Abstract
Pre-frontal limbic circuitry is vulnerable to effects of stress and injury. We examined microstructure of pre-frontal limbic circuitry after traumatic brain injury (TBI) or extracranial injury (EI) and its relation to post-traumatic stress symptoms (PTSS). Participants aged 8 to 15 years who sustained mild to severe TBI (n = 53) or EI (n = 26) in motor vehicle incidents were compared with healthy children (n = 38) in a prospective longitudinal study. At the seven-week follow-up, diffusion tensor imaging was obtained in all groups; injured children completed PTSS ratings using a validated scale. Using probabilistic diffusion tensor tractography, pathways were seeded from bilateral amygdalae and hippocampi to estimate the trajectory of white matter connecting them to each other and to targeted pre-frontal cortical (PFC) regions. Microstructure was estimated using fractional anisotropy (FA) in white matter and mean diffusivity (MD) in gray matter. Pre-frontal limbic microstructure was similar across groups, except for reduced FA in the right hippocampus to orbital PFC pathway in the injured versus healthy group. We examined microstructure of components of pre-frontal limbic circuitry with concurrently obtained PTSS cluster scores in the injured children. Neither microstructure nor PTSS scores differed significantly in the TBI and EI groups. Across PTSS factors, specific symptom clusters were related positively to higher FA and MD. Higher hyperarousal, avoidance, and re-experiencing symptoms were associated with higher FA in amygdala to pre-frontal and hippocampus to amygdala pathways. Higher hippocampal MD had a central role in hyperarousal and emotional numbing symptoms. Age moderated the relation of white and gray matter microstructure with hyperarousal scores. Our findings are consistent with models of traumatic stress that implicate disrupted top-down PFC and hippocampal moderation of overreactive subcortical threat arousal systems. Alterations in limbic pre-frontal circuitry and PTSS place children with either brain or body injuries at elevated risk for both current and future psychological health problems.
Keywords: brain injury, diffusion tensor imaging, injury, limbic, post-traumatic stress, pre-frontal
Introduction
Exposure to significant stressful experiences during childhood may influence adversely acute psychosocial and health outcomes and contribute to a long-term cascade of adverse experiences.1,2 Traumatic injury is a major source of acute and chronic stress. As the leading cause of death and morbidity in the pediatric age range, traumatic injury is a significant public health concern and accounts for annual hospitalization of more than 135,000 children under 16 years of age in the United States.3
Traumatic injury is linked to dysregulation of the hypothalamic-pituitary-adrenal (HPA) and noradrenergic stress systems.4 Further, traumatic injury contributes to chronic negative physical and/or psychological health symptoms,5 such as post-traumatic stress disorder (PTSD) or significant subclinical post-traumatic stress symptoms (PTSS).6 The PTSS consist of four different symptom dimensions, including intrusive thoughts, avoidance, negative changes in mood and cognitions, and alteration in arousal and reactivity that persist more than one month after exposure.7 Significant PTSS occur in 25–57% of injured children8 and appear to be more strongly related to an individual's cognitive and emotional response to an injury event than to the type or severity of injury.9,10
Chlildren and adolescents in whom PTSS develop after traumatic brain injury (TBI) have additional risk for long-term psychosocial problems above and beyond the impact of TBI.11,12 At present, little is known about how different types of injuries and age at injury affect neural structures associated with PTSS. This gap in knowledge is particularly troubling because stress exposure may delay, accelerate, or prolong the normal trajectory of brain development.13 The purpose of the present article is to investigate the impact of brain and body injury on pathways connecting subcortical and cortical stress system components and their relation to age at injury and dimensions of PTSS.
Models of stress regulation
Neurobiological models of emotion emphasize the interrelation of several core structures that exert bottom-up and top-down influences to shape the generation and regulation of emotions, including fear.14 Bottom-up structures such as the amygdala initially encode the emotional salience of cues to allow rapid reactivity to stress or fear. The amygdalae then interact with control systems in the pre-frontal cortex (PFC) that engage cognitive control and appraisal strategies to manage emotional and behavioral responses.15 Under conditions of stress, the amygdala can activate arousal and stress reactivity through stimulation of the HPA axis and activation of the sympathetic nervous system via the hypothalamus.16 Importantly, the amygdala has bidirectional connectivity with PFC regions,17 which exert top-down regulatory and inhibitory control over excitatory amygdala activity.15,18
Components of the PFC implicated in the stress response include dorsolateral PFC (dlPFC), which regulates cognitive control functions including inhibition, allocation of attention, and working memory, and ventromedial PFC (vmPFC), which regulates emotional control. The parahippocampal and rostral divisions of the cingulum bundle (rACB) connect the entorhinal and anterior cingulate cortex (ACC) and project to and from the amygdala.19,20 The subgenual ACC has been related to assessment of emotion and active regulation of emotion. The hippocampus moderates activity of both the amygdala and PFC14 and contributes to consolidation and extinction of emotionally salient memories.15
The implications of childhood PTSS for bottom-up and top-down brain circuitry are not well understood. In typically developing children, control of PFC over subcortical structures increases throughout childhood, particularly during the transition through adolescence.14 With development, PFC pathways play a salient top-down role in moderating reactivity of the subcortical structures via greater activation of dorsomedial than vmPFC, resulting in greater cognitive control over emotional reactivity.21 The subcortical and cortical structures involved in stress regulation are sensitive to a wide range of environmental stress exposures that may have different impacts depending on timing of exposure.18,22 It is unknown, however, whether the effects of traumatic injury on the structures mediating stress regulation differ depending on developmental stage at injury or type of injury.
Structural neuroimaging of stress systems in children with PTSS and TBI
As noted above, evidence is accumulating that PTSD affects brain structures specifically engaged in recognition and regulation of fear. Structural neuroimaging studies of children with PTSS largely have examined volumes in children exposed to significant maltreatment and interpersonal violence. Recent meta-analyses of children with PTSS reported reduced intracranial, gray matter, and corpus callosum volumes relative to healthy children23; reduced volume of the amygdala, hippocampus, and PFC approached significance levels.24 Studies specifically evaluating fear circuitry in youth with PTSD noted reduced volume in bilateral hippocampi, left amygdala, and vmPFC relative to healthy or maltreated comparison groups without PTSD; no differences were reported in the ACC.25,26
In children with TBI, frontal and temporal lobes are common sites of injury. Consequently, the hippocampus27 and PFC28 are vulnerable particularly to disruption. Smaller total or regional hippocampal volumes have been noted during both subacute29 and long-term follow-up.30 Volume reduction is pronounced in the hippocampus relative to other regions across a broad range of injury severity.31 In a 10-year follow-up study, relative to healthy children, those experiencing TBI during early and middle childhood had reduced hippocampal volume irrespective of TBI severity, while amygdala volumes were only reduced in severely injured children.30 After pediatric TBI, widespread reduction in cortical thickness was reported bilaterally in superior, dorsolateral, and orbitofrontal PFC as well as the ACC.32
Structural neuroimaging studies using diffusion tensor imaging (DTI) allow quantitation of the nature and extent of microstructural changes in both white and gray matter in relation to developmental changes, stress, and physical injury. The DTI metrics include fractional anisotropy (FA), which is influenced by a number of factors including myelin thickness, directionality of movement of water molecules, and the density of white matter (WM) tracts.33 Increased myelination during childhood and adolescence causes axons to be more tightly packed together, which increases FA. Mean diffusivity (MD) may index several variables, including fiber density, axonal diameter, myelination, and expansion of extracellular space, which may indicate loss of neurons or glia.34 The MD generally decreases with normal neural maturation. Pediatric TBI is associated with decreased FA and/or increased MD in diverse commissural, projection, and association pathways, as well as in limbic pathways such as the cingulum bundle and frontal lobe white matter.35,36 The MD is also elevated in subcortical gray matter.36,37
Even though limbic structures, particularly frontal-subcortical circuits, appear to be at high risk for shearing and stretching forces generated by rotational acceleration,38 few structural neuroimaging studies have investigated the integrity of limbic structures after pediatric TBI. The DTI studies showed reduced FA in TBI patients compared with control groups in bilateral vmPFC, dlPFC, and the cingulum bundle.39 The few studies examining DTI metrics in relation to psychological health outcomes noted the onset of new psychiatric disorders after pediatric TBI was associated with lower FA in bilateral frontal lobe WM, uncinate fasciculus, and the centrum semiovale relative to youth with orthopedic injury (OI).40 Lower FA in the uncinate fasciculi predicted greater emotional control problems41 while lower FA and elevated MD in the ventral striatum were associated with lower cognitive control.42
Regarding gray matter structures, Juranek and colleagues37 found increased MD of the hippocampus and amygdala during the subacute stage of recovery after pediatric TBI; elevated MD in the amygdala correlated with self-reported anxiety.
Present study
Substantial overlap is evident in neuroimaging studies examining core components of altered limbic circuitry in TBI and PTSS, with altered structure and/or function reported in the amygdala, hippocampus, ACC, dlPFC, and orbital PFC (oPFC), and middle frontal gyrus.43–45 No studies have examined how injury to the brain or body occurring at different developmental stages, however, may influence structural connectivity of components of the fear circuit or their relation to specific components of PTSS. The purpose of the present study was to explore the impact of injury on brain circuitry related to fear and stress reactivity in children and adolescents experiencing single-incident trauma in vehicle accidents. The primary aim used diffusion tensor tractography (DTT) to compare the integrity of pathways from the amygdala and hippocampus to targets in the PFC in children with TBI or injury to other body regions relative to healthy comparison children.
Because of the vulnerability of the hippocampus to disruption by TBI, we expected that FA in the hippocampus and connecting pathways would differ in the healthy group compared with both injury groups and that FA would be lower in the TBI than in the extracranial injury (EI) group. Given the overlap in structural changes in persons exposed to TBI and to traumatic stress, we anticipated that amygdalar pathway FA would differ in the healthy group compared with both injury groups but would not differ between the TBI and EI groups.
The second aim was exploratory and investigated the relation of pathway integrity with different PTSS clusters. Specifically, hyperarousal symptoms were expected to relate to amygdala diffusivity and FA of pathways connected to the amygdala because of its role in activating autonomic stress responses. Based on the role of the hippocampus in emotional memory formation, hippocampal MD and FA of pathways connecting with the hippocampus were expected to correlate with re-experiencing, avoidance, and emotional numbing clusters.
Methods
Participants and recruitment
Youth ages eight to 15 years injured in vehicular accidents and treated in the Emergency Department or Level 1 Pediatric Trauma Center at Children's Memorial Hermann Hospital/University of Texas Health Science Center at Houston for either a TBI or EI were screened for enrollment. Children with EI were included to account for possible pre-injury characteristics, such as risk-taking behavior, that may influence outcomes, to examine the stresses of injury and hospitalization, and to allow comparison of the effects of brain injury above and beyond that of body injury. Children with EI were excluded if they had evidence of blunt head trauma or concussion symptoms. A healthy typically developing comparison group (TDC) was recruited from the community to allow assessment of possible differences on neuroimaging between the injury groups and healthy youth.
All participants in the current study met the following inclusion criteria: (1) proficiency in English or Spanish, (2) residence within a 125 mile catchment radius, (3) no previous history of major neuropsychiatric disorder (intellectual deficiency or low functioning autism spectrum disorder) that would complicate assessment of the impact of injury on behavioral outcomes, (4) no metabolic disorders, (5) no previous medically attended TBI, and (6) usable neuroimaging data. Criteria 3–5 were assessed during screening using a brief parent interview.
Of 277 children meeting study inclusion criteria, 150 were enrolled and completed baseline procedures. Of these children, 117 had a usable magnetic resonance imaging (MRI) scan and 33 had unusable scans (n = 19 motion artifact, n = 8 scanner equipment failure, n = 3 data did not meet quality control, n = 2 scan artifact; n = 1 withdrew from study). The final sample consisted of 117 participants (TBI, n = 53, EI, n = 26, TDC, n = 38). Informed written consent was obtained from each child's guardian according to Institutional Review Board guidelines; written assent was obtained from all participants.
Injury characteristics
Demographic characteristics of the TBI, EI, and TDC groups and injury variables for the TBI and EI groups are provided in Table 1. The external cause of injury in a vehicle accident was selected to meet the Diagnostic and Statistical Manual of Mental Disorders7 (DSM-V) criterion A for a PTSD diagnosis that specifies exposure to a potentially life-threatening situation. For TBI participants, severity of brain injury was rated using the lowest post-resuscitation Glasgow Coma Scale (GCS) score at admission, which evaluates eye opening, motor, and verbal responses to stimulation.46 Mild, moderate, and severe TBI were indicated by total GCS scores of 13–15, 9–12, and 3–8, respectively.
Table 1.
Demographic, Injury, and Psychological Health Information for Traumatic Brain Injury, Extracranial Injury, and Healthy Comparison Groups
| Group | ||||
|---|---|---|---|---|
| Traumatic brain injury (n = 53) | Extracranial injury (n = 26) | Healthy comparison (n = 38) | Statistic (df) (p) | |
| Demographic variables | ||||
| Age M (SD) | 12.4 (2.2) | 11.8 (2.4) | 12.3 (2.3) | F (2, 114) = 0.749 (0.475) |
| Age Range (years) | 8.1 – 15.9 | 8.6 – 16.2 | 8.1 – 16 | |
| Sex % Male | 60 | 69 | 61 | X2(2, N = 117) = 0.666 (0.717) |
| Race (n) | X2(4, N = 117) = 3.90 (0.419) | |||
| Caucasian | 37 | 22 | 26 | |
| African American | 14 | 4 | 9 | |
| Other/Multiracial | 2 | 0 | 3 | |
| Ethnicity (n) | ||||
| Hispanic | 25 | 15 | 24 | X2(2, N = 117) = 2.40 (0.301) |
| Maternal education (n) | X2(4, N = 117) = 8.35 (0.080) | |||
| < High school | 24 | 15 | 9 | |
| < College | 26 | 10 | 27 | |
| Graduate degree | 3 | 1 | 2 | |
| Injury variables | ||||
| Age at injury to testing interval (d) –M (SD) | 47.83 (16.36) | 50.62 (10.87) | — | t(77) = 0.786 (0.434) |
| Age at injury to scan interval (d) – M (SD) | 51.06 (21.42) | 57.04 (23.23) | — | t(77) = 1.134 (0.260) |
| Injury Severity Score (ISS) M (SD) | 12.62 (7.99) | 11.12 (6.30) | t(77) = −.842 (0.403) | |
| Modified ISS M (SD) | 3.58 (4.38) | 10.73 (6.39) | t(77) = 5.83 (0.000) | |
| Treatment intensity (n) | X2(2, N = 79) = 18.74 (0.000) | |||
| Released from ED | 14 | 9 | ||
| Admitted to hospital | 8 | 14 | ||
| Admitted to PICU | 31 | 3 | ||
| Length of stay (d) M (SD) | 4.08 (3.39) | 4.59 (4.45) | t(54) = 0.471 (0.639) | |
| Surgery n (%) | 8 | 12 | X2(1, N = 84) = 6.05 (.014) | |
| Glasgow Coma Score (n) | ||||
| 3–8 | 12 | – | ||
| 9–12 | 5 | – | ||
| 13–15 | 36 | – | ||
| Psychological variables | ||||
| Pre-injury or enrollment | ||||
| Preinjury diagnoses (n) | ||||
| ADHD | 10 | 3 | 2 | |
| Anxiety | 0 | 1 | 0 | |
| Depression | 0 | 1 | 0 | |
| Psychosocial Adversity Index M (SD) | 1.36 (1.09) | 1.31 (1.12) | 1.39 (1.30) | X2(2) = 0.067 (0.967) |
| Child Behavior Checklist (T score) M (SD) | ||||
| Internalizing | 51.06 (12.16) | 52.96 (12.98) | 49.13 (10.60) | F (2, 114) = 0.818 (0.444) |
| Externalzing | 49.53 (11.80) | 54.92 (12.42) | 45.95 (9.86) | F (2, 114) = 4.823 (0.010) |
| 7 week follow-Up | ||||
| Pubertal Development Scale M (SD) | 2.44 (.935) | 2.25 (1.07) | 2.49 (.888) | F (2, 114) = 0.542 (0.583) |
| Child PTSD Symptom Scale M (SD) | 10.04 (9.27) | 10.46 (7.07) | — | X2(1) = 0.537 (0.464) |
SD. standard deviation; ED, emergency department; PICU, pediatric intensive care unit; ADHD, attention-deficit/hyperactivity disorder; PTSD. Post-traumatic stress disorder.
The GCS scores were abstracted from the medical record by trained assistants. Severity of bodily injury was based on the Abbreviated Injury Scale (AIS), which classifies injury to specified anatomical regions on a scale from 1 to 6 (minor to life-threatening). The AIS scores were obtained from the hospital trauma registry. In children with mild or moderate TBI, skeletal or body AIS scores were limited to ≤2 to minimize any confounding influence of severe extracranial injury on accurate assessment of GCS scores. We calculated the Injury Severity Score (ISS), which incorporates the highest AIS scores from three anatomical regions and ranges from 0 to 75.47 For TBI participants, the modified ISS score excluding injuries to the head was also used.48
Study procedures
Parents completed questionnaires and interviews at baseline to describe the child's psychosocial and behavioral functioning just prior to enrollment in the study, which is standard procedure in pediatric injury studies.49 Estimates of PTSS and pubertal status, as well as neuroimaging studies, were obtained at the seven-week follow-up.
Pre-injury questionnaire and interview
Child Behavior Checklist (CBCL)50
The internalizing and externalizing T-scores normed for age and sex were examined to characterize pre-injury behavioral problems.
Pre-injury problems
Parents indicated whether the child had a diagnosis of attention-deficit hyperactivity disorder, anxiety, or depression before injury or study enrollment.
Psychosocial adversity
Exposure to adversity was assessed by trained interviewers based on six categories defined by Biederman and colleagues.51 The following common indicators were assessed: 1() severe marital discord defined as divorce or separation, (2) low social status defined as levels IV or V on the Hollingshead Index; (3) large family size, defined as three or more children living in the child's primary home; (4) history of investigation by protective service agency regarding this child; (5) parental criminal conviction; and (6) treatment of parental mental health problems. Items were scored yes/no and summed to yield a total score.
Seven-week follow-up questionnaires
Pubertal status
Children and parent independently rated changes associated with puberty, including growth in height, body hair, and skin changes using the Petersen Pubertal Development Scale (PDS).52 Sex-specific changes included breast development and menstruation for females and deepening of voice and facial hair for males. Each item was then coded on a 5-point scale similar to Tanner staging53; ratings were averaged to yield a score ranging from 1 (pre-pubertal) to 5 (post-pubertal). The scale has favorable internal consistency: α = 0.77. If ratings of parents and children differed by more than 1 point, they were asked to discuss and come to consensus; the consensus rating was used in analyses.
Post-traumatic stress symptoms
Consistent with the National Institute of Mental Health Research Domain Criteria framework,54 we measured PTSS dimensionally using a validated self-report scale. This approach allows a focus on the spectrum of symptoms that impact children who experience trauma and subsequent impairment. We administered the Child PTSD Symptom Scale55 (CPSS), a validated self-report scale yielding a total PTSS score created from summing 17 items rated on a 4-point scale (range 0–51). Based on factor analysis and the four factor model of PTSD, we divided items into re-experiencing, active avoidance, emotional numbing, and hyperarousal factors.56 An impairment rating assessed impact on daily activities. Children with TBI and EI completed the CPSS. A score ≥11 suggests high PTSS. The total score has high internal consistency, α = 0.89, and test-retest reliability, r = 0.84.
Psychological health interview:
Parents completed a structured interview regarding their child's current DSM-V PTSD symptoms.
Image acquisition and processing
Scans were acquired on a Philips 3T MR scanner with a 32-channel head coil at the University of Texas McGovern Medical School. Diffusion-weighted data collection was completed using an echo planar imaging sequence (TR = 8700 ms; TE = 67 ms; 65 slices total; square FOV = 240 mm2; slice thickness = 2.5 mm; 32 diffusion-weighted volumes with b = 1,000 s/mm2 and 1 volume with b = 0) lasting 6:11 minutes. High resolution T1-weighted anatomical scans were collected (TR = 8.1, TE = 3.7, flip angle = 6°, matrix = 256 X 256, slice thickness = 1mm, and voxel size = 1 × 1 × 1); the scan duration was 4:47 minutes. The scanning facility replaced the scanner with a Philips 3T Ingenia toward the end of data collection and scans for 48 of our participants were collected after the upgrade; scanner change was accounted for in all analyses. Fidelity analysis was performed to match diffusion-weighted and T1-weighted scanning protocols.
Seed and target regions for DTI tractography were generated on a single subject basis to reflect the morphology of each individual. An automated image analysis suite, FreeSurfer 5.3.0 (http://surfer.nmr.mgh.harvard.edu), was used for parcellation of cortical and subcortical structures.57 Following standard processing procedures in Freesurfer, all data were visually inspected and edited to correct pial surfaces, white/gray matter boundaries, and accuracy of subcortical segmentation. Any error in parcellation resulting from lesions was corrected during manual editing.
Following manual correction, data were re-processed in Freesurfer. Quality of parcellation results was then reviewed and registered to the corresponding diffusion-weighted images for each subject using FSL's Linear Image Registration Tool (FSL: FLIRT). This process resulted in the creation of the seed regions for DTI analysis, restricted to each individual's anatomy, corresponding to all of the regions listed in Table 2. The same process was used to generate exclusion masks used in DTI analysis corresponding to ventricular regions.
Table 2.
Spearman Partial Correlation Coefficients of Regional Diffusion Tensor Tractography Microstructure with Injury Severity for Traumatic Brain and Extracranial Injury Groups
| Traumatic brain injury (n = 53) | ||||
|---|---|---|---|---|
| Diffusion tensor tractography metrics | Admission GCS Score df (49) | Modified Injury Severity Score df (49) | Extracranial injury (n = 26) Modified Injury Severity Score df (22) | |
| Pathway fractional anisotropy | r (p) | r (p) | r (p) | |
| Origin | Termination | |||
| Left hemisphere | ||||
| Amygdala | ||||
| oPFC | 0.108 (0.450) | −0.162 (0.257) | −0.006 (0.978) | |
| rACB | 0.464 (0.001) | 0.001 (0.996) | 0.168 (0.433) | |
| Hippocampus | ||||
| Amygdala | 0.155 (0.277) | −0.169 (0.236) | −0.033 (0.113) | |
| oPFC | 0.208 (0.144) | 0.103 (0.470) | 0.263 (0.214) | |
| Right hemisphere | ||||
| Amygdala | ||||
| oPFC | 0.294 (0.036) | 0.156 (0.275) | −0.041 (0.850) | |
| rACB | 0.102 (0.477) | 0.012 (0.931) | 0.081 (0.706) | |
| Hippocampus | ||||
| Amygdala | −0.113 (.430) | 0.131 (0.358) | −0.018 (0.933) | |
| oPFC | 0.348 (0.012) | −0.141 (0.325) | 0.520 (0.009) | |
| Gray matter mean diffusivity | ||||
| Left hemisphere | ||||
| Amygdala | −0.362 (0.009) | 0.038 (0.793) | 0.133 (0.534) | |
| Hippocampus | −0.278 (0.048) | 0.223 (0.116) | 0.156 (0.468) | |
| hight Hemisphere | ||||
| Amygdala | −0.235 (0.097) | 0.227 (0.109) | −0.100 (0.642) | |
| Hippocampus | −0.425 (0.002) | 0.132 (0.357) | 0.181 (0.397) | |
GCS, Glasgow Coma Scale; oPFC, orbital prefrontal cortex; rACB, rostral anterior cingulate bundle.
Note: Age at scan and scanner upgrade partialled.
Processing of DTI data was performed using the standard DTI pipeline in the FMRIB Software Library (FSL) 5.0.1 (http://www.fmrib.ox.ac.uk/fsl). Eddy Correct was used to reduce eddy current distortions and head movement.58 Skull-stripping of non-brain tissue was performed using BET (Brain Extraction Tool)59 and brain-extracted images were inspected and corrected to ensure their accuracy. DTIFIT was then used to calculate FA and MD at each brain voxel, and in preparation for tractography, Bayesian Estimation of Diffusion Parameters Obtained using Sampling Techniques (BedpostX) was then applied.
A probabilistic tractography algorithm (FSL: probtrack × 2) was utilized for seed-to-target fiber tracking in diffusion space from each seed region individually. For each voxel in a seed mask, 5000 samples were drawn from the probability distribution of the principal fiber direction, proceeding every 0.5 mm. Tracking was stopped if the streamline reached a target mask or any of the exclusion masks; in the latter case, the path was discarded. Pathway distribution maps were then normalized to account for the numbers of voxels in the seed masks by dividing the total number of streamline samples initiated from a seed region by the “waytotal,” the total number of streamlines that reach the target mask.
To restrict each tract to only white matter, pathway distribution maps were thresholded to include those voxels with FA >0.2. Seed-to-target combinations were: (1) amygdala to ipsilateral oPFC, (2) amygdala to ipsilateral rostral anterior cingulum bundle (rACB), (3) hippocampus to ipsilateral amygdala, and (4) hippocampus to ipsilateral oPFC. The MD of bilateral amygdalae and hippocampi was also obtained. Amygdala to oPFC pathways largely overlap with white matter tracts of the uncinate fascicles.
Statistical approach
After examination of variable distributions, comparability of demographic and psychosocial variables for the TBI, EI, and healthy groups was examined using analysis of variance or chi square. Psychosocial variables were examined to determine whether the three groups had comparable life adversity as well as internalizing and externalizing behavior problems before study enrollment. Bivariate relations of age, sex, and pubertal development with the tractography metrics were examined to assess whether the demographic variables should be considered as covariates.
To account for possible differences related to scanner upgrading, we included scanner upgrade as a covariate in all analyses using tractography data. To assess group differences, general linear models examined effects of group, age (continuous), and their interactions on tractography values. Orthogonal planned comparisons examined the relation of (1) both injury groups versus the healthy group and (2) the TBI versus the EI group with FA from each pathway and MD from the amygdala and hippocampus. Non-significant interactions of age by the group contrasts were trimmed from each model. Effect sizes were calculated using Hedges g for group comparisons with p < 0.1.
To address the exploratory second aim, Spearman partial correlation coefficients controlling for age and scanner upgrade examined the relation of the tractography pathway FA and gray matter MD with injury variables. We then examined whether the relation of pathway with CPSS factor scores was similar across TBI and EI groups. To assess whether hippocampal microstructure differed, generalized linear models with a negative binomial distribution and log link function examined the effects of group (TBI, EI), FA of pathways seeded in the hippocampus or hippocampal MD, and their interaction on CPSS scores controlling for age and scanner upgrade. Non-significant group by pathway interactions were trimmed. Finally, generalized linear models were fitted to examine the relation of region microstructure and age to the CPSS scores for all injured participants irrespective of injury group.
Results
Participant characteristics
Demographic, injury, and psychosocial variables were compared across the healthy comparison group and the TBI and EI groups (Table 1). The groups did not differ significantly on age, sex, race, ethnicity, or maternal education. Regarding injury variables, the type of vehicular accident was similar across TBI and EI groups; most patients were injured in vehicle or vehicle-pedestrian collisions (Table 1). The ISS did not differ significantly in the TBI and EI groups. On the modified ISS scale excluding the head, the EI group had significantly greater severity of body injury than the TBI group.
Although the length of hospital stay was similar across groups, the patients with TBI had greater treatment intensity based on level of hospital care. For the TBI group, 18.9% sustained extremity injuries and approximately 21% sustained injury to the abdomen or chest. For the EI group, 81% sustained an extremity injury while 58% had internal injuries involving the abdomen or chest. The severity of TBI ranged from mild to severe; 23% of the sample sustained severe TBI and 68% sustained mild TBI. Twenty-one of the 53 participants with TBI had lesions visualized on clinical reads of the MRI scan. The type and number of lesions were encephalomalaica (n = 8), gliosis (n = 5), and shear (n = 14). One participant with a large lesion was excluded from analysis.
Psychosocial adversity ratings and CBCL internalizing and externalizing T-scores did not differ across groups, suggesting comparable child adjustment and trauma exposure before enrollment. At the follow-up, pubertal status was similar across groups. Pubertal status and age at baseline assessment were highly correlated, r = 0.78, p < 0.001. Because of the substantial overlap in these variables, only age was included in analyses.
The mean CPSS score did not differ across TBI and EI groups; more than half of the participants in each group reported moderate to high levels of PTSS. Based on the parent interview, 23% of each injury group met criteria for Trauma and Stressor Related Disorders. Three children with TBI and two with EI met DSM-V criteria for diagnosis with PTSD; nine children with TBI and four with EI had other specified trauma- and stressor-related disorders because their symptoms did not meet full criteria.
Relation of injury with limbic microstructure and PTSS
Measures of injury severity were related to microstructural integrity. For the TBI group, the admission GCS score was significantly positively related to FA from several pathways and negatively related to MD of the left amygdala and bilateral hippocampi (Table 2). The modified ISS was not related to any DTT metrics in the TBI group and was only significantly positively related to FA from the right hippocampus to oPFC in the EI group.
For the orthogonal planned group comparisons, the group X age interactions were not significant and were trimmed from each model. Table 3 shows the least squares mean FA values for each trimmed model and statistical tests organized by pathway origin and MD values for the amygdala and hippocampus for each hemisphere. For pathways, FA from the right hippocampus to oPFC was significantly lower in injured relative to healthy children, F(1,112) = 5.46, p = 0.021, with a moderate effect size (Hedges g = 0.48), but did not differ significantly in the TBI versus EI participants. None of the other group comparisons examining either FA or MD values was significant.
Table 3.
Least Squares Means and Statistical Tests for Planned Group Comparisons of Pathway and Gray Matter Microstructure
| Injury groups | |||||||
|---|---|---|---|---|---|---|---|
| Pathways | TBI | EI | Group effect F (P) | ||||
| Origin | Termination | Fractional anisotropy Least squares mean | Healthy comparison | Injury vs. healthy | TBI vs. EI | Age effect F (p) | |
| Left hemisphere | |||||||
| Amygdala | |||||||
| oPFC | 0.33 | 0.32 | 0.33 | 0.26 (0.611) | 0.70 (0.404) | 0.95 (0.333) | |
| rACB | 0.35 | 0.37 | 0.35 | 0.88 (0.350) | 2.08 (0.152) | 0.30 (0.584) | |
| Hippocampus | |||||||
| Amygdala | 0.30 | 0.30 | 0.30 | 0.03 (0.858) | 0.04 (0.837) | 0.01 (0.910) | |
| oPFC | 0.35 | 0.35 | 0.36 | 3.61 (0.060) | 0.27 (0.605) | 2.68 (0.105) | |
| Right hemisphere | |||||||
| Amygdala | |||||||
| oPFC | 0.33 | 0.33 | 0.33 | 0.48 (0.491) | 0.85 (0.360) | 2.22 (0.139) | |
| rACB | 0.34 | 0.36 | 0.36 | 3.46 (0.066) | 3.56 (0.062) | 1.71 (0.194) | |
| Hippocampus | |||||||
| Amygdala | 0.30 | 0.30 | 0.30 | 0.92 (0.341) | 0.00 (0.964) | 3.59 (0.061) | |
| oPFC | 0.36 | 0.35 | 0.37 | 5.46 (0.021) | 0.27 (0.604) | 5.21 (0.024) | |
| Subcortical gray matter | Mean diffusivity Least squares mean | ||||||
| Left hemisphere | |||||||
| Amygdala | 0.85 | 0.85 | 0.85 | 0.84 (0.361) | 0.04 (0.841) | 2.38 (0.126) | |
| Hippocampus | 1.03 | 1.02 | 1.01 | 0.61 (0.434) | 0.11 (0.744) | 0.72 (0.397) | |
| Right hemisphere | |||||||
| Amygdala | 0.83 | 0.84 | 0.83 | 0.89 (0.348) | 2.42 (0.122) | 8.47 (0.004) | |
| Hippocampus | 1.00 | 1.00 | 0.99 | 0.30 (0.585) | 0.06 (0.800) | 2.38 (0.126) | |
TBI, traumatic brain injury; EI, extracranial injury; oPFC, orbital pre-frontal cortex; rACB, rostral anterior cingulate bundle.
Small effect sizes were obtained for healthy versus injury group comparisons for left hippocampal to oPFC (g = 0.33) and right amygdala to rACB (g = 0.37). The effect size for the latter pathway between TBI and EI groups was negligible (g = 0.05). With increasing age, FA of the right hippocampus to oPFC tract increased significantly and MD of the right amygdala decreased.
For aim 2, we first assessed whether TBI influenced the relation of hippocampal microstructure to PTSS factor scores. Generalized linear models examined FA from hippocampal pathways, group (TBI and EI), and their interaction on PTSS factor scores. The group by region effect was significant only for the left hippocampus to amygdala pathway predicting re-experiencing, χ2(1) = 4.82, p = 0.028, and approached significance for active avoidance, χ2(1) = 3.71, p = 0.054. Higher FA was associated with lower re-experiencing levels in the EI group, r (22) = -0.526, p = 0.008, and was unrelated to symptom burden in the TBI group, r (48) = 0.005, p > 0.1. Higher FA was associated with elevated avoidance symptoms only in the TBI group, r (48) = 0.349, p = 0.013. For the remaining pathways, neither the group × region interaction nor main effect of group was significant for FA of pathways originating in the hippocampus or hippocampal MD.
Generalized linear models were then used to examine the relation of pathway or gray matter microstructure, age, and their interaction on the PTSS subscores independent of group. Table 4 provides the age × region interaction effects for significant analyses; non-significant interaction terms were trimmed from the remaining models, and main effects for region and age are provided. Figure 1 shows the pattern of relations of regional limbic microstructure with the PTSS scores. Hyperarousal symptoms increased with age.
Table 4.
Generalized Linear Model Main Effects for Pathway Fractional Anisotropy or Gray Matter Mean Diffusivity and Age Predicting Post-Traumatic Stress Cluster Scores
| Left hemisphere | Right xemisphere | |||||||
|---|---|---|---|---|---|---|---|---|
| Pathway | Region | Age | Region x Age | Region | Age | Region x Age | ||
| Factor Score | Origin | Termination | X2 (p) | X2 (p) | X2 (p) | X2 (p) | X2 (p) | X2 (p) |
| Pathway fractional anisotropy | ||||||||
| Arousal | ||||||||
| Amygdala | oPFC | 3.99 (0.046) | 5.23 (0.022) | 4.99 (0.026) | 6.43 (0.011) | |||
| rACB | 3.30 (0.069) | 6.74 (.0055) | 4.80 (0.029) | 0.48 (0.489) | 6.44 (0.011) | |||
| Hippocampus | Amygdala | 0.59 (0.467) | 6.69 (0.010) | 0.83 (0.364) | 6.45 (0.011) | |||
| oPFC | 0.95 (0.331) | 5.71 (0.017) | 0.06 (0.805) | 6.01 (0.014) | ||||
| Active avoidance | ||||||||
| Amygdala | oPFC | 0.96 (0.328) | 0.01 (0.916) | 0.64 (0.409) | 0.02 (0.886) | |||
| rACB | 0.30 (0.584) | 0.03 (0.868) | 0.47 (0.494) | 0.00 (0.965) | ||||
| Hippocampus | Amygdala | 0.01 (0.905) | 0.02 (0.896) | 4.68 (0.031) | 0.06 (0.811) | |||
| oPFC | 0.06 (0.807) | 0.01 (0.925) | 0.14 (.0704) | 0.00 (0.976) | ||||
| Emotional numbing | ||||||||
| Amygdala | oPFC | 1.19 (0.275) | 0.52 (0.473) | 0.59 (.0443) | 0.53 (0.467) | |||
| rACB | 0.38 (0.538) | 0.62 (0.432) | 2.75 (0.097) | 0.08 (0.773) | ||||
| Hippocampus | Amygdala | 0.36 (0.549) | 0.81 (0.368) | 1.10 (0.294) | 0.53 (0.465) | |||
| oPFC | 0.15 (0.704) | 0.57 (0.452) | 0.95 (0.329) | 1.11 (.0292) | ||||
| Re-experiencing | ||||||||
| Amygdala | oPFC | 1.26 (0.263) | 1.36 (0.243) | 0.83 (0.362) | 0.81 (0.368) | |||
| rACB | 0.01 (0.755) | 0.88 (0.348) | 0.64 (0.425) | 1.07 (0.301) | ||||
| Hippocampus | Amygdala | 1.25 (0.264) | 0.80 (0.372) | 0.38 (0.539) | 0.94 (0.333) | |||
| oPFC | 0.85 (0.355) | 0.98 (0.322) | 0.05 (0.826) | 0.90 (0.344) | ||||
| Gray matter mean diffusivity | ||||||||
| Arousal | ||||||||
| Amygdala | 0.08 (0.781) | 6.38 (0.012) | 0.03 (0.857) | 5.84 (0.016) | ||||
| Hippocampus | 5.11 (0.024) | 4.76 (0.029) | 4.21 (0.040) | 5.20 (0.023) | 5.20 (0.023) | 4.55 (0.033) | ||
| Active avoidance | ||||||||
| Amygdala | 0.49 (0.482) | 0.00 (0.965) | 0.28 (0.599) | 0.09 (0.759) | ||||
| Hippocampus | 0.30 (0.584) | 0.00 (0.966) | 0.18 (0.676) | 0.00 (0.994) | ||||
| Emotional numbing | ||||||||
| Amygdala | 0.07 (0.785) | 0.72 (0.396) | 0.01 (0.932) | 0.55 (0.458) | ||||
| Hippocampus | 4.27 (0.039) | 1.46 (0.227) | 1.47 (0.226) | 1.19 (0.275) | ||||
| Re-experiencing | ||||||||
| Amygdala | 0.13 (0.724) | 0.96 (0.326) | 0.61 (0.436) | 1.33 (0.249) | ||||
| Hippocampus | 0.19 (0.661) | 0.95 (0.329) | 0.08 (0.773) | 0.93 (0.335) | ||||
oPFC, orbital prefrontal cortex; rACB, rostral anterior cingulate bundle.
Scanner upgrade included as a covariate.
FIG. 1.
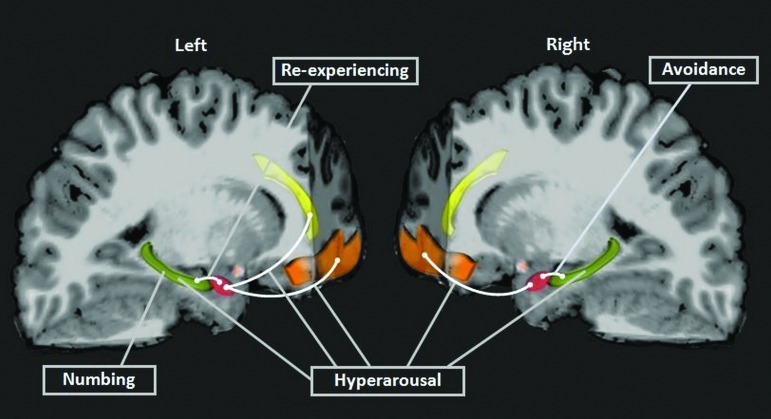
Post-traumatic stress symptoms clusters showed specific relations with limbic microstructure. Higher fractional anisotropy (FA) in the left hippocampus to amygdala pathway was associated with lower re-experiencing in the exracranial injury (EI) group and with higher avoidance in the traumatic brain injury (TBI) group. For both TBI and EI groups combined, hyperarousal was linked to higher FA of bilateral pathways connecting the amygdalae with orbital pre-frontal cortical (PFC), left amygdala to rostral anterior cingulate bundle, as well as to mean diffusivity (MD) of bilateral hippocampi. Avoidance was also related to higher FA of the right hippocampus to amygdala tract. Numbing was predicted specifically by elevated left hippocampal MD. Amygdala-red, hippocampus-green, orbital PFC-orange, rostral anterior cingulate bundle-yellow. Pathways indicated in white.
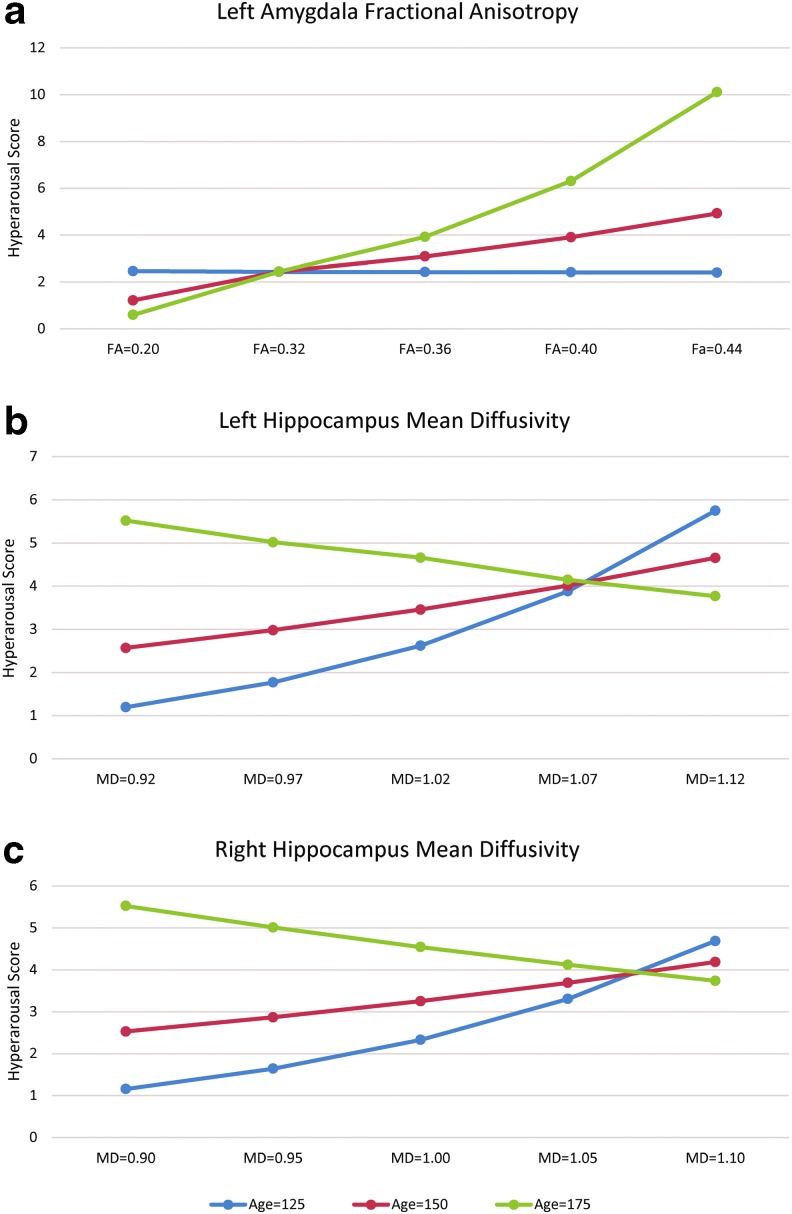
For hyperarousal scores, the region × age interaction was significant for the left amygdala to rACB FA and for bilateral MD. The interactions of age and DTI metrics on hyperarousal scores were decomposed by estimating FA values with age at the mean and ±1 standard deviation around the mean (Fig. 2). For the left amygdala to rACB pathway, FA values were not related to arousal scores in younger patients (p > 0.5) but approached significance at the mean age (χ2(1) = 3.57; p = 0.059), and were significant at older ages (χ2(1) = 7.97; p = 0.005). The interactions of age with hippocampal MD were significant at younger ages (left, χ2(1) = 5.64; p = 0.018; right, χ2(1) = 4.64; p = 0.031) but not at mid- and older ages (ps > 0.1).
FIG. 2.
Age × region interactions for hyperarousal scores. At older ages, hyperarousal increased as fractional anisotropy (FA) increased in the pathway connecting the left hippocampus and amygdala (a). In younger participants, hyperarousal scores increased as mean diffusivity (MD) of the left (b) and right (c) hippocampi increased.
In main effects models, hyperarousal was also predicted by higher FA in bilateral pathways linking the amygdala to oPFC. Active avoidance was predicted by higher FA of the right hippocampus to amygdala pathway. Emotional numbing was predicted by higher left hippocampal MD.
Discussion
We used DTT during the subacute stage of recovery to investigate the relation of pediatric brain and body injury with microstructure of core limbic regions that support bottom-up and top-down regulation of stress responses. Analyses of pre-frontal limbic structural connectivity indicated FA was significantly lower in injured children relative to the healthy group in the pathway connecting the hippocampus with oPFC. The FA was also lower in specific pathways connecting hippocampus and amygdala with PFC based on small effect sizes.
Overall, we did not find an additive impact of brain injury on either PTSS or on the tractography measures. Neither DTT microstructure nor PTSS differed significantly in the TBI and EI groups. The lack of difference in PTSS across injury groups is consistent with previous work suggesting that the subjective experience of being injured, rather than the type of injury, is a primary predictor of PTSS.9 Exploratory analyses indicated that regional microstructure was associated with specific PTSS factor scores in injured children.
Hyperarousal increased with age and was associated with microstructural metrics in pathways emanating from both amygdalae to pre-frontal targets and to bilateral hippocampal MD. Avoidance and re-experiencing clusters were associated specifically with FA of pathways connecting the hippocampus and amygdala while emotional numbing correlated with hippocampal MD. Higher re-experiencing was associated with higher FA in the hippocampus to amygdala pathway for the EI, but not TBI group.
These findings underscore the central role of both the amygdala and hippocampus in regulating the expression of PTSS. Both brain and body injury showed relations with the neural systems related to regulation of stress, placing children with a range of traumatic injuries at elevated risk for future psychological health problems and reduced health-related quality of life.2,60
Relation of traumatic injury with limbic circuitry
Consistent with our hypothesis, both injury groups showed lower FA relative to the healthy group in several pathways connecting the hippocampus and amygdala with pre-frontal targets. Our findings extend previous studies of pediatric TBI that noted reduced FA in a variety of limbic pre-frontal pathways, including the cingulum bundle,35,61 uncinate fasciculus,41 frontal lobe WM,62 the centrum semiovale, corona radiata, and temporal lobe WM relative to either typically developing children63 or those with orthopedic injuries.64–67 Because of the vulnerability of the hippocampus to disruption by TBI,27,29,68–70 we anticipated that FA from pathways seeded in the hippocampus and hippocampal MD would be reduced in the TBI group compared with the EI group. Based on the GCS score, severity of TBI was significantly associated with decreased FA in a number of pathways and increased MD in the hippocampus and amygdala.
Despite the impact of TBI severity on microstructure, the effects of TBI and EI did not differ significantly from each other on any pathway or subcortical gray matter. Few previous studies have used DTI to examine microstructure of subcortical limbic gray matter structures in injured children. Increased MD in the bilateral hippocampi and right amygdala during subacute stages of recovery37 and in the left ventral striatum 10 years after moderate to severe TBI42 have been reported in relation to orthopedic injury and healthy comparison groups, respectively.
Compared with previous studies, our sample has broader representation of TBI severity because two-thirds sustained mild or complicated-mild TBI. Microstructural changes are more prominent in patients with greater severity of injury and are not consistently found in studies of mild TBI.71 Scanning at longer intervals after injury may also highlight degenerative changes that are easier to detect in long-term rather than subacute stages of recovery.
Based on effect sizes, injury was associated with altered microstructure of pathways from the amygdala and hippocampus to targets in the PFC, but not to MD of these structures. Our findings suggest vulnerability of subcortical/PFC circuitry to the effects of both TBI and EI that did not interact with age of trauma exposure. With increasing age, we found increased FA of right hippocampal projections to the amygdala and oPFC as well as decreased MD of the right amygdala.
Although microstructural maturation of PFC WM and the cingulum bundle extend into adulthood,72 very little is known about microstructural maturation of the amygdala and hippocampus. This is a major gap in the literature because the amygdalae and hippocampi are particularly sensitive to effects of a wide range of environmental insults,73,74 especially at ages corresponding to pubertal transition.75 Resting state (rsfMRI) and task-based functional MRI (fMRI) studies indicated that although the amygdale mature relatively early, their development of functional connections with pre-frontal lobe structures is protracted.76 Further, functional connectivity of bilateral amygdalae as a whole becomes more constrained with increasing age except for increasing connectivity with bilateral hippocampi,77 which is consistent with a shift to greater PFC engagement and top-down control between childhood and adolescence.21,78
Limbic circuitry in relation to PTSS
The majority of neuroimaging studies examining changes associated with pediatric PTSS are based on samples exposed to interpersonal trauma or adversity. The few whole brain and region of interest studies employing DTI in maltreated children indicated reduced FA or increased diffusivity of the cingulum bundle, fornix, uncinate fasciculus, and corpus callosum.79,80–82 Recent application of graph theory to DTI-derived data suggested that maltreatment reduces the proportion of fiber streams connecting frontal lobes and limbic regions with basal ganglia and occipital regions and disrupts connectivity in local and global networks.83,84
Similar findings were noted based on graph theory analysis of DTI tractography in children exposed to natural disasters. Pre-frontal-limbic-striatal network abnormalities were found predominantly in the left hemisphere in youth in whom PTSD developed compared with trauma-exposed controls.85 Although additional studies are needed to parse how various types of trauma sustained at different ages impact neural architecture and connectivity assessed at different points in time, both single-incident and more protracted interpersonal traumas appear to impact the microstructure and connectivity of frontal-limbic networks as well as their integration with more posterior network components.
Given the limited research on DTT-based structural changes in youth with PTSD, alterations examined via functional imaging and volumetric studies can inform current findings. Task-based and rsfMRI studies converge with structural studies in showing abnormalities in pre-frontal limbic circuitry as well as alterations in activation and functional connectivity with age in youth with PTSD. Recent task-based studies identified hyperactivation of the dACC, but not the amygdala, to threat.86 Developmental differences were apparent in amygdalar-vmPFC connectivity because youth with PTSD had greater connectivity at younger ages and reduced connectivity at older ages compared with healthy controls.86
Relative to healthy youth, rsfMRI network analysis revealed that youth with PTSD had increased connectivity within the default mode network, including medial PFC and posterior cingulate cortex, which is involved in internally directed thought. Connectivity of the default mode network was decreased with both the salience and attentional control networks that are implicated in threat detection and cognitive control, respectively.87
Herringa88 inferred that several factors may place trauma-exposed children at increased risk for heightened stress sensitivity. These factors include hyperactivity in components of the salience network, including the dorsal ACC and amygdala, which monitor both internal and external threat cues, and increased anticorrelation or functional competition of the default mode network with salience and executive control networks. The effects of PTSD in youth, including reduced hippocampal volume, increased amygdala reactivity, and decreased amygdala-medial PFC connectivity, emerge over time and differ from commonly observed patterns in adults with PTSD.88
Relation of limbic structures and age with PTSS clusters
In our sample of children exposed to the stress of injury in a vehicle incident, we completed exploratory analyses of the relation of PTSS clusters with FA of core limbic pre-frontal pathways, as well as with diffusivity of the amygdalae and hippocampi based on a model of emotion regulation. Hyperarousal symptoms were the only PTSS that increased with age. This symptom cluster was also the only PTSS associated with DTT metrics from both hemispheres. As predicted, increased hyperarousal was associated with increased FA of pathways connecting both amygdalae to oPFC and the left amygdala to rACB, but not with amydgalar diffusivity.86
In a previous study with the same sample, we identified elevated reactivity of salivary alpha amylase, a surrogate marker of autonomic nervous system arousal, in response to a stressor six months after injury in adolescents with TBI but not EI.4 High levels of noradrenergic signaling may contribute to emotion dysregulation by inhibiting pre-frontal regulatory systems, resulting in increased activity and reactivity in the amygdala.89
Our finding that hyperarousal symptoms increased with age supports the hypothesis that the transition to adolescence involves increasing amygdala activation of autonomic and HPA stress response systems.60,90 Hyperarousal symptoms were also influenced jointly by age and hippocampal microstructure; children with elevated hippocampal MD had higher self-reported arousal, while there was no relation among adolescents. It is possible that alteration in hippocampal MD during the subacute stage of physical injury may be a precursor to the reduced volume often noted in long-term outcome studies of children and adults with diverse childhood trauma exposures75,91 as well as those with TBI.69
The hippocampi appear to play a central role in active avoidance, emotional numbing, and re-experiencing. Active avoidance was related specifically to higher FA in the right hippocampus to amygdala pathway; emotional numbing was associated with elevated MD in the left hippocampus. Exploring across imaging modalities, structural MRI studies found that both symptoms were related to reduced volume of the right anterior hippocampus and right subgenual ACC.26 Further, pediatric fMRI studies found that the severity of both avoidance and numbing symptoms was correlated with lower activation of the left hippocampus.92 Wolf and Heringa86 found an inverse relation between amygdala-medial PFC functional connectivity and avoidance.
Greater re-experiencing symptoms were predicted by higher FA in the left hippocampus to amygdala pathway for the EI, but not TBI, participants. The FA was unrelated to symptom burden in the TBI group and negatively associated with re-experiencing symptoms in the EI group. Higher re-experiencing cluster scores were related to reduced volume of the right subgenual ACC and right anterior hippocampus in children.26 Higher re-experiencing symptoms were related to increased connectivity of the posterior cingulate cortex and the inferior parietal gyrus.87,93 Our findings extend those of previous studies by suggesting that avoidance, numbing, and re-experiencing PTSS are also associated with the microstructural architecture of bottom-up subcortical gray matter structures regulating threat reactivity and emotional memory consolidation, particularly the hippocampus, that may contribute to alterations in the top-down components of functional networks regulating stress responses.
The mechanisms through which stress influences pediatric brain development are just beginning to be investigated. Severe stress may alter the timing and trajectory of normal brain development.94 Across PTSS factors, we found a positive relation of specific symptom clusters with white matter architecture. Higher hyperarousal, avoidance, and re-experiencing symptoms were associated with higher FA in core limbic pre-frontal and hippocampus to amygdala pathways. It is possible that this association reflects acceleration of pathway development or other alteration of microstructure. In contrast, TBI has been associated with reduced pathway FA likely reflecting stunting of subsequent brain development.35,62 Additional longitudinal studies are needed to disentangle the impact of both TBI and stress on brain structure and function and the trajectory of brain development.
As emphasized by Weems and colleagues,13 PTSS rarely has been examined within a neurodevelopmental network framework that examines potential moderators of associations between stress exposure, age, and neural outcomes. The present study used a neurodevelopmental framework and tested interactions of age with brain metrics on PTSS. We found that age at trauma exposure did not interact with TBI or EI, suggesting that the impact of subacute injury in both groups was similar across age. Age interacted with DTT metrics on hyperarousal symptoms, however, such that greater FA of the left amygdala to rACB pathway was related to greater hyperarousal symptoms at higher ages and hyperarousal symptoms increased at younger ages as MD increased in both hippocampi.
A number of variables, including type of trauma and developmental stage at the time of stress exposure, may shape the brain's response to injury and/or stress. Future studies examining interactions of age at traumatic exposure and age at assessment with time are needed to understand fully the interplay of these variables in relation to brain development and the expression of PTSS at different developmental stages.
Limitations
Our study findings should be interpreted in light of some limitations. Our sample was exposed to threat from vehicular incidents and received treatment or hospitalization. Consequently, our findings may not generalize to children with different types of injury mechanisms or those not seeking treatment. We examined the impact of injury on a pre-determined group of limbic structures based on a model of emotion regulation.11 It is possible that other structures and pathways that were not included also have salient associations with PTSS. The data are from a single time point after injury; longitudinal investigation of microstructure and symptom burden may show different effects of injury on the development of fear circuitry and allow determination of whether certain patterns of brain-PTSS relations are adaptive or maladaptive.
Even though our groups did not differ on pre-injury measures of psychological health, it is possible that relations of imaging and PTSS factors reflect pre-injury characteristics. Given the heterogeneity in injury outcomes, a larger sample would provide more power to detect injury-related changes as well as potential differences between brain and body injury.
Our scanner was upgraded at the midpoint in data collection. Although significant efforts were made to maintain the same high signal-to-noise ratio before and after the scanner upgrade, which included within-subject fidelity analysis of DTI and T1 scans, scanner upgrade was included as a covariate in all analyses to account for any remaining signal variability.
Despite its limitations, our article also has several strengths, including selection of targeted pre-frontal limbic regions based on a model of emotion regulation. We also examined both white and gray matter regions, including pathways connecting the hippocampus and amygdala. We used a dimensional approach to assessment of PTSS, which allowed us to investigate the spectrum of PTSS. To our knowledge, ours is the first article to explore the possible interaction of age at injury and DTI metrics from limbic pre-frontal regions on PTSD or PTSS factors in children.
Conclusions
Our findings at seven weeks after pediatric injury suggest that traumatic stress from even a single incident may affect core components of limbic pre-frontal networks, resulting in reduced top-down inhibition of emotional reactivity. Limbic microstructure and PTSS did not differ between participants with brain injuries compared with those with body injuries. Higher hyperarousal, avoidance, re-experiencing, and emotional numbing scores were related to increased FA or MD of specific components of pre-frontal limbic circuitry.
Our findings are consistent with recent models of PTSS that implicate overactivity and hyperconnectivity of components of the salience network, including the amygdala and dorsal ACC, that are believed to exaggerate threat arousal and disrupt top-down regulation by the central executive and default mode network in adults95 and to a lesser degree in children.88 Because of the high incidence of pediatric injury, the elevated PTSS after both TBI and EI is of great concern.
Longitudinal follow-up is essential to characterize the relation between longer-term psychological health and microstructural changes after pediatric injury. Future studies should refine our understanding of brain structures and networks contributing to specific PTSS at different developmental stages. This is critically important to guide creation of developmentally tailored interventions that specifically address core PTSS.15
Acknowledgments
This work was funded by the National Institutes of Health R01 NS046308 awarded to LEC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting institute. Support was also provided by the Nicole and Evan Katz Pediatric Neurodevelopmental Fund.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. McEwen B.S., Gray J., and Nasca C. (2015). Recognizing resilience: learning from the effects of stress on the brain. Neurobiol. Stress 1, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allwood M.A., Bell D.J., and Horan J. (2011). Posttrauma numbing of fear, detachment, and arousal predict delinquent behaviors in early adolescence. J. Clin. Child Adolesc. Psychol. 40, 659–667 [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention, N.C.f.I.P.a.C. (2016). Web-based injury statistics query and reporting system (WISQARS)
- 4. Ewing-Cobbs L., Prasad M.R., Cox C.S., Jr, Granger D.A., Duque G., and Swank P.R. (2017). Altered stress system reactivity after pediatric injury: Relation with post-traumatic stress symptoms. Psychoneuroendocrinology 84, 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ewing-Cobbs L., Bloom D.R., Prasad M.R., Waugh J.K., Cox C.S., Jr, and Swank P.R. (2014). Assessing recovery and disability after physical trauma: the Pediatric Injury Functional Outcome Scale. J. Pediatr. Psychol. 39, 653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zatzick D.F., Rivara F.P., Jurkovich G.J., Hoge C.W., Wang J., Fan M.Y., Russo J., Trusz S.G., Nathens A., and Mackenzie E.J. (2010). Multisite investigation of traumatic brain injuries, posttraumatic stress disorder, and self-reported health and cognitive impairments. Arch. Gen. Psychiatry 67, 1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Publishing: Washington, D.C [Google Scholar]
- 8. Schreier H., Ladakakos C., Morabito D., Chapman L., and Knudson M.M. (2005). Posttraumatic stress symptoms in children after mild to moderate pediatric trauma: a longitudinal examination of symptom prevalence, correlates, and parent-child symptom reporting. J. Trauma 58, 353–363 [DOI] [PubMed] [Google Scholar]
- 9. Brosbe M.S., Hoefling K., and Faust J. (2011). Predicting posttraumatic stress following pediatric injury: a systematic review. J. Pediatr. Psychol. 36, 718–729 [DOI] [PubMed] [Google Scholar]
- 10. Price J., Kassam-Adams N., Alderfer M.A., Christofferson J., and Kazak A.E. (2016). Systematic review: a reevaluation and update of the integrative (trajectory) model of pediatric medical traumatic stress. J. Pediatr. Psychol. 41, 86–97 [DOI] [PubMed] [Google Scholar]
- 11. Kenardy J., Le Brocque R., Hendrikz J., Iselin G., Anderson V., and McKinlay L. (2012). Impact of posttraumatic stress disorder and injury severity on recovery in children with traumatic brain injury. J. Clin. Child Adolesc. Psychol. 41, 5–14 [DOI] [PubMed] [Google Scholar]
- 12. O'Connor S.S., Zatzick D.F., Wang J., Temkin N., Koepsell T.D., Jaffe K.M., Durbin D., Vavilala M.S., Dorsch A., and Rivara F.P. (2012). Association between posttraumatic stress, depression, and functional impairments in adolescents 24 months after traumatic brain injury. J. Trauma Stress 25, 264–271 [DOI] [PubMed] [Google Scholar]
- 13. Weems C.F., Russell J.D., Neill E.L., and McCurdy B.H. (2018). Annual Research Review: pediatric posttraumatic stress disorder from a neurodevelopmental network perspective. J. Child Psychol. Psychiatry. Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 14. Casey B.J. (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu. Rev. Psychol. 66, 295–319 [DOI] [PubMed] [Google Scholar]
- 15. Arnsten A.F., Raskind M.A., Taylor F.B., and Connor D.F. (2015). The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol. Stress 1, 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davis M. and Whalen P.J. (2001). The amygdala: Vigilance and emotion. Mol. Psychiatry 6, 13–34 [DOI] [PubMed] [Google Scholar]
- 17. Nicholson A.A., Rabellino D., Densmore M., Frewen P.A., Paret C., Kluetsch R., Schmahl C., Theberge J., Neufeld R.W., McKinnon M.C., Reiss J., Jetly R., and Lanius R.A. (2017). The neurobiology of emotion regulation in posttraumatic stress disorder: amygdala downregulation via real-time fMRI neurofeedback. Hum. Brain Mapp. 38, 541–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lupien S.J., McEwen B.S., Gunnar M.R., and Heim C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445 [DOI] [PubMed] [Google Scholar]
- 19. Amaral D.G. and Price J.L. (1984). Amygdalo-cortical projections in the monkey (Macaca fascicularis). J. Comp. Neurol. 230, 465–496 [DOI] [PubMed] [Google Scholar]
- 20. Morawetz C., Bode S., Baudewig J., and Heekeren H.R. (2017). Effective amygdala-prefrontal connectivity predicts individual differences in successful emotion regulation. Soc. Cogn. Affect. Neurosci. 12, 569–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silvers J.A., Insel C., Powers A., Franz P., Helion C., Martin R., Weber J., Mischel W., Casey B.J., and Ochsner K.N. (2017). The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-to-dorsal shift in medial prefrontal recruitment. Dev. Cog. Neurosci. 25, 128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teicher M.H., Samson J.A., Anderson C.M., and Ohashi K. (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 17, 652–666 [DOI] [PubMed] [Google Scholar]
- 23. Daniels J.K., Lamke J.P., Gaebler M., Walter H., and Scheel M. (2013). White matter integrity and its relationship to PTSD and childhood trauma—a systematic review and meta-analysis. Depress. Anxiety 30, 207–216 [DOI] [PubMed] [Google Scholar]
- 24. Milani A.C., Hoffmann E.V., Fossaluza V., Jackowski A.P., and Mello M.F. (2017). Does pediatric post-traumatic stress disorder alter the brain? Systematic review and meta-analysis of structural and functional magnetic resonance imaging studies. Psychiatry Clin. Neurosci. 71, 154–169 [DOI] [PubMed] [Google Scholar]
- 25. Morey R.A., Haswell C.C., Hooper S.R., and De Bellis M.D. (2016). Amygdala, hippocampus, and ventral medial prefrontal cortex volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Neuropsychopharmacology 41, 791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keding T.J. and Herringa R.J. (2015). Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacology 40, 537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leh S.E., Schroeder C., Chen J.K., Mallar Chakravarty M., Park M.T., Cheung B., Huntgeburth S.C., Gosselin N., Hock C., Ptito A., and Petrides M. (2017). Microstructural integrity of hippocampal subregions is impaired after mild traumatic brain injury. J. Neurotrauma 34, 1402–1411 [DOI] [PubMed] [Google Scholar]
- 28. Yeh P.H., Wang B., Oakes T.R., French L.M., Pan H., Graner J., Liu W., and Riedy G. (2014). Postconcussional disorder and PTSD symptoms of military-related traumatic brain injury associated with compromised neurocircuitry. Hum. Brain Mapp. 35, 2652–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DeMaster D., Johnson C., Juranek J., and Ewing-Cobbs L. (2017). Memory and the hippocampal formation following pediatric traumatic brain injury. Brain Behav. 7, e00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beauchamp M.H., Ditchfield M., Maller J.J., Catroppa C., Godfrey C., Rosenfeld J.V., Kean M.J., and Anderson V.A. (2011). Hippocampus, amygdala and global brain changes 10 years after childhood traumatic brain injury. Int. J. Dev. Neurosci. 29, 137–143 [DOI] [PubMed] [Google Scholar]
- 31. Wilde E.A., Chu Z., Bigler E.D., Hunter J.V., Fearing M.A., Hanten G., Newsome M.R., Scheibel R.S., Li X., and Levin H.S. (2006). Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J. Neurotrauma 23, 1412–1426 [DOI] [PubMed] [Google Scholar]
- 32. Wilde E.A., Merkley T.L., Bigler E.D., Max J.E., Schmidt A.T., Ayoub K.W., McCauley S.R., Hunter J.V., Hanten G., Li X., Chu Z.D., and Levin H.S. (2012). Longitudinal changes in cortical thickness in children after traumatic brain injury and their relation to behavioral regulation and emotional control. Int. J. Dev. Neurosci. 30, 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pierpaoli C., Barnett A., Pajevic S., Chen R., Penix L.R., Virta A., and Basser P.J. (2001). Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. NeuroImage 13, 1174–1185 [DOI] [PubMed] [Google Scholar]
- 34. Pierpaoli C. and Basser P.J. (1996). Toward a quantitative assessment of diffusion anisotropy. Magn. Reson. Med. 36, 893–906 [DOI] [PubMed] [Google Scholar]
- 35. Ewing-Cobbs L., Johnson C.P., Juranek J., DeMaster D., Prasad M., Duque G., Kramer L., Cox C.S., and Swank P.R. (2016). Longitudinal diffusion tensor imaging after pediatric traumatic brain injury: Impact of age at injury and time since injury on pathway integrity. Hum. Brain Mapp. 37, 3929–3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilde E.A., Ayoub K.W., Bigler E.D., Chu Z.D., Hunter J.V., Wu T.C., McCauley S.R., and Levin H.S. (2012). Diffusion tensor imaging in moderate-to-severe pediatric traumatic brain injury: changes within an 18 month post-injury interval. Brain Imaging Behav. 6, 404–416 [DOI] [PubMed] [Google Scholar]
- 37. Juranek J., Johnson C.P., Prasad M.R., Kramer L.A., Saunders A., Filipek P.A., Swank P.R., Cox C.S., Jr, and Ewing-Cobbs L. (2012). Mean diffusivity in the amygdala correlates with anxiety in pediatric TBI. Brain Imaging Behav. 6, 36–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McAllister T.W. (2011). Neurobiological consequences of traumatic brain injury. Dialogues Clin. Neurosci. 13, 287–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roberts R.M., Mathias J.L., and Rose S.E. (2014). Diffusion tensor imaging (DTI) findings following pediatric non-penetrating TBI: a meta-analysis. Dev. Neuropsychol. 39, 600–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Max J.E., Keatley E., Wilde E.A., Bigler E.D., Schachar R.J., Saunders A.E., Ewing-Cobbs L., Chapman S.B., Dennis M., Yang T.T., and Levin H.S. (2012). Depression in children and adolescents in the first 6 months after traumatic brain injury. Int. J. Dev. Neurosci. 30, 239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Johnson C.P., Juranek J., Kramer L.A., Prasad M.R., Swank P.R., and Ewing-Cobbs L. (2011). Predicting behavioral deficits in pediatric traumatic brain injury through uncinate fasciculus integrity. J. Int. Neuropsychol. Soc. 17, 663–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Faber J., Wilde E.A., Hanten G., Ewing-Cobbs L., Aitken M.E., Yallampalli R., MacLeod M.C., Mullins S.H., Chu Z.D., Li X., Hunter J.V., Noble-Haeusslein L., and Levin H.S. (2016). Ten-year outcome of early childhood traumatic brain injury: diffusion tensor imaging of the ventral striatum in relation to executive functioning. Brain Inj. 30, 1635–1641 [DOI] [PubMed] [Google Scholar]
- 43. Brenner L.A. (2011). Neuropsychological and neuroimaging findings in traumatic brain injury and post-traumatic stress disorder. Dialogues Clin. Neurosci. 13, 311–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lopez K.C., Leary J.B., Pham D.L., Chou Y.Y., Dsurney J., and Chan L. (2017). Brain volume, connectivity, and neuropsychological performance in mild traumatic brain injury: the impact of post-traumatic stress disorder symptoms. J. Neurotrauma 34, 16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simmons A.N. and Matthews S.C. (2012). Neural circuitry of PTSD with or without mild traumatic brain injury: a meta-analysis. Neuropharmacology 62, 598–606 [DOI] [PubMed] [Google Scholar]
- 46. Teasdale G. and Jennett B. (1974). Assessment of coma and impaired consciousness: a practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 47. Baker S.P., O'Neill B., Haddon W., Jr., and Long W.B. (1974). The injury severity score: a method of describing patients with multiple injuries and evaluating emergency care. J. Trauma 14, 187–196 [PubMed] [Google Scholar]
- 48. Mayer T., Matlak M.E., Johnson D.G., and Walker M.L. (1980). The modified injury severity scale in pediatric multiple trauma patients. J. Pediatr. Surg. 15, 719–726 [DOI] [PubMed] [Google Scholar]
- 49. Max J.E., Lopez A., Wilde E.A., Bigler E.D., Schachar R.J., Saunders A., Ewing-Cobbs L., Chapman S.B., Yang T.T., and Levin H.S. (2015). Anxiety disorders in children and adolescents in the second six months after traumatic brain injury. J. Pediatr. Rehabil. Med. 8, 345–355 [DOI] [PubMed] [Google Scholar]
- 50. Achenbach T. (1991). Manual for the Child Behavior Checklist/4-18 and 1991 Profile. University of Vermont College of Medicine: Burlington, VT [Google Scholar]
- 51. Biederman J., Faraone S.V., and Monuteaux M.C. (2002). Differential effect of environmental adversity by gender: Rutter's index of adversity in a group of boys and girls with and without ADHD. Am. J. Psychiatry 159, 1556–1562 [DOI] [PubMed] [Google Scholar]
- 52. Petersen A.C., Crockett L., Richards M., and Boxer A. (1998). A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adol. 17, 117–131 [DOI] [PubMed] [Google Scholar]
- 53. Shirtcliff E.A. and Essex M.J. (2008). Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev. Psychobiol. 50, 690–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stover C.S. and Keeshin B. (2018). Research domain criteria and the study of trauma in children: implications for assessment and treatment research. Clin. Psychol. Rev. 64, 77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Foa E.B., Johnson K.M., Feeny N.C., and Treadwell K.R. (2001). The child PTSD Symptom Scale: a preliminary examination of its psychometric properties. J. Clin. Child Psychol. 30, 376–384 [DOI] [PubMed] [Google Scholar]
- 56. Kassam-Adams N., Marsac M.L., and Cirilli C. (2010). Posttraumatic stress disorder symptom structure in injured children: functional impairment and depression symptoms in a confirmatory factor analysis. J. Am. Acad. Child Adolesc. Psychiatry 49, 616–625 [DOI] [PubMed] [Google Scholar]
- 57. Fischl B., van der Kouwe A., Destrieux C., Halgren E., Segonne F., Salat D., Busa E., Seidman L.J., Goldstein J., Kennedy D., Caviness V., Makris N., Rosen B., and Dale A.M. (2004). Automatically parcellating the human cerebral cortex. Cereb. Cortex 14, 11–22 [DOI] [PubMed] [Google Scholar]
- 58. Andersson J.L., Skare S., and Ashburner J. (2003). How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870–888 [DOI] [PubMed] [Google Scholar]
- 59. Smith S.M. (2002). Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tottenham N. and Galvan A. (2016). Stress and the adolescent brain: amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci. Biobehav. Rev. 70, 217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Levin H.S., Wilde E.A., Chu Z., Yallampalli R., Hanten G.R., Li X., Chia J., Vasquez A.C., and Hunter J.V. (2008). Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J. Head Trauma Rehabil. 23, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wilde E.A., Ramos M.A., Yallampalli R., Bigler E.D., McCauley S.R., Chu Z., Wu T.C., Hanten G., Scheibel R.S., Li X., Vasquez A.C., Hunter J.V., and Levin H.S. (2010). Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Dev. Neuropsychol. 35, 333–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Caeyenberghs K., Leemans A., Geurts M., Taymans T., Linden C.V., Smits-Engelsman B.C., Sunaert S., and Swinnen S.P. (2010). Brain-behavior relationships in young traumatic brain injury patients: DTI metrics are highly correlated with postural control. Hum. Brain Mapp. 31, 992–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Caeyenberghs K., Leemans A., Geurts M., Taymans T., Vander Linden C., Smits-Engelsman B.C., Sunaert S., and Swinnen S.P. (2010). Brain-behavior relationships in young traumatic brain injury patients: fractional anisotropy measures are highly correlated with dynamic visuomotor tracking performance. Neuropsychologia 48, 1472–1482 [DOI] [PubMed] [Google Scholar]
- 65. Kurowski B., Wade S.L., Cecil K.M., Walz N.C., Yuan W., Rajagopal A., and Holland S.K. (2009). Correlation of diffusion tensor imaging with executive function measures after early childhood traumatic brain injury. J. Pediatr. Rehabil. Med. 2, 273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Levin H.S., Wilde E.A., Hanten G., Li X., Chu Z.D., Vasquez A.C., Cook L., Yallampalli R., and Hunter J.V. (2011). Mental state attributions and diffusion tensor imaging after traumatic brain injury in children. Dev. Neuropsychol. 36, 273–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Scheibel R.S., Newsome M.R., Wilde E.A., McClelland M.M., Hanten G., Krawczyk D.C., Cook L.G., Chu Z.D., Vasquez A.C., Yallampalli R., Lin X., Hunter J.V., and Levin H.S. (2011). Brain activation during a social attribution task in adolescents with moderate to severe traumatic brain injury. Soc. Neurosci. 6, 582–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rangaprakash D., Deshpande G., Daniel T.A., Goodman A.M., Robinson J.L., Salibi N., Katz J.S., Denney T.S., Jr, and Dretsch M.N. (2017). Compromised hippocampus-striatum pathway as a potential imaging biomarker of mild-traumatic brain injury and posttraumatic stress disorder. Hum. Brain Mapp. 38, 2843–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Beauchamp M.H., Anderson V.A., Catroppa C., Maller J.J., Godfrey C., Rosenfeld J.V., and Kean M. (2009). Implications of reduced callosal area for social skills after severe traumatic brain injury in children. J. Neurotrauma 26, 1645–1654 [DOI] [PubMed] [Google Scholar]
- 70. Wilde E.A., Bigler E.D., Hunter J.V., Fearing M.A., Scheibel R.S., Newsome M.R., Johnson J.L., Bachevalier J., and Levin H.S. (2007). Hippocampus, amygdala, and basal ganglia morphometrics in children after moderate-to-severe traumatic brain injury. Dev. Med. Child Neurol. 49, 294–299 [DOI] [PubMed] [Google Scholar]
- 71. Goodrich-Hunsaker N.J., Abildskov T.J., Black G., Bigler E.D., Cohen D.M., Mihalov L.K., Bangert B.A., Taylor H.G., and Yeates K.O. (2018). Age- and sex-related effects in children with mild traumatic brain injury on diffusion magnetic resonance imaging properties: a comparison of voxelwise and tractography methods. J. Neurosci. Res. 96, 626–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lebel C., Walker L., Leemans A., Phillips L., and Beaulieu C. (2008). Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40, 1044–1055 [DOI] [PubMed] [Google Scholar]
- 73. Braver T.S., Barch D.M., Gray J., Molfese D.L., and Snyder A. (2001). Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb. Cortex 11, 825–836 [DOI] [PubMed] [Google Scholar]
- 74. McEwen B.S., Nasca C., and Gray J.D. (2016). Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41, 3–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Teicher M.H., Anderson C.M., Ohashi K., Khan A., McGreenery C.E., Bolger E.A., Rohan M.L., and Vitaliano G.D. (2018). Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. Neuroimage 169, 443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Qin S., Young C.B., Supekar K., Uddin L.Q., and Menon V. (2012). Immature integration and segregation of emotion-related brain circuitry in young children. Proc. Natl. Acad. Sci. U. S. A. 109, 7941–7946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Saygin Z.M., Osher D.E., Koldewyn K., Martin R.E., Finn A., Saxe R., Gabrieli J.D., and Sheridan M. (2015). Structural connectivity of the developing human amygdala. PLoS One 10, e0125170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E., Hare T., and Tottenham N. (2014). The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage 95, 193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Eluvathingal T.J., Chugani H.T., Behen M.E., Juhasz C., Muzik O., Maqbool M., Chugani D.C., and Makki M. (2006). Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics 117, 2093–2100 [DOI] [PubMed] [Google Scholar]
- 80. Jackowski A.P., Douglas-Palumberi H., Jackowski M., Win L., Schultz R.T., Staib L.W., Krystal J.H., and Kaufman J. (2008). Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Res. 162, 256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Seckfort D.L., Paul R., Grieve S.M., Vandenberg B., Bryant R.A., Williams L.M., Clark C.R., Cohen R.A., Bruce S., and Gordon E. (2008). Early life stress on brain structure and function across the lifespan: a preliminary study. Brain Imaging Behav. 2, 49–58 [Google Scholar]
- 82. Rinne-Albers M.A., van der Werff S.J., van Hoof M.J., van Lang N.D., Lamers-Winkelman F., Rombouts S.A., Vermeiren R.R., and van der Wee N.J. (2016). Abnormalities of white matter integrity in the corpus callosum of adolescents with PTSD after childhood sexual abuse: a DTI study. Eur. Child Adolesc. Psychiatry 25, 869–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ohashi K., Anderson C.M., Bolger E.A., Khan A., McGreenery C.E., and Teicher M.H. (2017). Childhood maltreatment is associated with alteration in global network fiber-tract architecture independent of history of depression and anxiety. Neuroimage 150, 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Puetz V.B., Parker D., Kohn N., Dahmen B., Verma R., and Konrad K. (2017). Altered brain network integrity after childhood maltreatment: a structural connectomic DTI-study. Hum. Brain Mapp. 38, 855–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Suo X., Lei D., Chen F., Wu M., Li L., Sun L., Wei X., Zhu H., Li L., Kemp G.J. and Gong Q. (2017). Anatomic insights into disrupted small-world networks in pediatric posttraumatic stress disorder. Radiology 282, 826–834 [DOI] [PubMed] [Google Scholar]
- 86. Wolf R.C. and Herringa R.J. (2016). Prefrontal-amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology 41, 822–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Patriat R., Birn R.M., Keding T.J., and Herringa R.J. (2016). Default-mode network abnormalities in pediatric posttraumatic stress disorder. J. Am. Acad. Child Adolesc. Psychiatry 55, 319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Herringa R.J. (2017). Trauma, PTSD, and the developing brain. Curr. Psychiatry Rep. 19, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hendrickson R.C. and Raskind M.A. (2016). Noradrenergic dysregulation in the pathophysiology of PTSD. Exp. Neurol. 284, 181–195 [DOI] [PubMed] [Google Scholar]
- 90. Scherf K.S., Smyth J.M., and Delgado M.R. (2013). The amygdala: an agent of change in adolescent neural networks. Horm. Behav. 64, 298–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Paquola C., Bennett M.R., Hatton S.N., Hermens D.F., Groote I., and Lagopoulos J. (2017). Hippocampal development in youth with a history of childhood maltreatment. J. Psychiatr. Res. 91, 149–155 [DOI] [PubMed] [Google Scholar]
- 92. Carrion V.G., Garrett A., Menon V., Weems C.F., and Reiss A.L. (2008). Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depress. Anxiety 25, 514–526 [DOI] [PubMed] [Google Scholar]
- 93. Weis C.N., Belleau E.L., Pedersen W.S., Miskovich T.A., and Larson C.L. (2018). Structural connectivity of the posterior cingulum is related to reexperiencing symptoms in posttraumatic stress disorder. Chronic Stress Epub before print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Weems C.F. (2017). Severe stress and the development of the amygdala in youth: a theory and its statistical implications. Dev. Rev. 46, 44–53 [Google Scholar]
- 95. Akiki T.J., Averill C.L., Wrocklage K.M., Scott J.C., Averill L.A., Schweinsburg B., Alexander-Bloch A., Martini B., Southwick S.M., Krystal J.H., and Abdallah C.G. (2018). Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage 176, 489–498 [DOI] [PMC free article] [PubMed] [Google Scholar]