Abstract
Cannabis is one of the most widely used plant drugs in the world today. In spite of the large number of scientific reports on medical marijuana, there still exists much controversy surrounding its use and the potential for abuse due to the undesirable psychotropic effects. However, recent developments in medicinal chemistry of novel non-psychoactive synthetic cannabinoids have indicated that it is possible to separate some of the therapeutic effects from the psychoactivity. We have previously shown that treatment with the endocannabinoid 2-AG, which binds to both CB1 and CB2 receptors 1 h after traumatic brain injury in mice, attenuates neurological deficits, edema formation, infarct volume, blood–brain barrier permeability, neuronal cell loss at the CA3 hippocampal region, and neuroinflammation. Recently, we synthesized a set of camphor-resorcinol derivatives, which represent a novel series of CB2 receptor selective ligands. Most of the novel compounds exhibited potent binding and agonistic properties at the CB2 receptors with very low affinity for the CB1 receptor, and some were highly anti-inflammatory. This selective binding correlated with their intrinsic activities. HU-910 and HU-914 were selected in the present study to evaluate their potential effect in the pathophysiology of traumatic brain injury (TBI). In mice and rats subjected to closed-head injury and treated with these novel compounds, we showed enhanced neurobehavioral recovery, inhibition of tumor necrosis factor α production, increased synaptogenesis, and partial recovery of the cortical spinal tract. We propose these CB2 agonists as potential drugs for development of novel therapeutic modality to TBI.
Keywords: cannabinoids, CB2 receptor, HU-910, HU-914, traumatic brain injury
Introduction
Today, cannabis is one of the most widely used plant drugs in the world. In line with therapeutic benefits, smoking cannabis produces a number of acute, dose-dependent psychotropic effects, such as “feeling high,” relaxation, and euphoria. In spite of more than 3500 citations in the PubMed on medical marijuana, there still exists much controversy surrounding its medical use and the potential for abuse due to the unwanted psychotropic effects. However, recent developments in medicinal chemistry of novel non-psychoactive synthetic cannabinoids have indicated that it is possible to separate some of the therapeutic effects from the undesirable psychoactivity.
At least two types of cannabinoid receptors, CB1 and CB2, have been identified in mammals.1,2 Both receptors belong to the class of G-protein–coupled receptors and have a seven-transmembrane domain structure. Activation of either receptor blocks forskolin-induced accumulation of intracellular cyclic adenosine 3′,5′-monophosphate and involves linkage to Gi and/or Go-proteins.3 CB2 receptor shares 44% protein identity with CB1,4 but they display a distinct tissue distribution, pharmacological profile, and physiological roles pattern.
In the central nervous system (CNS), the CB1 receptor is located at the terminals of central and peripheral neurons, where it usually mediates inhibition of several neurotransmissions.5 CB2 receptor is widely distributed in cells of the immune system and was for many years referred to as “peripheral receptor” since it was initially cloned from a leukocyte cell line.2 Upon activation, CB2 receptor has been reported to alter immune functions like cell migration and cytokines release6 and the mechanisms underlying the CB1-induced psychotropic effects have not been attributed to the CB2 receptor. CB1 receptor is also shown to be associated with on-demand protection against acute excitotoxicity in central nervous system neurons and after brain injury.7,8 However a number of more recent studies have shown that in contrast to the previous claims, CB2 is also expressed in low levels in the CNS, where it appears more in microglia rather than in neurons.9 Thus, activation of CB2 with full agonists reduced, while antagonists worsened, post-traumatic inflammation.10 Surprisingly, modulating microglia activation with a CB2 inverse agonist was recently shown to rescue neuronal loss after mild TBI.11 These findings may have a significant impact on drug discovery and on our understanding of the biology of the endocannabinoid (eCB) system.
The cannabinoid receptors can be activated not only by plant-derived and synthetic ligands, but also by endogenous derivatives of fatty acids, particularly arachidonic acid, produced in mammalian tissues and often referred to as “endocannabinoids.” The first endocannabinoids to be discovered were N-arachidonoyl ethanolamine (anandamide) and 2-arachidonoyl glycerol (2-AG),12–14 which activate, with different affinities, both types of receptors. Later, a novel family of fatty-acids, amino acids (e.g., arachidonoyl-serine), also was identified as endocannabinoid-like compounds,15 which exhibit no direct binding to these receptors, but probably have an indirect or allosteric effect,16,17 probably mediated via the CB receptors.18–21
Although considered to be located mostly in the immune system, CB2 receptors are now well recognized on resident inflammatory cells within the CNS, on microglial and dendritic cells,22,23 and on brain endothelial cells.24 Activation of these receptors attenuates the inflammatory response by inhibiting the release of pro-inflammatory mediators and by diminishing leukocyte chemotaxis and extravasation into the brain parenchyma.25 CB2 agonists also were found to decrease cytochrome-C release, inhibit apoptosis, and exert anti-inflammatory effects in a diverse range of animal models.26,27
Traumatic brain injury (TBI) is a major cause of mortality and disability worldwide, followed by long-term physical and cognitive consequences. TBI-induced deleterious cerebral effects involve a complex of primary and secondary damages due to glutamate toxicity, oxidative stress, ionic and metabolic imbalance, inflammation, and ischemia. The observation of the TBI-induced multi-factorial pathology, along with the pharmacological profile of the eCBs, prompted us to investigate the effects of 2-AG after TBI. Our studies revealed that TBI-induces a 10-fold increase in 2-AG in the injured hemisphere 4 h after TBI, and that treatment with synthetic 2-AG attenuated edema formation, infarct volume, blood-brain barrier permeability, neuronal cell loss at the CA3 hippocampal region, and neuroinflammation.8,21,28,29
Recently, we synthesized a series of camphor-resorcinol derivatives, which represent a novel series of CB2 receptor selective ligands. The synthesis of the novel ligands, interaction with CB receptors, and anti-inflammatory properties are described in details in the PhD thesis by Magid,30 as well as in patents,31–32 and its protective effects in hepatic ischemia/reperfusion injury were reported by Horvath and colleagues.33
Most of the novel compounds exhibited potent binding affinities at the CB2 receptors in the low micromolar range, with up to 200-fold selectivity over the CB1 receptor. This binding discrimination correlated with their intrinsic activities in activating the CB2 receptors. We report now an evaluation of the effects of two compounds in this series, HU-910 and HU-914 (Fig. 1), in the pathophysiology of TBI.
FIG. 1.
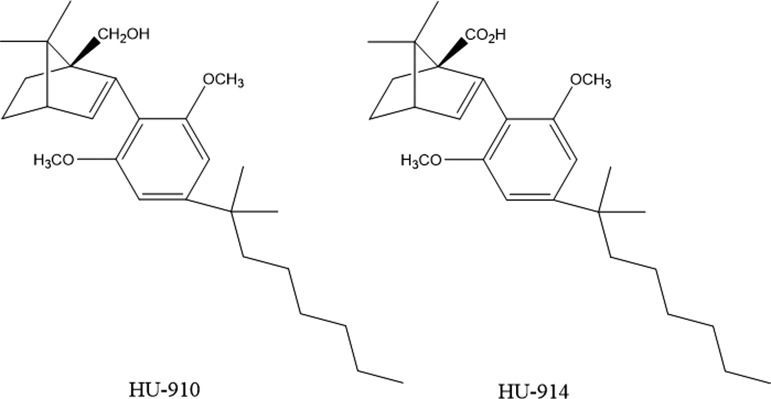
Chemical structure of HU-910 and HU914. HU-910: (1S,4R)-(2-(2,6-dimethoxy-4-(2-methyloctan-2-yl)phenyl)-7,7-dimethylbicyclo[2.2.1] hept-2-en-1-yl)methanol. HU-914: (1S,4R)-2-(2,6-dimethoxy-4-(2-methyloctan-2-yl)phenyl)-7,7-dimethylbicyclo[2.2.1] hept-2-ene-1-carboxylic acid.
Unlike the endogenous 2-AG, mentioned above, HU-910 is highly selective towards CB2 and was shown to attenuate oxidative stress, inflammation, and cell death associated with hepatic ischemia/reperfusion injury. HU-914 is a low-affinity partial selective CB2 agonist, which demonstrated high anti-inflammatory properties in lipopolysaccharide (LPS)-stimulated primary macrophages.30,33 Hence, we chose to evaluate these two compounds, which possess the target engagement and pharmacological requirements for the potential neuroprotective effect following TBI.
Methods
Animals
The study was performed according to the Institutional Animal Care and Use Committee guidelines in compliance with National Institutes of Health (NIH; Bethesda, MD) guidelines. Adult Sabra male mice (Harlan, Jerusalem, Israel) ages 6 to 8 weeks and weighing 40–45 g were used, unless otherwise stated. The animals were housed in individual cages during the testing period under controlled temperature and light conditions. During the entire experiment, they had free access to food and water. Knockout experiments were performed in male C57Bl/6 wild type (WT) mice (age 6–8 weeks; Harlan), which served as controls for CB2 receptor–deficient mice (CB2-/-) with C57Bl/6 strain background, generously provided by Professor Itai Bab. A dose–response experiment was performed in C57Bl/6 WT mice, which was designed to evaluate the therapeutic dose of the tested compound, HU-914, in the particular mice strain. Male Sprague-Dawley rats (250–300 g; Harlan) were used for the corticospinal tract study.
Closed-head injury model
Experimental closed-head injury (CHI) was induced by using a weight-drop device previously developed in our laboratory.34,35 Under isoflurane anesthesia, a midline longitudinal incision was performed, the skin was retracted, and the skull was exposed. The left anterior frontal area was identified, a tipped Teflon cone was placed 2 mm lateral to the midline and 1 mm caudal to the left coronal suture, and a metal rod (200 g for rats and 95 g for mice) was dropped down on the cone from a pre-adjusted height (according to body weight of each animal), resulting in a focal injury to the left hemisphere. After trauma, the mice received supporting oxygenation with 95% O2 for no longer than 2 min and were then returned to their cages. Sham controls were anesthetized and their scalps were incised, but they were not subjected to CHI. It is noteworthy that <10% of the injured mice were excluded from the study, mostly because of death by apnea within minutes of injury.
Neurological Severity Score
The functional neurological status of mice after CHI was determined by Neurological Severity Score (NSS), a 10-point scale based on the ability to perform motor and behavioral tasks.36 One point is awarded for failing to perform a task; scores range from 0 (complete success) to 10 (complete failure). The severity of injury was determined by the NSS evaluated at 1 h after CHI and referred to as NSS (1 h). Only mice with NSS (1 h) of 6–8 points (moderate TBI) were included in the study (< 10% were excluded on that basis). Immediately after evaluation of NSS, mice were randomly assigned to treatment groups and NSS was reevaluated during a time course indicated for each experiment. The extent of the recovery, ΔNSS, was used to present the neurological improvement and is calculated as the difference between the NSS at 1 h and NSS evaluated at any subsequent time-point after the trauma. Animals were scored by one observer who was blinded to the treatment groups. It should be noted that the functional neurological status of rats was determined by NSS on a 16-point scale (and not by the 10-point scale used for mice).
Drug administration
HU-910 and HU-914 were synthesized by a procedure described by Magid and colleagues30–32 Dose–response experiments were performed in the range of 0.1–10 mg/kg for HU-910, and 5 and 10 mg/kg for HU-914, unless otherwise stated. To evaluate whether the CB2 receptor was involved in the neuroprotective effects exerted by HU-910 and HU-914, CB2 selective antagonists/inverse agonists SR144528 1 mg/kg37,38 and AM630 1 mg/kg39,40 were administered alone or together with the tested compounds. The time window for the drug administration was determined in the preliminary experiments. All drugs, dissolved in ethanol:cremophor:saline 1:1:18, or vehicle alone, were injected intraperitoneally (i.p.) 1 h after injury. In the experiment with AM630, due to insolubility of the compound in ethanol, the drugs were dissolved in 0.75% Tween 20, 0.75% dimethylsulfoxide and saline.
Evaluation of tumor necrosis factor-α levels
Tumor necrosis factor (TNF)-α levels were analyzed 4 h after injury or sham. Cortical (∼50 mg) and hippocampal (∼15 mg) tissue segments from the ipsilateral hemisphere were immersed in liquid nitrogen and stored at −78°C until assayed. Tissue segments were homogenized in ice-cold buffer, 10 w/v ratio, containing 50 mM Tris-HCl, 1 mM EDTA, 1 mM EGTA, 0.5 mM Na3VO4, 0.1% 2-mercaptoethanol, 1% Triton X-100, 50 mM NaF, 5 mM sodium pyrophosphate, 10 mM sodium β-glyceropyrophosphate, 0.1 mM phenylmethanesulfonyl fluoride and protease inhibitor mixture (Roche Diagnostics). The homogenates were centrifuged at 10,000 rpm for 20 min at 4°C. Following determination of protein content according to Bradford method (BioRad Laboratories, Munich, Germany), the resulting supernatants were frozen at −78°C until enzyme-linked immunosorbent assay (ELISA) analysis. TNF-α levels were measured in triplicates using a Duo Set ELISA (R&D Systems, Minneapolis, MN).
Evaluation of synaptophysin levels: subcellular fractionation and Western blot analysis
Both cortex and hippocampus were dissected and homogenized 1:10 in homogenization buffer (320 mM sucrose, 10 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA, protease inhibitor mixture; Sigma). Subcellular fractionation was performed as described previously.41 Briefly, homogenates were centrifuged at 1000 g for 5 min at 4°C to remove nuclei and large debris. The supernatant was then centrifuged at 10,000 g for 30 min to obtain a crude synaptosomal fraction (pellet denominated P2 and supernatant denominated S2). A total of 5 μg protein of S2 fraction were loaded in 10% SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were incubated overnight at 4°C with synaptophysin 1:10,000 primary antibody (Chemicon, MAB 5258-20 uG) and then incubated (1 h at room temperature) with anti-mouse secondary antibody (Jackson ImmunoResearch 315-035003).
Magnetic resonance imaging studies
Magnetic resonance imaging (MRI) studies were performed on a 7.0 T/30 cm horizontal bore Bruker Biospec (Karlsruhe, Germany) MRI scanner equipped with a gradient system capable of producing gradient pulses of up to 400 mT/m. A body coil was used as the transmit coil and a quadrature coil (Bruker, Karlsruhe, Germany) dedicated for the mouse brain was used as the receiving coil. Anesthesia was induced with ∼4% isoflurane (Vetmarket Ltd., Petah Tikva, Israel) and maintained with 1–2% isoflurane in 95% O2 at a flow rate of 0.3–0.5 L/h. Respiratory rate was monitored throughout the entire MRI experiments and was maintained between 30–60 breaths/min. Body temperature was maintained by a feedback system of circulating water at 39°C. MRI experiments were performed on Days 1 and 36 post-trauma. The MRI experiments consisted of T2 weighted images acquired with the following parameters: RARE12 repetition time/echo time = 3500/70 msec, field of view of 1.8 × 1.8 cm2, matrix size of 256 × 128 zero filled to 256 × 256 resulting in an in-plane resolution of 70 × 70 (μm)2, and the number of scans was eight. Nine contiguous coronal slices of 0.9 mm thickness were collected from each animal. The collected images were analyzed by summation of all hypo-intense and hyperintense pixels in the ipsilateral hemisphere relative to the contralateral hemisphere. The brain damage was reported by expressing the percentage difference in the hypo-intense and hyperintense pixels observed at Day 1 and Day 36 ((d1-d36)/d1)*100. Damage evaluation was performed blindly by two independent examiners.
Anterograde tracing of corticospinal tract
Fourteen days after induction of CHI, rats were anesthetized with ketamine/xylazine (0.85/0.15, 0.1 mL/100 g body weight). To expose the right uninjured sensorimotor cortex, a 4 mm2 craniotomy was performed in an area bordered anteriorly by the bregma suture and medially by the central suture.42 Biotinylated dextran amine (BDA 10,000 MW, 10% w/v; Invitrogen) in phosphate-buffered saline was injected stereotaxically using a 10 μL Hamilton syringe (26 G). The injections were conducted into 12 points, one mm distant from one another. At each point the BDA was injected at depths of 0.5, 1.0, and 2.0 mm and the volume injected was 0.2, 0.4, and 0.2 μL respectively. The skin was sutured in place and 5 mL of saline was subcutaneously injected.
Tissue preparation and histology
Twenty-one days after BDA injection, the rats were re-anesthetized with ketamine/xylazine and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde (PFA). The brain and spinal cord were removed as one unit and post-fixed in PFA at 4°C for 24 h, then transferred to 30% sucrose solution at 4°C for at least 1 week. Tissue from the lumbar area of the spinal cord was embedded in OCT (Tissue-Tek, USA), 50 mm free-floating coronal sections were cut using a microtome cryostat (Microm HM 505E, Microm International, GmBH), and sections were transferred into cryobuffer solution (potassium acetate, polyvinylpyrrolidone 40K and ethylene glycol in DDW). To visualize the trajectory of the corticospinal tract (CST) axons, avidin-biotin complex conjugated to horseradish peroxidase (Vectastain Elite ABC Kit, Vector Laboratories) was used and further reacted with the glucose oxidase-nickel DAB technique. Stained sections were mounted on glass slides (Super Frost plus, Fisher). BDA-labeled axons were identified ipsilaterally to the injection site. The number and length of these midline-crossing axons were quantified using ImageJ software. The central canal and dorsal median fissure were used as midline landmarks.
Statistical analysis
The statistical analysis was performed using GraphPad Prism 4.0 (GraphPad Software Inc.). The data are expressed as the mean ± standard error of the mean. ΔNSS values expressing the neurobehavioral evaluation were compared between the experimental groups and statistical significance was determined at the selected time-points using the non-parametric Mann-Whitney test. Statistical significance for the quantification of TNF-α was calculated using one-way analysis of variance (ANOVA) followed by Dunnett's test. A value of p < 0.05 was considered statistically significant.
Results
Effect on neurobehavioral function (NSS)
Among a series of 20 novel compounds synthesized,30–32 HU-910 was found to be the most potent activator of the CB2 receptor with 6 nM binding affinity (vs. 1.37 μM for CB1) and was chosen to be tested as a single dose treatment, administered intraperitoneally at 1 h after the trauma. The chemical structure of these molecules is depicted in Figure 1.
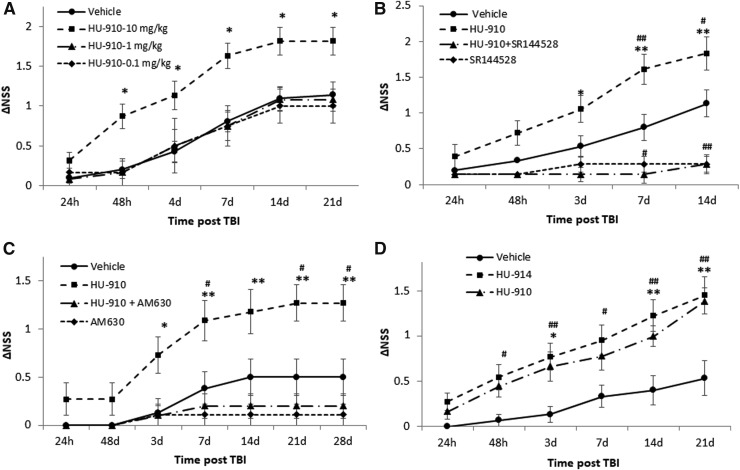
The recovery of neurobehavioral deficits was assessed as ΔNSS, and as can be seen from the dose–response study, a dose of 10 mg/kg yielded a significant recovery 48 h after the injury, which sustained for 3 weeks (Fig. 2A). The two lower doses (1 and 0.1 mg/kg) failed to produce any beneficial effect. To examine whether the beneficial effect achieved by HU-910 was CB2-dependent, the drug (10 mg/kg) was injected 10 min after the administration of the CB2 receptor selective antagonist/inverse agonist, SR144528 1 mg/kg. The beneficial effect of the drug was completely reversed upon its co- administration with SR144528 as observed at 3, 7, and 14 days after CHI (Fig. 2B). Interestingly, mice treated with either SR144528 alone or along with HU-910 lost the ability of the spontaneous neurological recovery when compared with the vehicle-treated group, attesting to the role of CB2 receptors in endogenous mechanisms of recovery.
FIG. 2.
Novel CB2 agonist improves functional recovery after traumatic brain injury (TBI). (A) Dose–response effects of HU-910. Mice were subjected to closed-head injury (CHI) and assigned to four treatment groups at the following doses: 0.1, 1.0, or 10.0 mg/kg or vehicle 1 h after CHI. The extent of the recovery, change in Neurological Severity Score (ΔNSS) is shown during 21 days. A significantly beneficial effect on neurological outcome relative to the vehicle-treated control group was found with 10 mg/kg. Data were expressed as mean ± standard error of the mean (SEM; n = 12–21/ group). *p ≤ 0.01 vs. vehicle-treated control group. (B) Administration of SR144528 abrogates the effect of HU-910. Mice were treated intraperitoneally 1 h after CHI with: vehicle, HU-910 10 mg/kg, SR144528 1 mg/kg followed by HU-910 10 mg/kg or SR144528 1 mg/kg alone. Administration of CB2 antagonist/inverse agonist, SR144528, 10 min before the administration of HU-910 completely abolished the beneficial effect of HU-910. Further, administration of SR144528 alone or together with HU-910 significantly exacerbated spontaneous neurological recovery compared with the vehicle-treated mice. Data were expressed as mean ± SEM (n = 8–11/group). *p ≤ 0.05 and **p ≤ 0.005 vs. HU-910 + SR144528 treated group; #p ≤ 0.05 and ##p ≤ 0.01 vs. vehicle-treated control group. (C) Administration of AM630 abrogates the effect of HU-910. Mice were treated with vehicle, HU-910 10 mg/kg, AM630 1 mg/kg followed by HU-910 10 mg/kg or AM630 1 mg/kg alone. Administration of CB2 antagonist/inverse agonist, AM630, 10 min prior to the administration of HU-910 completely reversed the beneficial effect of HU-910. Further, administration of AM630 alone or together with HU-910 tended to exacerbate the spontaneous neurological recovery, although not achieving statistical significance. Data were expressed as mean ± SEM (n = 8–11/group). *p ≤ 0.05 and **p ≤ 0.01 vs. HU-910 + AM630-treated group, #p ≤ 0.05 vs. vehicle-treated control group. (D) Similar therapeutic efficacies by HU-910 and HU-914 after TBI. Mice were treated with HU-914 5 mg/kg or HU-910 5 mg/kg. Both drugs showed greater recovery compared with vehicle-treated mice. Data are expressed as mean ± SEM (n = 15–22/group). #p < 0.05 and ##p < 0.01 HU-914 vs. vehicle-treated control group, *p < 0.05 and **p < 0.01 HU-910 vs. vehicle-treated control group.
To corroborate the involvement of the CB2 receptor in HU-910–induced beneficial effect, we designed a comparable experiment in which SR144528 was replaced by a structurally distinguishable CB2 receptor selective antagonist/inverse agonist, AM630 (Fig. 2C). In HU-910-treated mice, the recovery of the neurological function steadily continued over 28 days, reaching a plateau at Day 21. In vehicle-treated mice, spontaneous recovery reached the plateau already after 2 weeks. The beneficial effect of HU-910 was completely abolished when administered together with AM630.
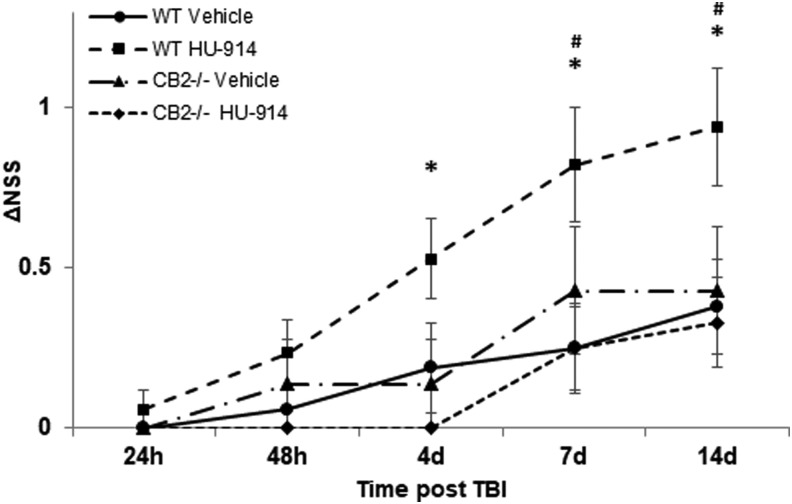
HU-914 is one of the 20 camphor-resorcinols which we have synthesized. It bears a carboxylic acid in the terpenoid moiety. Although this ligand binds with low affinity to CB2 (Ki 1500 nM) acting as partial agonist, the ligand is highly selective, as no activity at CB1 receptor was detected at concentrations up to 50 μM. Moreover, HU-914 was found to demonstrate the most potent inhibitory effects on TNF-α release in vitro, which is a modulator of the post-TBI inflammatory response.30 Therefore, a dose–response study was performed and the dose of 5 mg/kg was selected as it produced the maximal significant effect (data not shown). The neurobehavioral recovery in TBI model following administration of either HU-914 5 mg/kg or HU-910 5 mg/kg was compared (Fig. 2D). Both ligands were equally potent in ameliorating the functional recovery after TBI. To further emphasize the role of CB2 in the recovery process after TBI, CB2 receptor deficient (CB2-/-) mice and their WT littermates were subjected to CHI and treated with HU-914 or vehicle. Whereas HU-914 significantly improved the neurobehavioral function of WT mice, this beneficial effect was not observed in CB2-/- mice (Fig. 3). It should be noted that while the experiments described in Figure 2 were performed on the outbred Sabra mice, here, in order to use the genetically knockout CB2 mice, the comparison was made to their WT control of C57bl/6 strain. The difference in mice strain may account for the smaller effect observed here in the WT mice, which were treated with HU-914.
FIG. 3.
HU-914 is not effective in CB2 receptor deficient mice. CB2 receptor deficient (CB2-/-) mice with C57Bl/6 strain background and their wild type (WT) litter mates were treated intraperitoneally 1 h after closed-head injury with either HU-914 5 mg/kg or vehicle. HU-914 significantly improved the neurobehavioral function of WT but not of CB2-/- mice. Data are expressed as mean ± standard error of the mean (n = 7–17/group). *p ≤ 0.05 vs. HU-914-treated CB2-/- group, #p ≤ 0.05 vs. vehicle-treated WT control group.
Effect on infarct size—MRI
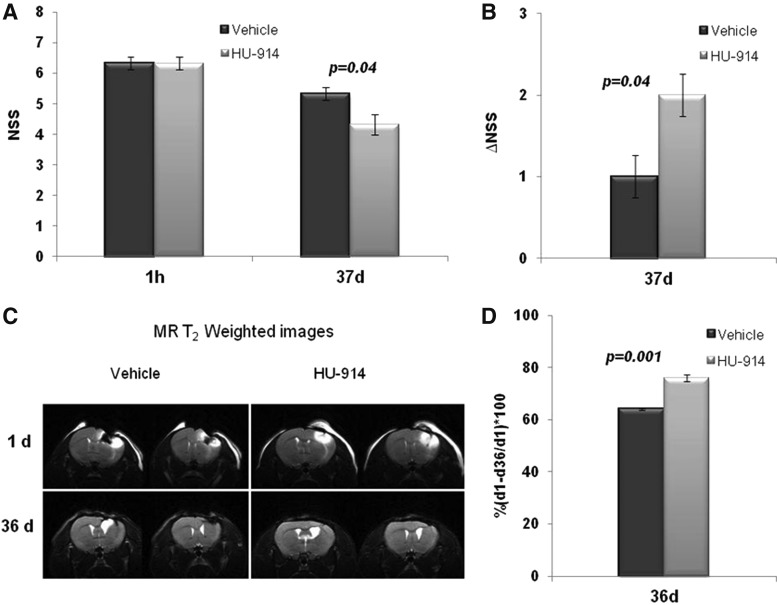
We next examined whether the treatment with HU-914 reduces the size of the brain damage scanned by MRI. Since NSS measured 1 h after CHI (NSS [1 h]) reflects the initial severity of injury and has been shown to correlate with the extent of damage seen on MRI,43 we examined whether the extent of the damage showed on MRI correlates with the extent of neurological recovery after the treatment with HU-914. Mice were assigned to two groups with equal initial mean NSS (1 h) of 6.3 ± 0.2. Both groups were treated i.p. 1 h after the injury with either vehicle (n = 6) or HU-914 (5 mg/kg). First MRI scan was performed in both groups within a few hours after the trauma (Day 0) and subsequent brain scans were collected on Day 1 and Day 36. The last NSS was measured at Day 37 after the injury. The extent of the recovery (ΔNSS) was calculated as the difference between NSS at 1 h following CHI and NSS on Day 37 (Fig. 4A, 4B). At 24 h after injury, the T2 weighted image could demonstrate brain edema and hemorrhage, contusions, and depressed skull fractures. Brain scans collected at Day 36 clearly visualized the size of the damaged area. Quantification of the damage was performed by measuring the area of total pathological signal in the ipsilateral hemisphere relative to the contralateral hemisphere. To evaluate the change in the size of damage, the value obtained at Day 36 was subtracted from the value obtained at Day 1 and the difference was expressed as a percentage of the initial area of damage (Fig. 4C, 4D). These results demonstrate that the administration of HU-914 significantly improved the neurological recovery of the mice 37 days after the injury. This improvement was correlated to the higher extent of attenuation of the brain damaged area in the HU-914-treated group (76.05 ± 2.3%) relative to the vehicle-treated group (64.05 ± 1.2%).
FIG. 4.
HU-914 reduces brain tissue damage after traumatic brain injury. Mice were treated intraperitoneally 1 h after closed-head injury with either HU-914, 5 mg/kg or vehicle. (A) Neurological assessment (Neurological Severity Score [NSS]) was performed at 1 h and 37 days. (B) Extent of the recovery at Day 37 post-injury ΔNSS = NSS (1 h) – NSS (37 days). (C) Magnetic resonance imaging (MRI) scans collected at 1 day and 36 days and presented as T2-weighted images. (D) MRI images were analyzed for the change in the size of the damage. The difference between the total pathological signals obtained at 1 day and 36 days was presented as a percentage of the initial area of damage (xy-axis). Data are expressed as mean ± standard error of the mean; n = 6/group.
Effect on inflammation
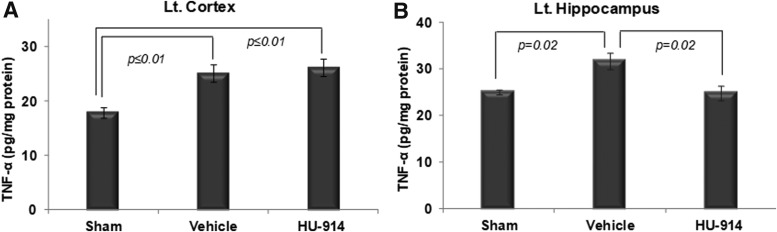
In our preliminary studies, we found that HU-910 and HU-914 modulate the secretion of pro-inflammatory cytokines and chemokines in LPS-stimulated murine peritoneal macrophages. Good correlation was found between the anti-inflammatory activity and the ligand-receptor relationship. These compounds bind with high affinity to the CB2 receptor, and most potently inhibit TNF-α, interleukin (IL)-6, and monocyte chemoattractant protein (MCP)-1 via the CB2 receptor pathway.30 We therefore examined the levels of the pro-inflammatory cytokine TNF-α in the cortex and hippocampus of the contused hemisphere, in order to have a mechanistic clue to the neuroprotective effects exert by these novel CB2 agonists.28 TNF-α levels were significantly elevated in the vehicle-treated ipsilateral cortex 25.15 ± 1.6 and hippocampus 31.67 ± 3.7 pg/mg tissue protein versus sham left side cortex 17.92 ± 1 and hippocampus 25.04 ± 1.1 pg/mg tissue protein. Treatment with HU-914 inhibited 22% of TNF-α release in the ipsilateral hippocampus, dropping to 24.87 ± 0.9 pg/mg tissue protein (Fig. 5B). However, it did not affect TNF-α level in the ipsilateral cortex 26.27 ± 1.6 pg/mg tissue protein as compared to the vehicle-treated control group (Fig. 5A). TNF-α protein content in the brain extracts of contralateral hemisphere did not differ between the sham, vehicle, and HU-914-treated groups.
FIG. 5.
Effect of HU-914 on tumor necrosis factor (TNF)-α levels in the brain after traumatic brain injury (TBI). Bars illustrate the protein concentrations of TNF-α, determined at 4 h after TBI in cortex (A) and hippocampus (B) of both hemispheres using enzyme-linked immunosorbent assay. Mice were treated intraperitoneally 1 h after closed-head injury with either vehicle or HU-914 5 mg/kg. (A) TNF-α levels were significantly elevated in vehicle ipsilateral (left) cortex (p ≤ 0.01) and (B) hippocampus (p = 0.02) vs. sham. When compared with vehicle, administration of HU-914 significantly reduced TNF-α secretion in hippocampus (p = 0.02), but not in cortex; n = 5–6/group.
Synaptogenesis
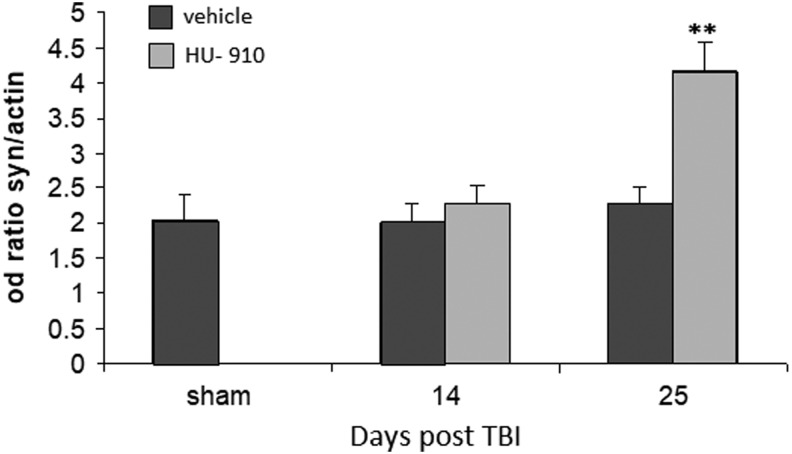
The endocannabinoid system has key roles in many aspects of nervous system development that includes neurogenesis, glia formation, migration and lengthening of axons. CB2 receptors are present on progenitor cells from embryo origin and from adult brain, and it is assumed that it mediates acceleration of neurogenesis.44 Since the improved clinical status after treatment with HU-910 was long lasting (at least 21 days), we examined the changes in synaptogenesis by quantifying the levels of synaptophysin (SYP) protein, which is located in the pre-synaptic vesicles and indicates synaptic density. Only in the cytosol fraction of sub-lesion cortical tissue at 25 days post-TBI, a 2-fold increase in SYP levels was found after the drug treatment (Fig. 6).
FIG. 6.
HU-910 increased cortical synaptogenesis 25 days after injury. Extraction of proteins from the cytosolic fraction prepared from cortical tissue adjacent to the site of injury was analyzed by Western blot, the levels of synaptophysin (SYP) was quantified, and a 2-fold increase in SYP levels was found after drug treatment (n = 4–5/group). **p < 0.001 vs. vehicle-treated mice.
Axonal sprouting in denervated CST
The CST, the largest axonal tract system leading from the frontal motor cortex to the contralateral motor neurons of the spinal cord, was shown in other TBI models to be impaired. Here, we used rats subjected to controlled CHI that received supporting oxygenation with 95% O2 for no longer than 2 min post-injury, and were then returned to their cages. No rats were excluded due to apnea, as they all regained breathing.
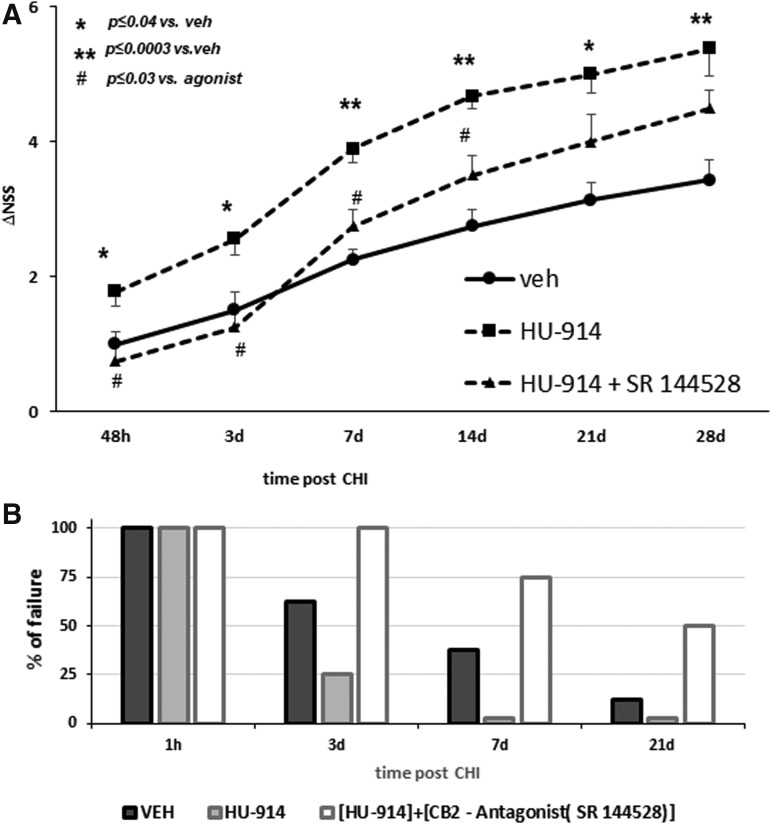
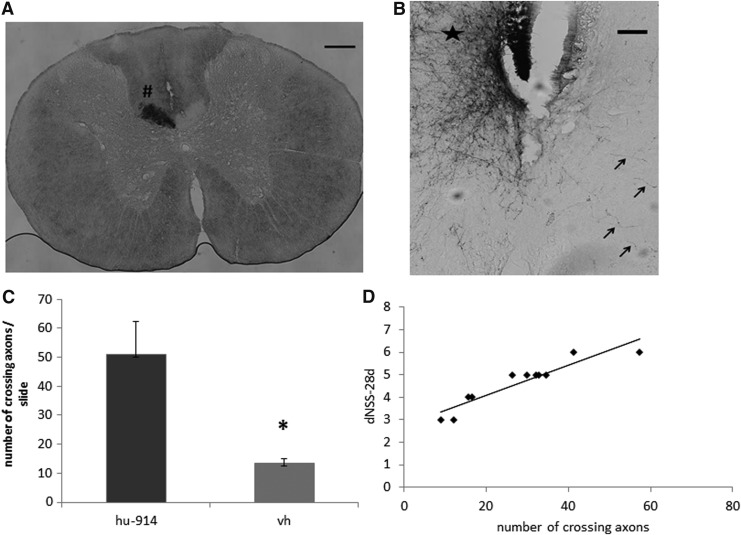
HU-914 was given at a single i.p. dose 1 h after the injury, as described for the mice above. The functional recovery of the rats was followed for 28 days using an adjusted NSS scale. Similar to mice, the rats treated with HU-914 displayed significant greater recovery, relative to the vehicle-treated rats, which was partially lost upon treatment with the antagonist SR144528 (Fig. 7A). Walk over an 8 cm wide beam is one of the tested motor functions in the NSS scale. At 1 h after the injury, all the rats failed to perform this task, and a gradual recovery to regain this ability was found in the vehicle-treated rats from Day 3 on (62.5% failure). Recovery of the rats treated with HU-914 was significantly faster, and on Day 3 only 25% failed this task, and all were able to walk the beam from Day 7 onward. The effect of the drug was abolished by the CB2 antagonist (Fig. 7B). Using the BDA labeling, we found that the CST was strongly labeled contralateral to the injection site, with some axons crossing the midline to innervate structures ipsilateral to the injection site (Fig. 8A). The number of midline-crossing axons in the lumbar segment of the CST was significantly higher in the HU-914 group compared with the control group (Fig. 8B, 8C). However, there were no significant differences between the groups in terms of the crossing fibers length.
FIG. 7.
HU-914 increases the extent of recovery after closed-head injury in rats. (A) Rats were treated with vehicle or HU-914 1 h after injury, and their functional recovery, change in Neurological Severity Score (ΔNSS adjusted to rats) was followed for 4 weeks. (A) Significantly higher extent of recovery was found in the HU-914 group from 48 h post-injury, and sustained the whole follow-up period. The effect was abolished by co-administration of HU-914 with CB2 antagonist SR144528. *p < 0.04; **p < 0.0003 HU-914 vs. vehicle and #p < 0.03 HU-914 + antagonist vs. vehicle) compared with the control group during 28 days of follow-up. (B) Percent of rats failing to complete the 8-cm wide walk task. While all rats failed at 1 h, from Day 3 on there was a significantly lower percent of failure among the HU-914 treated rats, compared with vehicle-treated controls. CB2 antagonist not only abolished this effect but led to increase rates of failure.
FIG. 8.
HU-914 induces midline-crossing axons in the lumbar segment of the CST. (A) Biotinylated dextran amine (BDA; 10,000 MW, 10% W/V; Invitrogen) in phosphate-buffered saline was injected stereotaxically into 12 points, 1 mm distant from one another at depths of 0.5, 1.0, and 2.0 mm. BDA-labeled corticospinal tract in the dorsal funiculus (#) was robust contralateral to the injection site. Scale bar = 200 μm. (B) High density of BDA-labeled axons is shown on the intact side of spinal cord (asterisk) and a number of BDA-labeled axons crossing the midline to the denervated side of the spinal cord (arrows) is also abundant in the treated mice. Scale bar 100 μm. (C) The number of axons crossing the midline was significantly higher in the HU-914 treated rats, compared with the vehicle-treated rats (p < 0.03). (D) Correlation between functional recovery and repair of the CST. ΔNSS score at 28 days after traumatic brain injury was significantly and directly correlated with the number of midline-crossing axons in the lumbar segment of the CST. R2 = 0.9315; p < 0.0001; n = 11.
Pearson correlation was performed to investigate the relationship between clinical recovery and anatomical reorganization. The ΔNSS score at 28 days after TBI was significantly and directly correlated with the number of midline-crossing axons in the lumbar segment of the CST demonstrating Pearson's value of R2 = 0.9315 (p < 0.0001; Fig. 8D). This is corroborating evidence that HU-914 has a potential neuroregenerative effect along with an inherent improvement in neurological status.
Discussion
2-Arachidonoyl-glycerol (2-AG), the most abundant endocannabinoid in the brain, binds and activates both CB1 (58–472nM) and CB2 (145–1400nM) receptors. We have previously shown that its levels are elevated in the ipsilateral hemisphere from 1 to 24 h after injury and that treatment with synthetic 2-AG attenuated edema, infarct volume, and inflammation, and protected the blood–brain barrier permeability. Part of these effects were absent in CB1-/- mice and were abolished by CB1 antagonist, albeit at a relatively high dose, suggesting that they are not solely mediated via the CB1 receptor.8,28,45 Therefore, our goal was to test whether the selective activation of the CB2 receptor by novel camphor-resorcinols will lead to neuroprotection following TBI.
We have previously shown that a pinene-resorcinol dimethyl ether derivative, HU-308, binds specifically to the CB2 receptor.37 Recently, an extensive characterization of the molecular pharmacology of the most widely used CB2 receptor ligands to date was reported. Marked differences in the ability of certain agonists to activate distinct signaling pathways and to cause off-target effects were noted. HU-910 and HU-308 were recommended as highly selective CB2 receptor agonists to study further the role of CB2 receptors in biological and disease processes.46
A newly designed and synthesized set of molecules based on camphor-resorcinols were assayed in search for selective CB2 agonists with pronounced anti-inflammatory properties. The two molecules selected for the present study on TBI were the hydroxyl derivative HU-910 and the corresponding carboxylic acid HU-914, both possessing substantial affinity and efficacy toward the CB2 receptor and which most efficiently reduced the levels of TNF-α, IL-6, and MCP-1, in LPS-stimulated macrophages.30 The therapeutic doses were chosen based on evaluation of neurological recovery. Both drugs exerted a long-lasting effect after a single administration at 1 h post-TBI, and increased recovery was still evident 3–6 weeks later (Fig. 2D and 4B), which was associated with reduction in the infarct volume as assessed by MRI (Fig. 4D). This corroborates our previous finding on the reduced infarct volume by 2-AG, assessed by TTC staining at 24 h post-injury.8
To corroborate the major hypothesis of the present study, Magid has shown that treatment with HU-914 demonstrated an improved neurological recovery over 28 days of follow-up after the injury. This effect was completely reversed upon co-administration of HU-914 and SR144528.30 Here, we showed that the neuroprotective effect of HU-910 is mediated by the CB2 receptor. Moreover, the two structurally similar CB2 antagonists/inverse agonists SR144528 and AM630 not only completely reversed the beneficial effect of HU-910, but significantly exacerbated the spontaneous neurological recovery demonstrated by the vehicle-treated mice, presumably due to blocking endogenous CB2 activity. To further support this notion, we showed (Fig, 3) that as expected, HU-914 had no effect on ΔNSS in CB2(-/-) mice, indicating the CB2 as mediator of an endogenous neuroprotective mechanism, which is set in motion after brain injury and is mediated via the endocannabinoid system. In our CHI model, a transient minor hypothermia (of approximately 2°C) is noticed between 1–4 h post-injury. From the previous studies (by us and others), it is well established that CB1 agonists induce hypothermia, which is a mechanism for neuroprotection,47 yet no such effect is reported for CB2.48 It should be noted that the endocannabinoid arachidonoyl-serine, the neuroprotective effect of which is mediated via both CB1 and CB2 receptors, was found to abolish the spontaneous hypothermia following injury.18 In the present experiments, we did not monitor the temperature.
Neuroinflammation is one of the major secondary mechanisms that is activated after TBI and is characterized by the invasion of peripheral immune cells and activation of glial cells, triggering the secretion of inflammatory mediators by those cells.49 Activated microglia and peripheral infiltrated macrophages release several cytokines, such as TNF-α, IL-1α, IL-1β, and IL-6, which increase the blood–brain barrier permeability and facilitate the invasion of peripheral immune cells and the secretion of toxic molecules. Following TBI, elevation of TNF-α was detected at an early phase in postmortem human brains and diverse experimental models.50 The release of TNF-α, following acute injury, plays dual and opposing roles, on the one hand enhancing brain damage through the release of neurotoxic substances at acute post-traumatic phase,51 but on the other hand facilitating long-term behavioral recovery and tissue repair.52 Here, we showed that the elevation of TNF-α in the ipsilateral cortex at 2 and 4 h and in the hippocampus at 4 h after CHI, was inhibited slightly, yet significantly, by HU-914, corroborating its anti-inflammatory properties.
Another consequence of TBI is dendritic damage which is reflected, among other ways, as a decrease in synaptophysin expression. Gomez and colleagues53 have proposed that glial CB2 receptors are involved in neuritic elongation and guidance, and in synaptogenesis. Although we did not find reduced expression of this pre-synaptic marker at any time after injury, 25 days post-injury, mice treated with HU-910 showed nearly 2-fold higher levels of synaptophysin in the cortical cytosolic fraction. These findings agree with the report by Tchantchou and colleagues54 in a mouse model of TBI. They used an inhibitor of fatty acid amide hydrolase, the degrading enzyme of the endocannabinoid anandamide that activates both CB1 and CB2 receptors, and demonstrated that elevating the levels of the endogenous eCB promotes neuronal survival, including increasing the expression of hippocampal synaptophysin.
Diffuse damage to white matter tracts, which are particularly susceptible to the shearing forces, often occur with TBI.55 The CST is the largest axonal tract system leading from the frontal motor cortex to the contralateral motor neurons of the spinal cord. Previous studies in rats after TBI demonstrated that sprouting of CST axons originating from the contralateral hemisphere into the denervated side of the spinal cord is associated with functional recovery.56,57 Benowitz and colleagues58 have showed that treatment with inosine improved the function following TBI by enhancing the ability of spared neurons to form new circuits that might compensate for ones that have been lost. Here, we showed that the number of midline-crossing axons from the intact to the injured side of the spinal cord was significantly increased in HU-914 treated rats as compared to control group. These rats also experienced partial spontaneous clinical recovery (ΔNSS) over a period of 1 month. HU-914 significantly improved recovery, which was completely reduced by co-administration with a specific CB2 antagonist.
The correlation between sensorimotor recovery and contra-lesion CST sprouting suggests that neuronal reorganization is one of the mechanisms by which the endocannabinoid system via activation of the CB2 receptor encourages recovery after TBI. Although previous studies have demonstrated sprouting of axons into the denervated side of the spinal cord after injury,56–58 this is, to the best of our knowledge, the first study to show this effect in response to cannabinoid administration. The decision to examine the CST was taken because of its importance in controlling voluntary movement and because it had been shown to undergo axonal plasticity after TBI. However, it is not inconceivable that there are other white matter tracts, as well as intercortical and intracortical axonal connections, that also demonstrate neuronal rearrangement after TBI. An interesting question to be studied in future research is whether the observed phenomenon is the result of sprouting and formation of new synapses or unmasking of existing but functionally inactive pathways, and what role the CB2 receptor plays in this setting.
Conclusion
It is conceivable that CB2 receptor plays an important role in neuroprotection following brain injury. The endogenous ligands, anandamide and 2-AG, which bind to both CB1 and CB2 receptors, contribute to the natural defense of the CNS. However, since CB1 is the receptor that mediates the psychotropic effects of cannabinoids, having a pure and selective CB2 agonist with no undesirable side effects is of high importance for translational studies in the setting of TBI.
Acknowledgments
This study was supported by a NIH grant DA-9789 to RM; RM thanks the Kessler Foundation for financial support. Part of this study was supported by grants from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (AMRF) to ES.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Matsuda L.A., Lolait S.J., Brownstein M.J., Young A.C., and Bonner T.I. (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564 [DOI] [PubMed] [Google Scholar]
- 2. Munro S., Thomas K.L., and Abu-Shaar M. (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65 [DOI] [PubMed] [Google Scholar]
- 3. Howlett A.C. (2005). Cannabinoid Receptor Signaling. Handb. Exp. Pharmacol. 168, 53–79 [DOI] [PubMed] [Google Scholar]
- 4. Howlett A.C., Barth F., Bonner T.I., Cabral G., Casellas P., Devane W.A., Felder C.C., Herkenham M., Mackie K., Martin B.R., Mechoulam. R., and Pertwee R.G. (2002). International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 54, 161–202 [DOI] [PubMed] [Google Scholar]
- 5. Szabo B. and Schlicker E. (2005) Effects of cannabinoids on neurotransmission, in: Cannabinoids. Pertwee R.G. (ed). Springer Publishing: Berlin Heidelberg, pps. 327–365 [DOI] [PubMed] [Google Scholar]
- 6. Cabral G.A., Pertwee R.G., and Staab A. (2005) Effects on the immune system, in: Cannabinoids. Pertwee R.G. (ed). Springer Publishing: Berlin Heidelberg, pps. 385–423 [Google Scholar]
- 7. Marsicano G., Goodenough S., Monory K., Hermann H., Eder M., Cannich A., Azad S.C., Cascio M.G., Gutiérrez S.O., van der Stelt M., López-Rodriguez M.L., Casanova E., Schütz G., Zieglgänsberger W., Di Marzo V., Behl C., and Lutz B. (2003). CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88 [DOI] [PubMed] [Google Scholar]
- 8. Panikashvili D., Simeonidou C., Ben-Shabat S., Hanus L., Breuer A., Mechoulam R., and Shohami E. (2001) An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature 413, 527–531 [DOI] [PubMed] [Google Scholar]
- 9. Stella N. (2010). Cannabinoid and cannabinoid-like receptors in microglia, astrocytes and astrocytomas. Glia 58, 1017–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amenta P.S., Jallo J.I., Tuma R.F., Hooper D.C., and Elliott M.B. (2014). Cannabinoid receptor type-2 stimulation, blockade, and deletion alter the vascular inflammatory responses to traumatic brain injury. J. Neuroinflammation 22, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bu W., Ren H., Deng Y., Del Mar N., Guley N.M., Moore B.M., Honig M.G., and Reiner A. (2016). Mild traumatic brain injury produces neuron loss that can be rescued by modulating microglial activation using a CB2 receptor inverse agonist. Front. Neurosci. 10, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Devane W., Hanus L., Breuer A., Pertwee R.G., Stevenson L.A., Griffin G., Gibson D., Mandelbaum A., Etinger A., and Mechoulam R. (1992). Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 [DOI] [PubMed] [Google Scholar]
- 13. Mechoulam R., Ben-Shabat S., Hanus L. L.igumsky M., Kaminski N.E., Schatz A.R., Gopher A., Almog S., Martin B.R., Compton D.R., Pertwee R.G., Griffin G., Bayewitch M., Barg J., and Vogel Z. (1995). Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50, 83–90 [DOI] [PubMed] [Google Scholar]
- 14. Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., and Waku K. (1995). 2-Arachidonoylgylcerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 215, 89–97 [DOI] [PubMed] [Google Scholar]
- 15. Milman G., Maor Y., Abu-Lafi S., Horowitz M., Gallily R., Batkai S., Mo F.M., Offertaler L., Pacher P., Kunos G., and Mechoulam R. (2006). N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc. Natl. Acad. Sci. 103, 2428–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan B., O'Dell D.K., Yu Y.W., Monn M.F., Hughes H.V., Burstein S., and Walker J.M. (2010). Identification of endogenous acyl amino acids based on a targeted lipidomics approach. J. Lipid Res. 51, 112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanuš L., Shohami E., Bab I., and Mechoulam R. (2014). N-Acyl amino acids and their impact on biological processes. Biofactors 40, 381–388 [DOI] [PubMed] [Google Scholar]
- 18. Cohen-Yeshurun A., Trembovler V., Alexandrovich A., Ryberg E, Greasley P.J., Mechoulam R., Shohami E., and Leker R.R. (2011). N-arachidonoyl-L-serine is neuroprotective after traumatic brain injury by reducing apoptosis. J Cereb. Blood Flow Metab. 31, 1768–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cohen-Yeshurun A., Willner D., Trembovler V., Alexandrovich A., Mechoulam R., Shohami E., and Leker R.R. (2013). N-arachidonoyl-L-serine (AraS) possesses proneurogenic properties in vitro and in vivo after traumatic brain injury. J. Cereb. Blood Flow Metabo. 33, 1242–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mann A., Smoum R., Trembovler V., Alexandrovich A., Breuer A., Mechoulam R., and Shohami E. (2015). Palmitoyl serine: an endogenous neuroprotective endocannabinoid-like entity after traumatic brain injury. J. Neuroimmune Pharmacol. 10, 356–363 [DOI] [PubMed] [Google Scholar]
- 21. Mann A., Cohen-Yeshurun A., Trembovler V., Mechoulam R., and Shohami E. (2016). Are the endocannabinoid-like compounds N-acyl aminoacids neuroprotective after traumatic brain injury? J. Basic Clin. Physiol. Pharmacol. 27, 209–216 [DOI] [PubMed] [Google Scholar]
- 22. Maresz K., Carrier E.J., Ponomarev E.D., Hillard C.J., and Dittel B.N. (2005). Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 95, 437–445 [DOI] [PubMed] [Google Scholar]
- 23. Pertwee R.G. (2008). Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict. Biol. 13, 147–159 [DOI] [PubMed] [Google Scholar]
- 24. Golech S.A., McCarron R.M., Chen Y., Bembry J., Lenz F., Mechoulam R., Shohami E., and Spatz M. (2004). Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res. Mol. Brain Res. 132, 87–92 [DOI] [PubMed] [Google Scholar]
- 25. Pacher P. and Hasko G. (2008). Endocannabinoids and cannabinoid receptors in ischaemia reperfusion injury and preconditioning. Br. J. Pharmacol. 153, 252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ashton J.C. and Glass M. (2007). The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr. Neuropharmacol. 5, 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benito C., Tolón R.M., Pazos M.R., Núñez E., Castillo A.I., and Romero J. (2008). Cannabinoid CB2 receptors in human brain inflammation. Br. J. Pharmacol. 153, 277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Panikashvili D., Shein N.A., Mechoulam R., Trembovler V., Kohen R., Alexandrovich A., and Shohami E. (2006). The endocannabinoid 2-AG protects the blood–brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol. Dis. 22, 257–264 [DOI] [PubMed] [Google Scholar]
- 29. Shohami E., Cohen-Yeshurun A., Magid L., Elgali M., and Mechoulam R. (2011) Endocannabinoids and traumatic brain injury. Br. J. Phram. 163, 1402–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Magid L., (2012). Development and pharmacological evaluation of novel CB2 receptor selective agonists as anti-inflammatory and neuroprotective agents [PhD dissertation]. Israel. Hebrew University of Jerusalem, Jerusalem, Israel [Google Scholar]
- 31. Mechoulam R., Magid L., Shohami E., Bab I., inventor; Arylated camphenes, processes for their preparation and uses thereof. US patent 8722938. November 18, 2010
- 32. Mechoulam R., Magid L., Shohami E., Bab I., inventor; Arylated camphenes, processes for their preparation and uses thereof. US patent 9365534. March 31, 2014
- 33. Horváth B., Magid L., Mukhopadhyay P., Bátkai S., Rajesh M., Park O., Tanchian G., Gao R.Y., Goodfellow C.E., Glass M., Mechoulam R., and Pacher P. (2012). A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury. Br. J. Pharmacol. 165, 2462–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Y., Constantini S., Trembovler V., Weinstock M., and Shohami E. (1996). An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma 13, 557–568 [DOI] [PubMed] [Google Scholar]
- 35. Flierl M.A., Stahel P.F, Beauchamp K.M., Morgan S.J., Smith W.R., and Shohami E. (2009). Mouse closed head injury model induced by a weight-drop device. Nat. Protoc. 4, 1328–1337 [DOI] [PubMed] [Google Scholar]
- 36. Beni-Adani L., Gozes I., Cohen Y., Assaf Y., Steingart R.A., Brenneman D.E., Eizenberg O., Trembolver V., and Shohami E. (2001). A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J. Pharmacol. Exp. Ther. 296, 57–63 [PubMed] [Google Scholar]
- 37. Hanuš L., Breuer A., Tchilibon S., Shiloah S., Goldenberg D., Horowitz M., Pertwee R.G., Ross R.A., Mechoulam R., and Fride E. (1999). HU-308: a specific agonist for CB2, a peripheral cannabinoid receptor. Proc Natl. Acad. Sci. 96, 14228–14233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beltramo M., Bernardini N., Bertorelli R., Campanella M., Nicolussi E., Fredduzzi S., and Reggiani A. (2006). CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur. J. Neurosci. 23, 1530–1538 [DOI] [PubMed] [Google Scholar]
- 39. Di Filippo C., Rossi F., Rossi S., and D'Amico M. (2004). Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: involvement of cytokine/chemokines and PMN. J. Leukoc. Biol. 75, 453–459 [DOI] [PubMed] [Google Scholar]
- 40. Ibrahim M.M., Deng H., Zvonok A., Cockayne D.A., Kwan J., Mata H.P., Vanderah T.W., Lai J., Porreca F., Makriyannis A., and Malan T.P., Jr. (2003). Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc. Natl. Acad. Sci. 100, 10529–10533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schumann J., Michaeli A., and Yaka R. (2009). Src-protein tyrosine kinases are required for cocaine-induced increase in the expression and function of the NMDA receptor in the ventral tegmental area. J. Neurochem. 108, 697–706 [DOI] [PubMed] [Google Scholar]
- 42. Dachir S., Shabashov D., Trembovler V., Alexandrovich A.G., Benowitz L.I., and Shohami E. (2014). Inosine improves functional recovery after experimental traumatic brain injury. Brain Res. 1555, 78–88 [DOI] [PubMed] [Google Scholar]
- 43. Tsenter J., Beni-Adani L., Assaf Y., Alexandrovich A.G., Trembovler V., and Shohami E. (2008). Dynamic changes in the recovery after traumatic brain injury in mice: effect of injury severity on T2-weighted MRI abnormalities, and motor and cognitive functions. J. Neurotrauma 25, 324–333 [DOI] [PubMed] [Google Scholar]
- 44. Prendervill J.A., Kelly A.M., and Downer E.J. (2015). The role of cannabinoids in adult neurogenesis. Br. J. Pharmacol. 172, 3950–3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Panikashvili D., Mechoulam R., Beni S.M., Alexandrovich A., and Shohami E. (2005). CB1 cannabinoid receptors are involved in neuroprotection via NF-kappa B inhibition. J. Cereb. Blood Flow Metab. 25, 477–484 [DOI] [PubMed] [Google Scholar]
- 46. Soethoudt M., Grether U., Fingerle J., Grim T.W., Fezza F., de Petrocellis L., Ullmer C., Rothenhäusler B., Perret C., van Gils N., Finlay D., MacDonald C., Chicca A., Gens M.D., Stuart J., de Vries H., Mastrangelo N., Xia L., Alachouzos G., Baggelaar M.P., Martella A., Mock E.D., Deng H., Heitman L.H., Connor M., Di Marzo V., Gertsch J., Lichtman A.H., Maccarrone M., Pacher P., Glass M., and van der Stelt M. (2017). Cannabinoid CB2 receptor ligand profiling reveals biased signaling and off-target activity. Nat. Commun. 8:13958, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dietrich W.D., Bramlett H.M. (2016). Therapeutic hypothermia and targeted temperature management in traumatic brain injury: Clinical challenges for successful translation. Brain Res. 1640(Pt A), 94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Malan T.P., Jr., Ibrahim M.M., Deng H., Liu Q., Mata H.P., Vanderah T., Porreca F., Makriyannis A. (2001). CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 93, 239–245 [DOI] [PubMed] [Google Scholar]
- 49. Morganti-Kossmann M.C., Satgunaseelan L., Bye N., and Kossmann T. (2007). Modulation of immune response by head injury. Injury 38, 1392–1400 [DOI] [PubMed] [Google Scholar]
- 50. Frugier T., Morganti-Kossmann M.C., O'Reilly D., and McLean C.A. (2009). In situ detection of inflammatory mediators in postmortem human brain tissue after traumatic injury. J. Neurotrauma 27, 497–507 [DOI] [PubMed] [Google Scholar]
- 51. Shohami E., Ginis I., and Hallenbeck J.M., (1999). Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev. 10, 119–130 [DOI] [PubMed] [Google Scholar]
- 52. Scherbel U., Raghupathi R., Nakamura M., Saatman K.E., Trojanowski J.Q., Neugebauer E., Marino M.W., and McIntosh T.K. (1999). Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc. Natl. Acad. Sci. 96, 8721–8726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gómez M., Hernández M., and Fernández-Ruiz J. (2008). Cannabinoid signaling system: does it play a function in cell proliferation and migration, neuritic elongation and guidance and synaptogenesis during brain ontogenesis? Cell Adh. Migr. 2, 246–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tchantchou F., Tucker L.B., Fu A.H., Bluett R.J., McCabe J.T., Patel S., and Zhang Y. (2014). The fatty acid amide hydrolase inhibitor PF-3845 promotes neuronal survival, attenuates inflammation and improves functional recovery in mice with traumatic brain injury. Neuropharmacology 85, 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Graham D., Gennarelli T., and McIntosh T. (2002). Trauma, in: Greenfield's Neuropathology. D. Graham and P. Lantos (eds). Arnold Hodder Publications: London, pps. 821–898 [Google Scholar]
- 56. Zhang Y., Xiong Y., Mahmood A., Meng Y., Liu Z., Qu C., and Chopp M. (2010). Sprouting of corticospinal tract axons from the contralateral hemisphere into the denervated side of the spinal cord is associated with functional recovery in adult rat after traumatic brain injury and erythropoietin treatment. Brain Res. 1353, 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mahmood A., Wu H., Qu C., Xiong Y., and Chopp M. (2013). Effects of treating traumatic brain injury with collagen scaffolds and human bone marrow stromal cells on sprouting of corticospinal tract axons into the denervated side of the spinal cord. J. Neurosurg. 118, 381–389 [DOI] [PubMed] [Google Scholar]
- 58. Benowitz L.I., Goldberg D.E., Madsen J.R., Soni D., and Irwin N. (1999). Inosine stimulates extensive axon collateral growth in the rat corticospinal tract after injury. Proc. Natl. Acad. Sci. 96, 13486–13490 [DOI] [PMC free article] [PubMed] [Google Scholar]