Abstract
Mental health problems, such as depression and anxiety, are often associated with functional limitations after traumatic brain injury (TBI), prompting researchers to explore which of these TBI-related sequelae tends to precede the other. Past studies among patients with injuries ranging in severity have predominantly reported that functional impairments predict subsequent psychological concerns, rather than the other way around; however, it remains unclear whether this directionality holds for individuals with mild TBI (mTBI). The present study utilized a cross-lagged panel design within a structural equation modeling analytical framework to explore the longitudinal relationships of symptoms of depression and anxiety to functional status among 717 adult mTBI patients, with assessments occurring at 2 weeks and 3 months post-injury. Symptoms of both depression and anxiety significantly predicted subsequent functional limitations (λs = −0.21 and −0.25), whereas the reverse effects were nonsignificant (λs = −0.05 and −0.03); thus, psychological concerns appeared to function as a precursor to functional impairment. This pattern was particularly pronounced among patients with normal head computed tomography (CT) results; however, results were less clear cut among those subjects whose injuries were accompanied by intracranial abnormalities detected on CT imaging, suggesting the possibility of a more reciprocal relationship in the case of CT-positive mTBI. These results may serve to partially explain the incidence of persistent functional limitations observed among subsets of mTBI patients in past studies. Findings likewise highlight the importance of assessment and treatment for mental health problems after mTBI as an important factor to promote psychological well-being and functional recovery.
Keywords: brain injuries, mental health, patient outcome assessment, traumatic
Introduction
Traumatic brain injury (TBI) is a widespread cause of death and disability in the United States, with recent estimates of incidence as high as 2.8 million cases per year.1 In addition to physical and cognitive complaints,2,3 elevated rates of mental health problems, such as depression and anxiety, are also observed in the aftermath of TBI.4–7 These psychological difficulties are associated with poor functional status after TBI,8,9 which has led researchers to explore the question of whether mental health problems operate as a cause or a consequence of functional limitations in the context of recovery from brain injury. On the one hand, depression and anxiety could certainly be expected to arise in reaction to the experience of functional deficits post-injury. At the same time, it seems feasible that depression and anxiety might also negatively impact functioning (or reported functioning) among survivors of TBI. Thus, a small number of studies have set out to evaluate the directionality of this association—or, in other words, to answer the question: Which comes first?
In attempting to address this topic, a useful methodological approach is cross-lagged panel analysis, a statistical technique that is used to infer causal relationships among variables measured at multiple time points without direct experimental intervention.10 Through this approach, reciprocal predictive impacts of two variables on one another over time (in this case, mental health problems and functional status) can be evaluated and directly compared against each other in order to determine which appears to be the more causal factor. To date, we are aware of three studies that have explored the directionality of the relationship between psychological difficulties and functional limitations after TBI. One study implemented a cross-lagged panel design and found that functional limitations appeared to precede depressive symptomology, but not the other way around11; a second study also utilized this analytical approach and found similar results for the association of functional status to both depression and anxiety.12 A more recent, large-scale research effort drawn from the TBI Model Systems national study found evidence for a similar pattern utilizing a latent variable structural equation modeling approach to reflect the constructs of functional status and mental health, although effect-size considerations led the researchers to conclude that the relationship is likely at least somewhat reciprocal.13
Importantly, all of these studies to date have explored the directionality of this relationship among populations either including a range of complicated mild-to-severe TBIs11,12 or exclusively comprising patients with moderate-to-severe brain injuries.13 Thus, it remains unclear whether mental health problems and functional status exhibit a similar relationship in the aftermath of mild TBI (mTBI), and particularly for those with mTBIs without intracranial abnormalities as determined by computed tomography (CT) scan (sometimes termed “uncomplicated” mTBI). Recovery post-mTBI has been a topic of some controversy, as researchers studying hospital-based patient samples have frequently identified sizable minorities of mTBI patients who evidence persistent post-traumatic symptoms14,15 or functional limitations16,17 that are difficult to explain given the absence of structural brain injury on standard clinical neuroimaging. Some studies cite psychological difficulties as potential causal factors for the persistence of injury-related complaints post-mTBI18–20; thus, it is particularly important to clarify the temporal direction of the relationship between functional status and mental health problems among this population.
The present study sought to address this area in need of further study, utilizing a cross-lagged panel framework to accomplish three main goals: 1) to assess the predictive impact of functional status at 2 weeks post-mTBI on symptoms of depression and anxiety at 3 months post-injury; 2) to evaluate the predictive effects of depression and anxiety symptoms on functional status over the same interval; and 3) to compare the strength of these reciprocal predictive impacts over time to determine whether functional status or mental health problems appear to precede the other in the aftermath of mTBI.
Methods
Subjects and study design
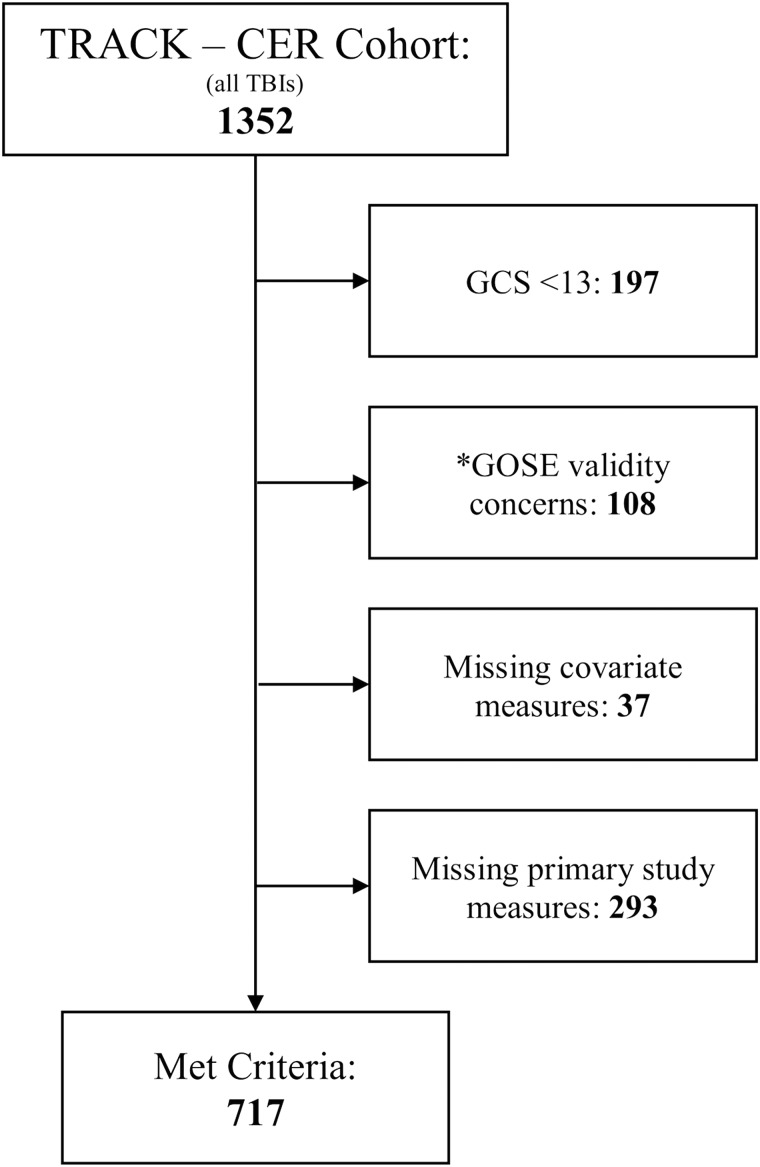
Participants included a subset of the larger subject pool enrolled in the Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study, a large-scale longitudinal, observational, multi-center research effort evaluating recovery and outcomes associated with closed-head TBIs of all severities (http://tracktbi.ucsf.edu/; complete details of study inclusion and exclusion criteria can be found in Supplemental Materials [see online supplementary material at http://www.liebertpub.com] accompanying the online publication of this report). The present study included patients seen at the emergency department (ED) across 10 of 11 study sites between March 2014 and May 2016. (Participants from one study site were excluded from this report following the TRACK-TBI Data Acquisition and Quality Committee's assessment that the GOSE was not administered according to standard study procedures.) Included subjects for this report were participants with a Glasgow Coma Scale (GCS) score of 13–15 upon admission to the ED, for whom a clinical head CT scan was obtained, and for whom complete data were collected for all study measures at the scheduled 2-week and 3-month follow-up points. Participant selection and inclusion criteria are represented in STROBE diagram format in Figure 1. This resulted in a final sample of 717 adults (mean age [Mage] = 39.97 [standard deviation {SD} = 16.77; range = 17–88]; 34.6% female; 74.5% white, 25.1% racial minority, 0.4% did not identify racial background).
FIG. 1.
Participant inclusion STROBE flow diagram. *Note: Participants from one study site were excluded from this report because of concerns specific to that site that the GOSE was not administered according to study procedures. GCS, Glasgow Coma Scale; GOSE, Glasgow Outcome Scale-Extended;
Comparisons between the final sample and those potential participants not selected for inclusion (per the criteria described above) demonstrated no significant group differences between included and excluded subjects in terms of age, sex, racial background, CT findings (i.e., positive or negative), presence or absence of a premorbid psychiatric diagnosis, or average ED-admission GCS score (all ps > 0.088). As such, the subjects included in this study are thought to be largely representative of the overall TRACK-TBI study's participant pool. The great majority (79.4%) of included participants had ED-admission GCS scores of 15, and most (70.4%) evidenced negative CT findings (i.e., results not indicative of intracranial abnormalities). Thus, this report's sample was primarily composed of patients who had sustained mTBIs unaccompanied by intracranial abnormalities on CT. Nearly one quarter (23.2%) of participants reported a psychiatric history (i.e., the presence of at least one pre-injury psychiatric diagnosis); as such, and given this variable's demonstrated role in predicting post-mTBI symptoms, psychiatric history was included as a covariate in our statistical analyses as described below.
Measures
Depression and anxiety
These domains of psychological dysfunction were assessed by the Brief Symptom Inventory (BSI) 18-item version (BSI-18),21 an abbreviated adaptation of the original BSI.22 This measure includes subscales representing depression, anxiety, and somatization, of which the former two (henceforth “BSI-depression” and “BSI-anxiety”) were utilized here. BSI-18 scores included for the present study were standardized T-scores derived from normative data for this measure,21 facilitating a descriptive evaluation of depression and anxiety symptom levels within this sample of mTBI patients as compared with symptom levels typically observed among the general population.
Functional status
The GOSE,23 a widely used measure of global outcome after TBI, was administered to study participants to assess their functional status at both time points post-injury. This measure provides a classification of an individual's functional status on an 8-point scale ranging from 1 (“dead”) to 8 (“upper good recovery”). The GOSE can be administered so as to assess functional limitations attributed to TBI alone (GOSE-TBI) or attributed to the TBI plus other system injuries sustained in the same event (GOSE-all). In order to isolate the specific context of recovery after mTBI, the GOSE-TBI score was utilized to represent functional status for the present study.
Statistical analysis
Cross-lagged panel analyses were conducted using a structural equation modeling (SEM) framework24 via the LISREL software package (version 8.80; Scientific Software International, Inc., Chicago, IL).25 SEM was chosen over the alternative approach utilizing a series of standard multiple regressions to allow for statistical comparisons between cross-variable predictive impacts over time. Two separate models were specified, one each to test the reciprocal predictive impacts of 1) depressive symptoms and functional status and 2) symptoms of anxiety and functional status. All parameters were estimated by maximum likelihood estimation.
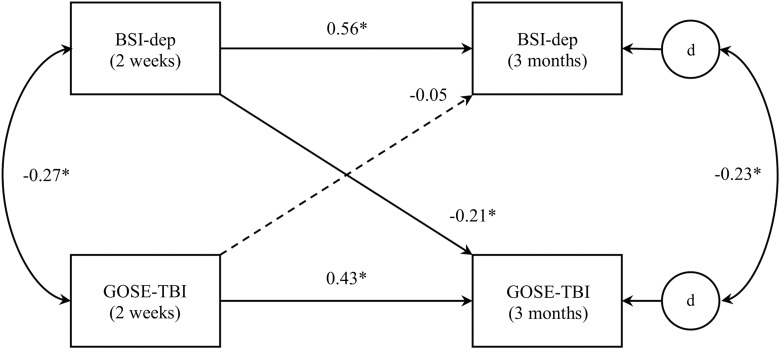
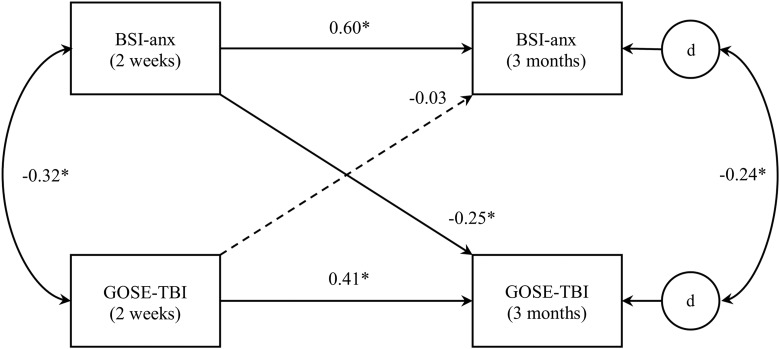
The first of these models (model 1, represented in Fig. 2) was structured such that independent variables included GOSE-TBI and BSI-depression scores at 2 weeks post-injury, dependent variables included the same measures at 3-month follow-up, and the longitudinal predictive effects of both independent variables on both dependent variables were evaluated. Additionally, as is customary in cross-lagged panel analysis,10,24 cross-sectional associations between variables (i.e., between GOSE-TBI and BSI-depression scores at 2-week follow-up and between the same at 3-month follow-up) were also evaluated. Because SEM approaches do not permit the specification of dependent variables to correlate directly, the error (“disturbance”) terms for the GOSE-TBI and BSI-depression subscale at 3 months post-injury were specified to correlate instead, as in other cross-lagged panel models tested via SEM.12,13 Covariates, including age, sex, CT findings (negative or positive), and the presence or absence of a premorbid psychiatric diagnosis, were also included in this model as predictors of GOSE-TBI and BSI-depression scores at 3 months and correlated with these variables at 2-week follow-up. (Of note, the inclusion of pre-injury psychiatric history allowed for the evaluation of the predictive relationships among symptoms of depression and anxiety and functional status above and beyond any impacts of possessing a pre-morbid psychiatric diagnosis. Preliminary runs of these models without pre-injury psychiatric history as a covariate yielded highly similar results.) A second model was tested that was identical to the first, except with BSI-anxiety scores substituted in place of the BSI-depression subscale (model 2; see Fig. 3).
FIG. 2.
Cross-lagged panel analysis model for depression and functional status (model 1). Note: “BSI-dep” = BSI depression subscale. “d” represents the unique error (“disturbance”) term corresponding to its respective dependent variable. Curved lines represent correlations; straight lines represent predictive paths. Correlation and standardized path coefficients are displayed. Statistically significant effects are presented in solid lines; dashed lines indicate nonsignificant effects. Age, sex, computed tomography findings, and psychiatric history were also included in the model as covariates, but, for clarity purposes, are not pictured here. *p < 0.001. BSI, Brief Symptom Inventory.
FIG. 3.
Cross-lagged panel analysis model for anxiety and functional status (model 2). Note: “BSI-anx” = BSI anxiety subscale, “d” represents the unique error (“disturbance”) term corresponding to its respective dependent variable. Curved lines represent correlations; straight lines represent predictive paths. Correlation and standardized path coefficients are displayed. Statistically significant effects are presented in solid lines; dashed lines indicate nonsignificant effects. Age, sex, computed tomography findings, and psychiatric history were also included in the model as covariates, but, for clarity purposes, are not pictured here. *p < 0.001. BSI, Brief Symptom Inventory.
Of particular interest in each model were the cross-variable longitudinal effects—that is, those that reflected the predictive impacts of symptoms of depression or anxiety on functional status over time and those that represented the reverse impacts. A common approach for comparing the strength of distinct predictive effects (“paths”) in SEM is to estimate a model wherein the paths of interest are specified to be equal in strength, with the overall fit of this equality-constrained model then compared to the fit of the original model in which the relevant paths were permitted to vary freely. This methodology was used in a previous study evaluating the association between psychological symptoms and functional limitations among moderately to severely brain-injured patients, with findings demonstrating that the impact of functional limitations on depression and anxiety over time was significantly stronger than the reverse predictive effect.12
However, as statistical methods researchers have pointed out,26 this approach can be problematic when the variables of interest are measured by differing scales, as is the case for the GOSE and BSI. In short, this presents an issue because it results in comparisons between effects that are represented in disparate units of variance, a form of false comparison that can lead to untrustworthy results.26 Therefore, instead of utilizing overall model fit comparisons that could be subject to this confounding effect, direct comparisons were made between paths of interest using phantom variable nonlinear constraints.27,28 This approach allowed for the evaluation of an additional (“phantom”) parameter specified to reflect the difference between standardized path coefficients (i.e., effects defined in shared standardized units) corresponding to the cross-variable paths of interest. Tests of these additional parameters were such that a model returning a statistically significant “phantom” parameter would indicate that the two paths of interest differed significantly from one another in strength, whereas a nonsignificant additional parameter would indicate that the two effects were not significantly different from one another. For the two cross-lagged panel models evaluated, this approach was used to compare the reciprocal predictive effects of depressive symptoms and functional status over time (model 1) and those of symptoms of anxiety and functional status over time (model 2).
To facilitate the use of SEM in evaluating the models described above, the GOSE-TBI was treated as a continuous variable to parallel the interval-scale BSI-depression and BSI-anxiety scores. This is consistent with previous cross-lagged panel analysis studies of functional status and psychological concerns, which have specified continuous variables so as to evaluate linear predictive effects over time.11,13 Nonetheless, because it was uncertain how specifying GOSE-TBI scores as a continuous indicator might affect results, sensitivity analyses were conducted wherein the models above were tested with GOSE-TBI scores dichotomized at cutpoints of 7 or below and 6 or below representing poor outcome. Results for models specified with each of these dichotomization points were then compared with those of the primary study models described above in order to establish the extent to which evaluating GOSE-TBI scores as a continuous variable might affect findings.
Results
Descriptive statistics and inter-correlations for study variables at both time points are presented in Table 1. A sizable minority of subjects reported depression and anxiety symptom levels above the clinical cut-off score recommended by the BSI manual (i.e., T-scores ≥ 63)21 at 2-week follow-up (20.8% for both depression and anxiety) and at 3-month follow-up (15.6% and 15.3% for depression and anxiety, respectively). Approximately one fourth (24.8%) of subjects reported “upper good recovery” (i.e., GOSE-TBI = 8) at 2-week follow-up; this proportion increased to 41.7% at 3 months post-injury.
Table 1.
Descriptive Statistics and Intercorrelations for Study Variables
| Variable | M | SD | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|
| 1. BSI-depression (2 weeks) | 52.06 | 10.50 | — | |||||
| 2. BSI-anxiety (2 weeks) | 52.30 | 11.74 | 0.71* | — | ||||
| 3. GOSE-TBI (2 weeks) | 6.30 | 1.37 | −0.27* | −0.32* | — | |||
| 4. BSI-depression (3 months) | 50.30 | 10.65 | 0.56* | 0.47* | −0.21* | — | ||
| 5. BSI-anxiety (3 months) | 49.11 | 11.37 | 0.51* | 0.61* | −0.21* | 0.74* | — | |
| 6. GOSE-TBI (3 months) | 6.95 | 1.11 | −0.32* | −0.38* | 0.50* | −0.45* | −0.48* | — |
p < 0.001.
BSI, Brief Symptom Inventory; GOSE, Glasgow Outcome Scale-Extended; TBI, traumatic brain injury; M, mean; SD, standard deviation.
Cross-lagged panel analyses
Correlation and standardized path coefficients for model 1, representing the cross-lagged predictive impacts of depressive symptoms and functional status, are presented in Figure 2. Depressive symptoms and functional status were cross-sectionally correlated with one another at both 2-week and 3-month follow-ups. Depressive symptoms at 3 months were significantly predicted by symptoms depression at 2 weeks, as was 3-month report of functional status significantly predicted by functional status at 2 weeks. Depressive symptoms at 2-week follow-up also significantly predicted functional status at 3 months, such that greater report of depression predicted poorer functional outcome over time. However, the reverse relationship was not statistically significant (i.e., functional status at 2 weeks did not predict depressive symptom levels at 3-month follow-up). Further, phantom variable-based comparison of these two effects indicated that the effect of depressive symptoms on functional status over time was significantly stronger than that of functional status on subsequent depressive symptoms (z-statistic = −3.08; p < 0.001). Sensitivity analyses demonstrated that these results were quite consistent whether the GOSE-TBI was treated as a continuous variable or dichotomized at either 7 or below or 6 or below as a proxy for poor outcome.
Coefficients for model 2, representing the cross-lagged predictive impacts of symptoms of anxiety and functional status, are presented in Figure 3. Results of this model were very similar to those of model 1. Symptoms of anxiety and functional status were significantly correlated with one another at both 2-week and 3-month follow-ups, and each variable was significantly predictive of itself over time. Anxiety symptom levels at 2 weeks significantly predicted functional status at 3-month follow-up, such that greater report of anxiety symptoms predicted poorer functional outcome over time. The reverse relationship was once again nonsignificant, such that functional status at 2-week follow-up did not predict subsequent anxiety symptom levels. Comparison of these two effects indicated that the effect of anxiety symptoms on functional status over time was significantly stronger than that of functional status on subsequent anxiety (z-statistic = −4.46; p < 0.001). Once again, sensitivity analyses demonstrated that these results were quite consistent whether the GOSE-TBI was treated as a continuous variable or dichotomized at either cutpoint.
Finally, these same models were tested separately for participants with negative CT results versus those for whom CT scans demonstrated evidence of intracranial abnormalities. Results for the former group (n = 505) were very similar to those of the sample overall: Symptoms of both depression and anxiety significantly predicted functional status over time (λs = −0.23 and −0.29; ps < 0.001), whereas the reverse effects were not statistically significant (λs = −0.03 and −0.02; ps = 0.477 and 0.561). Likewise, the predictive effects of depression and anxiety symptoms on functional status were significantly stronger than their corresponding reverse effects (z-statistics = −3.45 and −4.44; ps < 0.001).
Conversely, for subjects who had sustained mTBIs with intracranial abnormalities on CT (n = 212), results were somewhat less clear. For the cross-lagged reciprocal effects of depressive symptoms and functional status among the CT-positive mTBI group, it was once again found that symptoms of depression predicted functional limitations over time (λ = −0.15; p = 0.016), whereas functional status did not significantly predict depressive symptoms (λ = −0.11; p = 0.087). However, these cross-lagged predictive effects did not differ significantly from one another in strength (z-statistic = −0.46; p = 0.644). Thus, the predictive effect of depressive symptoms on functional status over time was not significantly stronger than the reverse effect.
Similarly, for the cross-lagged predictive effects of anxiety symptoms and functional status among the CT-positive mTBI group, anxiety symptoms were significantly predictive of functional limitations over time (λ = −0.17; p = 0.004), whereas functional status did not significantly predict anxiety symptom levels (λ = −0.04; p = 0.481). However, these cross-lagged predictive effects once again did not differ significantly in strength (z-statistic = −1.40; p = 0.161). This indicated that the predictive effect of anxiety symptoms on functional limitations was not significantly stronger than the reverse longitudinal effect. (Conceptual figures featuring coefficients for study models separated by CT status can be found in Supplementary Materials accompanying the online publication version of this report, noted as Figs. S1–S4.) (See online supplementary material at http://www.liebertpub.com)
Discussion
In this study exploring the temporal directionality of the documented link between mental health problems and functional limitations after mTBI in adult patients, cross-lagged panel analysis models indicated that symptoms of depression and anxiety appear to precede functional impairments post-mTBI, and not the other way around. This pattern was particularly pronounced for patients who had suffered mTBIs without intracranial abnormalities identified by CT scan, whereas findings for subjects with CT-positive mTBIs were somewhat less clear cut. Implications of these findings for TBI research and treatment are discussed in the following sections.
Depression and anxiety symptoms predict functional limitations after mild traumatic brain injury
Past research has largely reported that for those with more severe brain injuries, functional limitations post-TBI appear to predict subsequent psychological difficulties, and not the other way around.11–13 The present results are particularly interesting in the context of these past studies, given that the post-injury mental health problems reported by our participants were significantly predictive of subsequent functional limitations, whereas functional status did not predict the same psychological concerns over time. This pattern held true for models evaluating the relationships of both depression and anxiety symptoms to functional status, offering consistent and clear support for the directionality of these effects. Overall, these results suggest that the temporal relationship of depression and anxiety symptoms to functional impairments may operate in the opposite direction for patients with mTBIs as compared with those who have sustained more-severe injuries.
This pattern was especially prominent for patients with CT-negative mTBIs, who comprised the large majority (70.4%) of patients included in the present study. However, when those with CT-positive mTBIs were analyzed separately, results among this latter subset of participants were less straightforward, such that the reciprocal predictive effects of mental health problems and functional status were not significantly different from one another. In the case of depressive symptoms and functional status, this finding was accompanied by standardized coefficients that were quite similar to one another in magnitude (λs = −0.15 and −0.11). This is suggestive of a longitudinal relationship between these sequelae of brain injury that may be at least somewhat reciprocal for those with CT-positive mTBIs, in contrast to the apparently unidirectional nature of this association after CT-negative mTBI.
However, although the reciprocal effects of anxiety symptoms and functional limitations also did not differ from one another in statistical terms, the standardized coefficients for these paths appeared much more different from one another (λs = −0.17 and −0.04). This suggests that the lack of statistical differentiation in this case may have occurred because of limited statistical power resulting from smaller sample size (n = 212 for CT-positive mTBI), rather than truly bidirectional effects. Still, these findings offer potential evidence of a trend wherein those with CT-positive mTBIs may “bridge” the gap between observations made among those with moderate-to-severe brain injuries and the findings of the present study for patients with mTBIs without intracranial abnormalities identified by CT. Future research including larger samples of patients with CT-positive mTBIs is needed to further explore this possibility.
The findings reported here also offer important insights into the trajectory of functional recovery after mTBI, which has been a subject of some controversy in the literature. In particular, studies have repeatedly identified subsets of mTBI patients who report persistent functional impairment after their injury,16,17 a phenomenon that is difficult to explain given the absence of documented structural brain injury or lasting neuropsychological deficits. Our findings—which indicate that functional limitations after mTBI may occur subsequent to mental health problems such as depression and anxiety—suggest a partial explanation for the persistent functional impairments experienced by a minority of the patients assessed in other studies. As such, results described here are consistent with other accounts of recovery post-mTBI, which have often implicated psychological problems as a causal factor in report of other injury-related complaints.18–20 Of note, these effects of depression and anxiety symptoms on functional status after mTBI were significant even accounting for pre-morbid psychiatric history, which was included as a covariate. Thus, our findings indicate that mental health problems arising after injury appear to exert a unique impact on subsequent functional limitations above and beyond an individual's pre-injury psychiatric history.
Importantly, we recognize that the effects of symptoms of depression and anxiety on subsequent functional limitations, although significant, were nonetheless in the relatively small range. Thus, mental health problems such as depression and anxiety should not be interpreted as fully explaining the path by which functional deficits occur after mTBI. Rather, these findings suggest that mental health problems occurring post-mTBI are noteworthy contributors to the larger picture of personal and injury-related factors that may influence post-injury functional deficits.
Implications for treatment and recovery
The potential for mental health problems to limit functional status after mTBI is an important consideration related to functional recovery post-injury, given that such concerns are quite frequently observed in survivors of TBI.4–7 Depression is a particularly common concern post-brain injury, with studies estimating that rates of major depressive disorder may be as high as 42% after TBI.29 Likewise, research has identified anxiety disorders as commonly occurring psychiatric diagnoses after brain injury.6
These psychological difficulties certainly constitute an important treatment target for providers working with brain injury patients, given that relief from symptoms of depression and anxiety is a worthwhile treatment goal in and of itself. In addition, the findings of the present study suggest that an added benefit of effective treatment for these concerns in the aftermath of mTBI may be that improvements in psychological functioning could also contribute to improvements in functional status, enabling individuals who have sustained mTBIs to continue to recover their day-to-day functioning across life domains. Thus, one very important way in which healthcare providers can help their mTBI patients is by providing referrals and other resources to aid patients in finding the treatment they need to manage mental health problems such as depression and anxiety as they arise after injury. This necessitates the implementation of effective and regular assessment for these psychological difficulties, given that providers will only be able to offer appropriate support in finding treatment to the extent that they are aware that mental health problems exist. However, substantial gaps in care currently remain, with a majority of mTBI patients failing to receive appropriate medical follow-up wherein such assessments might occur.30 As such, a key implication of the present study is the supreme importance of routine follow-up to ensure assessment and treatment for mental health problems in promoting recovery after mild brain injury.
Limitations of the present study
Several limitations to the present study merit consideration. First is the specification of the GOSE-TBI as a continuous variable, a decision that was made to facilitate the use of this measure of functional status in conjunction with other continuous variables within a structural equation modeling framework. Although this approach was consistent with past studies of psychological problems and functional status post-TBI,11,13 it is nonetheless worth noting that utilizing the measure in this way makes assumptions about equal intervals between GOSE-TBI scores that may or may not be appropriate. As noted above, we included a series of sensitivity analyses evaluating two separate GOSE dichotomization cutpoints to partially address this concern. Nonetheless, future work to incorporate the ordinal nature of GOSE score data will offer additional support for our findings.
Additionally, although the large sample size represents a notable strength of this study, TRACK-TBI is not an epidemiological study and thus the sample assessed here should not be viewed as necessarily representative of the larger population of those with mTBIs in the United States. The TRACK-TBI study enrolls subjects presenting to EDs at level 1 trauma centers. Thus, our findings may not be generalizable to patients who do not warrant ED assessment at a high-level trauma center or receive less intensive care in other medical settings (or no medical care at all). Replication of the present study's methods among more representative samples of those with mTBIs—and particularly including those patients who are likely under-represented among the TRACK-TBI sample—is needed to further support the generalizability of the findings reported here.
Further, we note a few potential confounds that may have influenced our results. First, although we included pre-morbid psychiatric diagnosis as a covariate in our models, the measure of premorbid psychiatric functioning (i.e., presence or absence of a non-specific pre-injury diagnosis) included in this study is somewhat limiting. Future studies exploring the directionality of the relationships assessed here would be well advised to collect specific information regarding premorbid symptoms of depression and anxiety and, ideally, utilizing dimensional measures of these constructs rather than merely assessing their presence or absence. Additionally, other possible confounding variables (e.g., the severity of post-traumatic symptoms, pain, and/or fatigue post-injury) were not included in our models and thus could be influencing our results in unknown ways. Further research that accounts for these factors is needed in order to isolate the associations of depression and anxiety to functional status post-mTBI in the context of these other common post-injury factors.
Finally, it is worth noting that this study does not include measures of symptom and/or performance validity, a limitation inherent to the larger TRACK-TBI study (which does not feature such measures within its protocol). As such, we were unable to assess for the possibility of invalid reporting with regard to functional status and psychological concerns as reported by the present study's subjects. Given that validity concerns can be prevalent among patients who have sustained TBIs,31 it will be important for future research to address questions of performance validity in implementing further study of functional status and mental health problems following traumatic brain injury.
Conclusions and Future Directions
Despite the limitations noted above, this report offers a substantial contribution to scientific understanding of psychological and functional sequelae of mTBI. In contrast to findings reported in the literature for patients with brain injuries ranging in severity from complicated mild to severe, our results suggest that functional impairments may often follow mental health concerns that are frequently observed in the aftermath of mTBI. Notably, this pattern appears to be most prominent for those with mTBIs that do not feature intracranial abnormalities as determined by CT. Findings underscore the importance both of routine follow-up to monitor psychological well-being and of treatment for concerns such as depression and anxiety in order to facilitate recovery after CT-negative mild brain injury.
As we consider directions for future research to build on these findings, one important avenue is the exploration of how post-traumatic symptoms may relate to the other sequelae of mTBI discussed here. Just as past studies have identified minorities of subjects who experience persistent functional limitations after mild brain injury,16,17 so too has research evidenced that some mildly brain-injured individuals may report post-traumatic symptoms that linger in the aftermath of mTBI.14,15 Future studies would be well served to explore the reciprocal longitudinal associations of post-traumatic symptoms and functional limitations post-mTBI, and likewise the cross-lagged effects of symptoms and mental health problems such as depression and anxiety. As research provides further exploration of the interactions of these various sequelae of mTBI, findings will continue to advance our understanding of factors influencing prognosis, treatment, and successful recovery from mild traumatic brain injury.
Supplementary Material
Acknowledgments
This study was supported by funding from the following grants: D.O.D. U.S. Army W81XWH-14-2-0176 and NIH/NINDS 1U01NS086090.
The TRACK-TBI investigators include the following researchers, listed in alphabetical order by surname: Opeolu Adeoye, MD, University of Cincinnati; Neeraj Badjatia, MD, University of Maryland; Kim Boase, University of Washington; Yelena Bodien, PhD, Massachusetts General Hospital; M. Ross Bullock, MD, PhD, University of Miami; Randall Chesnut, MD, University of Washington; John D. Corrigan, PhD, ABPP, Ohio State University; Karen Crawford, University of Southern California; Ramon Diaz-Arrastia, MD, PhD, University of Pennsylvania; Ann-Christine Duhaime, MD, Mass General Hospital; Richard Ellenbogen, MD, University of Washington; V. Ramana Feeser, MD, Virginia Commonwealth University; Adam Ferguson, PhD, University of California, San Francisco; Brandon Foreman, MD, University of Cincinnati; Raquel Gardner, University of California, San Francisco; Etienne Gaudette, PhD, University of Southern California; Joseph Giacino, PhD, Spaulding Rehabilitation Hospital; Luis Gonzalez, TIRR Memorial Hermann; Shankar Gopinath, MD, Baylor College of Medicine; Rao Gullapalli, PhD, University of Maryland; J. Claude Hemphill, MD, University of California, San Francisco; Gillian Hotz, PhD, University of Miami; Sonia Jain, PhD, University of California, San Diego; Frederick Korley, MD, PhD, University of Michigan; Joel Kramer, PsyD, University of California, San Francisco; Natalie Kreitzer, MD, University of Cincinnati; Harvey Levin, MD, Baylor College of Medicine; Chris Lindsell, PhD, Vanderbilt University; Christopher Madden, MD, UT Southwestern; Alastair Martin, PhD, University of California, San Francisco; Thomas McAllister, MD, Indiana University; Michael McCrea, PhD, Medical College of Wisconsin; Randall Merchant, PhD, Virginia Commonwealth University; Pratik Mukherjee, MD, PhD, University of California, San Francisco; Florence Noel, PhD, Baylor College of Medicine; David Okonkwo, MD, PhD, University of Pittsburgh; Eva Palacios, PhD, University of California, San Francisco; Daniel Perl, MD, Uniformed Services University; Ava Puccio, PhD, University of Pittsburgh; Miri Rabinowitz, PhD, University of Pittsburgh; Claudia Robertson, MD, Baylor College of Medicine; Jonathan Rosand, MD, MSc, Massachusetts General Hospital; Angelle Sander, PhD, Baylor College of Medicine; Gabriella Satris, University of California, San Francisco; David Schnyer, PhD, UT Austin; Seth Seabury, PhD, University of Southern California; Mark Sherer, PhD, TIRR Memorial Hermann; Sabrina Taylor, PhD, University of California, San Francisco; Arthur Toga, PhD, University of Southern California; Alex Valadka, MD, Virginia Commonwealth University; Mary Vassar, RN, MS, University of California, San Francisco; Paul Vespa, MD, University of California, Los Angeles; Kevin Wang, PhD, University of Florida; John Yue, CCRC, PMP, University of California, San Francisco; and Ross Zafonte, Harvard Medical School.
Contributor Information
Collaborators: the TRACK-TBI Investigators, Opeolu Adeoye, Neeraj Badjatia, Kim Boase, Yelena Bodien, M. Ross Bullock, Randall Chesnut, John D. Corrigan, Karen Crawford, Ramon Diaz-Arrastia, Ann-Christine Duhaime, Richard Ellenbogen, V. Ramana Feeser, Adam Ferguson, Brandon Foreman, Raquel Gardner, Etienne Gaudette, Joseph Giacino, Luis Gonzalez, Shankar Gopinath, Rao Gullapalli, J. Claude Hemphill, Gillian Hotz, Sonia Jain, Frederick Korley, Joel Kramer, Natalie Kreitzer, Harvey Levin, Chris Lindsell, Christopher Madden, Alastair Martin, Thomas McAllister, Michael McCrea, Randall Merchant, Pratik Mukherjee, Florence Noel, David Okonkwo, Eva Palacios, Daniel Perl, Ava Puccio, Miri Rabinowitz, Claudia Robertson, Jonathan Rosand, Angelle Sander, Gabriella Satris, David Schnyer, Seth Seabury, Mark Sherer, Sabrina Taylor, Arthur Toga, Alex Valadka, Mary Vassar, Paul Vespa, Kevin Wang, John Yue, and Ross Zafonte
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Taylor C.A., Bell J.M, Breiding M.J., and Xu L. (2017). Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMRW Surveill. Summ. 66, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walter W.C., and Pickett T.C. (2007). Motor impairment after severe traumatic brain injury: a longitudinal multicenter study. J. Rehabil. Res. Dev. 44, 975–982 [DOI] [PubMed] [Google Scholar]
- 3. Dikmen S.S., Machamer J., Temkin N., and McLean A. (1990). Neuropsychological recovery in patients with moderate to severe traumatic brain injury. Arch. Phys. Med. Rehabil. 84, 1449–1475 [DOI] [PubMed] [Google Scholar]
- 4. Hibbard M.R., Uysal S., Kepler K., Bogdany J., and Silver J. (1998). Axis I psychopathology in individuals with traumatic brain injury. J. Head Trauma Rehabil. 13, 24–39 [DOI] [PubMed] [Google Scholar]
- 5. Deb S., Lyons I., Koutzoukis C., Ali I., and McCarthy G. (1999). Rate of psychiatric illness 1 year after traumatic brain injury. Am. J. Psychiatry 156, 374–378 [DOI] [PubMed] [Google Scholar]
- 6. Ashman T.A., Spielman L.A., Hibbard M.R., Silver J.M., Chandna T., and Gordon W.A. (2004). Psychiatric challenges in the first 6 years after traumatic brain injury: cross-sequential analyses of Axis I disorders. Arch. Phys. Med. Rehabil. 85, Suppl., 36–42 [DOI] [PubMed] [Google Scholar]
- 7. Whelan-Goodinson R., Ponsford J., Johnston L., and Grant F. (2009). Psychiatric disorders following traumatic brain injury: their nature and frequency. J. Head Trauma Rehabil. 24, 324–332 [DOI] [PubMed] [Google Scholar]
- 8. Temkin N.R., Machamer J.E., and Dikmen S.S. (2003). Correlates of functional status 3–5 years after traumatic brain injury with CT abnormalities. J. Neurotrauma 20, 229–241 [DOI] [PubMed] [Google Scholar]
- 9. McCleary C., Satz P., Forney D., Light R., Zaucha K., Asarnow R., and Namerow N. (1998). Depression after traumatic brain injury as a function of Glasgow Outcome Score. J. Clin. Exp. Neuropsychol. 20, 270–279 [DOI] [PubMed] [Google Scholar]
- 10. Pelz D.C., and Andrews F.M. (1964). Detecting causal priorities in panel study data. Am. Sociol. Rev. 29, 836–848 [Google Scholar]
- 11. Pagulayan K.F., Hoffman J.M., Temkin N.R., Machamer J.E., and Dikmen S.S. (2008). Functional limitations and depression after traumatic brain injury: examination of the temporal relationship. Arch. Phys. Med. Rehabil. 89, 1887–1892 [DOI] [PubMed] [Google Scholar]
- 12. Schönberger M., Ponsford J., Gould K.R., and Johnston L. (2011). The temporal relationship between depression, anxiety, and functional status after traumatic brain injury: a cross-lagged analysis. J. Int. Neuropsychol. Soc. 17, 781–787 [DOI] [PubMed] [Google Scholar]
- 13. Perrin P.B., Stevens L.F., Sutter M., Lequerica A.H., Krch D., Kolakowsky-Hayner S.A., and Arango-Lasprilla J.C. (2017). Reciprocal causation between functional independence and mental health 1 and 2 years after traumatic brain injury: a cross-lagged panel structural equation model. Am. J. Phys. Med. Rehabil. 96, 374–380 [DOI] [PubMed] [Google Scholar]
- 14. Dikmen S., Machamer J., and Temkin N. (2017). Mild traumatic brain injury: longitudinal study of cognition, functional status, and post-traumatic symptoms. J. Neurotrauma 34, 1524–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hiploylee C., Dufort P.A., Davis H.S., Wennberg R.A., Tartaglia M.C., Mikulis D., Hazrati L., and Tator C.H. (2017). Longitudinal study of postconcussion syndrome: not everyone recovers. J. Neurotrauma 34, 1511–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Korley F.K., Diaz-Arrastia R., Falk H.J., Peters M.E., Leoutsakos J.S., Roy D., Rao V., Sair H.I., Ofoche U., Hall A.J., Akbari F., Van Meter T.E., Everett A.D., Van Eyk J.E., and Bechtold K.T. (2017). Prevalence of incomplete functional and symptomatic recovery among patients with head injury but brain injury debatable. J. Neurotrauma 34, 1531–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pagulayan K.F., Temkin N.R., Machamer J., and Dikmen S.S. (2006). A longitudinal study of health-related quality of life after traumatic brain injury. Arch. Phys. Med. Rehabil. 87, 611–618 [DOI] [PubMed] [Google Scholar]
- 18. Kay T. (1993). Neuropsychological treatment of mild traumatic brain injury. J. Head Trauma Rehabil. 8, 74–85 [Google Scholar]
- 19. Ponsford J., Willmott C., Rothwell A., Cameron P., Kelly A., Nelms R., Curran C., and Ng K. (2000) Factors influencing outcome following mild traumatic brain injury in adults. J. Int. Neuropsychol. Soc. 6, 568–579 [DOI] [PubMed] [Google Scholar]
- 20. Ruff R.M., Camenzuli L., and Mueller J. (2009). Miserable minority: emotional risk factors that influence the outcome of a mild traumatic brain injury. Brain Inj. 10, 551–556 [DOI] [PubMed] [Google Scholar]
- 21. Derogatis L.R. (2000). Brief Symptom Inventory (BSI) 18: Administration, Scoring, and Procedures Manual, 3rd ed. NCS Pearson: Minneapolis, MN [Google Scholar]
- 22. Derogatis L.R., and Melisaratos N. (1983). The Brief Symptom Inventory: an introductory report. Psychol. Med. 13, 595–605 [PubMed] [Google Scholar]
- 23. Wilson J.T.L., Pettigrew L.E.L., and Teasdale G.M. (1998). Structured interviews for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale: guidelines for their use. J. Neurotrauma 15, 573–585 [DOI] [PubMed] [Google Scholar]
- 24. Kline R.B. (2005). Principles and Practice of Structural Equation Modeling. Guilford: New York [Google Scholar]
- 25. Jöreskog K.G., and Sörbom D. (1993). LISREL 8: Structural Equation Modeling with the SIMPLIS Command Language. Erlbaum: Hillsdale, NJ [Google Scholar]
- 26. Kwan J.L.Y., and Chan W. (2011). Comparing standardized coefficients in structural equation modeling: a reparameterization approach. Behav. Res. Methods 43, 730–745 [DOI] [PubMed] [Google Scholar]
- 27. Rindskopf D. (1984). Using phantom and imaginary latent variables to parameterize constraints in linear structural models. Psychometrika 49, 37–47 [Google Scholar]
- 28. Cheung M.W.L. (2009). Constructing approximate confidence intervals for parameters with structural equation models. Struct. Equ. Modeling 16, 267–294 [Google Scholar]
- 29. Kreutzer J.S., Seel R.T., and Gourley E. (2001). The prevalence and symptom rates of depression after traumatic brain injury: a comprehensive evaluation. Brain Inj. 15, 563–576 [DOI] [PubMed] [Google Scholar]
- 30. Seabury S.A., Guadette E., Goldman D.P., Markowitz A.J, Brooks J., McCrea M.A., Okonkwo D.O., and Manley G.T.; TRACK-TBI Investigators. (2018). Assessment of follow-up care after emergency department presentation for mild traumatic brain injury and concussion: results from the TRACK-TBI study. JAMA Netw. Open 1, E180210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sherer M., Davis L.C., Sander A.M., Nick T.G., Luo C., Pastorek N., and Hanks R. (2015). Factors associated with word memory test performance in persons with medically documented traumatic brain injury. Clin. Neuropsychol. 29, 522–541 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.