin to make a recommendation for a premeal bolus or a user-initiated correction. The recommendation must be confirmed by the user before delivery; if not confirmed, premeal insulin is not delivered automatically. The bolus calculator is activated on demand by the user before meals or manual corrections.
System components
The devices that were used to implement the USS Virginia closed-loop system included the Diabetes Assistant (DiAs) smart phone medical platform, Dexcom G4® Platinum CGM (Dexcom, Inc., San Diego, CA) connected to DiAs via CGM receiver and USB-Bluetooth relay hardware, a Roche Accu-Chek® Spirit Combo insulin pump (Roche Diabetes Care, Inc., Mannheim, Germany) connected to DiAs via wireless Bluetooth, and a remote monitoring server connected to DiAs via 3G or local WiFi. Subjects also received a Roche Accu-Chek Aviva Combo glucometer to use for all calibrations and SMBG assessments during the study. DiAs was upgraded to the new inControl system in 2016 (TypeZero Technologies, Inc., Charlottesville, VA), which also runs on a smart phone with the USS Virginia CLC algorithm. Hence, the final two experimental subjects at UVA used a smartphone running inControl (TypeZero Technologies, Inc.) in place of DiAs. Subjects in the sensor-augmented pump (SAP) group used a study-provided Dexcom G4 Platinum CGM and Roche Accu-Chek Aviva glucometer along with their home insulin pump.
Outcomes and statistical analysis
Sample size determination was based on literature data3 and on results from our previous studies of hypoglycemia unawareness.4 We estimated, conservatively, that the effect size of using DiAs+USS Virginia versus CGM in terms of limiting hypoglycemic risk (as assessed by LBGI) would be moderate, ∼0.35. Power calculations assuming α = 0.05, and power of 95%, for within/between interaction, with two groups and two measurements (assumed correlation of 0.5) yielded a required sample size of N = 30 (using G*Power 3.1.9.2 Software19). Assuming a proportion of noncompliance, R = 33% for intention-to-treat analysis,20 44 completed subjects were anticipated to adequately address the specific aims of this study. Per-protocol analysis was also performed. To achieve 44 completed subjects, up to 70 subjects were approved to sign consent, given an expected screen failure, withdrawal, and dropout rate of ∼40% due to the rigorous exclusion criteria and requirements imposed for DiAs system use in the subject's local environment. All glucose outcomes were computed based on CGM records as described in a recent review.18 The primary outcome was the LBGI—an established metric of the frequency and extent of hypoglycemia.17,18 The choice of secondary metrics is consistent with the recommendations of the recent International Consensus on Interpretation of CGM data.21 Repeated-measures general linear model was used to compare HCLC versus SAP data collected during the baseline week versus the last week of study.
Results
Study population
A total of 85 subjects signed consent at UVA (70) and Stanford (15). Of those, 20 did not meet eligibility criteria, and 21 subjects withdrew or dropped out (18 prerandomization, 1 control, and 2 experimental). Reasons for withdrawal included scheduling conflicts (5), travel cost considerations (1), interest in a different CLC trial (2), dislike of DiAs (1), alarm fatigue (1), need for a steroid injection (1), and unresponsiveness to study team communications (10). Therefore, a total of 44 subjects completed the study at the two study centers (UVA 33, Stanford 11). CGM data were missing for 2 of the 44 subjects (1 control and 1 experimental) and outcomes could not be reliably computed. Therefore, 42 subjects were included in the final analysis. Baseline study subject characteristics are presented in Table 1.
Table 1.
Baseline Characteristics of the Completed Study Participants
| Characteristic | SAP (n = 21) | USS Virginia HCLC (n = 21) |
|---|---|---|
| Male (%) | 28.6 | 52.4 |
| CGM users' pretrial (%) | 66.7 | 71.4 |
| Age (years)a | 38.0 ± 3.3 | 38.3 ± 3.3 |
| Duration of diabetes (years)a | 18.2 ± 2.3 | 21.2 ± 2.6 |
| BMIa,b | 27.0 ± 1.0 | 26.0 ± 1.2 |
| Total daily insulin (units)a | 43.7 ± 3.6 | 49.0 ± 4.8 |
| BG for low symptoms mg/dL (mmol/L) | 54.77 ± 2.22 (3.03 ± 0.13) | 50.68 ± 1.45 (2.84 ± 0.08) |
| LBGI from baseline CGMa | 2.1 ± 0.23 | 2.51 ± 0.26 |
| Clarke scorea | 3.19 ± 0.28 | 3.81 ± 0.32 |
| Screening HbA1c% (mmol/mol)a | 7.5 ± 0.2 (59 ± 1.5) | 7.2 ± 0.2 (55 ± 1.5) |
Mean ± SD.
BMI is weight in kilograms divided by height in meters squared.
BG, blood glucose; BMI, body mass index; CGM, continuous glucose monitoring; HbA1c, hemoglobin A1c; LBGI, Low Blood Glucose Index; SAP, sensor-augmented pump; SD, standard deviation.
Overall glycemic control
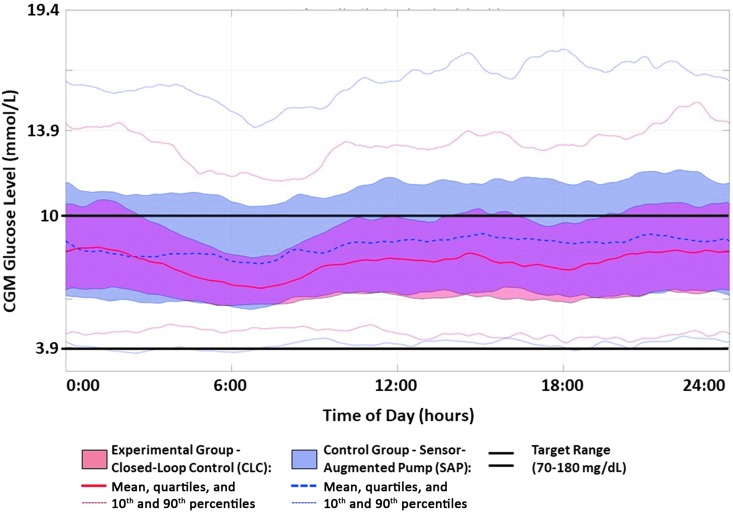
Figure 1 presents the data envelope of an average day for the experimental (HCLC) and control (SAP) groups, together with mean lines, quartiles, and 90th percentiles of the data spread. It is evident that HCLC reduced significantly the spread of the data, with the most prominent difference overnight and in the early morning hours, which is consistent with the design of the USS Virginia algorithm. In addition to narrowing the overall spread of the data, the HCLC system reduced substantially both the excursions into hypoglycemia (lower 90th percentile of the data) and hyperglycemia (upper 90th percentile of the data). The statistical significance of these effects is confirmed by the metrics and the P-values are presented in Table 2.
FIG. 1.
Twenty-four-hour CGM data envelope on closed-loop control (red) versus sensor-augmented pump therapy (blue). The data clouds represent 25th and 75th percentiles. The solid/dashed lines within the clouds are the mean CGM traces, and the dotted outer lines are the 10th and 90th percentile envelope of the data. Closed-loop control results in markedly narrowed data envelope, signifying reduced volatility of glucose control. This effect is particularly prominent in late-night and prebreakfast hours, which is consistent with the intent of the control algorithm, designed to gradually lower its target and tighten control overnight. CGM, continuous glucose monitoring.
Table 2.
Glycemic Performance of USS Virginia Hybrid Closed-Loop Control Versus Sensor-Augmented Pump Therapy
| USS Virginia HCLC | SAP | Relative improvement post vs. pre | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | F | P | |
| LBGI | 2.51 ± 1.17 | 1.28 ± 0.5 | 2.1 ± 1.05 | 1.79 ± 0.98 | 15.2 | <0.001 |
| Percent below 54 mg/dL (3.0 mmol/L) | 2.1% ± 2.2% | 0.3% ± 0.4% | 1.3% ± 1.8% | 0.9% ± 1.1% | 5.8 | 0.02 |
| Percent below 60 mg/dL (3.3 mmol/L) | 3.4% ± 3.3% | 0.8% ± 0.7% | 2.6% ± 2.8% | 2.1% ± 2.5% | 8.5 | 0.006 |
| Percent below 70 mg/dL (3.9 mmol/L) | 7.2% ± 5.3% | 2% ± 1.4% | 5.8% ± 4.7% | 4.8% ± 4.5% | 11.8 | 0.001 |
| Percent between 70–180 mg/dL (3.9–10 mmol/L) | 67.8% ± 13.5% | 78.2% ± 10% | 65.6% ± 12.9% | 59.6% ± 16.5% | 14.8 | <0.001 |
| Percent above 180 mg/dL (10 mmol/L) | 25.1% ± 15.3% | 19.8% ± 10.1% | 28.6% ± 14.6% | 35.6% ± 17.6% | 7.5 | 0.009 |
| Percent above 250 mg/dL (13.9 mmol/L) | 6.8% ± 9.8% | 3.6% ± 3.9% | 8.5% ± 8.1% | 11.2% ± 9.4% | 5.5 | 0.024 |
| Percent above 300 mg/dL (16.7 mmol/L) | 2.5% ± 4.8% | 1.1% ± 1.8% | 2.7% ± 3.3% | 3.6% ± 5.6% | 3.3 | 0.077 |
| Mean glucose mg/dL (mmol/L) | 144.9 ± 27.9 (8.1 ± 1.6) | 143.8 ± 14.4 (8.0 ± 0.8) | 152.5 ± 24.3 (8.5 ± 1.4) | 162.4 ± 28.2 (9.0 ± 1.6) | 2.2 | ns |
| Glucose variability (coefficient of variation) | 0.38 ± 0.06 | 0.32 ± 0.04 | 0.38 ± 0.05 | 0.37 ± 0.07 | 7.1 | 0.011 |
| High blood glucose index | 5.7 ± 4.1 | 4.5 ± 2.1 | 6.6 ± 3.6 | 8.1 ± 4.3 | 6.1 | 0.018 |
| ADRR | 8.1 ± 3.6 | 6.2 ± 2.6 | 7.9 ± 2.3 | 8.1 ± 3.4 | 2.8 | 0.102 |
| Daytime (7 am–11 pm) | ||||||
| LBGI | 2.55 ± 1.07 | 1.28 ± 0.51 | 1.92 ± 0.98 | 1.72 ± 1.03 | 21.6 | <0.001 |
| Percent below 54 mg/dL (3.0 mmol/L) | 1.8% ± 1.8% | 0.3% ± 0.4% | 1.1% ± 1.3% | 0.9% ± 1.3% | 9.8 | 0.003 |
| Percent below 60 mg/dL (3.3 mmol/L) | 3.4% ± 2.8% | 0.8% ± 0.7% | 2.2% ± 2.3% | 2.1% ± 2.5% | 15.2 | <0.001 |
| Percent below 70 mg/dL (3.9 mmol/L) | 7.4% ± 5% | 2.1% ± 1.6% | 5.1% ± 4.2% | 4.7% ± 4.3% | 19.1 | <0.001 |
| Percent between 70–180 mg/dL (3.9–10 mmol/L) | 69.2% ± 12.5% | 77.9% ± 11% | 66.1% ± 12.9% | 59.3% ± 15.7% | 12.3 | 0.001 |
| Percent above 180 mg/dL (10 mmol/L) | 23.3% ± 14.1% | 20% ± 11.1% | 28.8% ± 14.6% | 36% ± 16.8% | 5.8 | 0.020 |
| Percent above 250 mg/dL (13.9 mmol/L) | 6.8% ± 9.7% | 3.5% ± 3.3% | 7.2% ± 6.9% | 11.3% ± 9.1% | 9.8 | 0.003 |
| Percent above 300 mg/dL (16.7 mmol/L) | 2.5% ± 4.6% | 1% ± 1.6% | 1.6% ± 2.1% | 4% ± 5.8% | 9.0 | 0.005 |
| Mean glucose mg/dL (mmol/L) | 142.9 ± 26.9 (7.9 ± 1.5) | 144.1 ± 15.5 (8.0 ± 0.9) | 152.2 ± 22.5 (8.5 ± 1.3) | 163.9 ± 27 (9.1 ± 1.5) | 2.3 | ns |
| Glucose variability (coefficient of variation) | 0.38 ± 0.05 | 0.32 ± 0.04 | 0.36 ± 0.05 | 0.37 ± 0.08 | 15.3 | <0.001 |
| High blood glucose index | 5.5 ± 4 | 4.5 ± 2.1 | 6.3 ± 3.2 | 8.3 ± 4.2 | 7.8 | 0.008 |
| Overnight (11 pm–7 am) | ||||||
| LBGI | 2.34 ± 1.79 | 1.23 ± 0.71 | 2.34 ± 1.51 | 1.87 ± 1.12 | 2 | ns |
| Percent below 54 mg/dL (3.0 mmol/L) | 2.5% ± 3.9% | 0.4% ± 0.5% | 1.9% ± 3.2% | 0.8% ± 1.1% | 1.5 | ns |
| Percent below 60 mg/dL (3.3 mmol/L) | 3.5% ± 5% | 0.8% ± 1.2% | 3.4% ± 4.4% | 2.2% ± 3.1% | 1.4 | ns |
| Percent below 70 mg/dL (3.9 mmol/L) | 6.6% ± 7.4% | 1.9% ± 2.3% | 7.2% ± 6.6% | 5.3% ± 6.1% | 2.0 | ns |
| Percent between 70–180 mg/dL (3.9–10 mmol/L) | 64.9% ± 19.1% | 78.7% ± 12.6% | 64.5% ± 16.6% | 60% ± 22% | 8.4 | 0.006 |
| Percent above 180 mg/dL (10 mmol/L) | 28.5% ± 21.7% | 19.4% ± 12.6% | 28.3% ± 18.8% | 34.7% ± 23.4% | 4.8 | 0.034 |
| Percent above 250 mg/dL (13.9 mmol/L) | 6.7% ± 10.7% | 3.7% ± 6.1% | 10.8% ± 11.7% | 10.9% ± 12.1% | 0.7 | ns |
| Percent above 300 mg/dL (16.7 mmol/L) | 2.4% ± 5.4% | 1.3% ± 3.3% | 4.7% ± 6.1% | 2.6% ± 6.1% | 0.3 | ns |
| Mean glucose mg/dL (mmol/L) | 148.9 ± 34.2 (8.3 ± 1.9) | 143.5 ± 19.3 (8.0 ± 1.1) | 153.3 ± 32.5 (8.5 ± 1.8) | 159.2 ± 35.3 (8.8 ± 2.0) | 1.1 | ns |
| Glucose variability (coefficient of variation) | 0.34 ± 0.09 | 0.31 ± 0.06 | 0.38 ± 0.08 | 0.34 ± 0.06 | 0.3 | ns |
| High blood glucose index | 6.2 ± 4.9 | 4.4 ± 2.9 | 7.1 ± 4.9 | 7.6 ± 5.4 | 2.1 | ns |
Values are reported as mean ± SD. The repeated-measures general linear model was used to compare HCLC versus SAP data collected during the baseline week (pre) versus the last week of study (post).
ADRR, average daily risk range; HCLC, hybrid closed-loop control.
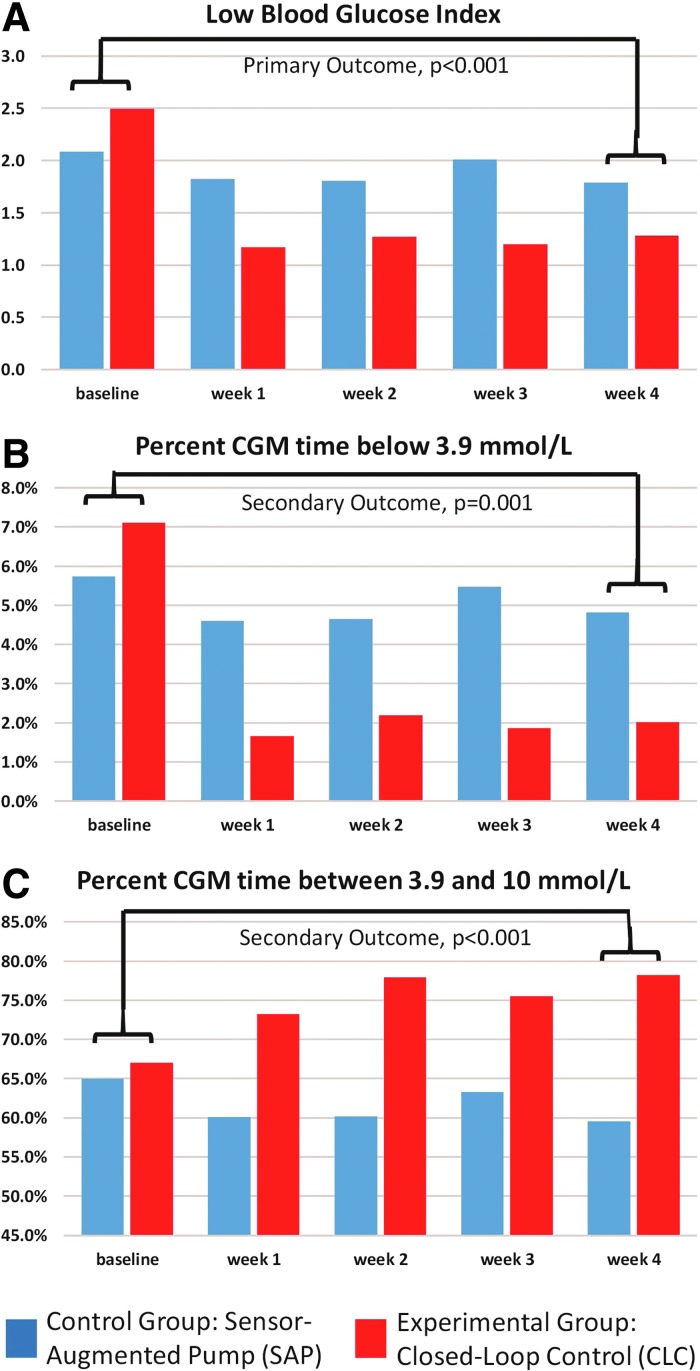
Primary outcomes
Figure 2, upper panel A, presents the weekly changes in the LBGI observed throughout the study. The LBGI decreased significantly more on HCLC from 2.5 to 1.3 from baseline to the last week of study, than on SAP (2.1–1.8). It is evident that this change was rather fast, occurred during the first week of active HCLC, and was sustained during the remaining 3 weeks of active HCLC. The statistical significance of the contrast between baseline versus last week of study was high: F = 15.5, P < 0.001.
FIG. 2.
Primary and key secondary outcomes plotted weekly throughout the study. The study was designed to assess differences between the experimental (CLC) and control (SAP) groups in their improvement from the baseline week to the last week of the trial. However, it is evident that the differences due to closed-loop control occurred immediately after turning on the system—during the first week of study—and were sustained thereafter. CLC, closed-loop control; SAP, sensor-augmented pump.
Secondary outcomes
As presented in Figure 2, panel B, the percent time below 70 mg/dL (3.9 mmol/L) decreased more on HCLC (7.2%–2.0%) than on SAP (5.8%–4.8%), P = 0.001. Figure 2, Panel C, presents the percent time within the target range 70–180 mg/dL (3.9–10 mmol/L), which increased on HCLC (67.8%–78.2%) but decreased on SAP (65.6%–59.6%), P < 0.001. Similar to the LBGI, these effects were evident during the first week of activating HCLC and were sustained thereafter. Table 2 presents several secondary outcomes, including time in ranges and coefficient of variation, as recommended by the International Consensus on Use of CGM.19 In particular, the percent time above 180 mg/dL (10 mmol/L) decreased on HCLC (25.1%–19.8%) but increased on SAP (28.6%–35.6%), P = 0.009, which is consistent with the upper 75th and 90th percentiles of the data depicted in Figure 1. Taken together with the overall reduction in glucose variability (as quantified by the coefficient of variation), these data suggest that HCLC reduced significantly the overall volatility of glucose control and simultaneously reduced the risk for hypoglycemia.
Adverse events
There were no serious adverse events during the study. Table 3 provides details of all adverse events and subject treatment group.
Table 3.
Adverse Events
| Subject no. | Treatment group | Event |
|---|---|---|
| 36102 | Control | Moderate hyperglycemia, mild ketonemia |
| 36106 | Experimental | Soccer injury |
| Upper respiratory illness | ||
| 36112 | Control | Moderate hyperglycemia, moderate ketonemia |
| 36114 | Experimental | Mild ketonemia |
| Mild ketonemia | ||
| 36117 | Experimental | Gastroenteritis with dehydration |
| IV site bruising | ||
| IV site bruising | ||
| 36121 | Prerandomization | Upper respiratory tract infection with asthma exacerbation requiring steroid |
| 36123 | Control | Outpatient surgery for spiral fracture of right arm resulting from a fall |
| Mild fever | ||
| 36125 | Experimental | Moderate hyperglycemia, moderate ketonemia |
| 36127 | Experimental | Moderate hyperglycemia, moderate ketonemia |
| Fever and flu-like symptoms | ||
| 36130 | Experimental | Ear infection |
| 36137 | Prerandomization | Bronchitis |
| 36149 | Control | Vasovagal event during CGM sensor insertion |
| IV site bruising | ||
| Vasovagal event after IV insertion | ||
| 36161 | Control | Gastroenteritis |
| Mild hyperglycemia, mild ketonemia | ||
| 36162 | Experimental | Upper respiratory tract infection |
| 36163 | Control | Moderate hyperglycemia, moderate ketonemia |
| 36167 | Experimental | Moderate hyperglycemia, moderate ketonemia |
| Vasovagal event during IV insertion |
Discussion
This study focused on the effects of CLC in a subpopulation of people with type 1 diabetes, who are at moderate to high risk for hypoglycemia due to hypoglycemia unawareness or other factors of their glycemic control. This subgroup has been typically excluded from previous HCLC studies because automated insulin delivery was deemed too risky for these vulnerable subjects. On the contrary, it is intuitively clear that this is the subgroup that could benefit most from the use of HCLC, particularly if the HCLC system is designed with the primary objective to safeguard against hypoglycemia. Thus, we used the USS Virginia—a modular hybrid closed-loop system, which has the unique property of having an SSM exclusively dedicated to prevention of insulin stacking and low blood glucose levels. Since the SSM can cancel any insulin delivery commands coming from other system components and this feature was extensively tested in previous studies, the USS Virginia was deemed safe for prolonged at-home deployment in moderate- to high-risk patients. Therefore, the primary objective of this randomized trial was to assess whether risk and frequency of CGM-measured hypoglycemia can be reduced by HCLC, compared with sensor-augmented pump therapy.
Relative weaknesses of this study include use of SAP in the control group rather than a low-glucose suspend (LGS) or predictive LGS system, of which several are now currently available. At the time of this study, the only system approved for use was the MiniMed 530G (Medtronic Diabetes, Northridge, CA). This system used the Enlite sensor, which has different accuracy characteristics from the Dexcom G4 Platinum CGM (Dexcom, Inc.) that was used in both treatment groups. For this study, we chose to equip the control (SAP) group with the same treatment means—a CGM sensor and an insulin pump—as the experimental HCLC group. The only difference between the study groups was a control algorithm specifically tuned to prevent hypoglycemia, allowing the specific effect of the control algorithm to be isolated from the effect of CGM use alone. An ongoing multicenter trial is currently comparing SAP, Basal IQ Predictive Low-Glucose Suspend, and Control IQ Hybrid Closed-Loop (NCT03591354). An additional weakness of the present study is the relatively short 4-week treatment period. Longer trials would be useful to assess whether similar beneficial effects from HCLC are sustainable in this population.
There was a high penetrance of baseline CGM use in the study population. Despite this, baseline time <70 mg/dL (3.9 mmol/L) was higher than most previously studied cohorts6–8,10–13 and subjects had no adrenergic symptoms at a glucose level of 60 mg/dL (3.3 mmol/L), with inability to detect hypoglycemia until a glucose level of 55–57 mg/dL (3.03–3.16 mmol/L). However, Clarke scores on average were slightly less than 4 at baseline.
All CGM-based metrics, including the LBGI, time in various ranges, and coefficient of variation, confirmed significant simultaneous reduction of both the frequency and extent of hypoglycemic episodes, and the extent of hyperglycemia. With a sample size of N = 42 subjects, randomized to HCLC versus SAP and participating in the study for 4 weeks, these results are statistically and clinically significant. For example, the time below 70 mg/dL (3.9 mmol/L) in the experimental group was reduced more than threefold on HCLC but was not reduced significantly in the control group. This improvement was accompanied by increased percent time in the target range of 70–180 mg/dL (3.9–10 mmol/L) in the experimental group, but decrease of this time in the control group and an increase in percent time >180 mg/dL (10 mmol/L) and >250 mg/dL (13.9 mmol/L). Thus, HCLC reduced the overall variability of the CGM traces, both visually (Fig. 1) and numerically, as assessed by the time in target range and the coefficient of variation (Table 2).
We can therefore conclude that automated insulin delivery by a hybrid closed-loop system is safe in the vulnerable subpopulation of people who are at moderate to high risk for hypoglycemia.
Acknowledgments
The authors acknowledge all of the volunteers who participated in the study for their help on this project. Funding: The study was funded by NIH NIDDK grant DP3 DK 101055. Material support was provided by Roche Diabetes Care, Inc. (insulin pumps and glucometers); Dexcom, Inc. (CGM sensors), and TypeZero Technologies, Inc. (control system and technical support).
Authors' Contribution
S.M.A. is the protocol principal investigator and lead clinician of the UVA site with contributions to protocol development and conduct, regulatory compliance, data collection and interpretation, and writing and editing of this article. B.A.B. is the coprincipal investigator of grant DP3 DK 101055 and lead clinician of the Stanford site with contributions to study design, data interpretation, and writing and editing of this article. M.D.B. is the lead engineer of the project with contributions to technology development, study design, regulatory approval, data analysis and interpretation, and writing and editing of this article. J.L.R. is the study coordinator of the UVA site with contributions to study conduct, data collection and management, regulatory submissions, and writing and editing of this article. C.L.B. provided technical support and training, data collection and management, and editing of this article. C.A.W. conducted data analysis, interpretation, and presentation, and editing of this article. M.C.O. was the project manager at the UVA site and assisted with regulatory submissions and approvals and editing of this article. S.A.B. is a subinvestigator at the UVA site with contributions to study conduct and editing of this article. T.T.L. and L.M.N. are subinvestigators at the Stanford site and contributed to study conduct and editing of this article. P.K.C., L.J.H., R.S.K., S.E.L., and S.R.-D.V. are study coordinators at the Stanford site and contributed to study conduct, data collection and management, and editing of this article. B.P.K. is the principal investigator of grant DP3 DK 101055 with contributions to technology development, study design, regulatory approval, data interpretation, and writing and editing of this article. Statement of guarantor: S.M.A. is the guarantor of the content of this article.
Author Disclosure Statement
S.M.A. has research support from Medtronic and has served as a consultant for Senseonics. B.A.B. is on medical advisory boards for Sanofi, Novo Nordisk, BD Biosciences, Unomedical, and Medtronic and has received research grant and/or material support from Medtronic, Dexcom, LifeScan, Insulet, Bayer, Unomedical, Tandem Diabetes Care, and Roche Diagnostics. M.D.B. has served on an advisory panel for Ascensia; had speaking engagements for Ascensia and Roche; received research grant/material support (to the University of Virginia) from Dexcom, Roche Diagnostics, Sanofi, and Tandem; and is receiving patent royalties managed by the University of Virginia Licensing and Ventures Group. J.L.R., C.L.B., C.A.W., M.C.O., P.K.C., L.J.H., R.S.K., L.M.N., S.E.L., and S.R.-D. have nothing to disclose. S.A.B. has received research grant/material support (to the University of Virginia) from Dexcom, Roche Diagnostics, Tandem, and Medtronic. T.T.L. has received research funding from Medtronic and Tandem and is currently employed by Insulet. B.P.K. has served on an advisory panel for Sanofi; had speaking engagements for Dexcom and Sanofi; received research grant/material support (to the University of Virginia) from Dexcom, Roche Diagnostics, Sanofi, and Tandem; and is receiving patent royalties managed by the University of Virginia Licensing and Ventures Group.
References
- 1. The DCCT Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2. Nathan DM, Cleary PA, Backlund JY, et al. : Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Boer IH DC.CT/EDIC Research Group: Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014;37:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gubitosi-Klug RA, Braffett BH, White NH, et al. : Risk of severe hypoglycemia in type 1 diabetes over 30 years of follow-up in the DCCT/EDIC study. Diabetes Care 2017;40:1010–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weinstock RS, Xing D, Maahs DM, et al. : Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab 2013;98:3411–3419 [DOI] [PubMed] [Google Scholar]
- 6. Leelarathna L, Dellweg S, Mader JK, et al. : Assessing the effectiveness of 3 months day and night home closed-loop insulin delivery in adults with suboptimally controlled type 1 diabetes: a randomised crossover study protocol. BMJ Open 2014;4:e006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruan Y, Bally L, Thabit H, et al. : Hypoglycaemia incidence and recovery during home use of hybrid closed-loop insulin delivery in adults with type 1 diabetes. Diabetes Obes Metab 2018;20:2004–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tauschmann M, Allen JM, Wilinska ME, et al. : Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2016;39:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tauschmann M, Thabit H, Leelarathna L, et al. : Factors associated with glycemic control during free-living overnight closed-loop insulin delivery in children and adults with type 1 diabetes. J Diabetes Sci Technol 2015;9:1346–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thabit H, Lubina-Solomon A, Stadler M, et al. : Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study. Lancet Diabetes Endocrinol 2014;2:701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thabit H, Tauschmann M, Allen JM, et al. : Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015;373:2129–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kovatchev B, Cheng P, Anderson SM, et al. : Feasibility of long-term closed-loop control: a multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther 2017;19:18–24 [DOI] [PubMed] [Google Scholar]
- 13. Garg SK, Weinzimer SA, Tamborlane WV, et al. : Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kovatchev BP: The artificial pancreas in 2017: the year of transition from research to clinical practice. Nat Rev Endocrinol 2018;14:74–76 [DOI] [PubMed] [Google Scholar]
- 15. Clarke WL, Cox DJ, Gonder-Frederick LA, et al. : Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 1995;18:517–522 [DOI] [PubMed] [Google Scholar]
- 16. Kovatchev BP, Otto E, Cox DJ, et al. : Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care 2006;29:2433–2438 [DOI] [PubMed] [Google Scholar]
- 17. Kovatchev BP, Cox DJ, Gonder-Frederick LA, et al. : Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care 1998;21:1870–1875 [DOI] [PubMed] [Google Scholar]
- 18. Kovatchev BP: Metrics for glycaemic control: from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol 2017;13:425–436 [DOI] [PubMed] [Google Scholar]
- 19. Faul F, Erdfelder E, Lang A-G, et al. : G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–191 [DOI] [PubMed] [Google Scholar]
- 20. Lachin JM: Statistical considerations in the intent-to-treat principle. Control Clin Trials 2000;21:167–189 [DOI] [PubMed] [Google Scholar]
- 21. Danne T, Nimri R, Battelino T, et al. : International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]