Abstract
Aims: Oxidative stress is implicated in cardiomyocyte cell death and cardiac remodeling in the failing heart. The role of NADPH oxidase 4 (NOX4) in cardiac adaptation to pressure overload is controversial, but its function in myocardial ischemic stress has not been thoroughly elucidated. This study examined the function of NOX4 in the pathogenesis of ischemic heart failure, utilizing mouse models, cell culture, and human heart samples.
Results: Nox4−/− mice showed a protective phenotype in response to permanent left anterior descending coronary artery ligation with smaller infarction area, lower cardiomyocyte cross-sectional area, higher capillary density, and less cell death versus wild-type (WT) mice. Nox4−/− mice had lower activity of soluble epoxide hydrolase (sEH), a potent regulator of inflammation. Nox4−/− mice also showed a 50% reduction in the number of infiltrating CD68+ macrophages in the peri-infarct zone versus WT mice. Adenoviral overexpression of NOX4 in cardiomyoblast cells increased sEH expression and activity and CCL4 and CCL5 levels; inhibition of sEH activity in NOX4 overexpressing cells attenuated the cytokine levels. Human hearts with ischemic cardiomyopathy showed adverse cardiac remodeling, increased NOX4 and sEH protein expression and CCL4 and CCL5 levels compared with control nonfailing hearts.
Innovation and Conclusion: These data from the Nox4−/− mouse model and human heart tissues show for the first time that oxidative stress from increased NOX4 expression has a functional role in ischemic heart failure. One mechanism by which NOX4 contributes to ischemic heart failure is by increasing inflammatory cytokine production via enhanced sEH activity.
Keywords: NADPH oxidase 4, soluble epoxide hydrolase, ischemic heart failure, inflammation, LAD ligation
Introduction
Heart failure, a condition in which the heart is unable to efficiently pump blood, currently affects ∼6.5 million Americans and causes 1 in 8 deaths in the United States (7). Coronary heart disease is characterized by the buildup of plaque in the coronary arteries, thus impeding blood flow to the heart. Complete blockage of coronary arteries causes myocardial infarction and results in massive cell death within the heart. Cardiac remodeling after infarction leads to worsening cardiac performance termed ischemic cardiomyopathy (ICM), which is the leading cause of heart failure, contributing to ∼20% of all cases (7).
Reactive oxygen species (ROS) production and oxidative stress markers are increased during heart failure (24, 46, 54). Excessive ROS induce cellular damage, including lipid peroxidation of cellular membranes (65), DNA damage (61), and protein modification, causing enzyme inactivation or denaturation (37). In coronary artery disease, ROS play a role in the origin and progression of plaque buildup by increasing oxidized low-density lipoproteins and stimulating matrix degradation (30, 50, 51). After occlusion, cardiomyocyte cell death and remodeling in the heart is exacerbated by increased ROS levels (31, 34, 53, 77). Thus, in ICM, ROS play a role in both the genesis and progression of heart failure.
One of the major sources of ROS is the NADPH oxidase (Nox) family of enzymes, which consists of seven isoforms, NOX1-5 (12), and two dual oxidases (DUOXs), DUOX 1 and 2. The NADPH oxidase 4 (NOX4) isoform is expressed in many cell types, including cardiomyocytes (1, 13). NOX4 is unique among the Nox family members because it produces H2O2, whereas others produce superoxide (63). H2O2 is more stable than superoxide, can diffuse across cell membranes, and, hence, can cause biologic effects beyond the cellular compartment where it is generated. Also, NOX4, unlike other Nox family members, is constitutively active as it does not require cytosolic subunits for activation; its activity level is primarily regulated by expression (40, 55). NOX4 is ubiquitously expressed, including kidneys, heart, and blood vessels; endothelial cells, vascular smooth muscle cells, and fibroblasts in the vascular wall and cardiomyocytes and cardiac fibroblasts express NOX4 (1, 5, 34).
In cardiomyocytes, NOX4 is primarily located in the mitochondria, and the effects of NOX4 activity are cell-type dependent (1). Increased NOX4 expression/activity is implicated in cardiac fibrosis, cardiomyocyte hypertrophy, and cell death during pressure overload, in cardiac remodeling (34, 77), and in inflammatory cytokine production in atherosclerosis (39, 66). In contrast, NOX4-dependent protection against chronic load-induced stress in mouse hearts was attributed to preservation of myocardial capillary density from increased paracrine angiogenic activity mediated by induction of hypoxia inducible factor 1 and activation and release of vascular endothelial growth factor (74).
Innovation
Myocardial ischemia, induced by permanent left anterior descending (LAD) coronary artery ligation, increased peri-infarct zone NADPH oxidase 4 (NOX4) expression, cardiac oxidative stress, and soluble epoxide hydrolase (sEH) activity, causing adverse cardiac remodeling and impaired cardiac function in the wild-type mice, effects that were significantly attenuated in Nox4−/− mice. H9c2 cell culture experiments indicate NOX4-dependent regulation of sEH as the effector of increased inflammatory cytokine production. Data from analysis of ischemic failing versus nonfailing human hearts corroborate evidence from H9c2 cell culture and Nox4−/− mice that NOX4 plays a role in ischemic heart failure by increasing myocardial oxidative stress and inflammation via upregulation of sEH.
Chronic inflammation, a hallmark of heart failure, worsens as failure progresses and is often accompanied by increased cytokine production (56). As inflammatory cells migrate to the heart to remove necrotic and apoptotic cardiomyocytes after myocardial ischemic damage, a cytokine cascade ensues that exerts deleterious effects on the surrounding tissue, worsening myocardial performance.
Soluble epoxide hydrolase (sEH) increases inflammation by inactivating anti-inflammatory epoxyeicosatrienoic acids (EETs), molecules that inhibit the production and activity of cytokines (21, 47, 76). sEH activity is increased in animal models of myocardial infarction and hypertension, suggesting that it might play a role in heart failure progression by prolonging the cytokine cascade (33, 45). NOX4 has been shown to play a role in regulating sEH expression though the results have been cell-type dependent: In vascular smooth muscle cells, NOX4 positively regulated sEH (66); wheresa in endothelial cells, NOX4 negatively regulated sEH (29). Both studies used overexpression of dominant negative Nox4 in cell culture to determine the regulation of sEH by NOX4 and at this point, it is unclear how NOX4 differentially regulates sEH in these cell types.
In this study, we explored the hypothesis that Nox4−/− mice would be protected from ischemic heart injury after left anterior descending artery (LAD) ligation. We also used cardiomyoblast cell culture to investigate the relationship between NOX4 expression and sEH activity. Our results show that the Nox4 knockout mice had reduced ischemic heart damage by attenuating sEH activity and decreasing inflammation. Data from failing human hearts corroborate our findings, showing a positive correlation between NOX4 and sEH expression compared with healthy controls.
Results
Nox4 deletion in mice protects against ischemic heart injury
The function of NOX4 during heart failure in mouse pressure overload models has been controversial: Kuroda et al. found that a cardiac-specific knockout of Nox4 was protective against myocardial fibrosis and cardiac dysfunction (34), whereas Zhang et al. reported that mice with global deletion of Nox4 had impaired cardiac function and cardiac-specific Nox4 overexpression was protective (74). However, not much is known about the role of NOX4 in ischemic heart injury.
To address this issue, we evaluated cardiac function in wild-type (WT) and Nox4−/− mice 2 weeks after permanent LAD ligation. There was no significant difference in mortality during this period between WT (8.9%) and Nox4−/− mice (4.9%). Echocardiographic measurements showed that both WT and Nox4−/− mice subjected to LAD ligation had reduced ejection fraction compared with sham-operated mice but that after LAD ligation the ejection fraction in WT mice was significantly reduced compared with Nox4−/− mice (p < 0.001; Supplementary Table S1). Both end-systolic and end-diastolic areas were increased in mice undergoing LAD ligation compared with sham-operated mice (Supplementary Table S1). After LAD ligation, however, Nox4−/− mice had significantly lower areas than WT mice (systolic p < 0.001; diastolic p < 0.01). Heart weight in LAD-ligated WT was significantly increased compared with sham-operated mice (p < 0.01), but not in Nox4−/− mice (Supplementary Table S1). In addition, two-way ANOVA demonstrated a significant interaction between LAD ligation and NOX4 deficiency for ejection fraction (p < 0.05) and left ventricle (LV) systolic area (p < 0.05). Infarct area in 2 weeks post–LAD-ligated hearts was measured in five to six short-axis slices and was greater in the WT compared with Nox4−/− mice (41.9% increase; Fig. 1A, B).
FIG. 1.
Nox4 deficiency attenuates cardiac injury after LAD ligation. (A) Infarction size was assessed at the midline circumference of five to six cross-sections of LAD-ligated hearts stained with Masson's Trichrome. Representative cross-sections from the site of ligation to the apex. Scale is 1 mm. (B) Infarct size quantified from sections shown in A (mean ± SEM; n = 9 in each group). **p < 0.01. LAD, left anterior descending artery; NOX4, NADPH oxidase 4; WT, wild-type.
Infarction area, measured 1 day after LAD ligation, by Evans blue perfusion and triphenyltetrazolium chloride (TTC) staining showed that WT and Nox4−/− mice had similar infarct size (Supplementary Fig. S1A, B), which suggests that differences seen at 14 days post-LAD ligation reflect the contribution of NOX4 regulated inflammatory processes to the infarct size. Together, these results indicate that NOX4 has an important role in regulating myocardial damage in response to ischemia and that regulating its expression/activity may exert a cardiac protective effect.
Nox4 expression, sEH activity, and proinflammatory cytokine expression are increased after LAD ligation
Because NOX2 and NOX4 expression is increased in ischemia (38), NOX4 is implicated in the regulation of sEH activity (29, 66), and sEH regulates postischemic recovery of myocardial function (57), we investigated NOX2 and NOX4 expression and the relationship between NOX4 expression and sEH expression/activity after LAD ligation in our mouse model. NOX4 protein expression in the peri-infarct zone of the heart tissue was increased ∼1.7-fold in WT after LAD ligation compared with sham-operated mice (p < 0.05, Fig. 2A, B). NOX1 protein levels were unaffected by ischemia and were not different between the genotypes (Supplementary Fig. S2A, B), which indicated a lack of compensatory upregulation of NOX1 in Nox4−/− mice. NOX2 protein expression in the peri-infarct region was increased in both WT and Nox4−/− mice after LAD ligation; however, NOX2 expression was significantly greater in the WT mice (Supplementary Fig. S2C, D).
FIG. 2.
Ischemia enhances NOX4 expression, sEH activity, and cytokine levels in the hearts of WT mice and Nox4 deletion attenuates ischemia-induced increase in sEH activity. Heart tissues from the peri-infarct region of LAD-ligated hearts were used for analysis and compared with sham-operated hearts. (A) A representative Western blot of NOX4 protein in sham-operated and LAD-ligated hearts from WT mice. (B) Densitometric quantification of NOX4 levels normalized to β-tubulin levels. (C) A representative Western blot of sEH protein in WT and Nox4−/− mice with sham or 3 day post-LAD ligation. (D) Densitometric quantification of sEH levels normalized to β-tubulin levels. (E) Myocardial sEH activity in the hearts of WT and Nox4−/− mice 3 days after LAD ligation. (F) CCL4 and (G) CCL5 protein levels in mouse heart lysates 3 days after LAD ligation. (H) A representative Western blot of sEH protein in WT and Nox4−/− mice with sham and 2 weeks post-LAD ligation. (I) Densitometric quantification of sEH levels normalized to β-tubulin levels. (J) sEH activity in the hearts of WT and Nox4−/− mice 2 weeks after LAD ligation. (K) CCL4 and (L) CCL5 protein levels in mouse heart lysates 2 weeks after LAD ligation. Data are the mean ± SEM; n = 6 (A, H–L), n = 9 (C–G). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. CCL4, C-C motif chemokine ligand 4 (or MIP-1β, macrophage inflammatory protein-1β); CCL5, C-C motif chemokine ligand 5 (or RANTES, regulated on activation, normal T cell expressed and secreted); sEH, soluble epoxide hydrolase.
sEH protein expression was similar in the WT and Nox4−/− hearts at the basal level and did not change after LAD ligation, at either 3 days (Fig. 2C, D) or 2 weeks (Fig. 2H, I), consistent with reports of no change in sEH protein expression after LAD ligation in the WT mice (2, 45). However, sEH activity was significantly increased in WT, whereas no change was observed in Nox4−/− mice 3 days post-LAD ligation (Fig. 2E). Two-way ANOVA showed a significant interaction between LAD ligation and NOX4 deficiency for sEH activity (p < 0.001). In contrast, sEH activity was significantly increased in both the WT and Nox4−/− hearts 2 weeks post-LAD ligation (p < 0.001 vs. respective controls; Fig. 2J); however, the enzyme activity in WT was significantly greater than in Nox4−/− hearts (p < 0.05; Fig. 2J).
The myocardial levels of inflammatory cytokine CCL4 (C-C motif chemokine ligand 4 [or MIP-1β, macrophage inflammatory protein-1β]), which are reportedly rapidly induced during ischemia (48), were significantly increased in both WT and Nox4−/− hearts 3 days after LAD ligation, but to a greater extent in WT hearts (p < 0.01 vs. Nox4−/− hearts; Fig. 2F). CCL5 (C-C motif chemokine ligand 5 [or RANTES, regulated on activation, normal T cell expressed and secreted]) levels were markedly increased in WT hearts 3 days after LAD ligation (p < 0.0001; Fig. 2G), whereas the levels were unchanged in Nox4−/− hearts. A two-way ANOVA analysis showed a significant interaction between LAD ligation and Nox4 deficiency for CCL4 (p < 0.01) and CCL5 (p < 0.05) levels. CCL4 levels remained significantly higher in WT hearts 2 weeks post-LAD ligation, but returned to basal levels in Nox4−/− hearts (Fig. 2K). However, CCL5 levels returned to basal levels in WT hearts 2 weeks post-LAD ligation (Fig. 2L). These results suggest that NOX4 contributes to sEH activation early after ischemic injury whereas other, as-yet unknown factors also regulate sEH activity in the resolution phase of ischemic injury as Nox4−/− mice also had a significantly increased enzyme activity 2 weeks post-LAD ligation. Further, oxidative stress resulting from increased NOX4 expression promotes inflammation in the ischemic heart.
Increase in NOX4 expression during ischemia enhances myocardial cellular and mitochondrial oxidative stress and DNA damage
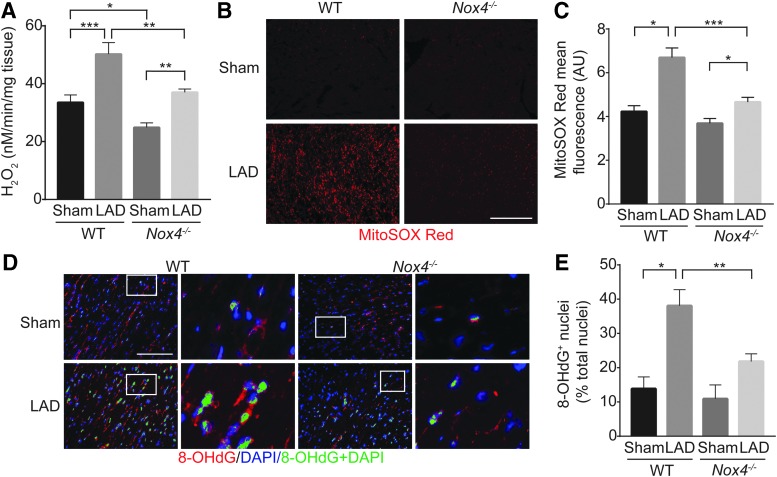
To correlate NOX4 levels with increased oxidative stress during ischemic stress, we first measured H2O2 levels in mouse heart tissues by using Amplex Red assay. Myocardial H2O2 levels were significantly increased in both the WT and Nox4−/− mice after LAD ligation (Fig. 3A); however, congruent with increased myocardial NOX4 protein expression (Fig. 2A, B), myocardial H2O2 levels were the maximum in WT mice after LAD ligation (p < 0.001, Fig. 3A). The sham (p < 0.05) as well as post-LAD ligation (p < 0.01) myocardial H2O2 levels were significantly lower in Nox4−/− mice compared with WT mice. Because NOX4 localizes to mitochondria (1), we examined mitochondrial oxidative stress by staining mouse myocardial sections with MitoSOX Red, a mitochondria-targeted superoxide indicator. Although MitoSOX Red fluorescence was markedly increased in both WT and Nox4−/− hearts after LAD ligation, the increase in mitochondrial ROS levels was significantly higher in the WT versus Nox4−/− hearts (p < 0.001; Fig. 3B, C), correlating with higher NOX4 levels.
FIG. 3.
H2O2 levels and DNA damage, increased in the WT, are attenuated in Nox4−/− mice in the peri-infarct region 2 weeks after LAD ligation. (A) H2O2 levels in mouse heart tissue were assessed by Amplex Red assay. (B) Representative fluorescent images of heart sections stained with MitoSOX Red (C). Quantification of MitoSOX Red fluorescence. (D) Representative fluorescent images of heart sections stained for 8-OHdG and counterstained with DAPI (8-OHdG-red, DAPI-blue, 8-OHdG+ nuclei-pseudo-colored green). High magnification of insets (white rectangle) to the right show nuclear localized 8-OHdG+ staining. (E) Quantification of 8-OHdG+ nuclei. Data are the mean ± SEM, n = 9. Scale is 100 μm, n = 9, *p < 0.05, **p < 0.01, and ***p < 0.001. 8-OHdG, 8-hydroxy-2′-deoxyguanosine; DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride.
To further determine the role of NOX4 during ischemic stress, mouse heart sections were stained with 8-hydroxy-2′-deoxyguanosine (8-OHdG), an oxidized nucleoside of DNA and a surrogate marker of enhanced oxidative stress (68, 70). Sham Nox4−/− had lower, but not statistically significant, 8-OhdG-positive nuclei compared with sham WT mice (Fig. 3D, E). However, LAD-ligated Nox4−/− had significantly less 8-OhdG-positive nuclei compared with LAD-ligated WT mice (p < 0.01), confirming the role of NOX4 in increased myocardial oxidative stress during ischemia. These results indicate that NOX4 might play a role in myocardial dysfunction during ischemia by enhancing oxidative stress and DNA damage.
Adverse cardiac remodeling and macrophage infiltration is attenuated in Nox4−/− mice during ischemic stress
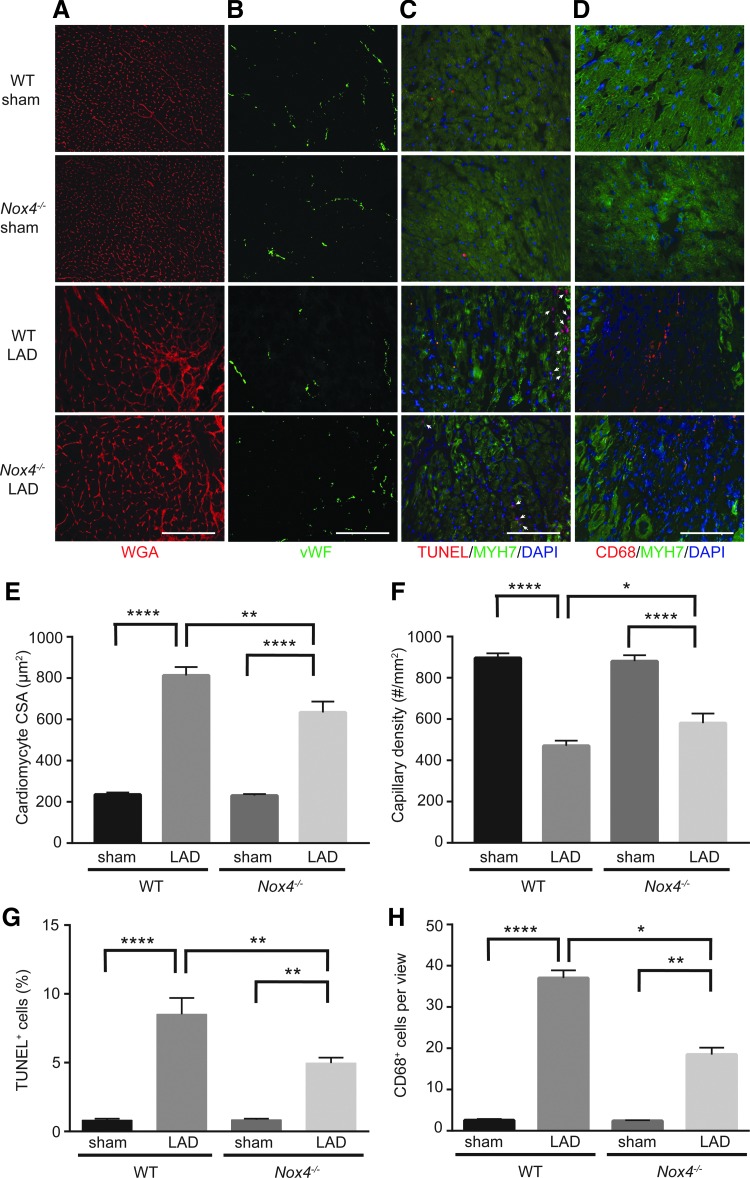
Next, we investigated left ventricular remodeling in LAD-ligated mice to determine the effect of NOX4 expression on cardiac function during ischemic stress. Wheat germ agglutinin (WGA) staining was used to measure cardiomyocyte cross-sectional area (CSA) in the peri-infarct zone, and both WT and Nox4−/− mice exhibited cardiomyocyte hypertrophy after LAD ligation; however, the effect was more pronounced in WT mice (p < 0.01; Fig. 4A, E). Capillary density was decreased in LAD-ligated mice compared with sham-operated mice, but the decrease was greater in WT versus Nox4−/− mice (p < 0.05; Fig. 4B, F). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining showed that LAD ligation increased the number of apoptotic myocardial cells in both WT and Nox4−/− mice; however, TUNEL-positive cells were significantly decreased in the heart tissue of mice that lack NOX4 (p < 0.05; Fig. 4C, G).
FIG. 4.
Cardiac remodeling and macrophage infiltration is reduced in Nox4−/− mice after LAD ligation. WT and Nox4−/− mice hearts were analyzed by immunofluorescence microscopy 2 weeks after sham or from the peri-infarct region after LAD ligation (A) Cardiomyocyte CSA was measured by WGA staining. (B) Capillary density was measured by vWF staining. (C) Cell death was measured in cardiomyocytes by co-staining with TUNEL and MYH7, a cardiomyocyte cell marker. (arrowheads indicate TUNEL+ nuclei). (D) Macrophages were identified by CD68 staining in sham-operated mice and in the border region of the infarct zone of LAD-ligated mice. MYH7 was used to identify the border region. (E) Quantification of cardiomyocyte CSA, (F) quantification of capillary density, (G) quantification of TUNEL+ cells, and (H) quantification of CD68+ cells. Data are the mean ± SEM, n = 9. Scale is 100 μm. *p < 0.05, **p < 0.01, and ****p < 0.0001. CSA, cross-sectional area; MYH7, myosin heavy chain beta; TUNEL, terminal deoxynucleotidyl transferase; vWF, von Willebrand Factor; WGA, wheat germ agglutinin.
Since reduction of sEH activity has been associated with reduced inflammation (2, 33), we hypothesized that macrophage infiltration into the border region of the infarct zone after LAD ligation would be lower in Nox4−/− mice. Sham-operated WT and Nox4−/− mice contained a few macrophages whereas LAD-ligated mice showed an increase in CD68+ macrophages at the infarct border zone 2 weeks post-LAD ligation (Fig. 4D, H). However, Nox4−/− LAD ligated hearts had significantly fewer macrophages compared with WT (p < 0.0001). In addition, two-way ANOVA demonstrated a significant interaction between LAD ligation and NOX4 deficiency for CD68+ macrophage infiltration (p < 0.0001). To confirm increased macrophage infiltration in WT mice after LAD ligation, we determined CD68 mRNA levels in sham and LAD-ligated hearts. A significant increase in CD68 mRNA was observed in WT hearts 2 weeks post-LAD ligation and the increase was markedly higher in WT compared with Nox4−/− hearts (p < 0.5; Supplementary Fig. S3). Together, these data suggest that absence of NOX4 protects against adverse ventricular remodeling by decreasing inflammation during ischemic stress.
NOX4 overexpression induced oxidative stress, sEH activity, and cytokine expression in the H9c2 rat cardiomyoblast cell line is attenuated by catalase
To further explore the relationship among NOX4, sEH, and inflammation, we transduced H9c2 rat cardiomyoblasts with an adenovirus expressing human NOX4 (3) in the presence and absence of polyethylene glycol conjugated catalase (PEG-catalase). We chose H9c2 cells for the studies as they exhibit several electrical and cellular signaling characteristics of adult cardiomyocytes (27) and are considered a good model for mechanistic studies investigating molecular pathways in cardiomyocytes exposed to ischemia and oxidative stress (11, 35).
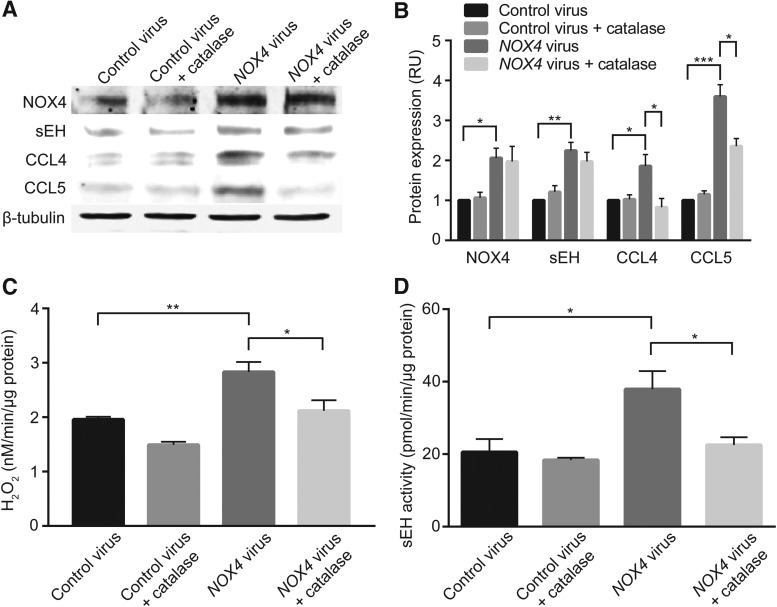
A marked increase in NOX4 expression in H9c2 cells transduced with human NOX4 adenoviral construct correlated with a significant increase in H2O2 production (Fig. 5A–C; p < 0.01 vs. control virus). Concomitant with increased NOX4 expression and activity, a significant increase in sEH expression (p < 0.01 vs. control virus; Fig. 5A, B) and activity (p < 0.05 vs. control virus; Fig. 5D) was observed in cells transduced with human NOX4 adenovirus. The positive correlation between NOX4 expression and sEH activity in WT mice after LAD ligation is similar to that observed in cardiomyoblast cells. The absence of positive correlation between NOX4 and sEH expression after LAD ligation might reflect the differential response of various cell types in the heart to ischemia. PEG-catalase treatment had no effect on NOX4 and sEH expression (Fig. 5A, B); however, increases in H2O2 levels and sEH activity in NOX4 adenovirus transduced cardiomyoblast cells were abrogated in the presence of catalase (p < 0.05 in each case; Fig. 5C, D). In addition, human NOX4 overexpressing cells showed increased expression of inflammatory cytokines CCL5 and CCL4 compared with cells transduced with the control virus, and cytokine production is attenuated with PEG-catalase treatment (Fig. 5A, B). These results suggest that H2O2 produced by NOX4 induces sEH activity, promoting cytokine production and they confirm the previous reports that both NOX4 and sEH positively regulate inflammatory cytokine production (39, 73).
FIG. 5.
Adenoviral overexpression of human NOX4 increases sEH expression and activity and cytokine production in H9c2 cells and is negated by addition of PEG-catalase. (A) Western blot analysis of NOX4, sEH, CCL4, and CCL5 protein levels. (B) Densitometric quantification of protein levels normalized to β-tubulin levels. (C) H2O2 production as measured by Amplex Red assay. (D) Enzymatic activity of sEH as measured by using sEH Cell-Based Assay Kit. Data are the mean ± SEM, n = 3 (A, B), n = 5 (C), and n = 3 (D). *p < 0.05, **p < 0.01, and ***p < 0.001. PEG-catalase, polyethylene glycol conjugated catalase.
Pharmacological inhibition of increased sEH activity in H9c2 cells overexpressing human NOX4 abrogates increased cytokine production
To further explore the role of sEH in NOX4-regulated inflammation, we treated H9c2 cells transduced with human NOX4 adenovirus and control virus with TPPU, a specific inhibitor of sEH (29, 66). TPPU had no marked effect on NOX4 and sEH protein expression (Fig. 6A, B) and H2O2 production (Fig. 6C) in H9c2 cells, but it significantly decreased sEH enzyme activity (p < 0.01 vs. NOX4 transduced cells, Fig. 6D). TPPU also significantly decreased CCL4 (p < 0.05 vs. human NOX4 transduced cells; Fig. 6B) and CCL5 (p < 0.01 vs. human NOX4 transduced cells; Fig. 6B) expression in NOX4-transduced cells, further supporting the notion that sEH activation might regulate NOX4-induced inflammation in myocardium.
FIG. 6.
Inhibiting increased sEH activity in H9c2 cell overexpressing human NOX4 abrogates cytokine production. (A) Western blot analysis of NOX4, sEH, CCL4, and CCL5 protein levels. (B) Densitometric quantification of protein levels normalized to β-tubulin levels. (C) H2O2 production as measured by Amplex Red assay. (D) Enzymatic activity of sEH as measured by using sEH Cell-Based Assay Kit. Data are the mean ± SEM, n = 3 (A, B), n = 5 (C), and n = 3 (D). *p < 0.05, **p < 0.01, and ***p < 0.001.
Ischemic human hearts show increased NOX4 expression, oxidative stress, and LV remodeling
To determine the clinical relevance of NOX4 in ischemic heart injury and inflammation, we examined NOX4 expression in heart tissue from patients with ischemic heart failure who previously had a myocardial infarction (ICM) as well as in control nonfailing (NF) heart tissue from donors whose hearts were not used for transplantation. Western blot analysis of LV tissue showed a significant increase in NOX4 protein expression in ICM versus NF hearts (p < 0.01, Fig. 7A, B). Double immunofluorescence staining of LV cross-sections for NOX4 and MYH7 (myosin heavy chain beta), a cardiomyocyte marker, confirmed increased NOX4 expression in cardiomyocytes in ICM versus NF hearts (p < 0.0001; Fig. 7C).
FIG. 7.
Human hearts from patients with ICM show increased NOX4 expression and oxidative stress. ICM hearts (n = 19) were compared with NF hearts (n = 24). (A) Representative Western blot analysis of NOX4 protein levels in human ventricular tissue. (B) Densitometric quantification of NOX4 levels normalized to GAPDH levels. (C) Representative immunofluorescence images of human ventricular tissue stained for immunoreactive NOX4 (red) and MYH7 (green), a cardiomyocyte marker, and counterstained with DAPI (blue) (colocalization of NOX4 and MYH7, yellow; left panel). NOX4 expression represented as fluorescence-integrated density for number of cells (right panel). (D) Representative fluorescent images of heart sections stained for 8-OHdG and counterstained with DAPI (8-OHdG-red, DAPI-blue, 8-OHdG+ nuclei-pseudo-colored green; n = 10 NF and ICM hearts; left panel). Quantification of 8-OHdG+ nuclei (right panel). (E) Representative fluorescent images of heart sections stained for 8-OHdG and ATP5G2, a mitochondrial marker (8-OHdG-red, ATP5G2-green, 8-OHdG+ mitochondria-pseudo-colored blue; n = 10 NF and ICM hearts; left panel). Quantification of 8-OHdG+ mitochondria (right panel). (F) Representative left ventricular sections were stained for immunoreactive nitrotyrosine (n = 6 NF and ICM hearts; left panel). Quantification of nitrotyrosine staining (right panel). Data are the mean ± SEM. Scale bar is 100 μm. *p < 0.05, **p < 0.01, and ****p < 0.0001. ATP5G2, ATP Synthase Membrane Subunit C Locus 2; ICM, ischemic cardiomyopathy; NF, nonfailing.
To determine whether increased NOX4 expression resulted in enhanced myocardial oxidative stress, LV cross-sections were stained with 8-OHdG. The number of 8-OHdG-positive nuclei was significantly higher in tissue from ICM versus NF patients (p < 0.05, Fig. 7D); 8-OHdG also detects mitochondrial DNA damage caused by oxidative stress (69, 71). Co-staining of 8-OHdG and ATP Synthase Membrane Subunit C Locus 2 (ATP5G2), a protein specific to mitochondria, showed significantly more mitochondrial DNA damage in ICM hearts (p < 0.05; Fig. 7E). Also, LV cross-sections of ICM patients had significantly increased nitrotyrosine staining (p < 0.05 vs. NF; Fig. 7F), an in situ marker of myocardial oxidative/nitrosative stress (78).
To determine the pathomechanistic relevance of our data from mouse LAD ligation experiments of NOX4 and ischemic heart damage, we evaluated LV remodeling in the human heart samples. Morphometric analysis of LV cross-sections stained with WGA showed a 2.3-fold increase in cardiomyocyte size in ICM versus NF hearts (p < 0.001, Fig. 8A), which was correlated with increased NOX4 expression (p < 0.05). In addition, myocardial capillary density, determined by von Willebrand factor staining, was significantly reduced in ICM versus NF patients (p < 0.001, Fig. 8B) and was negatively correlated with NOX4 protein expression (p < 0.05).
FIG. 8.
Adverse left ventricular remodeling in human hearts with ICM. ICM hearts (n = 19) were compared with NF hearts (n = 24). (A) Representative images of left ventricular cross-sections were stained with WGA (top panels). Cardiomyocyte cross-sectional area was measured by using Image J (n ∼ 500–600 cells; middle panel). (B) Representative images of left ventricular cross-sections stained with von Willebrand factor to determine capillary density (top panel). Quantification of capillary density (n ∼ 100–125 microscopic fields; middle panel) (C) Representative fluorescent microscopy images of TUNEL-stained left ventricular sections (arrowheads indicate TUNEL+ nuclei; upper panel). Quantification of TUNEL+ nuclei (n ∼ 100–125 cross-sectional areas, middle panel) (D) Representative images of left ventricular cross-sections stained with Verhoeff sirius red (upper panel), and collagen was quantified by using Image J (middle panel). Data are the mean ± SEM. Scale bar is 100 μm. Scatter plots showing correlation between NOX4 protein levels and specific left ventricular remodeling markers in all human heart samples (bottom panels). The regression lines in each graph show the 95% confidence intervals (dotted lines), and R2 values are shown for each plot. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Apoptosis, as determined by TUNEL staining, showed that LV cross-sections from ICM patients had more TUNEL-positive cells versus NF patients (p < 0.0001, Fig. 8C) and that NOX4 protein expression was positively correlated with myocardial cell death (p < 0.01). Finally, Verhoeff picrosirius red staining revealed a significant increase in LV fibrosis in ICM versus NF hearts (p < 0.05, Fig. 8D), which was positively correlated with NOX4 protein expression (p < 0.05). These data support the role of NOX4 in inducing ischemic injury shown in our mouse model and suggest that increased NOX4 expression contributes to human myocardial injury and dysfunction under ischemic conditions.
ICM patients had increased expression of sEH protein, matrix metalloproteinase 9, and inflammatory cytokines in the LV
To determine whether increased NOX4 expression in the LV of ICM patients is associated with enhanced sEH protein levels, we assessed sEH protein levels in human hearts by Western analysis; sEH protein expression was significantly increased in ICM compared with NF hearts (p < 0.01; Fig. 9A, B). Further, sEH expression levels were positively correlated with NOX4 protein expression levels in ICM (p < 0.001, R2 = 0.6197; Fig. 9C), but not in NF heart samples (Fig. 9D). These data further strengthen the notion that NOX4 regulates sEH expression in human heart failure with ischemic etiology.
FIG. 9.
sEH expression is increased in human hearts with ICM. ICM hearts (n = 19) were compared with NF hearts (n = 24). (A) A representative Western blot of sEH protein in left ventricular lysates. (B) Densitometric quantification of sEH protein levels normalized to GAPDH levels. Data are the mean ± SEM. Scatter plots showing correlation between NOX4 and sEH protein levels in left ventricular tissue in (C) ICM and (D) NF hearts. The regression lines in the graphs show the 95% confidence intervals (dotted lines), and R2 values are shown for each plot. **p < 0.01, ***p < 0.001; ns, not significant.
We next examined whether increased NOX4 and sEH expression in ischemic human heart is associated with increased inflammation, as observed in our mouse models of ischemic heart injury. MMP9 (matrix metalloproteinase 9), both a distal biomarker of inflammation and a biomarker of structural myocardial remodeling in response to injury or stress (25), was increased significantly in the LV of ICM versus NF patients as measured by both quantitative real-time polymerase chain reaction (qPCR) (p < 0.01; Fig. 10A) and Western blot analysis (p < 0.001, Fig. 10E). Similarly, CCL5 and CCL4, measured by qPCR and Bio-Plex multiplex immunoassay, were upregulated at both mRNA (CCL5 p < 0.0001, Fig. 10B; CCL4 p < 0.01, Fig. 10C) and protein levels (CCL5 p < 0.0001, Fig. 10F; CCL4 p < 0.01, Fig. 10G) in the LV of ICM versus NF patients.
FIG. 10.
Markers of structural remodeling and inflammation in left ventricle of human hearts with ICM. ICM hearts (n = 19) were compared with NF hearts (n = 24). Real-time PCR analysis of mRNA expression of (A) MMP9, (B) CCL5, (C) CCL4, and (D) IL16. Data are normalized to 18S RNA expression and relative to NF hearts (mean ± SEM). (E) MMP9 protein levels were measured by Western blots. Inflammatory cytokines (F) CCL5, (G) CCL4, and (H) IL16 protein levels were measured by using Bio-Plex multiplex assay kit. Data are the mean ± SEM. Scatter plots showing correlation between (I, J) NOX4 and CCL5 and (K, L) CCL4 in left ventricular tissue in ICM and NF hearts. The regression lines in the graphs show the 95% confidence intervals (dotted lines), and R2 values are shown for each plot. **p < 0.01, ***p < 0.001, and ****p < 0.0001; ns, not significant. IL16, interleukin 16; MMP9, matrix metalloproteinase 9; PCR, polymerase chain reaction.
Interleukin 16 (IL16) mRNA levels were 25% higher (Fig. 10D), whereas IL16 protein levels were significantly higher (p < 0.001, Fig. 10H) in ICM versus NF patients. Further, CCL5 and CCL4 expression levels were positively correlated with NOX4 protein expression levels in ICM (CCL5 p < 0.0001, R2 = 0.6725; Fig. 10I; CCL4 p < 0.0001, R2 = 0.7673; Fig. 10K), but not in NF heart samples (CCL5 Fig. 10J; CCL4 Fig. 10L). Tumor necrosis factor α (TNFα) protein levels were 75% higher in ICM hearts, but they were not statistically significant (p = 0.1637 vs. NF, Supplementary Fig. S4A); there were no significant differences in interleukin 6 (IL6) (Supplementary Fig. S4B), Monocyte chemoattractant protein 1 (MCP1) (Supplementary Fig. S4C), and interleukin 13 (IL13) (Supplementary Fig. S4D) protein levels between ICM and NF hearts. These results suggest that increased oxidative stress, mediated in part by increased NOX4 expression/activity, induces upregulation of chemoattractant cytokines such as CCL5, CCL4, and IL16, triggering the infiltration of inflammatory immune cells into the ischemic myocardium and exacerbating ischemic injury.
Discussion
Dysregulated ROS generation is implicated in the pathology of ICM. However, the most important sources of ROS in the ischemic myocardium, the mechanisms by which ROS generation results in sustained myocardial dysfunction and whether an opportunity exists for therapeutic intervention, remain unanswered questions. This study was designed to critically examine the mechanisms by which NOX4 NADPH oxidase impacts myocardial cell function in ischemic mouse models and then to determine whether correlatively aligned impact is seen in human hearts.
Our data demonstrate that: (i) cardiac NOX4 expression and activity are enhanced in WT mice after ischemic injury; (ii) genetic deletion of Nox4 in mice attenuates cardiac injury and dysfunction during myocardial ischemia; (iii) myocardial sEH activity, which increased in both WT and Nox4−/−mice under ischemic conditions, is significantly lower in Nox4−/− mice; (iv) deficiency of NOX4 reduced left ventricular remodeling and macrophage infiltration into peri-infarct areas and inhibited CCL4 expression; (v) inducing sEH expression and activity by overexpressing NOX4 augmented cytokine expression in H9c2 cells; catalase and TPPU abrogated the increased sEH activity and cytokine production in NOX4 overexpressing H9c2 cells; (vi) LV tissue of ICM patients had increased NOX4 expression along with evidence of oxidative stress and adverse cardiac remodeling; and (vii) myocardial NOX4 proteins levels were positively correlated with sEH expression, and LV heart tissue had increased MMP9 and inflammatory cytokine expression in ICM patients. Together, these studies indicate that NOX4 is an important profibrotic and proinflammatory mediator in ICM and heart failure.
Our results that increased NOX4 is a major contributor to profibrotic changes in ischemic human myocardium is supported by data from a recent study in which Nox4 is identified as one of the six co-regulated genes in HF pathogenesis (32). Affirming the role of NOX4 in myocardial fibrosis in pathophysiological conditions, Sadoshima and colleagues (34) showed that myocardial Nox4 expression regulated pathological cardiac remodeling in a pressure overload model of mouse heart failure; overexpression of Nox4 in the heart induced fibrosis, apoptosis, and cardiomyocyte hypertrophy whereas cardiac-specific Nox4 knockout mice had attenuated fibrosis, apoptosis, and cardiac hypertrophy.
Similarly, cardiac-specific overexpression of NOX4 increased cardiac interstitial fibrosis (77); angiotensin II (Ang II), which is elevated in ischemic heart failure (69), increased cardiac NOX4 expression and induced fibrosis and hypertrophy (77). Infusion of Ang II to cardiac Nox4 TG mice exacerbated cardiac remodeling, further supporting the role of NOX4 in cardiac remodeling. Interestingly, NOX4 was shown to physically bind with Ang II and Ang II receptor type 1 (AT1) and enhance their binding (59). It is possible that increased NOX4 expression in cardiac fibroblasts also may contribute to myocardial fibrosis under ischemic conditions by stimulating myofibroblast differentiation (18, 34, 59). Since we did not observe cardiac rupture or ventricular aneurysms in Nox4−/− mice after LAD ligation, other oxidative enzymes upregulated in ischemic stress, such as NOX2, may ensure that a threshold of oxidative stress preserves basic fibroblast repair mechanisms.
Inflammation is a major component in the development of heart failure, and inflammatory cytokine levels were positively associated with heart failure severity (9). Our current data showing increased inflammatory cytokine production in ischemic myocardium are consistent with this notion and previous reports of increased systemic and myocardial cytokine levels during chronic ischemia (14, 44). Further, these data extend the role of NOX4 in regulating cytokine and proinflammatory gene expression in the vasculature under pathophysiological conditions reported by us recently to the ischemic heart (39).
Our data showing an increase in CCL5 levels in WT versus Nox4−/− mice 3 days after LAD ligation are supported by the observation of Montecucco et al. (44), who reported increased serum and myocardial CCL5 levels in C57BL/6 mice 3 days after LAD ligation. And neutralizing the cytokine with a monoclonal antibody decreased the infarct size, circulating levels of chemokines, and myocardial MMP9 and collagen content, which correlated with reduced infiltration of neutrophils and macrophages into the infarcted myocardium. These data suggest that protection against ischemic myocardial injury in Nox4−/− mice reported here is likely partly mediated by decreased CCL5 levels (39), as evidenced by reduced macrophage infiltration into ischemic myocardium, and NOX4 may regulate human ischemic myocardial injury by inducing macrophage infiltration and chemokine and proinflammatory gene expression. The ischemic myocardial inflammation may be maintained or exacerbated as upregulation of CCL4 observed in LAD-ligated WT mice and in human ICM promotes continuous recruitment of macrophages and other inflammatory cells (75).
Similarly, IL16 levels were associated with adverse cardiac remodeling as fibrosis and LV stiffening followed by increased macrophage infiltration were reported in transgenic mice with cardiac specific overexpression of IL16 (64). Interestingly, macrophages stimulated with IL16 secrete TGFβ1, a potent inducer of NOX4 (39), suggesting a positive feedback loop among TGFβ1, NOX4, and IL16. Increased systemic and myocardial MMP9 levels in ischemic heart failure patients were previously reported (32, 72) and correlated with adverse LV remodeling, which supports our results of upregulated myocardial MMP9 levels in the heart tissue of ICM patients.
Our data showed NOX4-dependent upregulation of sEH expression/activity in heart failure during ischemic stress; however, increased sEH activity in WT and Nox4−/− hearts 2 weeks post-LAD ligation, along with increased NOX2 expression, suggests that NOX2 might also contribute to ischemic heart failure (28, 38, 49).
Relatively little is known about the mechanisms that control sEH expression and activity (22). Reactive nitrogen species may be involved as it has been recently reported that S-nitrosation of cysteine 141, a conserved cysteine residue in many species, induced sEH activity in H9c2 cells and increased sEH activity was correlated with endogenous sEH nitrosation after ischemia/reperfusion injury in mice (19). Interestingly, NOX4 and inducible nitric oxide synthase were significantly upregulated after hypoxia and ischemia/reperfusion injury (16) and inhibition of iNOS markedly reduced myocardial infarct size in mice after LAD ligation (71). It is noteworthy that cis allelic variants of sEH gene Ephx2 segregated with heart failure, with increased transcript, protein, and enzyme activity (45).
Further supporting the role of sEH in heart failure, increased cardioprotective EET levels in Ephx2 knockout mice protected against myocardial infarction and left ventricular dysfunction after ischemic injury (57). Interestingly, Kompa et al. (33) reported that GSK2188931B, an sEH inhibitor, not only attenuated fibrosis but also reduced macrophage infiltration into the peri-infarct zone in rats after myocardial infarction. Also, as discussed earlier regarding the role of NOX4 in inflammation, sEH inhibitors suppressed proinflammatory gene expression in cardiac fibroblasts after myocardial infarction (36) and in activated human monocytes (33), further illustrating the role of sEH in inflammation.
Similarly, pharmacological and genetic inactivation of sEH yielded less myocardial infarction and adverse cardiac remodeling after ischemic injury, which was attributed to preserved mitochondrial efficiency in the noninfarct region of the LV (2). This is in line with others and our data showing that upregulation of NOX4 increases mitochondrial oxidative stress and mitochondrial dysfunction (34, 70), causing cardiomyocyte death during heart failure (34). Lending further support to the role of NOX4 in mitochondrial bioenergetics and stress response, Thannickal and colleagues (8) reported that inhibition of NOX4 expression/activity by genetic and pharmacological methods stimulates mitochondrial biogenesis. Another mechanism by which increased sEH activity under ischemic conditions might affect mitochondrial function is via inactivation of sarcolemmal ATP-sensitive potassium channels, resulting in Ca2+ overload in cardiomyocytes (4, 53a). In this context, it is worth noting that defects in Ca2+ regulation underlie decreased contractility in heart failure and elevated H2O2 levels from increased NOX4 expression in ICM hearts may cause abnormal activation of RyR2 in cardiomyocytes, resulting in reduced systolic Ca2+ and contraction (6, 41, 62).
However, NOX4 may exert complex effects in the cardiovascular system depending on the cell type-specific expression. Although this article was in preparation, Shah and colleagues reported that mice with cardiomyocyte-targeted Nox4 overexpression had higher survival along with reduced cardiac remodeling and better contractile function after permanent LAD ligation compared with the WT and the beneficial effects were attributed to NOX4-mediated skewing of macrophages to the M2 phenotype, particularly in the nonischemic myocardium (43). This contradiction in data may reflect the effect of global increase in NOX4 expression in WT after MI and the cumulative effect of the contribution of NOX4 in various cell types in myocardial cell homeostasis and repair.
Increased expression of NOX4 and sEH and a positive correlation between their expression in ICM heart samples in this study along with increased activity of sEH in WT versus Nox4−/− mouse hearts after LAD ligation and Nox4-dependent upregulation of sEH expression/activity in H9c2 cells support the notion that NOX4 is upstream of sEH in cell signaling. Increased sEH activity metabolizes EETs, increasing inflammation. In addition, decreased EET levels might activate NF-κB and increase the expression of TGFβ1 (20, 26), both of which are upstream regulators of NOX4, constituting a feedforward cycle involving NOX4, sEH, nuclear factor kappa-ligh-chain-enhancer of activated B cells, and TGFβ1 (Supplementary Fig. S5) during ICM.
The limitations of the study are that human heart tissues obtained from Duke Human Heart Repository (DHHR) had incomplete medical history on cardiac function and comorbidities. Thus, it was not possible to probe the effect of NOX4 expression on cardiac function and rule out the contribution of comorbidities on myocardial infarction and heart failure. The area of the heart sampled relative to the site of myocardial infarction was variable. Thus, the samples may have been subject to inconsistent ischemic stress. Further, utilization of a global Nox4 knockout model does not distinguish the contribution of different myocardial cell types to ischemia-induced cardiac injury.
In summary, the data presented here suggest that NOX4 NADPH oxidase is an important contributor to myocardial damage and dysfunction, oxidative stress, adverse cardiac remodeling, and inflammation under chronic ischemic stress. Targeting NOX4 and sEH expression/activity may be a potential additional therapeutic option for improved cardiac function in ICM and heart failure patients.
Materials and Methods
Nox4−/− mouse model
Nox4 global knockout mice were generated as previously described (15). WT C57BL/6J mice were obtained from Jackson Laboratories. All mice used in in vivo experiments were males and between the ages of 6 and 7 months. All procedures were approved by the Institutional Animal Care and Use Committees (IACUC) at the University of Michigan.
LAD ligation
LAD ligations were performed by the Physiology Phenotyping Core at the University of Michigan. The surgeon was blinded to the genotype of mice during surgery. Myocardial infarction of the lower ventral region of the LV was produced by ligation of the LAD coronary artery. Surgical anesthesia was achieved with inhaled isoflurane. Induction was performed in a 50-mL cylindrical tube with 5% isoflurane in 2 L/min oxygen for 2 minutes 20 seconds, at which time the mouse was in lateral recumbence and the respiration rate was around 50 breaths per minute. The mouse was moved to a nose cone located on a tilt table that continues to provide isoflurane at 5% in oxygen at 2 L/min. The vocal folds were visualized with an otoscope, and a 20G intracatheter was guided into the trachea. The mouse was moved to the surgery table and ventilated at 90 rpm and 20 cmH2O. The mouse was secured in a lateral position with the left thorax facing up. Carprofen at 5 mg/kg was administered subcutaneously. The hair over the left thorax was shaved, and the skin was disinfected. The isoflurane was reduced to 1.75% in oxygen. A 1.5-cm longitudinal incision was made to the left of the sternum with the third and fourth ribs located at about the middle of the incision. The skin was teased away from the muscle for 1 cm around the incision and retracted. The pectoralis and rectus muscle were gently freed of connective tissue and retracted. The intercostal muscles between the third and fourth ribs were bluntly dissected, the pleura cavity was punctured, and the ribs were separated. The pericardium was opened with an attention made to not injure the phrenic nerve. The triangular region between the pulmonary cone and left auricle was observed, and the LAD was visualized. A 7-0 silk suture was passed under the LAD and permanently ligated. For sham operations, the suture was pulled all the way through and removed.
The ribs were re-approximated with two 6-0 silk ties. The inspiratory pressure was increased to 25–30 cmH2O. A conservative amount of tissue adhesive was applied to the intercostal muscles over the sutures. The rectus and pectorals were returned to their original position. The skin incision was stapled with two 9-mm wound clips and glued. The inspiratory pressure was returned to 20 cmH2O. Once voluntary respiration was confirmed, the mouse was removed from the ventilator. The isoflurane was reduced to 0.4% and when gag reflex returned the mouse was extubated and moved to a warm cage with care taken to prevent aspiration of the bedding. Once the mouse was adequately mobile and active, alert and grooming, it was then moved to its home cage for recovery. The mouse was observed daily until the wound clips were removed, usually 7 days after the surgery. The survival rate was >90% for WT and Nox4−/− mice.
Analysis of infarction 1 day after LAD ligation was performed by Evans' Blue perfusion and TTC staining, as previously outlined by others (10, 52). Briefly, mouse hearts were removed and the coronaries were washed with HBSS to remove blood by retrograde perfusion of the aorta. Then, the hearts were perfused with 250 μL 1% Evans blue dye (Cat No. E2129; Sigma, St. Louis, MO), wrapped in cling film, and stored at −20°C for 15 minutes. The semi-frozen heart was sliced into five to six short-axis sections by using a Zivic heart matrix. The slices were exposed to freshly prepared 1% TTC (Cat No. T8877; Sigma) in HBSS for 20 minutes at 37°C and then fixed in 10% neutral buffered formalin for 60 minutes before images were taken with a Canon PowerShot SX40 HS digital camera (Canon, Inc., Ota, Japan).
Mouse hearts were collected 3 days after LAD ligation or sham surgeries were dissected to isolate the peri-infarct region. Protein was isolated for analysis of sEH activity and cytokine production by ELISA. Mouse hearts were collected 2 weeks after LAD or sham surgeries were blotted of excess moisture and weighed. The tibia was collected from each mouse and tissue was dissected away from the bone to determine the tibial length, which was used to control for mouse body size when comparing heart weights. After weighing, hearts were cut into 1-mm slices by using a Zivic heart matrix (Zivic Instruments, Pittsburgh, PA), yielding five to six slices per heart. Heart slices were then embedded into a block of optimal cutting temperature compound (OCT), and sections were cut at a thickness of 5 μm and stored at −80°C. After sectioning, heart tissues from the peri-infarct zone were removed from OCT for downstream analysis of proteins by Western blots or sEH activity assay.
Cardiac function
Induction of anesthesia was performed in an enclosed container filled with 5% isoflurane. After induction, the mice were placed on a warming pad to maintain body temperature. An isoflurane level of 1%–1.5% was supplied via a nose cone to maintain a surgical plane of anesthesia. The hair was removed from the upper abdominal and thoracic area with depilatory cream. Electrocardiogram (ECG) was monitored via noninvasive resting ECG electrodes. Transthoracic echocardiography was performed in the supine or left lateral position. Two-dimensional long-axis and short-axis M-mode echocardiographic images were recorded by using a Visual Sonics' Vevo 2100 high-resolution in vivo micro-imaging system with an MS 550D transducer, which has a center frequency of 40 MHz and a bandwidth of 22–55 MHz. We measured LV ejection fraction, fractional shortening, systolic and diastolic areas, and wall thickness by M-mode from the two-dimensional long axis view at the level of the papillary muscles.
Cell culture
H9C2 immortalized rat cardiomyoblasts were obtained from ATCC (Cat No. CRL-1446; ATCC, Manassas, VA) and cultured in Dulbecco's minimum essential media (DMEM) (Cat No. 30-2002; ATCC) with 10% fetal bovine serum (FBS) (Cat No. 26140-079; ThermoFisher, Pittsburgh, PA) and 2% antibiotic/antimycotic solution (Cat No. 15240-062; ThermoFisher).
Adenoviral preparation and transduction
The Nox4 adenovirus construct (pAd/CMV-hNOX4/V5) was a generous gift from Dr. David J.R. Fulton (Georgia Regents University, Augusta, GA). Adenovirus was generated by using the Invitrogen Virapower system as previously described (17, 60, 67). The virus was amplified in a HEK293T cell line and concentrated by ViraBind™ Adenovirus purification kit (Cell Biolabs, Inc., San Diego, CA). Briefly, adenovirus was transduced into 50% confluent HEK293T cells in a T-75 flask and viral replication proceeded for 48 hours or until most of the cells were rounded but not detached. The cells and the supernatant were harvested, and the virus from cells was released by repeated freeze/thaw cycles. The supernatant thus obtained was loaded on to the equilibrated column. The column was then washed and eluted with the elution buffer. The virus was aliquoted and stored at −80°C until use. PFU (plaque-forming units) were calculated by QuickTiter™ adenovirus titer immunoassay kit (Cell Biolabs, Inc.). Briefly, HEK293 cells were seeded in a six-well plate to about 60% confluence. Various dilutions of viral titer between 10−4 and 10−9 were transduced into the HEK 293 cells. After 48 hours, cells were fixed and stained with anti-Hexon, a protein in the coat of the adenovirus particles, and the positive cells were counted to calculate PFU/mL of the titer. Control adenovirus (Ad5/CMV) was purchased from vector core (University of Michigan).
H9c2 cells were plated to ∼60% confluence in a six-well culture plate. The cells were transduced with 20 μL of 109 PFU/mL (133 MOI) of NOX4 adenovirus or control virus. The addition of an sEH inhibitor N-[1-(1-Oxopropyl)-4-piperidinyl]-N′-[4-(trifluoromethoxy)phenyl]-urea (TPPU, 0.1 μM) was performed 4 hours before NOX4 adenovirus treatment, and DMSO was added in equivalent volume to control and NOX4 adenovirus-treated groups. The addition of polyethylene glycol conjugated catalase (PEG-catalse) (Cat No. C4963, 100 U/mL; Sigma) was added to the appropriate wells along with the virus, and this treatment was continued every 16 hours. After 48 hours, cells were washed and lysed with M-PER buffer (ThermoFisher) in the presence of 1 × HALT protease and phosphatase inhibitor cocktail (ThermoFisher). Cellular lysates were used to measure protein levels. Adenovirus-transduced cells were also used to measure sEH activity and H2O2 levels. Adenoviral transduction and/or the addition of TPPU or catalase had no effect on cell survival in culture.
Human heart samples
All human samples were deidentified, and their use was approved by the University of North Carolina Institutional Review Board. Samples were obtained from the DHHR. ICM (n = 19) samples were from patients with a prior myocardial ischemia event. ICM samples were acquired from the left ventricular free wall of explanted hearts after cardiac transplantation or from heart tissue removed to put in place a left ventricular assist device. The ages of ICM patients ranged from 28 to 73 years, with an average age of 57.1 ± 2.9 years. The NF (n = 24) samples were acquired from organ donors whose hearts were not used for transplant. The NF patients ranged in age from 19 to 67 years, with an average age of 47.3 ± 2.9. All heart samples were immediately frozen in liquid nitrogen and stored at −80°C by the DHHR. Immediately on receipt, the samples were divided into at least three pieces on dry ice to prevent thawing and were used for downstream analysis of protein by Western blots, RNA quantification, and cryosectioning. Samples were embedded in OCT in the orientation necessary to obtain transverse sections of the myocardial tissue. Sections were cut at a thickness of 5 μm and stored at −80°C.
Histology and immunostaining
Slides with tissue samples were fixed with acetone. Fibrosis in human hearts was determined by staining with Verhoeff-Sirius red and measuring the percentage of red staining per given area by using ImageJ software (NIH, Bethesda, MD). Infarction area in mouse hearts was determined by staining with Masson's trichrome. ImageJ was used to determine the midline circumference of each section and the arc of infarcted tissue to determine the total infarction percentage for each heart after LAD ligation. Cardiomyocyte CSA was measured by staining heart sections with WGA (Cat No. RL-1022; Vector Labs, Burlingame, CA), which stains cell membranes. Five areas of each tissue section and five cells per area were chosen at random, and the CSA of each cell was measured by using ImageJ. Capillary density was measured by staining for von Willebrand Factor (Cat No. A-0082; Dako, Carpinteria, CA) with an Alexa Fluor 488 (Cat No. A11070; ThermoFisher) secondary antibody and counting the number of capillaries per given area. Noncellular, fibrotic areas of the slide were excluded from analysis. The TUNEL assay (Cat No. 12156792910; Roche Diagnostics, Indianapolis, IN) was used to determine cell death. Four areas per tissue section were chosen at random, and TUNEL-positive cells were determined by co-staining with DAPI (4′,6-diamidino-2-phenylindole). Image J was used to quantify the total number of cells; 8-OHdG staining was performed with a primary antibody (Cat No. PA1-84172; ThermoFisher) with Alexa Fluor 594 (Cat No. A11072; ThermoFisher) secondary antibody. For nuclear DNA damage, 8-OhdG-positive nuclei were determined by co-staining with DAPI and 8-OhdG-positive nuclei were expressed as a percentage of total nuclei. For mitochondrial DNA damage, localization of mitochondria was determined by ATP5G2 expression and co-staining with 8-OHdG was used to determine the percent of cells with mitochondrial DNA damage compared with the total number of cells. The primary antibody for nitrotryosine (Cat No. AB5411; Millipore, Billerica, MA) was used with Alexa Fluor 594 secondary antibody. Nitrotyrosine intensity and cell count per image was determined by using ImageJ. Mitochondrial superoxide was measured with MitoSOX Red reagent (Cat No. M36008; ThermoFisher), as previously described (70). Briefly, fresh frozen sections without fixation were incubated with 5 μM MitoSOX Red in the presence or absence of 200 U/mL SOD polyethylene glycol (Cat No. S9549; Sigma) in Hank's balanced salt solution containing 1 mM CaCl2 and 1 mM MgSO4 (HBSS/Ca/Mg) at 37°C for 15 minutes. MitoSOX Red fluorescence images were acquired by using a Nikon Microphot-SA microscope using 510-nm excitation/580-nm emission filters at the same photomultiplier tube voltage, exposure, gain, and offset. Images were converted to grayscale, and the integrated density per image area of interest was measured by using ImageJ.
Macrophages were stained by using an Alexa Fluor conjugated anti-CD68 primary antibody (Cat No. bs-1432R-A594; Bioss, Woburn, MA). Cardiomyocytes were identified by using an Alexa Fluor conjugated primary antibody for MYH7 (Cat No. bs-15444R-A488; Bioss). Images of stained tissue sections were acquired by using a Nikon Microphot-SA microscope or a Nikon Eclipse TE2000-E inverted microscope at 2 × and 20 × magnification. Fluorescence or bright-field images were taken at the same exposure, gain, and offset.
Protein analysis
Human and mouse tissue pieces were homogenized in T-PER Tissue Protein Extraction Reagent (Cat No. 78510; ThermoFisher) with 1X HALT protease and phosphatase inhibitors (Cat No. 78440; ThermoFisher) by using a 2-mL glass Dounce tissue grinder (Cat No. K885300-0002; ThermoFisher) for 30–50 strokes until the tissue was thoroughly homogenized. H9C2 cell protein lysates were prepared by using M-PER lysis buffer (Cat No. 78501; ThermoFisher) with 1 × HALT protease and phosphatase inhibitors. After incubation on ice for 30 minutes, the lysates were spun at 16,000 g for 10 minutes at 4°C and the supernatant was kept for protein analysis.
Protein content was measured by using the Pierce BCA protein assay kit (Cat No. 23227; ThermoFisher). Protein was mixed with 4 × Laemmli Sample buffer (Bio-Rad, Hercules, CA) with 15% β-mercaptoethanol (Sigma), and 10-μg protein samples were analyzed by polyacrylamide gel electrophoresis. After electrophoresis, the proteins were transferred onto an Amersham Protran nitrocellulose membrane (GE Healthcare, Pittsburgh, PA) by using a Trans-Blot Turbo Transfer System (Bio-Rad). Blocking buffer (1% BSA) was applied for 1 hour before the addition of primary antibodies for overnight incubation at 4°C. Secondary antibodies included either the Li-Cor IRDyes (Li-Cor, Lincoln, NE), which were imaged by using an Odyssey CLx imager, or HRP secondary antibodies (GE Healthcare), which were developed with Hy-Glo quick spray (Denville Scientific, Holliston, MA) and imaged by using Blue Devil autoradiography film (Cat No. 30-101; Genesee Scientific, San Diego, CA). Li-Cor Westerns were quantified by using Image Studio software (Li-Cor), whereas ECL Westerns were quantified by using Image J (NIH, Bethesda, MD). All unprocessed Western blots are presented in Supplementary Figures S6–S11.
Primary antibodies used in this study include NOX4 (human and mouse, Santa Cruz, Dallas, TX sc-30141, H9C2, Abcam ab109225), NOX1 (bs-3682R; Bioss), NOX2 (611414; BD Biosciences, Franklin Lakes, NJ) sEH (human, Santa Cruz sc-25797, mouse and H9C2, Santa Cruz sc-166961), MMP9 (3852S; Cell Signaling, Danvers, MA), CCL5 (710001; Invitrogen), CCL4 (AF6935; R&D systems, Minneapolis, MN), IL16 (ab180792; Abcam), GAPDH (ab9489; Abcam), and β-tubulin (T8328; Sigma).
Inflammatory cytokines (IL16, CCL5, CCL4, TNFα, IL6, IL13, and MCP1) were measured in human heart protein lysates by using a custom-selected Bio-Plex multiplex immunoassay and measured by using a Bio-Plex 200 reader (Bio-Rad). Mouse inflammatory cytokines CCL4 (Cat No. DY451; R&D systems) and CCL5 (Cat No. DY478-05; R&D systems) were measured by ELISA following the manufacturer's protocol.
RNA analysis by qPCR
Human heart tissue (10–20 mg) was mixed with 350 μL of RLT buffer from the RNeasy Micro kit (Cat No. 74004; Qiagen, Hilden, Germany) and lysed by using the TissueLyser II (Qiagen) set at 25 Hz for 4 minutes. RNA was extracted by using the RNeasy micro kit according to the manufacturer's instructions. The concentration and purity of the RNA was measured by using a Nanodrop 2000 instrument (ThermoFisher). The iScript™ cDNA Synthesis Kit (Cat No. 170-8891; Bio-Rad) was used to make cDNA by using 1 μg RNA. The cDNA was diluted 1:4 with RNase, DNase, and protease-free purified water (Cat No. 46-000-CV; Corning, Tewksbury, MA) before use. Quantification of cDNA was performed with 7500 Fast Real-Time PCR System (ThermoFisher). The qPCR buffer in each well is composed of 10 μL 2 × Universal PCR master mix (ThermoFisher), 5 μL RNase, DNase, protease-free purified water (Corning), 1 μL Taqman primer (ThermoFisher) of the gene being investigated, and 5 μL of the cDNA sample. The following Taqman primers were used in this study: MMP9 (Hs00234579_m1), CCL5 (Hs00982282_m1), CCL4 (Hs04421399_gH), and IL16 (Hs00189606_m1); 18S (Hs99999901_s1) was used for normalization of the data.
sEH activity assay
Activity of sEH enzyme was measured by using the Soluble Epoxide Hydrolase Cell-Based Assay Kit (Cat No. 60090; Cayman Chemical, Ann Arbor, MI). H9C2 cells were washed with assay buffer, scraped in 350 μL Digitonin lysis buffer, and homogenized in a 2-mL glass Dounce tissue grinder for 15 strokes. Mouse heart tissue was homogenized in 250-μL Digitonin lysis buffer in a 2-mL glass Dounce tissue grinder (ThermoFisher) for 30 strokes. The homogenates were centrifuged at 15,000 g for 10 minutes, and the supernatant was used in the sEH activity assay. sEH activity was normalized to protein concentration.
Measurement of H2O2
H9C2 cells were incubated in 24-well plates for 2 days with either control or NOX4 adenovirus. Media were replaced with Amplex Red (ThermoFisher) working solution (50 μM Amplex Red, 0.1 U/mL horseradish peroxidase) and collected after a 30-minute incubation at 37°C. Phenylmethylsulfonyl fluoride (PMSF, Cat No. 10837091001; Sigma) (100 μM) was added to Amplex Red working solution to prevent carboxylesterase activity (42). Fluorescence was measured at 530-nm excitation and 590-nm emission, by using a Spectramax M5 spectrophotometer (Molecular Devices, Sunnyvale, CA). A standard curve using fresh H2O2 was generated for comparison. Cells were then lysed with M-PER with protease inhibitors, and protein content was measured to control for the cellular content of each well.
H2O2 was measured in mouse hearts by using the method previously employed by Frazziano et al. and Kuroda et al. (23, 34). Fresh frozen mouse heart tissue (30–50 mg) was incubated in Amplex Red (ThermoFisher) working solution (100 μM Amplex Red, 1 U/mL horseradish peroxidase) in Krebs-HEPES buffer at 37°C protected from light. The supernatant was collected after 30 minutes and fluorescence was measured at 530-nm excitation and 590-nm emission, by using a Spectramax M5 spectrophotometer (Molecular Devices, Sunnyvale, CA). A standard curve using fresh H2O2 was generated for comparison. The tissue was then dried and weighed, and H2O2 measurements were normalized to dry tissue weight.
Statistical analysis
All analysis was performed by using GraphPad Prism and GraphPad InStat software (Graphpad Software, Inc., La Jolla, CA). Measurements are reported as the average ± SEM. All datasets were tested for normality by using the Kolmogorov–Smirnov test, and datasets were adjusted by using a logarithmic transformation as necessary. A comparison between groups was performed by using unpaired student's t-test or one-way ANOVA followed by a post hoc Fisher's comparison test. A two-way ANOVA followed by a post hoc Fisher's comparison test was used to test for interactions between LAD ligation and mouse genotype. A p value <0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported, in part, by NIH grants, AG024282 and HL111664. The authors also would like to thank Dr. Judith Connett for her help in preparing this article.
Abbreviations Used
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
- Ang II
angiotensin II
- ATP5G2
ATP Synthase Membrane Subunit C Locus 2
- CSA
cross-sectional area
- CCL4
C-C motif chemokine ligand 4 (a.k.a. MIP-1β, macrophage inflammatory protein-1β)
- CCL5
C-C motif chemokine ligand 5 (a.k.a. RANTES, regulated on activation, normal T cell expressed and secreted)
- DAPI
4′,6-diamidino-2-phenylindole, dihydrochloride
- DHHR
Duke Human Heart Repository
- DUOXs
two dual oxidases
- ECG
electrocardiogram
- EET
epoxyeicosatrienoic acids
- ICM
ischemic cardiomyopathy
- IL13
interleukin 13
- IL16
interleukin 16
- IL6
interleukin 6
- LAD
left anterior descending artery
- LV
left ventricle
- MCP1
monocyte chemoattractant protein 1
- MMP9
matrix metalloproteinase 9
- MYH7
myosin heavy chain beta
- NF
nonfailing
- NOX4
NADPH oxidase 4
- OCT
optimal cutting temperature compound
- PEG-catalase
polyethylene glycol conjugated catalase
- PFU
plaque-forming units
- qPCR
quantitative real-time polymerase chain reaction
- ROS
reactive oxygen species
- sEH
soluble epoxide hydrolase
- TNFα
tumor necrosis factor α
- TTC
triphenyltetrazolium chloride
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- WGA
wheat germ agglutinin
- WT
wild-type
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Ago T, Kuroda J, Pain J, Fu C, Li H, and Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106: 1253–1264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akhnokh MK, Yang FH, Samokhvalov V, Jamieson KL, Cho WJ, Wagg C, Takawale A, Wang X, Lopaschuk GD, Hammock BD, Kassiri Z, and Seubert JM. Inhibition of soluble epoxide hydrolase limits mitochondrial damage and preserves function following ischemic injury. Front Pharmacol 7: 133, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, Orfi L, Szantai-Kis C, Keri G, Szabadkai I, Barabutis N, Rafikova O, Rafikov R, Black SM, Jonigk D, Giannis A, Asmis R, Stepp DW, Ramesh G, and Fulton DJ. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol 34: 1704–1715, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Batchu SN, Lee SB, Samokhvalov V, Chaudhary KR, El-Sikhry H, Weldon SM, and Seubert JM. Novel soluble epoxide hydrolase inhibitor protects mitochondrial function following stress. Can J Physiol Pharmacol 90: 811–823, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Belevych AE, Radwański PB, Carnes CA, and Györke S. ‘Ryanopathy’: causes and manifestations of RyR2 dysfunction in heart failure. Cardiovasc Res 98: 240–247, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135: e146–e603, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bernard K, Logsdon NJ, Miguel V, Benavides GA, Zhang J, Carter AB, Darley-Usmar VM, and Thannickal VJ. NADPH Oxidase 4 (Nox4) Suppresses mitochondrial biogenesis and bioenergetics in lung fibroblasts via a nuclear factor erythroid-derived 2-like 2 (Nrf2)-dependent pathway. J Biol Chem 292: 3029–3038, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Biasucci LM, La Rosa G, Pedicino D, D'Aiello A, Galli M, and Liuzzo G. Where does inflammation fit? Curr Cardiol Rep 19: 84, 2017 [DOI] [PubMed] [Google Scholar]
- 10. Bohl S, Medway DJ, Schulz-Menger J, Schneider JE, Neubauer S, and Lygate CA. Refined approach for quantification of in vivo ischemia-reperfusion injury in the mouse heart. Am J Physiol Heart Circ Physiol 297: H2054–H2058, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borchi E, Parri M, Papucci L, Becatti M, Nassi N, Nassi P, and Nediani C. Role of NADPH oxidase in H9c2 cardiac muscle cells exposed to simulated ischaemia-reperfusion. J Cell Mol Med 13: 2724–2735, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brandes RP, Weissmann N, and Schröder K. Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic Biol Med 76: 208–226, 2014 [DOI] [PubMed] [Google Scholar]
- 13. Brewer AC, Murray TV, Arno M, Zhang M, Anilkumar NP, Mann GE, and Shah AM. Nox4 regulates Nrf2 and glutathione redox in cardiomyocytes in vivo. Free Radic Biol Med 51: 205–215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cappuzzello C, Di Vito L, Melchionna R, Melillo G, Silvestri L, Cesareo E, Crea F, Liuzzo G, Facchiano A, Capogrossi MC, and Napolitano M. Increase of plasma IL-9 and decrease of plasma IL-5, IL-7, and IFN-γ in patients with chronic heart failure. J Transl Med 9: 28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, Guichard C, Arbiser JL, Banfi B, Pache JC, Barazzone-Argiroffo C, and Krause KH. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal 15: 607–619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coucha M, Abdelsaid M, Li W, Johnson MH, Orfi L, El-Remessy AB, Fagan SC, and Ergul A. Nox4 contributes to the hypoxia-mediated regulation of actin cytoskeleton in cerebrovascular smooth muscle. Life Sci 163: 46–54, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crosswhite P. and Sun Z. Molecular mechanisms of pulmonary arterial remodeling. Mol Med 20: 191–201, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, and Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97: 900–907, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Ding Y, Li Y, Zhang X, He J, Lu D, Fang X, Wang Y, Wang J, Zhang Y, Qiao X, Gan LM, Chen C, and Zhu Y. Soluble epoxide hydrolase activation by S-nitrosation contributes to cardiac ischemia-reperfusion injury. J Mol Cell Cardiol 110: 70–79, 2017 [DOI] [PubMed] [Google Scholar]
- 20. Dong L, Zhou Y, Zhu ZQ, Liu T, Duan JX, Zhang J, Li P, Hammcok BD, and Guan CX. Soluble epoxide hydrolase inhibitor suppresses the expression of triggering receptor expressed on myeloid cells-1 by inhibiting NF-kB activation in murine macrophage. Inflammation 40: 13–20, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fang X, Weintraub NL, Oltman CL, Stoll LL, Kaduce TL, Harmon S, Dellsperger KC, Morisseau C, Hammock BD, and Spector AA. Human coronary endothelial cells convert 14,15-EET to a biologically active chain-shortened epoxide. Am J Physiol Heart Circ Physiol 283: H2306–H2314, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Fleming I. The pharmacology of the cytochrome P450 epoxygenase/soluble epoxide hydrolase axis in the vasculature and cardiovascular disease. Pharmacol Rev 66: 1106–1140, 2014 [DOI] [PubMed] [Google Scholar]
- 23. Frazziano G, Al Ghouleh I, Baust J, Shiva S, Champion HC, and Pagano PJ. Nox-derived ROS are acutely activated in pressure overload pulmonary hypertension: indications for a seminal role for mitochondrial Nox4. Am J Physiol Heart Circ Physiol 306: H197–H205, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115: 500–508, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halade GV, Jin YF, and Lindsey ML. Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther 139: 32–40, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He Z, Zhang X, Chen C, Wen Z, Hoopes SL, Zeldin DC, and Wang DW. Cardiomyocyte-specific expression of CYP2J2 prevents development of cardiac remodelling induced by angiotensin II. Cardiovasc Res 105: 304–317, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, and Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res 69: 1476–1486, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, and Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 41: 2164–2171, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Hu P, Wu X, Khandelwal AR, Yu W, Xu Z, Chen L, Yang J, Weisbrod RM, Lee KSS, Seta F, Hammock BD, Cohen RA, Zeng C, and Tong X. Endothelial Nox4-based NADPH oxidase regulates atherosclerosis via soluble epoxide hydrolase. Biochim Biophys Acta 1863: 1382–1391, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung HJ, Rong Y, and Galis ZS. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation 109: 520–525, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, Utsumi H, and Takeshita A. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ Res 87: 392–398, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Koentges C, Pepin ME, Müsse C, Pfeil K, Alvarez SVV, Hoppe N, Hoffmann MM, Odening KE, Sossalla S, Zirlik A, Hein L, Bode C, Wende AR, and Bugger H. Gene expression analysis to identify mechanisms underlying heart failure susceptibility in mice and humans. Basic Res Cardiol 113: 8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kompa AR, Wang BH, Xu G, Zhang Y, Ho PY, Eisennagel S, Thalji RK, Marino JP, Jr, Kelly DJ, Behm DJ, and Krum H. Soluble epoxide hydrolase inhibition exerts beneficial anti-remodeling actions post-myocardial infarction. Int J Cardiol 167: 210–219, 2013 [DOI] [PubMed] [Google Scholar]
- 34. Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, and Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A 107: 15565–15570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. L'Ecuyer T, Horenstein MS, Thomas R, and Vander Heide R. Anthracycline-induced cardiac injury using a cardiac cell line: potential for gene therapy studies. Mol Genet Metab 74: 370–379, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Li N, Liu JY, Timofeyev V, Qiu H, Hwang SH, Tuteja D, Lu L, Yang J, Mochida H, Low R, Hammock BD, and Chiamvimonvat N. Beneficial effects of soluble epoxide hydrolase inhibitors in myocardial infarction model: insight gained using metabolomic approaches. J Mol Cell Cardiol 47: 835–845, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lockwood TD. Redox control of protein degradation. Antioxid Redox Signal 2: 851–878, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, and Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension 51: 319–325, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Lozhkin A, Vendrov AE, Pan H, Wickline SA, Madamanchi NR, and Runge MS. NADPH oxidase 4 regulates vascular inflammation in aging and atherosclerosis. J Mol Cell Cardiol 102: 10–21, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, and Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, and Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Miwa S, Treumann A, Bell A, Vistoli G, Nelson G, Hay S, and von Zglinicki T. Carboxylesterase converts Amplex red to resorufin: implications for mitochondrial H2O2 release assays. Free Radic Biol Med 90: 173–183, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mongue-Din H, Patel AS, Looi YH, Grieve DJ, Anilkumar N, Sirker A, Dong X, Brewer AC, Zhang M, Smith A, and Shah AM. NADPH oxidase-4 driven cardiac macrophage polarization protects against myocardial infarction-induced remodeling. JACC Basic Transl Sci 2: 688–698, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Montecucco F, Braunersreuther V, Lenglet S, Delattre BM, Pelli G, Buatois V, Guilhot F, Galan K, Vuilleumier N, Ferlin W, Fischer N, Vallee JP, Kosco-Vilbois M, and Mach F. CC chemokine CCL5 plays a central role impacting infarct size and post-infarction heart failure in mice. Eur Heart J 33: 1964–1974, 2012 [DOI] [PubMed] [Google Scholar]
- 45. Monti J, Fischer J, Paskas S, Heinig M, Schulz H, Gösele C, Heuser A, Fischer R, Schmidt C, Schirdewan A, Gross V, Hummel O, Maatz H, Patone G, Saar K, Vingron M, Weldon SM, Lindpaintner K, Hammock BD, Rohde K, Dietz R, Cook SA, Schunck WH, Luft FC, and Hubner N. Soluble epoxide hydrolase is a susceptibility factor for heart failure in a rat model of human disease. Nat Genet 40: 529–537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Münzel T, Gori T, Keaney JF, Jr, Maack C, and Daiber A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur Heart J 36: 2555–2564, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, and Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285: 1276–1279, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nossuli TO, Frangogiannis NG, Knuefermann P, Lakshminarayanan V, Dewald O, Evans AJ, Peschon J, Mann DL, Michael LH, and Entman ML. Brief murine myocardial I/R induces chemokines in a TNF-alpha-independent manner: role of oxygen radicals. Am J Physiol Heart Circ Physiol 281: H2549–H2558, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Parajuli N, Patel VB, Wang W, Basu R, and Oudit GY. Loss of NOX2 (gp91phox) prevents oxidative stress and progression to advanced heart failure. Clin Sci (Lond) 127: 331–340, 2014 [DOI] [PubMed] [Google Scholar]
- 50. Parthasarathy S, Raghavamenon A, Garelnabi MO, and Santanam N. Oxidized low-density lipoprotein. Methods Mol Biol 610: 403–417, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, and Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest 98: 2572–2579, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Redfors B, Shao Y, and Omerovic E. Myocardial infarct size and area at risk assessment in mice. Exp Clin Cardiol 17: 268–272, 2012 [PMC free article] [PubMed] [Google Scholar]
- 53. Sabri A, Hughie HH, and Lucchesi PA. Regulation of hypertrophic and apoptotic signaling pathways by reactive oxygen species in cardiac myocytes. Antioxid Redox Signal 5: 731–740, 2003 [DOI] [PubMed] [Google Scholar]
- 53. a.Samokhvalov V, Alsaleh N, El-Sikhry HE, Jamieson KL, Chen CB, Lopaschuk DG, Carter C, Light PE, Manne R, Falck JR, and Seubert JM. Epoxyeicosatrienoic acids protect cardiac cells during starvation by modulating an autophagic response. Cell Death Dis 4: e885, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sawyer DB, Siwik DA, Xiao L, Pimentel DR, Singh K, and Colucci WS. Role of oxidative stress in myocardial hypertrophy and failure. J Mol Cell Cardiol 34: 379–388, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Fórró L, Schlegel W, and Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seta Y, Shan K, Bozkurt B, Oral H, and Mann DL. Basic mechanisms in heart failure: the cytokine hypothesis. J Card Fail 2: 243–249, 1996 [DOI] [PubMed] [Google Scholar]
- 57. Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, Luria A, Newman JW, Hammock BD, Falck JR, Roberts H, Rockman HA, Murphy E, and Zeldin DC. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res 99: 442–450, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. This reference has been deleted
- 59. Somanna NK, Valente AJ, Krenz M, Fay WP, Delafontaine P, and Chandrasekar B. The Nox1/4 Dual inhibitor GKT137831 or Nox4 knockdown inhibits angiotensin-II-induced adult mouse cardiac fibroblast proliferation and migration. AT1 physically associates with Nox4. J Cell Physiol 231: 1130–1141, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stenmark KR, Fagan KA, and Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki N, and Takeshita A. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 107: 1418–1423, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Sun QA, Hess DT, Nogueira L, Yong S, Bowles DE, Eu J, Laurita KR, Meissner G, and Stamler JS. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc Natl Acad Sci U S A 108: 16098–16103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takac I, Schröder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, and Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286: 13304–13313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tamaki S, Mano T, Sakata Y, Ohtani T, Takeda Y, Kamimura D, Omori Y, Tsukamoto Y, Ikeya Y, Kawai M, Kumanogoh A, Hagihara K, Ishii R, Higashimori M, Kaneko M, Hasuwa H, Miwa T, Yamamoto K, and Komuro I. Interleukin-16 promotes cardiac fibrosis and myocardial stiffening in heart failure with preserved ejection fraction. PLoS One 8: e68893, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thollon C, Iliou JP, Cambarrat C, Robin F, and Vilaine JP. Nature of the cardiomyocyte injury induced by lipid hydroperoxides. Cardiovasc Res 30: 648–655, 1995 [PubMed] [Google Scholar]
- 66. Tong X, Khandelwal AR, Wu X, Xu Z, Yu W, Chen C, Zhao W, Yang J, Qin Z, Weisbrod RM, Seta F, Ago T, Lee KS, Hammock BD, Sadoshima J, Cohen RA, and Zeng C. Pro-atherogenic role of smooth muscle Nox4-based NADPH oxidase. J Mol Cell Cardiol 92: 30–40, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tuder RM, Stacher E, Robinson J, Kumar R, and Graham BB. Pathology of pulmonary hypertension. Clin Chest Med 34: 639–650, 2013 [DOI] [PubMed] [Google Scholar]
- 68. Valavanidis A, Vlachogianni T, and Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 27: 120–139, 2009 [DOI] [PubMed] [Google Scholar]
- 69. van de Wal RM, Plokker HW, Lok DJ, Boomsma F, van der Horst FA, van Veldhuisen DJ, van Gilst WH, and Voors AA. Determinants of increased angiotensin II levels in severe chronic heart failure patients despite ACE inhibition. Int J Cardiol 106: 367–372, 2006 [DOI] [PubMed] [Google Scholar]
- 70. Vendrov AE, Vendrov KC, Smith A, Yuan J, Sumida A, Robidoux J, Runge MS, and Madamanchi NR. NOX4 NADPH Oxidase-dependent mitochondrial oxidative stress in aging-associated cardiovascular disease. Antioxid Redox Signal 23: 1389–1409, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang D, Yang XP, Liu YH, Carretero OA, and LaPointe MC. Reduction of myocardial infarct size by inhibition of inducible nitric oxide synthase. Am J Hypertens 12: 174–182, 1999 [DOI] [PubMed] [Google Scholar]
- 72. Yan AT, Yan RT, Spinale FG, Afzal R, Gunasinghe HR, Arnold M, Demers C, McKelvie RS, and Liu PP. Plasma matrix metalloproteinase-9 level is correlated with left ventricular volumes and ejection fraction in patients with heart failure. J Card Fail 12: 514–519, 2006 [DOI] [PubMed] [Google Scholar]
- 73. Yang J, Bratt J, Franzi L, Liu JY, Zhang G, Zeki AA, Vogel CF, Williams K, Dong H, Lin Y, Hwang SH, Kenyon NJ, and Hammock BD. Soluble epoxide hydrolase inhibitor attenuates inflammation and airway hyperresponsiveness in mice. Am J Respir Cell Mol Biol 52: 46–55, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang M, Brewer AC, Schröder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, and Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A 107: 18121–18126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang T, Guo CJ, Li Y, Douglas SD, Qi XX, Song L, and Ho WZ. Interleukin-1beta induces macrophage inflammatory protein-1beta expression in human hepatocytes. Cell Immunol 226: 45–53, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang W, Yang AL, Liao J, Li H, Dong H, Chung YT, Bai H, Matkowskyj KA, Hammock BD, and Yang GY. Soluble epoxide hydrolase gene deficiency or inhibition attenuates chronic active inflammatory bowel disease in IL-10(−/−) mice. Dig Dis Sci 57: 2580–2591, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhao QD, Viswanadhapalli S, Williams P, Shi Q, Tan C, Yi X, Bhandari B, and Abboud HE. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFkappa B signaling pathways. Circulation 131: 643–655, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhu XY, Rodriguez-Porcel M, Bentley MD, Chade AR, Sica V, Napoli C, Caplice N, Ritman EL, Lerman A, and Lerman LO. Antioxidant intervention attenuates myocardial neovascularization in hypercholesterolemia. Circulation 109: 2109–2115, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.