Abstract
Severe traumatic brain injury (TBI) induces seizures or status epilepticus (SE) in 20–30% of patients during the acute phase. We hypothesized that severe TBI induced with lateral fluid-percussion injury (FPI) triggers post-impact SE. Adult Sprague-Dawley male rats were anesthetized with isoflurane and randomized into the sham-operated experimental control or lateral FPI-induced severe TBI groups. Electrodes were implanted right after impact or sham-operation, then video-electroencephalogram (EEG) monitoring was started. In addition, video-EEG was recorded from naïve rats. During the first 72 h post-TBI, injured rats had seizures that were intermingled with other epileptiform EEG patterns typical to non-convulsive SE, including occipital intermittent rhythmic delta activity, lateralized or generalized periodic discharges, spike-and-wave complexes, poly-spikes, poly-spike-and-wave complexes, generalized continuous spiking, burst suppression, or suppression. Almost all (98%) of the electrographic seizures were recorded during 0–72 h post-TBI (23.2 ± 17.4 seizures/rat). Mean latency from the impact to the first electrographic seizure was 18.4 ± 15.1 h. Mean seizure duration was 86 ± 57 sec. Analysis of high-resolution videos indicated that only 41% of electrographic seizures associated with behavioral abnormalities, which were typically subtle (Racine scale 1–2). Fifty-nine percent of electrographic seizures did not show any behavioral manifestations. In most of the rats, epileptiform EEG patterns began to decay spontaneously on Days 5–6 after TBI. Interestingly, also a few sham-operated and naïve rats had post-operation seizures, which were not associated with EEG background patterns typical to non-convulsive SE seen in TBI rats. To summarize, our data show that lateral FPI-induced TBI results in non-convulsive SE with subtle behavioral manifestations; this explains why it has remained undiagnosed until now. The lateral FPI model provides a novel platform for assessing the mechanisms of acute symptomatic non-convulsive SE and for testing treatments to prevent post-injury SE in a clinically relevant context.
Keywords: antiepileptic drugs, epileptogenesis, lateral fluid-percussion injury, seizure, video-EEG monitoring
Introduction
Studies in humans monitored by electroencephalography in the intensive care unit show that approximately 20–30% of patients with severe traumatic brain injury (TBI) have nonconvulsive seizures or nonconvulsive status epilepticus (NCSE),1–5 and 33% present in the first 3 days after injury.6 Seizure occurrence exhibits a bimodal distribution, peaking at 29 h and 140 h post-injury.3 Seizures are linked to increased intracranial pressure, cerebral metabolic distress, hippocampal atrophy, and increased mortality, implicating a therapeutic need to terminate the NCSE.2–5 To date, an animal model of post-TBI NCSE has not been reported, although an animal model could provide a valuable in vivo platform for investigating the mechanisms of NCSE and testing the efficacy of mechanism-based treatments to stop NCSE in this clinically challenging group of patients.
The lateral fluid-percussion injury (FPI)–induced rat model of TBI was characterized by Dixon and colleagues7 and McIntosh and colleagues.8 The model is extensively used to study the mechanisms of TBI and develop therapies for improving post-TBI recovery.9 Although immediate post-impact seizure-like behaviors occur in approximately 30% of rats8,10 and treatment with several antiepileptic drugs including topiramate11 and levetiracetam12 has recovery-enhancing effects after FPI, the contribution of acute post-injury epileptiform activity or its suppression on the outcome has received little attention. Most of the electroencephalogram (EEG) recording studies in the lateral FPI model have been performed weeks to months after TBI, focusing on detection of late unprovoked seizures to diagnose post-traumatic epilepsy, rather than on post-impact electrographic abnormalities.13–17
We recently began to monitor rats with severe lateral FPI immediately after the injury induction using video-EEG. Data from two independent animal cohorts revealed that all animals developed NCSE with subtle clinical manifestations. Similar to human post-TBI NCSE, rats with lateral FPI showed various patterns of epileptiform activities and a bimodal occurrence of electrographic seizures. Our observations revealed that severe lateral FPI-induced TBI represents the first animal model of acute symptomatic NCSE due to structural (TBI) etiology in adults,18 and provide a novel in vivo platform for studying the mechanisms and potential treatment strategies.
Methods
Animals
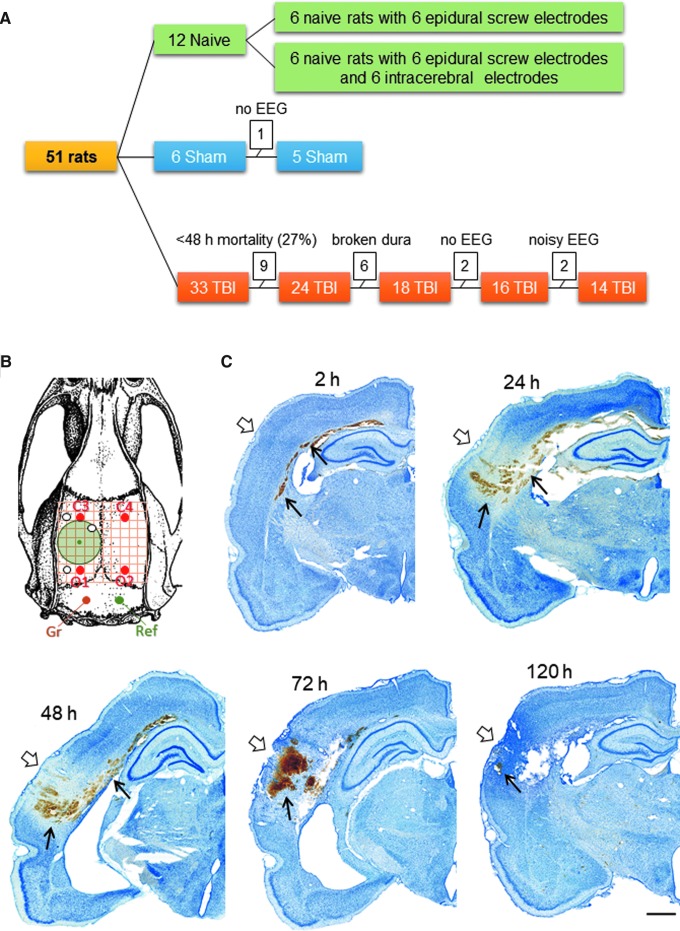
Figure 1A summarizes the number of animals in different treatment groups. Adult male Sprague-Dawley rats (n = 39, 12 weeks old at the time of TBI, weight 330 ± 19 g, median 329 g, range 299–384 g; Envigo Laboratories B.V., Melderslo, the Netherlands) were randomized by lottery to either the sham-operated experimental control (n = 6) or TBI group (n = 33). In addition, 12 naïve rats were included in the analysis to assess the effect of electrode implantation on post-operation EEG. The animals were housed in individual cages in a controlled environment (temperature, 22 ± 1°C, humidity 50–60%, lights on 07:00–19:00) and had free access to food and water. All the experiments were approved by the Animal Ethics Committee of the Provincial Government of Southern Finland and performed in accordance with the guidelines of the European Community Council Directives 2010/63/EU.
FIG. 1.
Animal numbers, electrode montage, and perilesional pathology during the first week after lateral fluid-percussion injury. (A) A flow-chart of the study that shows the number of animals in each group, at each phase. (B) Electrode montage used for the present analysis. Four epidural skull recording electrodes, a ground electrode (Gr), and a reference electrode (Ref). (C) Thionin-stained coronal sections from our tissue biobank revealed gross brain pathology at the level of the lesion at 2, 24, 48, 72, and 120 h after TBI. Note the clearance of massive hemorrhage (arrow) after the third post-injury day, when the epileptiform patterns in electroencephalogram also started to diminish. No EEG during the first 3 days after sham-operation or fluid-percussion injury. EEG, electroencephalography; TBI, traumatic brain injury. Color image is available online.
Lateral FPI-induced TBI
TBI was induced by lateral FPI.8,13 Briefly, the rats were placed on a heating pad and body temperature was continuously monitored using a rectal probe (maximum temperature was set to 38°C). Anesthesia was induced using 5% isoflurane (room air as carrier gas) and maintained with 1.9% isoflurane (Somnosuite # SS6069B; Kent Scientific). Each rat was then mounted into a stereotaxic frame with lambda and bregma at the same horizontal level. To monitor the physiologic parameters (pulse distension, heart rate, arterial O2 saturation), a foot sensor (MouseOxPlus # 72–8019; Starr Life Science Corp.) was clipped to the right hindpaw, and monitoring was started. Lidocaine (200 μL, 5 mg/mL, subcutaneously [s.c.]; Orion Pharma) was injected over the planned incision, and 3–5 min later a midline scalp incision was made. A craniotomy (diameter 5 mm) centered over the left cortex (center coordinate: anteroposterior (AP) −4.5 mm from bregma; mediolateral (ML) 2.5 mm) was performed using a hand-held trephine (#18004-50; Fine Science Tools GmbH, Germany). Care was taken to leave the dura intact and remove all bone fragments from the dural surface.
Then, a plastic female Luer Lock connector made from an 18 G needle hub was inserted into the craniotomy vertical to the skull surface, and its edges were carefully sealed with tissue adhesive (3M Vetbond; 3M Deutschland GmbH, Germany). The Luer Lock connector was anchored to the skull with dental acrylate (Selectaplus powder #10009210; Selectaplus liquid CN #D10009102; DeguDent, Germany) that also surrounded the frontally inserted anchoring dental screw (ø 1 mm, #BN82213; Bossard). TBI was induced with a fluid-percussion device equipped with a straight tip (Model FP 302; AmScience Instruments, Richmond, VA). The pressure level was adjusted to produce severe TBI with an expected post-impact mortality rate of 20–30% within the first 48 h.19 The occurrence of acute behavioral post-impact seizures and duration of apnea were monitored. Immediately after impact, the rat was removed from the device and placed on a heating pad. The dental cement, screw, and Luer Lock were detached from the skull. Time to righting was recorded.
Sham-operated experimental controls underwent all surgical procedures, including craniotomy, but were not exposed to FPI. Naïve animals underwent surgical procedures for electrode implantation only (no craniotomy).
Electrode implantation
To monitor the occurrence of electrographic seizures or status epilepticus (SE) during the acute post-TBI phase, the rats were mounted in a stereotaxic frame after the righting reflex returned, and re-anesthetized with isoflurane for electrode implantation as described above. In addition, electrodes were implanted in 12 naïve rats to monitor the effect of surgery and electrode implantation on EEG.
Electrode locations are summarized in Figure 1B. Four stainless steel epidural screw electrodes (EM12/20/SPC; PlasticsOne Inc.) were implanted in the skull, two ipsilaterally and two contralaterally. The epidural electrodes were positioned as follows: frontal cortex C3 (AP: −1.7; ML: left 2.5) and C4 (AP: right 1.7; ML: −2.5); parieto-occipital cortex O1 (AP: −7. 6; ML: left 2.5) and O2 (AP: −7.6; ML: right 2.5). In addition, three tungsten bipolar electrodes (EM12/3-2TW/SPC; Plastics One Inc.; tip separation 1.0 mm) were implanted in the ipsilateral perilesional cortex and one was implanted in the ipsilateral septal hippocampus. Positions of the lower tips of the intracerebral electrodes were: anterior perilesional cortex (AP: −1.72; ML: −4.0; DV: 1.8), hippocampus (AP: −3.0; ML: −1.4; DV: 3.6), and posterior perilesional cortex (AP: −7.56; ML: −4.0; DV: 1.8). One epidural stainless steel screw electrode placed ipsilaterally posterior to lambda served as a ground and another placed contralaterally served as a reference electrode (Fig. 1B). The electrodes were soldered to a multi-pin connector (MS12P; Plastics One Inc.), according to a monopolar referential montage. The whole assembly was then secured to the skull with dental acrylic.
Sham-operated experimental controls underwent similar electrode implantation procedures as TBI animals. Six of the 12 naïve rats were implanted with epidural electrodes (four recording electrodes, ground, and reference), and the remaining six animals with similar 12-electrode setup to the TBI and sham-operated control rats (Fig. 1B).
Post-impact monitoring and care
Buprenorphine (0.05 mg/kg, s.c.; Orion Pharma, Finland) was administered for post-operative analgesia after the electrode implantation, at 24 h post-surgery, and thereafter based on assessment of the animal's well-being. Rats received a powdered pellet diet (ad libitum) and 10 mL of 0.9% NaCl (twice a day, s.c.) for the first 3 days after FPI or until able to eat solid pellets and drink on their own. Physiologic parameters related to induction of the impact and electrode implantation were monitored during the surgeries. Arterial oxygen saturation (SpO2), heart rate and pulse distension were monitored before and after the impact for 5 min. Daily monitoring of animal's well-being included assessing the weight, temperature, signs of any disease or discomfort, general appearance (hair, coat and skin abnormalities), bowel and gastrointestinal function, body condition score, and external bleeding (if any).20
Video-EEG recording
Immediately after the electrode implantation, each rat was placed in a custom designed Plexiglas EEG recording chamber (29 [wide] × 44 [length] × 50 [height] cm; one rat per chamber). The electrode headset in the rat skull was connected to a 12-pin swivel commutator (SL12C; PlasticsOne Inc.) via a flexible shielded cable (M12C-363/2; PlasticsOne Inc.), allowing the rat to move freely during the EEG recordings. The commutator was connected to an amplifier using a flexible shielded cable 363/2-441/12 (PlasticsOne Inc.). High-definition electrical brain activity was monitored using a 320-channel Digital Lynx 16SX amplifier (Neuralynx) with a 10-kHz sampling rate. The amplifier had an analog bandwidth between 0.01 Hz to 80 kHz. It had 80 independent analog references, allowing for a configuration of independent references for each animal. Data from each channel were converted individually into 24 bits.
Each animal was video-monitored with a single high-resolution camera (Basler acA1300-75gm GigE; Basler, Germany) that was configured to record 30 frames per sec (fps; maximum 75 fps) with a resolution of 1.3 megapixels, and compressed using H.264. At night, cameras recorded under cage-specific infrared illumination (24 V, 150 mA). The EEG and video were synchronized at nanosecond resolution, using the precision time protocol IEEE-1588. The entire system generated approximately 1.5 TB of data every 24 h. For data storage, the video-EEG system was connected to a network attached storage comprising 200 TB of storage configured to RAID6 for data redundancy.
Video-EEG analysis
EEG recorded with epidural screw electrodes was used for the analysis. Each video-EEG raw data file was imported to Spike2 and analyzed visually by browsing through 30-sec recording epochs on the computer screen using the Spike2 analysis program (version 9: CED, UK).
The association of various behavioral manifestations with different epileptiform EEG patterns was analyzed from time-locked videos. Severity of behavioral seizures was scored according to Racine21: Score 0—wandering, walking around the cage (e.g., towards food); Score 1—facial movements (eye-blinking, chewing, facial muscle twitches); Score 2—head nodding and clonus of one of the extremities; Score 3—bilateral forelimb clonus; Score 4—forelimb clonus with rearing; Score 5—score 4 and falling.
EEG power spectrum
Four EEG channels (C3, C4, O1, O2) were selected to generate power spectrograms of representative epileptiform EEG activities in Spike2. Spike2 uses a fast-Fourier transform, a mathematical device that transforms a time series to the frequency base. To generate the heat maps from selected periods of recordings, we used an in-house created script in Spike2 (v9.02).
Statistical analysis
The data analysis was performed using Graph Pad Prism (5.0) and R (version 3.4.2)22 with RStudio (version 1.1.383).23 Distribution normality was tested with the Shapiro-Wilk normality test, and correlations were calculated using Spearman's correlation. The differences in physiologic measures between the animal groups were tested using the independent t-test or Mann-Whitney U Test. Differences between pre-impact and post-impact measures were assessed using the paired t-test or Wilcoxon signed-rank test. Seizure-related differences were analyzed using the Kruskal-Wallis test followed by post hoc analysis with Dunn's multiple comparison test or one-way analysis of variance (ANOVA) followed by post hoc analysis with Tukey's multiple comparison test. The difference was considered significant at p < 0.05.
Results
Severity of impact and post-impact apnea, time to righting reflex, observed seizures, mortality, duration of anesthesia, and physiologic measures
The mean impact pressure was 2.8 ± 0.2 atm (median 2.8 atm, range 2.4–3.0 atm). The mean post-impact time in apnea was 25.4 ± 17.4 sec (median 27.5 sec, range 0–85 sec). The mean post-impact time to righting reflex was 15.8 ± 12.8 min (median 14.0 min, range 2.0–70.0 min). Note that only one of our 14 TBI rats had a time to righting of 70 min. In the other TBI rats, it was 2–21 min. The longer the time to righting, the lower the seizure number (r = -0.59; p = 0.037). Immediate post-impact behavioral seizures were observed in 13/19 rats (68%). Acute mortality within 48 h after impact was 24% (8/33).
Post-impact physiological parameters are summarized in Supplementary Table S1. Analysis showed that the post-impact pulse-distension was remarkably increased in TBI animals (before impact 24.6 ± 11.5 μm [median 20.8 μm, range 9.8–46.3 μm] vs. after impact 86.3 ± 50.2 μm [median 72.9 μm, range 12.8–168.6 μm]; p < 0.01). The longer the post-impact apnea duration, the lower the post-impact mean arterial O2 saturation (r = -0.62, p < 0.01; Supplementary Fig. S1A) and the lower the post-impact mean heart rate (r = -0.61, p < 0.05). The shorter the time to righting reflex, the higher the post-impact arterial O2 saturation, (r = -0.74, p < 0.01; Supplementary Fig. 1B). The higher the pre-impact heart rate, the higher the post-impact heart rate (r = 0.67, p < 0.01). The higher the post-impact heart rate, the higher the post-impact mean arterial O2 saturation (r = 0.50, p = 0.05).
In the TBI group, the lower the pre-impact mean arterial O2 saturation, the higher the total number of electrographic seizures during NCSE (r = -0.65, p = 0.044). The higher the post-impact mean arterial O2 saturation, the higher the total number of electrographic seizures during NCSE (r = 0.66, p = 0.028). The higher the pre-impact heart rate, the higher the total number of electrographic seizures during NCSE (r = 0.67, p = 0.035). The higher the post-impact heart rate, the higher the total number of electrographic seizures during NCSE (r = 0.69, p = 0.018). The higher the pre-impact mean heart rate, the longer the latency to the last electrographic seizure during NCSE (r = 0.66, p = 0.039).
The mean duration of anesthesia before impact induction was 27 ± 3 min (median 26 min, range 22–34 min). The mean duration of anesthesia for electrode implantation was 68 ± 18 min (median 71 min, range 30–89 min). The total mean duration of anesthesia (impact induction plus electrode implantation) was 95 ± 23 min (median 99 min, range 51–119 min).
In the TBI group, the elapsed time between the impact to the connection of rats to video-EEG monitoring was 123 ± 71 min (n = 10, median 88 min, range 72–258 min). Due to technical difficulties, the remaining four rats were connected to video-EEG monitoring at 18–40 h post-TBI.
Figure 1C shows the resulting overall brain pathology during the first post-injury week in the lateral FPI model. Lateral FPI caused extensive intracortical damage and hemorrhage, with the epicenter being in the auditory cortex, and then extending dorsally along the external capsule. There was a significant clearance of blood from the brain tissue during Days 4–5 post-injury.
Post-TBI EEG patterns
Altogether, 14 rats with TBI and five sham-operated controls with high-quality EEG were included in the final analysis (Fig. 1A). There are no prior systematic descriptions of findings from EEGs recorded immediately after lateral FPI-induced mechanical impact in rats. To ensure accuracy of the description, we adopted the descriptions for EEG patterns presented by Kane and colleagues24 approved by the International Federation of Clinical Neurophysiology.
The nine major types of epileptiform activities that occurred between the electrographic seizures with subtle behavioral manifestations during the first 3 days post-TBI are presented in Figures 2-10 and are briefly summarized below:
FIG. 2.
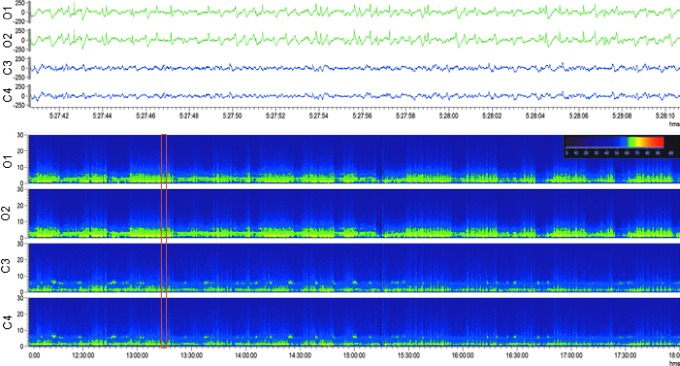
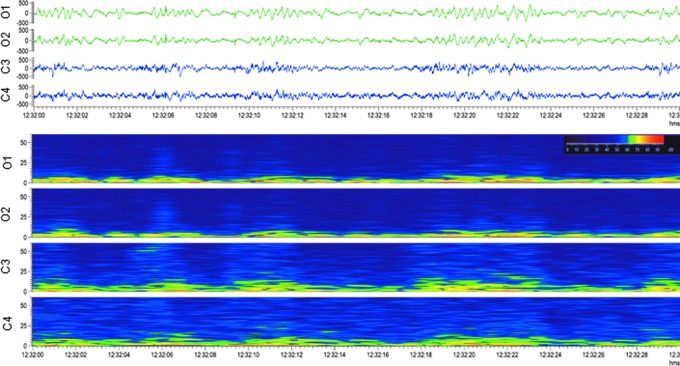
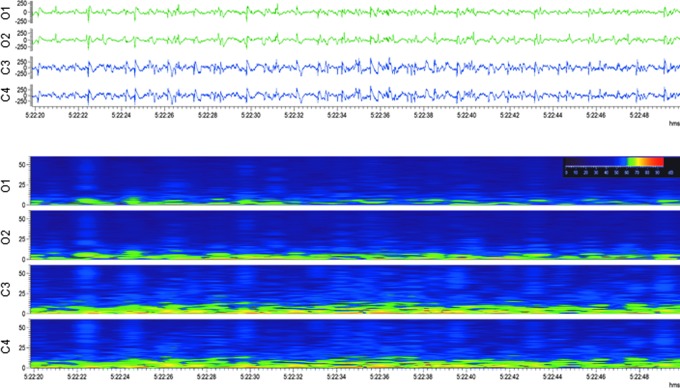
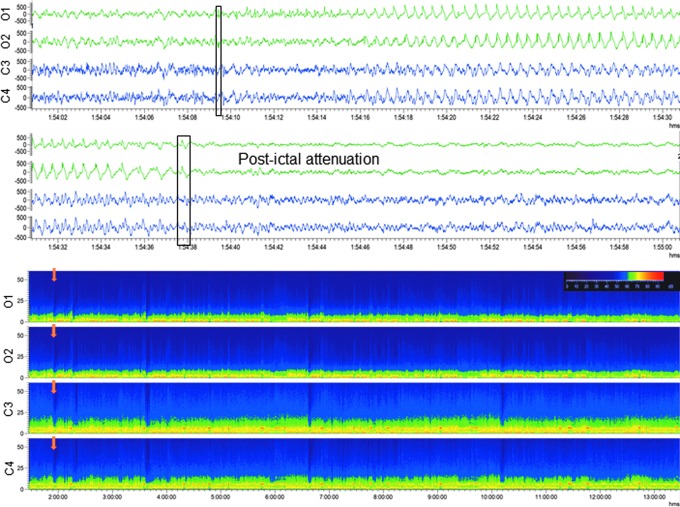
Occipital intermittent rhythmic delta activity (OIRDA). Upper panel: Electroencephalogram (30-sec epoch, low-frequency filter 0.5 Hz, high-frequency filter 100 Hz) showing occipital intermittent rhythmic delta activity after lateral fluid-percussion injury Note the marked attenuation in the anterior electrodes (C3, C4) with persistent polymorphic sharp delta activity in the posterior electrodes (O1, O2) with sporadic spikes and sharp waves. The activity was not associated with any apparent behavioral manifestation (animal was lying down). Lower panel: A heat map showing 6 h of continuous sharp delta activity in the same rat. Note the intermittent increase in occipital (O1, O2) delta band, followed by periods of silence. Orange box indicates the electroencephalogram epoch shown in the upper panel. For a longer period of OIRDA, see Supplementary Video S1. Color image is available online.
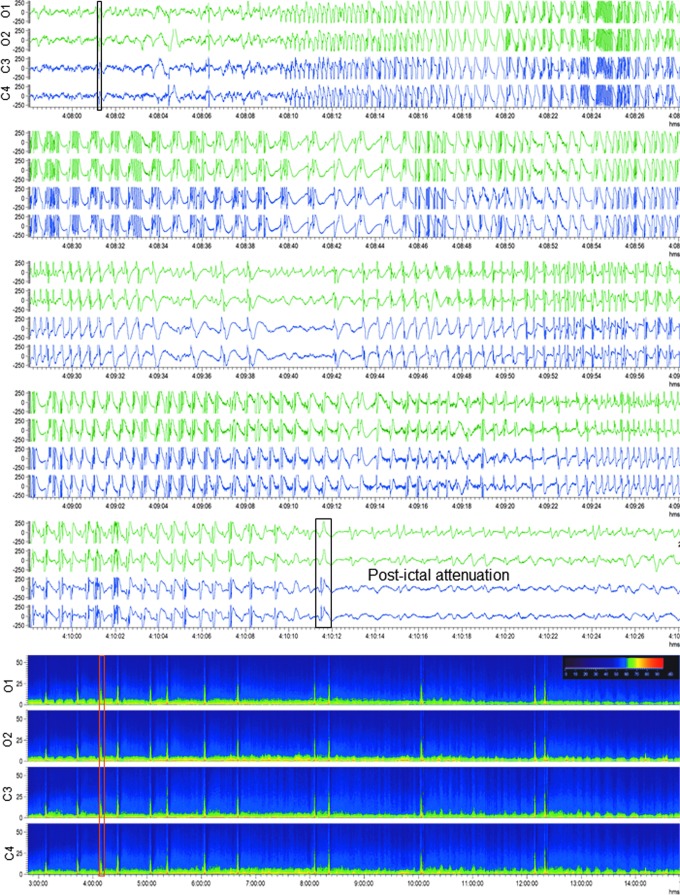
FIG. 10.
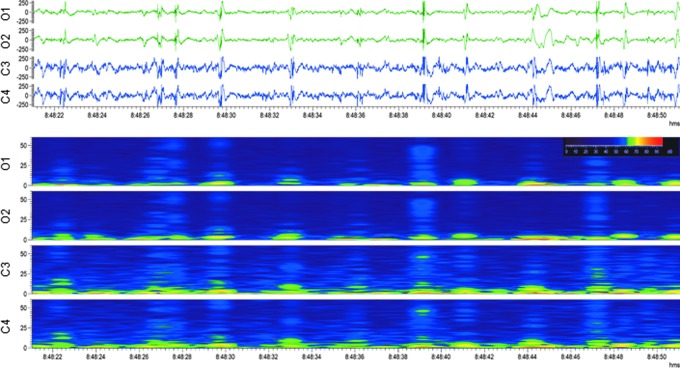
Generalized electroencephalographic seizure in a rat with traumatic brain injury. Upper panel: Electroencephalogram (EEG; 30-sec epoch, low-frequency filter 0.5 Hz, high-frequency filter 100 Hz) showing a generalized electroencephalographic seizure after lateral fluid-percussion injury–induced impact. Seizure duration was 130 sec. Note the frequency evolution, changing morphology and modulation of the activity, culminating in attenuation. Video recording indicated slight head-turning to the left. Seizure beginning and ending are marked with a black box. Lower panel: A heat map representing 12 h of continuous EEG in the same rat. Orange box indicates the seizure in upper panel. Note that the animal had 13 seizures within 12 h. On the heat map, each seizure is identifiable by a peak in power. For a video-EEG showing a non-convulsive seizure in a rat with TBI, see Supplementary Video S4. Color image is available online.
-
1.
Occipital intermittent rhythmic delta activity. This pattern is characterized by sinusoidal waves or fairly regular, mostly but not exclusively occurring in bursts at 2–3 Hz over the occipital areas in one or both sides of the brain (Fig. 2; Supplementary Video S1).
-
2.
Continuous slow activity. Uninterrupted ongoing slow activity (delta band), either regular or polymorphic that does not regress, although it can vary in amplitude and morphology (Fig. 3).
-
3.
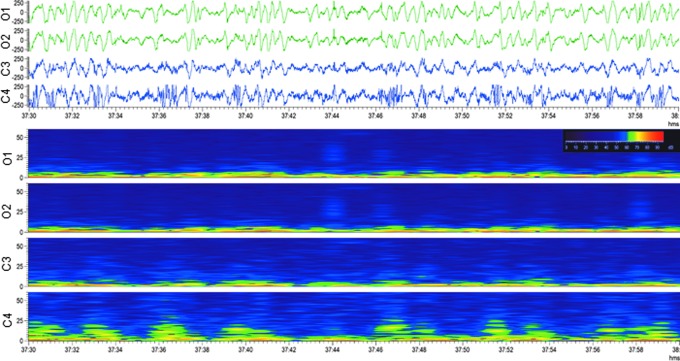
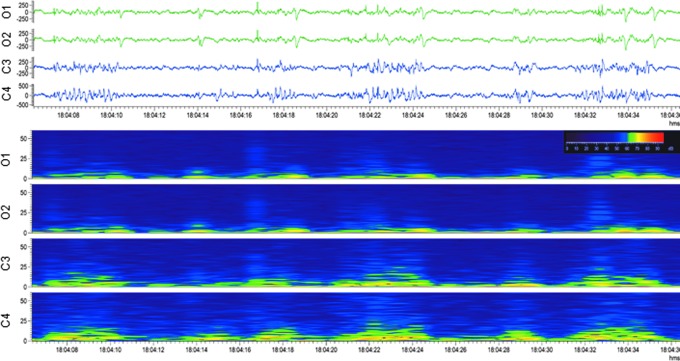
Periodic discharges (PDs). Repetitive waveform with a relatively uniform morphology and duration, a quantifiable inter-discharge interval between consecutive waveforms, and recurrence of the waveform at nearly regular intervals. After lateral FPI, PDs were either lateralized periodic discharges (LPDs), in which sharp or slow-waves with polyphasic morphology appeared at quasi-periodic intervals unilaterally (Fig. 3) or generalized (GPDs), in which they were observed in all four EEG channels (Fig. 4; Supplementary Video S2). Both LPDs and GPDs were typically followed by attenuation, referring to a reduction in the amplitude of the background, usually remaining greater than 10 μV, but lower than 50 μV.
-
4.
Burst suppression was observed in all injured animals. This pattern is characterized by paroxysmal bursts of delta and/or theta waves, sometimes mixed with sharp and/or faster activity/transients, alternating with periods of attenuation or suppression (Fig. 5).
-
5.
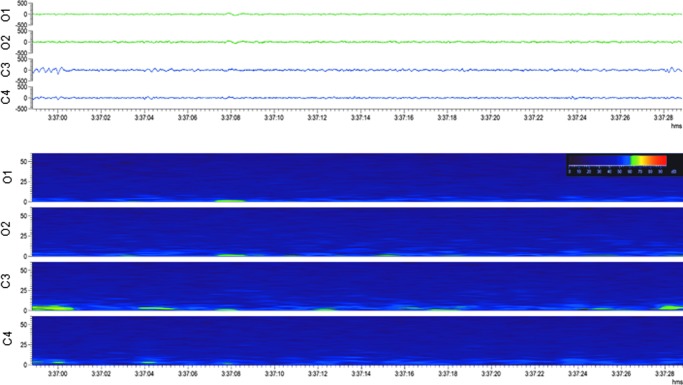
Suppression refers to an EEG recording showing activity below 10 μV throughout its duration (referential derivation; Fig. 6). In one rat, the suppression continued for approximately 10 min.
-
6.
Generalized continuous spiking. A spike is a transient signal that clearly differs from the background, with a pointed peak (conventional time scale), and a duration between 20 and 70 msec, variable amplitude, and usually a negative main component at the focal point. In some injured animals, generalized spiking continued for hours (Fig. 7).
-
7.
Spike-and-slow-wave complex. Pattern characterized by a spike followed by a slow wave, clearly distinguished from background activity. These grapho-elements appeared as either isolated or in clusters after lateral FPI (Fig. 8; Supplementary Video S3).
-
8.
Poly-spike-and-slow-wave complex. Pattern comprising at least two spikes followed by one or more slow waves (Fig. 9).
-
9.
Seizures (ictal EEG pattern). An electrographic seizure was defined as a phenomenon characterized by repetitive epileptiform discharges and/or a characteristic pattern with quasi-rhythmic spatio-temporal evolution (change in frequency, amplitude, morphology, and location), lasting at least 10 sec (Fig. 10; Supplementary Videos S4-S6).
FIG. 3.
Lateralized periodic discharges with attenuation. Upper panel: Electroencephalogram (30-sec epoch, low-frequency filter 0.5 Hz, high-frequency filter 100.0 Hz) showing lateralized periodic discharges with attenuation after lateral fluid-percussion injury. Electroencephalogram showed generalized polymorphic delta activity and lateralized periodic discharges in the contralateral anterior channel (C4). Lower panel: A heat map showing the same epoch, indicating lateralization. Note the intermittent increase in contralateral parietal (C4) fast activity. Color image is available online.
FIG. 4.
Generalized periodic discharges. Upper panel: Electroencephalogram (30-sec epoch, low-frequency filter 0.5 Hz, high-frequency filter 100.0 Hz) showing generalized periodic discharges after lateral fluid-percussion injury. No clear ictal behavior was associated with the activity. Lower panel: A heat map showing the same epoch, indicating periodicity. Note the generalized (C3, C4, O1, O2) intermittent activity, followed by attenuation. For a longer period of periodic discharges, see Supplementary Video S2. Color image is available online.
FIG. 5.
Burst suppression. Upper panel: Electroencephalogram (30-sec epoch, low-frequency filter 0.5 Hz, high-frequency filter 100.0 Hz) showing burst suppression after lateral fluid-percussion injury. No clear ictal behavior was associated with the activity. Lower panel: A heat map of the same epoch, indicating suppression between the bursts with higher power in the parietal (C3, C4) channels. Color image is available online.
FIG. 6.
Generalized suppression. Upper panel: Electroencephalogram (30-sec epoch, low-frequency filter 0.5 Hz, high-frequency filter 100.0 Hz) showing a complete suppression of the electroencephalogram after lateral fluid-percussion injury. Lower panel: A heat map of the same epoch, indicating suppression in all channels. Note that this was not preceded by a seizure. Color image is available online.
FIG. 7.
Generalized continuous spiking. Upper panel: Electroencephalogram (30-sec epoch, low-frequency filter 0.5 Hz, high-frequency filter 100.0 Hz) showing generalized continuous spiking after lateral fluid-percussion injury. No clear ictal behavior was associated with the activity. Lower panel: A heat map of the same epoch, indicating an increased power of spikes on an attenuated background. Color image is available online.
FIG. 8.
Spike-and-slow-wave complexes. Upper panel: Electroencephalogram (30-sec epoch, low-frequency filter 0.5 Hz, high-frequency filter 100.0 Hz) showing spike-and-slow-wave complexes after lateral fluid-percussion injury. No clear ictal behavior was associated with the activity. Lower panel: A heat map of the same epoch, indicating a typical shift in the band power when transitioning from a spike to a wave. The spike-and-slow-wave complexes were more distinct occipitally (O1, O2) than parietally (C3, C4), where they are mixed with other activities. For a longer period of spike-and-slow-wave complexes, see Supplementary Video S3. Color image is available online.
FIG. 9.
Poly-spike-and-waves. Upper panel: Electroencephalogram (30-sec epoch, low-frequency filter 0.5 Hz, high-frequency filter 100.0 Hz) showing poly-spike-and-waves after lateral fluid-percussion injury. No clear ictal behavior was associated with the activity. Lower panel: A heat map from the same epoch, showing the poly-spike and wave power profile. Note that poly-spikes-and-waves were generalized as being present both parietally (C3, C4) and occipitally (O1, O2). Color image is available online.
Seizure characteristics
Rats with TBI
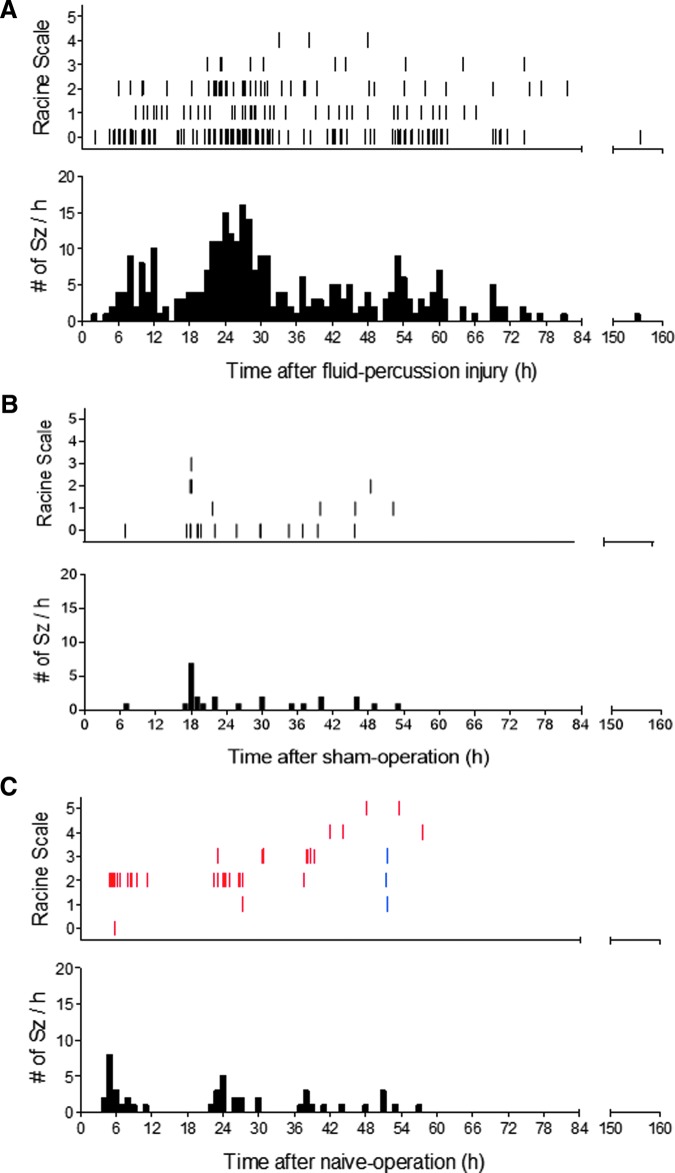
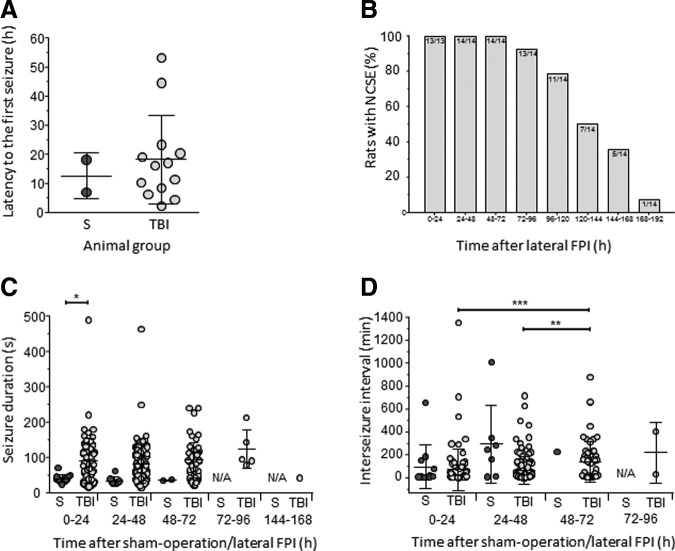
Altogether, 308 electrographic seizures were recorded in 13 of 14 rats with TBI (Fig. 10; Supplementary Video 4). Of these, 98% (n = 302) were recorded within 0–72 h post-TBI, showing the first peak between 6–8 h and the second peak between 20–30 h post-TBI (Fig. 11A). Mean latency to the first seizure was 18.4 ± 15.1 h (median 16.1 h, range 2.4–53.1 h; Fig. 12A). Mean number of seizures per injured rat was 23.7 ± 17.4 (median 17, range 2–56). The mean duration of electrographic seizures was 86 ± 57 sec (median 87.5 sec, range 13–490 sec; Fig. 12B). The mean cumulative duration of electrographic seizures per rat was 2051 ± 1717 sec (median 1732 sec, range 87–5563 sec). Mean seizure interval was 89 ± 170 min (median 16 min, range 0.2–1 349 min; Fig. 12C). Of all electrographic seizures, 66% (n = 202) occurred during lights-on period (mean 16.8 ± 14.1, median 10.5, range 2–45, p < 0.05) and 34% (n = 106) during lights-off period (mean 8.8 ± 5.4, median 7.5, range 1–19).
FIG. 11.
Distribution of occurrence of electrographic seizures during the first post-TBI week. (A) Top: A raster plot showing the distribution of occurrence of 308 electrographic seizures in 13 rats after lateral fluid-percussion induced traumatic brain injury (x-axis) and their behavioral severity on Racine's scale (y-axis).21 Note the peaks approximately 6–8 h and 20–30 h after injury. Bottom: A histogram showing the number of seizures per hour. (B) Top: A raster plot showing the distribution of occurrence of electrographic seizures in two of the five sham-operated experimental control rats. Note that all seizures occurred within the first 54 h after surgery. Bottom: Histogram showing the number of seizures per hour. (C) Top: A raster plot showing the distribution of occurrence of post-surgery electrographic seizures in four of the 12 naïve rats. The seizures in a naïve rat with six epidural screw electrodes are shown in blue; and those in three naïve animals implanted with six epidural screw electrodes and six intracerebral bipolar wire electrodes are shown in red. Note that all the seizures occurred within the first 57 h after electrode implantation. Bottom: Histogram showing the number of seizures per hour. Independent of animal numbers in (A-C), there was a peak in seizure occurrence approximately at 6 h (A and C) and 18–24 h (A-C) post-operation. Color image is available online.
FIG. 12.
Quantification of seizures and non-convulsive status epilepticus. (A) Latency to the appearance of the first electrographic seizure in sham-operated experimental controls (S, blue dots) and in rats with traumatic brain injury (TBI, light grey dots; x-axis). (B) Percentage of rats with non-convulsive status epilepticus (NCSE) at different time-points after traumatic brain injury. Electroencephalographic recordings showed that 100% of injured animals presented with NCSE during the first 72 h post-TBI. One rat was still in NCSE on Day 8 post-TBI. Numbers refer to animals in NCSE from all TBI rats with electroencephalogram available. (C) Duration of individual electrographic seizures (y-axis) in sham-operated experimental controls and in rats with TBI (x-axis). Each dot represents one seizure. (D) Inter-seizure interval. Data are shown as mean ± standard deviation. Statistical significance: *p < 0.05 in panel C (one-way analysis of variance, Tukey's multiple comparisons test); **p < 0.01, ***p < 0.001 in panel D (Kruskall-Wallis test, Dunn's multiple comparison test).
Altogether, 111 of 272 (41%) video-monitored electrographic seizures were associated with behavioral manifestations. Of these, 47 were associated with mouth and facial movements, 52 with head nodding, nine with unilateral forelimb clonus, and three with rearing and bilateral forelimb clonus. Other subtle behavioral manifestations included head turning, unilateral eye blinking, piloerection, “staring eyes,” unilateral whisker movements, wet-dog shakes, non-meaningful “eating,” climbing, and circling behavior. The mean severity of motor seizures in individual rats was 0.7 ± 0.9 (median 0, range 0–4). None of the video-monitored electrographic seizures reached stage 5 of Racine's scale. Note that 161 (59%) video-monitored electrographic seizures were not accompanied by any apparent behavioral manifestation in cage-specific high-resolution videos. Thus, in animals recovering from TBI, behavior appeared “normal” most of the time, despite the EEG showing continuous epileptiform patterns.
Sham-operated experimental controls
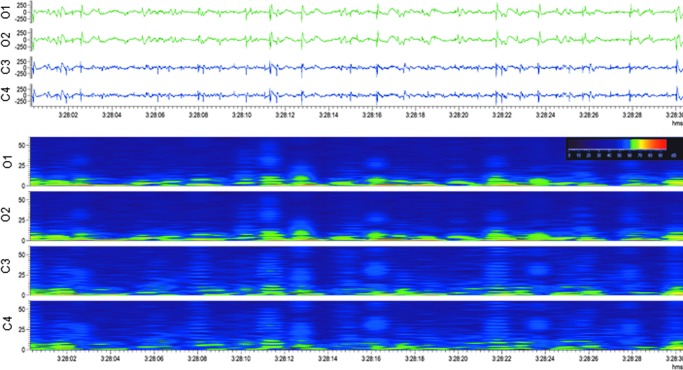
None of the five sham-operated experimental controls were considered to have SE. In two of the five sham-operated experimental controls, however, we detected 24 electrographic seizures between 0–72 h post-surgery with a mean latency to the first seizure of 12.6 ± 7.9 h (median 12.6 h, range 7–18 h; Fig. 11B; Fig. 12A; Fig. 13; Supplementary Video 5). The mean seizure duration was 37 ± 11 sec (median 37 sec, range 23–71 sec; Fig. 12B), and the mean behavioral severity score was 0.7 ± 0.9 (median 0, range 0–3). The mean cumulative duration of electrographic seizures was 442 ± 267 sec (median 442 sec, range 253–631 sec). The mean seizure interval was 171 ± 264 min (median 21 min, range 4-1007 min; Fig. 12C). Of all electrographic seizures, 75% (n = 18) occurred during lights-on period (mean 9 ± 2.8, median 9, range 7–11), while 25% (n = 6) were observed during the lights-off period (mean 6 ± 0.0, median 6). Importantly, in sham-operated experimental controls, the interictal EEG appeared to be relatively normal and did not show the same electrographic patterns detected in rats with TBI.
FIG. 13.
Upper panel: Electroencephalogram (30-sec epoch, low-frequency filter 0.5 Hz, high-frequency filter 100 Hz) showing a secondary generalized seizure in a sham-operated experimental control rat. Ictal activity was first detected in the occipital electrodes (O1, O2), and then secondarily generalized to the channels located anterior to the craniotomy (C3, C4) with changes in the frequency and morphology, ending in attenuation. The start and end of the seizure are marked with black boxes. The seizure started with a twitch of the right ear, followed by chewing, then by a tonic extension of the right hind-paw, evolving to tonic-clonic movements of the right forepaw and hindpaw, and eventually to clonus of both forepaws and whole body. Lower panel: A heat map representing 12 h of continuous electroencephalogram in the same rat. Orange arrows indicate the seizure in upper panel. Note that the animal had altogether five seizures within 12 h. Color image is available online.
Naïve rats with electrode implantation
One of the six naïve animals implanted with six epidural screw electrodes had three seizures between 48–72 h after electrode implantation (Fig. 11C; Supplementary Video 6). The mean latency to the first electrographic seizure was 51.4 h. The mean seizure duration was 87 ± 58 sec (median 100 sec, range 24–138 sec). The mean behavioral severity score was 2 ± 1 (median 2, range 1–3). The cumulative duration of electrographic seizures was 262 sec. The mean seizure interval was 3.7 ± 2.8 min (median 3.7 min, range 2–6 min). Of all electrographic seizures, 100% of them occurred during the lights-on period.
Three of the six naïve rats with 12 electrodes (both epidural and intracerebral) had 45 seizures altogether between 0 to 72 h after electrode implantation (Fig. 11C). The mean latency to the first seizure was 14.5 ± 9.5 h (median 14.7 h, range 5–24 h). The mean number of seizures per naïve rat was 15.3 ± 8.6 (median 17, range 6–23). The mean duration of electrographic seizures was 70 ± 47 sec (median 48 sec, range 13–206 sec), and the mean behavioral severity score was 2.4 ± 0.9 (median 2, range 0–5). The mean cumulative duration of electrographic seizures per rat was 1044 ± 457 sec (median 1036 sec, range 591–1504 sec). Mean seizure interval was 77 ± 122 min (median 16 min, range 1–501 min). Of all electrographic seizures, 64% (n = 29) occurred during the lights-on period (mean 9.7 ± 6.4, median 6, range: 6–17), while 36% (n = 16) were observed during the lights-off period (mean 8 ± 2.8, median 8, range 6–10).
Appearance, type, sequence, and duration of epileptiform activity after TBI
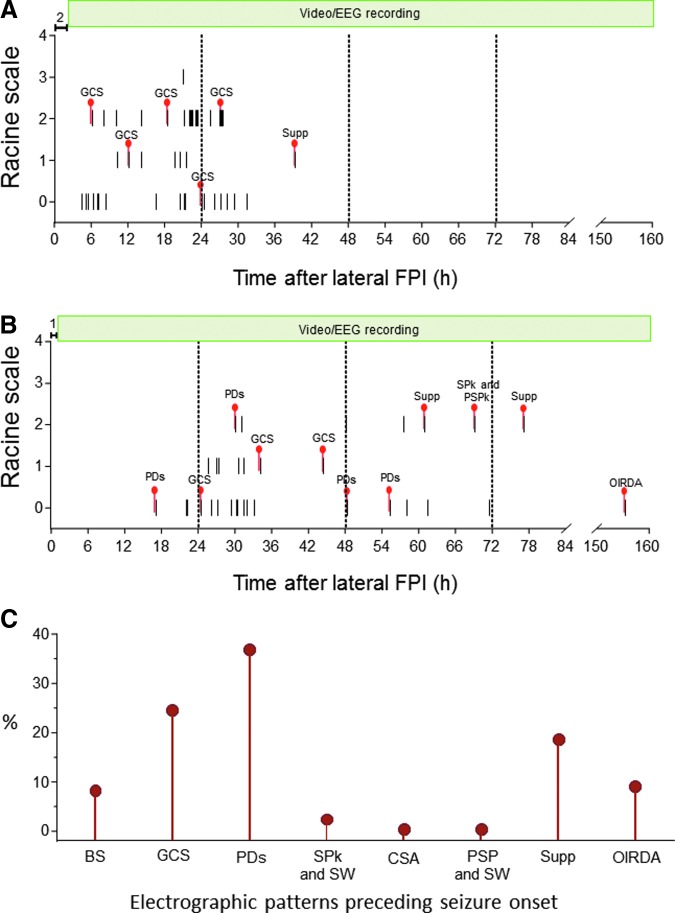
As summarized in Figure 14, the appearance, type, and sequence of various epileptiform patterns varied between animals with TBI. For example, seven of the 14 injured rats showed robust occipital intermittent rhythmic delta activity after waking-up from isoflurane anesthesia. Two rats showed continuous spiking when they were connected to EEG at 1.2 h and 2.1 h post-TBI, respectively. Over the first week follow-up, periodic epileptic discharges followed by attenuation were the most common EEG patterns observed in 10 of the 14 TBI rats. Interestingly, we noticed a “re-occurrence” or “cyclicity” of some epileptiform patterns during the follow-up (e.g., periodic discharges or suppression), eventually culminating in the occurrence of a seizure; Fig. 14B).
FIG. 14.
Epileptiform patterns observed in traumatic brain injury (TBI) rats at 1 min before the initiation of an electrographic seizure. Y-axis shows the behavioral severity of each seizure on Racine's scale. (A) Rat #1049 that had 56 seizures over a period of 40 h. Epileptiform activity largely comprised generalized continuous spiking (GCS; see Fig. 7). (B) Rat #1059 had 39 seizures over a period of 156 h. Preictal epileptiform activity largely comprised periodic discharges (PDs), GCS, spikes (SPk) and polyspikes (PSPk), suppression (Supp), and rhythmic occipital intermittent delta activity (OIRDA). (C) Percentage of different electrographic patterns preceding the electrographic seizure in rats with TBI. Color image is available online.
During the first 72 h post-TBI, all injured rats (14/14) showed epileptiform EEG patterns. Still, between 144–168 h post-TBI, 35.7% (5/14) of the injured rats continued to exhibit epileptiform activity (Fig. 12D). Unlike TBI rats, sham-operated experimental controls and naïve rats with electrode implantations, showed a return of awake-sleep EEG patterns interictally, and after the end of the last seizure.
Discussion
The present study assessed the occurrence of seizures and other epileptiform activities after lateral FPI-induced TBI, a commonly used rat model of human closed head injury. The data revealed six major findings. First, all 14 injured rats developed NCSE with subtle behavioral manifestations. Second, the electrographic patterns were comparable to those previously described in human NCSE and varied between animals as well as in a given animal during the course of NCSE. Third, the occurrence of electrographic seizures showed two peaks, one at 6–8 h and another 20–30 h post-TBI. Fourth, the subtle behavioral manifestations associated with electrographic seizures typically included facial muscle twitches, eye blinking, salivation, staring eyes, circling and wandering in the cage, occasional wet-dog shakes, and head turning. Fifth, none of the rats died from NCSE. Sixth, electrode implantation, with or without craniotomy, can cause unprovoked seizures during the first post-operative days.
Lateral FPI induces NCSE
Although lateral FPI is a commonly used experimental model of TBI, only a few previous studies demonstrated electrographic epileptiform activity during the acute post-injury period. In our earlier study, we implanted two screw electrodes into the contralateral frontal and parietal cortex before lateral FPI in three rats under pentobarbital-chloral hydrate anesthesia, and recorded EEG for 72 h starting immediately after TBI.13 The initial low voltage activity was followed by diffuse continuous slowing, high-voltage delta activity, bursts of sharp waves, and single spikes, and one of the three rats also had an electrographic seizure during the first 48 h.13 Recently, Bragin and colleagues16 recorded EEG in rats with severe lateral FPI during the acute post-TBI period and found electrographic seizures associated with freezing in five of 12 rats. Similarly, Reid and colleagues17 described one rat with severe lateral FPI that exhibited repeated seizures associated with freezing behavior for 2 days starting on Day 4 post-TBI. None of these studies, however, concluded that the rats were experiencing NCSE, which is the major finding of the present study.
Our initial post-TBI EEG analysis revealed that previous descriptions used to define and characterize convulsive SE induced with cobalt-homocysteine, kainate, pilocarpine, or electrical stimulation of the hippocampus or amygdala in normal rats25–27 were not applicable. The descriptions did not include the variety of electrographic patterns revealed by our initial analysis of EEG in rats with lateral FPI. Nor did the descriptions of EEG patterns in rats during NCSE induced with hippocampal stimulation28 or in rats with repeated unprovoked seizures and interictal epileptiform activity with subtle behavioral symptoms induced by ballistic TBI29 correspond to the EEG patterns observed in our animals. Rather, the electrographic patterns we observed corresponded to those described in human complex partial SE after brain injury.30–32 Therefore, we adopted the International League Against Epilepsy guidelines to define and classify NCSE18 and the International Federation of Clinical Neurophysiology guidelines used to describe EEG patterns24,33 in humans.
We analyzed the data from epidural electrodes to diagnose NCSE in a way that matches with the clinical diagnosis of NCSE, which relies on analysis of scalp EEG recordings. The four data axes proposed for classification of SE are semiology, etiology, EEG correlates, and age.
Regarding semiology, 82% of humans with NCSE have altered consciousness, which is the most common symptom, including confusion, coma, lethargy, and memory loss.34 We did not systematically assess the level of consciousness. Observation of the in-cage behavior in high-resolution cage-specific videos as well as responsiveness to grabbing when the rat was taken for blood sampling at 48 h post-TBI revealed that the responsiveness of rats to external stimuli was compromised. Previous studies showed that rats with lateral FPI perform poorly in the Morris water-maze, a hippocampus-dependent spatial memory test, on Day 2 post-TBI, which is linked to TBI-induced hippocampal damage. The possible contribution of NCSE-related altered consciousness to poor performance in spatial memory tests at the acute post-injury phase needs to be reconsidered as the principal cell damage in the septal hippocampus, particularly in the CA1 subfield, is mild.19 Other symptoms in humans include subtle facial or limb twitches, head and/or eye deviation, peculiar automatisms, speech arrest, sudden behavioral changes, and autonomic disturbances.34 These symptoms correspond well with our observations that all electrographic patterns had no or only subtle behavioral correlates in the rat model, including head turning, facial muscle twitches, eye blinking, salivation, purposeless walking in the cage, and piloerection.
Regarding etiology, our model represents acute symptomatic SE related to the structural (TBI) cause. Regarding EEG correlates, we report nine typical EEG patterns that were observed in all animals, including occipital intermittent rhythmic delta activity, continuous slow activity, lateralized or generalized PDs, burst suppression, suppression, generalized continuous spiking, spike-and-slow-wave complexes, poly-spike-and-slow-wave complexes, and seizures. These EEG patterns could be classified based on location, pattern, morphology, and time-related features, and showed great similarity to the EEG patterns previously described in humans with NCSE.30–32 The order of occurrence and duration of different EEG patterns, however, varied among the animals. Even in a given rat, it was difficult to predict the evolution of EEG patterns, although there seemed to be some cyclic electrographic evolving patterns, culminating in the occurrence of an electrographic seizure. Some rats showed long periods of generalized spiking, whereas other animals showed sharp delta activity intermingled with other activities, including electrographic seizures with subtle behavioral manifestations.
We have not yet systematically tested the effect of modulation or treatments on post-TBI electrographic patterns. There was no clear association, however, between the duration of anesthesia and the type of initial EEG pattern. Our preliminary observation indicated that when the animal in NCSE was taken for blood sampling, which was performed under light, ∼5-min long, isoflurane sedation at 48 h post-TBI and then returned back to its home cage, the EEG activity was slightly attenuated compared with the pre-sampling EEG. This suggests that isoflurane anesthesia modulates the EEG patterns, but further studies are needed to demonstrate the anesthesia effect and its possible specificity to various epileptiform patterns. With regard to age, all our rats were young adult males, corresponding to the human subjects at the highest risk of TBI.35
The duration of epileptiform EEG patterns and occurrence of recurrent seizures lasted substantially longer than 60 min in all cases. Thus, the two time dimensions included in the International League Against Epilepsy SE classification, that is, t1 10 min, indicating “need of treatment,” and t2 > 60 min, indicating “likelihood of long-term consequences after focal SE with impaired consciousness,”18 were exceeded in our animals.
Based on these observations, we conclude that lateral FPI associated with approximately 25% mortality in adult male rats induces “acute symptomatic NCSE of structural (TBI) etiology.”
Electrographic seizures during NCSE
Vespa and co-workers reported that in humans with TBI, electrographic seizures related to NCSE show two peaks: one at 20 h and another at 140 h post-impact.1–4 Ronne-Engstrom and colleagues6 reported a peak at 70 h post-TBI. After lateral FPI, the first peak occurred about 8–12 h and a later peak occurred at approximately 20–30 h post impact. Thus, the seizure peaks appeared faster in a rat model than in humans. It remains to be investigated whether the difference is real rather than methodologic, related to the delay in initiating scalp EEG monitoring in TBI patients in the intensive care unit, which in the Vespa and colleagues36 study was on average 17 h. In the present study, the elapsed time between the impact to the connection of rats to video-EEG monitoring was about 2 h, varying from 72 min to 258 min. Moreover, severe TBI patients in the intensive care unit are typically treated with lorazepam/midazolam, phenytoin, pentobarbital, and/or propofol,1–3,37 which also can influence seizure occurrence and their detection in scalp EEG. It is important to note that unlike humans, our animals in post-TBI NCSE were unmedicated.
Most of the electrographic seizures after lateral FPI occurred on Days 1–3 post-TBI. The mean duration of individual electrographic seizures during NCSE in the lateral FPI model varied from 13 to 490 sec with an average seizure duration of 2.8 min, comparable to that in humans.2 The cumulative seizure duration was about 34 min, varying from 1.5 min to 93 min and the average interval between the electrographic seizures varied from 0.2 to 1349 min. Thus, the overall duration of “classical electrographic seizures” occupied a relatively short period of the overall duration of NCSE, which lasted for days. We would like to note that due to technical difficulties, we connected four animals to video-EEG at 18–40 h post-TBI. The lack of early monitoring in these rats may lead to slight underestimation of the total number of seizures detected in the whole animal cohort, but does not affect the overall conclusions.
Whether the fluctuation of seizures and appearance of various epileptiform electrographic patterns could serve as biomarkers for ongoing progression in the pathology type and magnitude, including neurodegeneration and release of glutamate, vascular damage, and hemorrhage, axonal injury, evolution of inflammatory response, and oxidative stress during the first post-injury week remains to be explored. Interestingly, a retrospective analysis by Kim and co-workers38 indicated that subdural hemorrhage, epileptiform discharges, and focal polymorphic slowing in post-TBI scalp EEG recorded during the 30 days post-TBI predict the development of post-traumatic epilepsy. Whether comparable EEG patterns in rats serve as prognostic biomarkers for epileptogenesis remains a testable hypothesis. Also, further studies should assess whether the suppression of seizures or other epileptiform EEG patterns after lateral FPI improves functional outcome, including prevention of epileptogenesis.
Previous studies revealed that post-TBI seizures in humans are associated with elevated intracranial pressure, longer-lasting cerebral metabolic distress related to an increase in the lactate/pyruvate ratio, hippocampal atrophy, increased mortality, and the development of post-traumatic epilepsy.2,3,5,37 These studies concluded that post-TBI seizures, although largely non-convulsive, are therapeutic targets for improving post-TBI outcome. In rats, a 2.8-atm fluid-percussion impact at the end of isoflurane anesthesia into a 5-mm–diameter craniotomy with the center coordinate 4 mm posterior to bregma and 2.5 mm lateral to midline results in an approximately 20-mm2 cortical lesion with an epicenter in the left auditory cortex.39 This impact level increases intracranial pressure,40–43 particularly in association with intracerebral hemorrhage,44 as in our animals. The TBI-related acute mortality in the study cohort was 24%, corresponding to severe TBI in humans.41,45 None of our rats died from acute post-TBI NCSE. Our initial monitoring of physiological parameters did not reveal strong associations; for example, between the post-impact hypoxia and severity of NCSE. A higher post-impact arterial O2 level, however, was associated with faster righting time. Further studies, with a longer physiological monitoring, are needed to establish the possible links between the physiological parameters and evolution of brain pathology.
It is difficult to define when NCSE ended in each animal as the epileptiform patterns were not consistent between animals, and there is no consensus of the criteria defining the end of NCSE in humans, which could have been adapted to our animal model. One parameter that we are investigating is normalization of the sleep cycle. Our initial analysis suggested that most of the animals attempted to go from N3 sleep to REM sleep on Days 7–10 post-TBI.
Acute seizures in electrode-operated naive rats and in experimental controls with craniotomy
Unexpectedly, we also found electrographic seizures with behavioral manifestations in two out of five sham-operated experimental controls with epidural and intracerebral electrodes, three out of six naïve animals with epidural and intracerebral electrodes, and one of six naïve rats with epidural electrodes only. Importantly, the seizures were not associated with EEG patterns typical to NCSE found in TBI rats. Moreover, unlike the TBI rats, sham-operated controls and naïve animals showed sleep–wake cycle during longer interictal periods and after their last seizure.
One reason why the occurrence of post-implantation seizures in animal models has not yet been widely reported in the literature, may be due to the initiation of EEG recordings (typically) at 1 week after electrode implantation, to avoid anesthesia and stress effects on EEG. As we have shown, seizures occurred during the first 3 days post-implantation, and thus, had been unnoticed in previous studies.25 Further, as intensive long-lasting EEG-monitoring is typically done in models of epileptogenesis triggered by chemically or electrically induced SE, the possible occurrence of a few electrode-implantation seizures would easily become interpreted as a part of SE-induced activity.
There are several possible explanations for seizure occurrence after electrode implantation. Craniotomy induces a cortical inflammatory response46 that together with isoflurane anesthesia, disrupts the blood–brain barrier, which may have triggered the acute seizures.47,48 Placement of intracerebral electrodes inevitably causes dural penetration, possible intracerebral hemorrhages, neuronal damage and inflammation, which are aligned with the electrode path. Even though the skull electrodes are aimed to be epidural, their position together with the headset may slightly move in freely-moving animals during the follow-up, and break the meningeal vasculature, leading to hematoma49 and even pial and brain tissue inflammation of the underlying cortex, which may initiate seizure activity. However, so far, we have not seen a clear association of subpial penetration of a screw electrode with seizure occurrence (unpublished).
In humans, use of intracerebral electrodes for localization of epileptic focus for surgical removal, is generally considered safe,50 with benefits outweighing the risks. However, occurrence of electrode-implantation related seizures in patients with drug-refractory epilepsy is difficult to differentiate from epilepsy-related seizures, as electrodes are implanted in ictogenic regions. Studies on non-epileptic patients with movement disorders treated by deep brain stimulation have reported that the incidence of intra-operative seizures (i.e., during electrode implantation) varies between 0.3–2.3%,51–58 and during the post-operative period (≤ 2 weeks) incidence varies between 0.9–9.1%.51,55,57 Interestingly, Fenoy and Simpson52 recently reported that two of 728 patients had tonic–clonic seizures during electrode operation and three patients had them during the post-operative Days 1 or 2 (with negative CT findings), matching with the timeline in the present study.
In the present study, there appeared to be a “dose effect” in seizure occurrence, as 45% (4/11) of rats with both epidural and intracerebral electrodes and 16% (1/6) of rats with epidural electrodes, had unprovoked post-operative seizures. The cause for the occurrence of post-operative seizures, and their association with electrode implantation, requires further studies. As some of the seizures in naïve animals reached Racine scale 3–5, they could be detected even without EEG monitoring, that is, without electrode implantation. Even though it is laborious, post-operative seizure monitoring may be necessary, for example, when collecting samples for biomarker analysis, which can be affected by seizures, or when testing novel treatments which modulate seizure threshold.59
Conclusions and Future Directions
Previous studies demonstrated that lateral FPI causes somato-motor and spatial memory impairments that are detectable as early as 1–2 days post-injury.8 It has been well-described that lateral FPI also induces immediate post-impact “seizure-like” behaviors, and late (>7 days post-impact) unprovoked seizures (i.e., post-traumatic epilepsy).8,13,60 In this present study, we show that severe lateral FPI also induces acute days-long NCSE. Our data strongly suggest that the contribution of continuous seizure activity to poor performance in functional tests needs to be co-factored in future studies. Further, as antiepileptic drugs, including remacemide, topiramate, and levetiracetam, have recovery enhancing effects when administered during the acute post-TBI period in rats with FPI,11,12,61 the possible anti-seizure effect of recovery enhancing compounds as a co-factor leading to a favorable outcome should be analyzed. Detailed studies of the occurrence of NCSE in other TBI models are needed. Mechanisms of seizures related to electrode implantation and craniotomy need to be investigated in order to minimize their occurrence and contribution to data obtained in “control animals.”
Overall, inclusion of continuous video-EEG monitoring as part of the study protocol in acute TBI studies is warranted to better characterize the consequences of different types of TBI on brain electrical activity to assess the contribution of seizures on outcome and therapeutic effects, and to increase the rigor of analysis and reproducibility of data among laboratories. Occurrence of post-injury NCSE after lateral FPI -induced TBI strengthens the view that the rat model recapitulates the aftermath of human severe TBI.
Supplementary Material
Acknowledgments
We thank Jarmo Hartikainen for his excellent technical help. This study was supported by the Medical Research Council of the Academy of Finland (Grants 272249 and 273909), NINDS Center without Walls, U54 NS100064 (EpiBioS4Rx) and the CONACYT (CVU 333114).
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Vespa P.M., Nuwer M.R., Nenov V., Ronne-Engstrom E., Hovda D.A., Bergsneider M., Kelly D.F., Martin N.A., and Becker D.P. (1999). Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J. Neurosurg. 91, 750–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vespa P.M., Miller C., McArthur D., Eliseo M., Etchepare M., Hirt D., Glenn T.C., Martin N., and Hovda D. (2007). Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit. Care Med. 35, 2830–2836 [PMC free article] [PubMed] [Google Scholar]
- 3. Vespa P.M., McArthur D.L., Xu Y., Eliseo M., Etchepare M., Dinov I., Alger J., Glenn T.P., and Hovda D. (2010). Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology 75, 792–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vespa P.M., Nuwer M.R., Nenov V., Ronne-Engstrom E., Hovda D.A., Bergsneider P.D.M., Kelly D.F., Martin N.A., and Becker D.P. (1999). Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalograpic monitoring. J Neurosurg. 91, 750–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sandsmark D.K., Kumar M.A., Woodward C.S., Schmitt S.E., Park S., and Lim M.M. (2016). Sleep features on continuous electroencephalography predict rehabilitation outcomes after severe traumatic brain injury. J. Head Trauma Rehabil. 31, 101–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ronne-Engstrom E. and Winkler T. (2006). Continuous EEG monitoring in patients with traumatic brain injury reveals a high incidence of epileptiform activity. Acta Neurol. Scand. 114, 47–53 [DOI] [PubMed] [Google Scholar]
- 7. Dixon C.E., Lyeth B.G., Povlishock J.T., Findling R.L., Hamm R.J., Marmarou a, Young H.F., and Hayes R.L. (1987). A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 67, 110–119 [DOI] [PubMed] [Google Scholar]
- 8. McIntosh T.K., Vink R., Noble L., Yamakami I., Fernyak S., Soares H., and Faden A.L. (1989). Traumatic brain injury in the rat: Characterization of a lateral fluid-percussion model. Neuroscience 28, 233–244 [DOI] [PubMed] [Google Scholar]
- 9. Marklund N. and Hillered L. (2011). Animal modelling of traumatic brain injury in preclinical drug development: Where do we go from here? Br. J. Pharmacol. 164, 1207–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nissinen J., Andrade P., Natunen T., Hiltunen M., Malm T., Kanninen K., Soares J.I., Shatillo O., Sallinen J., Ndode-Ekane X.E., and Pitkänen A. (2017). Disease-modifying effect of atipamezole in a model of post-traumatic epilepsy. Epilepsy Res. 136, 18–34 [DOI] [PubMed] [Google Scholar]
- 11. Hoover R.C., Motta M., Davis J., Saatman K.E., Fujimoto S.T., Thompson H.J., Stover J.F., Dichter M.A., Twyman R., White H.S., and McIntosh T.K. (2004). Differential effects of the anticonvulsant topiramate on neurobehavioral and histological outcomes following traumatic brain injury in rats. J. Neurotrauma 21, 501–512 [DOI] [PubMed] [Google Scholar]
- 12. Browning M., Shear D.A., Bramlett H.M., Dixon C.E., Mondello S., Schmid K.E., Poloyac S.M., Dietrich W.D., Hayes R.L., Wang K.K.W., Povlishock J.T., Tortella F.C., and Kochanek P.M. (2016). Levetiracetam treatment in traumatic brain injury: operation brain trauma therapy. J. Neurotrauma 33, 581–594 [DOI] [PubMed] [Google Scholar]
- 13. Kharatishvili I., Nissinen J.P., McIntosh T.K., and Pitkänen A. (2006). A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience 140, 685–697 [DOI] [PubMed] [Google Scholar]
- 14. Shultz S.R., Cardamone L., Liu Y.R., Hogan R.E., Maccotta L., Wright D.K., Zheng P., Koe A., Gregoire M.-C., Williams J.P., Hicks R.J., Jones N.C., Myers D.E., O'Brien T.J., and Bouilleret V. (2013). Can structural or functional changes following traumatic brain injury in the rat predict epileptic outcome? Epilepsia 54, 1240–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang X., Wang Y., Zhang C., Liu C., Zhao B., Wei N., Zhang J.G., and Zhang K. (2016). CB1 receptor antagonism prevents long-term hyperexcitability after head injury by regulation of dynorphin-KOR system and mGluR5 in rat hippocampus. Brain Res. 1646, 174–181 [DOI] [PubMed] [Google Scholar]
- 16. Bragin A., Li L., Almajano J., Alvarado-Rojas C., Reid A.Y., Staba R.J., and Engel J. (2016). Pathologic electrographic changes after experimental traumatic brain injury. Epilepsia 57, 735–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reid A.Y., Bragin A., Giza C.C., Staba R.J., and Engel J. (2016). The progression of electrophysiologic abnormalities during epileptogenesis after experimental traumatic brain injury. Epilepsia 57, 1558–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trinka E., Cock H., Hesdorffer D., Rossetti A.O., Scheffer I.E., Shinnar S., Shorvon S., and Lowenstein D.H. (2015). A definition and classification of status epilepticus - Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 56, 1515–1523 [DOI] [PubMed] [Google Scholar]
- 19. Pitkänen A., and McIntosh T.K. (2006). Animal models of post-traumatic epilepsy. J. Neurotrauma 23, 241–61 [DOI] [PubMed] [Google Scholar]
- 20. Hickman D.L. and Swan M. (2010). Use of a body condition score technique to assess health status in a rat model of polycystic kidney disease. J. Am. Assoc. Lab. Anim. Sci. 49, 155–9 [PMC free article] [PubMed] [Google Scholar]
- 21. Racine R.J. (1972). Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 32, 281–294 [DOI] [PubMed] [Google Scholar]
- 22. (2017), R.C.T. ([date unknown]). A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna Austria. http://www.R-project.org (Last accessed January10, 2019)
- 23. RStudio Team. (2016). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA [Google Scholar]
- 24. Kane N., Acharya J., Benickzy S., Caboclo L., Finnigan S., Kaplan P.W., Shibasaki H., Pressler R., and van Putten M.J.A.M. (2017). A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin. Neurophysiol. Pract. 2, 170–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nissinen J., Halonen T., Koivisto E., and Pitkänen A. (2000). A new model of chronic temporal lobe epilepsy induced by electrical stimulation of the amygdala in rat. Epilepsy Res. 38, 177–205 [DOI] [PubMed] [Google Scholar]
- 26. Treiman D.M., Walton N.Y., and Kendrick C. (1990). A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Res. 5, 49–60 [DOI] [PubMed] [Google Scholar]
- 27. Lewczuk E., Joshi S., Williamson J., Penmetsa M., Shan S., and Kapur J. (2018). Electroencephalography and behavior patterns during experimental status epilepticus. Epilepsia 59, 369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Avdic U., Ahl M., Chugh D., Ali I., Chary K., Sierra A., and Ekdahl C.T. (2018). Nonconvulsive status epilepticus in rats leads to brain pathology. Epilepsia 59, 945–958 [DOI] [PubMed] [Google Scholar]
- 29. Lu X.-C.M., Hartings J.A, Si Y., Balbir A., Cao Y., and Tortella F.C. (2011). Electrocortical pathology in a rat model of penetrating ballistic-like brain injury. J. Neurotrauma 28, 71–83 [DOI] [PubMed] [Google Scholar]
- 30. Drislane F.W., Lopez M.R., Blum A.S., and Schomer D.L. (2011). Survivors and nonsurvivors of very prolonged status epilepticus. Epilepsy Behav. 22, 342–345 [DOI] [PubMed] [Google Scholar]
- 31. Beniczky S., Hirsch L.J., Kaplan P.W., Pressler R., Bauer G., Aurlien H., Broøgger J.C., and Trinka E. (2013). Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia 54, 28–29 [DOI] [PubMed] [Google Scholar]
- 32. Trinka E. and Leitinger M. (2015). Which EEG patterns in coma are nonconvulsive status epilepticus? Epilepsy Behav. 49, 203–222 [DOI] [PubMed] [Google Scholar]
- 33. (1999). Recommendations for the practice of clinical neurophysiology: guidelines of the International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 52, 1–304 [PubMed] [Google Scholar]
- 34. Sutter R., Semmlack S., and Kaplan P.W. (2016). Nonconvulsive status epilepticus in adults - Insights into the invisible. Nat. Rev. Neurol. 12, 281–293 [DOI] [PubMed] [Google Scholar]
- 35. Centers for Disease Control and Prevention. Traumatic Brain Injury and Concussion. http://www.cdc.gov/traumaticbraininjury (Last accessed January10, 2019)
- 36. Zanier E.R., Lee S.M., Vespa P.M., Giza C.C., and Hovda D. a. (2003). Increased hippocampal CA3 vulnerability to low-level kainic acid following lateral fluid percussion injury. J. Neurotrauma 20, 409–420 [DOI] [PubMed] [Google Scholar]
- 37. Vespa P., Tubi M., Claassen J., Buitrago-Blanco M., McArthur D., Velazquez A.G., Tu B., Prins M., and Nuwer M. (2016). Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann. Neurol. 79, 579–590 [DOI] [PubMed] [Google Scholar]
- 38. Kim J.A., Boyle E.J., Wu A.C., Cole A.J., Staley K.J., Zafar S., Cash S.S., and Westover M.B. (2018). Epileptiform activity in traumatic brain injury predicts post-traumatic epilepsy. Ann. Neurol. 83, 858–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ekolle Ndode-Ekane X., Kharatishvili I., and Pitkänen A. (2017). Unfolded maps for quantitative analysis of cortical lesion location and extent after traumatic brain injury. J. Neurotrauma 34, 459–474 [DOI] [PubMed] [Google Scholar]
- 40. Prins M.L., Lee S.M., Cheng C.L.Y., Becker D.P., and Hovda D.A. (1996). Fluid percussion brain injury in the developing and adult rat: a comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res. Dev. Brain Res. 95, 272–282 [DOI] [PubMed] [Google Scholar]
- 41. Clausen F. and Hillered L. (2005). Intracranial pressure changes during fluid percussion, controlled cortical impact and weight drop injury in rats. Acta Neurochir. (Wien). 147, 775–780 [DOI] [PubMed] [Google Scholar]
- 42. Kim H., Cam-Etoz B., Zhai G., Hubbard W.J., Zinn K.R., and Chaudry I.H. (2015). Salutary Effects of Estrogen Sulfate for Traumatic Brain Injury. J. Neurotrauma 32, 1210–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim H., Yu T., Cam-Etoz B., van Groen T., Hubbard W.J., and Chaudry I.H. (2017). Treatment of traumatic brain injury with 17α-ethinylestradiol-3-sulfate in a rat model. J. Neurosurg. 127, 23–31 [DOI] [PubMed] [Google Scholar]
- 44. Gabrielian L., Willshire L.W., Helps S.C., van den Heuvel C., Mathias J., and Vink R. (2011). Intracranial pressure changes following traumatic brain injury in rats: lack of significant change in the absence of mass lesions or hypoxia. J. Neurotrauma 28, 2103–11 [DOI] [PubMed] [Google Scholar]
- 45. Andriessen T.M.J.C., Horn J., Franschman G., van der Naalt J., Haitsma I., Jacobs B., Steyerberg E.W., and Vos P.E. (2011). Epidemiology, Severity Classification, and Outcome of Moderate and Severe Traumatic Brain Injury: A Prospective Multicenter Study. J. Neurotrauma 28, 2019–2031 [DOI] [PubMed] [Google Scholar]
- 46. Cole J.T., Yarnell A., Kean W.S., Gold E., Lewis B., Ren M., McMullen D.C., Jacobowitz D.M., Pollard H.B., O'Neill J.T., Grunberg N.E., Dalgard C.L., Frank J.A, and Watson W.D. (2011). Craniotomy: true sham for traumatic brain injury, or a sham of a sham? J. Neurotrauma 28, 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tétrault S., Chever O., Sik A., and Amzica F. (2008). Opening of the blood-brain barrier during isoflurane anaesthesia. Eur. J. Neurosci. 28, 1330–1341 [DOI] [PubMed] [Google Scholar]
- 48. Thal S.C., Luh C., Schaible E.V., Timaru-Kast R., Hedrich J., Luhmann H.J., Engelhard K., and Zehendner C.M. (2012). Volatile anesthetics influence blood-brain barrier integrity by modulation of tight function protein expression in traumatic brain injury. PLoS One 7, e50752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rowe R.K., Harrison J.L., Ellis T.W., Adelson P.D., and Lifshitz J. (2018). Midline (central) fluid percussion model of traumatic brain injury in pediatric and adolescent rats. J. Neurosurg. Pediatr. 22, 22–30 [DOI] [PubMed] [Google Scholar]
- 50. Wellmer J., von der Groeben F., Klarmann U., Weber C., Elger C.E., Urbach H., Clusmann H., and von Lehe M. (2012). Risks and benefits of invasive epilepsy surgery workup with implanted subdural and depth electrodes. Epilepsia 53, 1322–1332 [DOI] [PubMed] [Google Scholar]
- 51. Beric A., Kelly P.J., Rezai A., Sterio D., Mogilner A., Zonenshayn M., and Kopell B. (2001). Complications of deep brain stimulation surgery. Stereotact. Funct. Neurosurg. 77, 73–78 [DOI] [PubMed] [Google Scholar]
- 52. Fenoy A.J. and Simpson R.K. (2014). Risks of common complications in deep brain stimulation surgery: management and avoidance. J. Neurosurg. 120, 132–139 [DOI] [PubMed] [Google Scholar]
- 53. Boviatsis E.J., Stavrinou L.C., Themistocleous M., Kouyialis A.T., and Sakas D.E. (2010). Surgical and hardware complications of deep brain stimulation. A seven-year experience and review of the literature. Acta Neurochir. (Wien). 152, 2053–2062 [DOI] [PubMed] [Google Scholar]
- 54. Lyons K.E., Koller W.C., Wilkinson S.B., and Pahwa R. (2001). Long term safety and efficacy of unilateral deep brain stimulation of the thalamus for parkinsonian tremor. J. Neurol. Neurosurg. Psychiatry 71, 682–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pahwa R., Wilkinson S.B., Overman J., and Lyons K.E. (2003). Bilateral subthalamic stimulation in patients with Parkinson disease: long-term follow up. J. Neurosurg. 99, 71–77 [DOI] [PubMed] [Google Scholar]
- 56. Starr PA Sillay K. (2008). Complication avoidance and management in deep brain stimulation surgery, in: Deep Brain Stimulation in Neurological and Psychiatric Disorders (Current Clinical Neurology). Tarsy D., Vitek J.L., Star P.A., and Okun M.S. (eds). Humana Press: New York, pps. 135–150 [Google Scholar]
- 57. Umemura A., Jaggi J.L., Hurtig H.I., Siderowf A.D., Colcher A., Stern M.B., and Baltuch G.H. (2003). Deep brain stimulation for movement disorders: morbidity and mortality in 109 patients. J. Neurosurg. 98, 779–784 [DOI] [PubMed] [Google Scholar]
- 58. Voges J., Waerzeggers Y., Maarouf M., Lehrke R., Koulousakis A., Lenartz D., and Sturm V. (2006). Deep-brain stimulation: long-term analysis of complications caused by hardware and surgery—experiences from a single centre. J. Neurol. Neurosurg. Psychiatry 77, 868–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pitkänen A., Ekolle Ndode-Ekane X., Lapinlampi N., and Puhakka N. (2018). Epilepsy biomarkers—toward etiology and pathology specificity. Neurobiol. Dis. pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nissinen J., Andrade P., Natunen T., Hiltunen M., Malm T., Kanninen K., Soares J.I., Shatillo O., Sallinen J., Ndode-Ekane X.E., and Pitkänen A. (2017). Disease-modifying effect of atipamezole in a model of post-traumatic epilepsy. Epilepsy Res. 136, 18–34 [DOI] [PubMed] [Google Scholar]
- 61. Smith D.H., Perri B.R., Raghupathi R., Saatman K.E., and McIntosh T.K. (1997). Remacemide hydrochloride reduces cortical lesion volume following brain trauma in the rat. Neurosci. Lett. 231, 135–138 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.