Key Points
Question
Is the endocannabinoid system abnormal in people with psychosis?
Findings
In this systematic review and meta-analysis of 18 studies, a higher tone of the endocannabinoid system was observed in people with psychosis, a finding that was consistent across all stages of illness and independent of antipsychotic treatment and current cannabis use. This increased tone was inversely associated with the severity of psychosis symptoms and was normalized after remission of psychosis.
Meaning
The endocannabinoid system appears abnormal in people with psychotic symptoms and provides a useful target for illness measurement and treatment.
Abstract
Importance
The endocannabinoid system (ECS) is a lipid-based endogenous signaling system. Its relevance to psychosis is through the association between cannabis use and the onset and course of illness and through the antipsychotic properties of cannabidiol, a potential ECS enhancer.
Objective
To conduct a systematic review and meta-analysis of the blood and cerebrospinal fluid (CSF) measures of the ECS in psychotic disorders.
Data Sources
Web of Science and PubMed were searched from inception through June 13, 2018. The articles identified were reviewed, as were citations to previous publications and the reference lists of retrieved articles.
Study Selection
Original articles were included that reported blood or CSF measures of ECS activity in patients with psychotic illnesses and in healthy controls.
Data Extraction and Synthesis
PRISMA guidelines, independent extraction by multiple observers, and random-effects meta-analysis were used. Heterogeneity was assessed with the I2 index. Sensitivity analyses tested the robustness of the results.
Main Outcomes and Measures
The clinical relevance of ECS modifications in psychotic disorders was investigated by (1) a quantitative synthesis of the differences in blood and CSF markers of the ECS between patients and healthy controls, and (2) a qualitative synthesis of the association of these markers with symptom severity, stage of illness, and response to treatment.
Results
A total of 18 studies were included. Three individual meta-analyses were performed to identify the differences in ECS markers between people with schizophrenia and healthy controls. Five studies, including 226 patients and 385 controls, reported significantly higher concentrations of anandamide in the CSF of patients than controls (standardized mean difference [SMD], 0.97; 95% CI, 0.67-1.26; P < .001; I2 = 54.8%). In 9 studies, with 344 patients and 411 controls, significantly higher anandamide levels in blood were found in patients, compared with controls (SMD, 0.55; 95% CI, 0.05-1.04; P = .03; I2 = 89.6%). In 3 studies, involving 88 patients and 179 controls, a significantly higher expression of type 1 cannabinoid receptors on peripheral immune cells was reported in patients compared with controls (SMD, 0.57; 95% CI, 0.31-0.84; P < .001; I2 = 0%). Higher ECS tone was found at an early stage of illness in individuals who were antipsychotic naïve or free, and it had an inverse association with symptom severity and was normalized after successful treatment. Moderate to high level of heterogeneity in methods was found between studies.
Conclusions and Relevance
Testing clinically relevant markers of the ECS in the blood and CSF of people with psychotic illness appears possible, and these markers provide useful biomarkers for the psychotic disorder; however, not all studies accounted for important variables, such as cannabis use.
Trial Registration
PROSPERO identifier: CRD42018099863
This systematic review and meta-analysis examines articles indexed in Web of Science and PubMed to understand the advantages of identifying blood and cerebrospinal fluid markers in the endocannabinoid system in the diagnosis and prognosis of psychotic disorder.
Introduction
The endocannabinoid system (ECS) is an endogenous biological signaling system that encompasses lipid-based mediators, their synthesizing and degrading enzymes, and 2 main receptors (Figure 1).1,2 The ECS regulates a series of physiological functions throughout the body, including cognition,3 sleep,4 energy metabolism,5 and inflammation.6 In the brain, the ECS modulates different neurotransmitter systems, including dopamine,7 glutamate,8 and γ-aminobutyric acid.1 The ECS mainly uses 2 major lipid-based endogenous mediators, anandamide and arachidonoyl-sn-glycerol, that act through type 1 and type 2 cannabinoid (CB1 and CB2)2 receptors (Figure 1). The main endocannabinoid-synthesizing enzymes are the N-acyl phosphatidylethanolamine phospholipase D and the diacylglycerol lipase.2 Catabolism is primarily regulated by the fatty acid amide hydrolase and the monoacylglycerol lipase.2
Figure 1. The Endocannabinoid System.
A, Type 1 cannabinoid (CB1) receptors are mainly localized on the neuronal presynaptic terminal, possibly mediating retrograde inhibitory feedback and neurotransmitter release. B, CB1 receptors are also localized on the soma of cholecystokinin (CCK)-positive interneurons, contributing to the synchronization of pyramidal cells cortical firing, which is involved in the genesis of γ oscillations. C, Given their high expression on immune cells, type 2 cannabinoid (CB2) receptors are mainly associated with the peripheral functions of the endocannabinoid system, particularly the regulation of immune processes and vascular permeability. However, CB2 receptors are expressed in the human brain, not only on immunomodulatory components (microglia and blood vessels) but also on neural tissues in which they may regulate dopamine-enriched areas. AEA indicates anandamide; 2-AG, 2-arachidonoylglicerol; DGL, diacylglycerol lipase; FAAH, fatty acid amide hydrolase; MGL, monoacylglycerol lipase; NAPE-PLD, N-acyl-phosphatidylethanolamine phospholipase D; PV, parvalbumin.
The endogenous activity of the ECS is affected by exogenous cannabinoids, such as THC (delta-9-tetrahydrocannabinol), the main psychoactive components of cannabis, and cannabidiol (CBD).3,9,10,11 Impairment of the ECS after cannabis consumption has been associated with increased risk of psychotic illness.12,13,14,15 By contrast, enhancement of the ECS with CBD has shown anti-inflammatory and antipsychotic outcomes in both healthy study participants and in preliminary clinical trials on people with psychotic illness or at high risk of developing psychosis.11,16,17,18 Furthermore, the ECS modulates the expression of N-methyl-D-aspartate receptors, which play a key role in the onset of psychotic illnesses.19,20,21,22 For these reasons, the ECS may be an important system for understanding the factors associated with and effective treatments for psychosis. However, to date it has not been established whether measuring clinically meaningful ECS activity in the blood or cerebrospinal fluid (CSF) is possible.23,24,25 We explored this question with a systematic review and meta-analysis of the available evidence on blood and CSF markers of the ECS in psychotic illnesses.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline26 (eFigure in the Supplement). The protocol was registered in PROSPERO (eMethods 1-3 in the Supplement).
Search for published studies was conducted from inception to June 13, 2018, in Web of Science and PubMed. Titles and abstracts of retrieved publications were imported into Mendeley (https://www.mendeley.com). All studies that did not meet the inclusion criteria were excluded, with the reasons for exclusion documented in eTable 1 in the Supplement. The entire search process was conducted independently by 2 of us (M.S. and S.Z.), and disagreements at the final stage were resolved by consensus. One of us (A.M.) extracted data from all included studies into an electronic summary table, which was checked by another one of us (N.B.).
We used the Newcastle-Ottawa Scale that has been adapted for cross-sectional studies (eTable 2 in the Supplement) to assess the quality of included studies. Psychotic illnesses included schizophrenia, schizoaffective disorders, first-episode psychosis, and prodromal stage.
Outcomes and Meta-analyses
We investigated the clinical relevance of ECS modifications in psychosis by (1) comparing the differences in blood and CSF markers of the ECS between patients and healthy controls (primary outcome), (2) reporting data on the correlation analysis between severity of positive and negative symptoms and blood and CSF markers in patients, (3) reporting data on the stage-related modifications of blood and CSF markers in patients and healthy controls, and (4) reporting data on the association between the modifications of blood and CSF markers and the response to treatment (secondary outcomes).
A quantitative synthesis of the differences in ECS markers between healthy controls and patients was provided when 2 or more studies were available. Medians, SEs, and interquartile ranges were transformed to means and SDs after a validated procedure.27 When needed, data were obtained from graphs using a web-based validated tool (WebPlotDigitizer; Automeris).28 All meta-analyses were conducted in Stata, version 13.0 (StataCorp LLC). The standardized mean difference (SMD) was used as summary statistics. Between-study heterogeneity was assessed by calculating Higgins I2 on the basis of Cochrane Q indexes. Because meta-analyses of observational studies are supposed to be characterized by substantial heterogeneity, we used random-effects models.29 To assess the robustness of the results, we performed sensitivity analyses by sequentially removing single studies and rerunning the analysis.30 When feasible, we performed additional descriptive analyses to explore the putative association of a priori–identified clinical subgroups with findings.
Clinical subgroups were identified on the following basis: (1) illness stage, with 3 subgroups: prodromal, first-episode, and multiepisode; (2) illness phase, with 2 subgroups: acutely ill (ie, inpatients or outpatients clearly defined as acutely ill) and clinically stable (ie, outpatients not defined as acutely ill); (3) antipsychotic medication use, with 2 subgroups: antipsychotic-free or antipsychotic-naïve and antipsychotic-treated; and (4) cannabis use, with 2 subgroups: current user (ie, patients included in studies in which current cannabis use was not an exclusion criterion) and not current user (ie, patients with past use or no previous use).
Results
Eighteen studies11,24,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46 met the inclusion criteria (Table and eFigure in the Supplement). These articles used different methodologies to investigate the ECS. Studies on CSF markers consistently reported data on anandamide levels only. Studies on blood markers reported information on either blood levels of anandamide or expression (messenger RNA/protein) of ECS receptors and enzymes on peripheral immune cells.
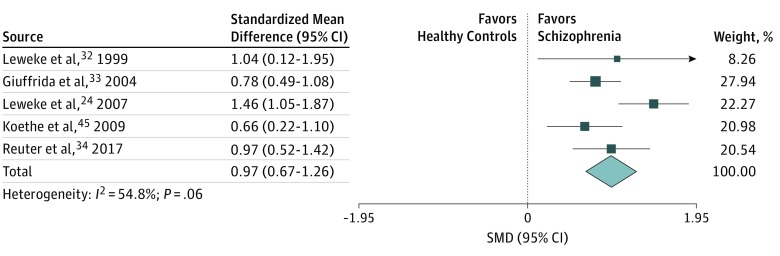
Five studies24,32,33,34,47 reported data on levels of anandamide in the CSF, including 226 patients and 385 controls. Across these 5 studies,24,32,33,34,47 significantly higher concentrations of anandamide were found in the CSF of patients compared with healthy controls (SMD, 0.97; 95% CI, 0.67-1.26; P < .001), with evidence of moderate heterogeneity (I2 = 54.8%) (Figure 2). This heterogeneity was explained by excluding 1 outlying study,24 with results remaining statistically significant (SMD, 0.81; 95% CI, 0.60-1.02; P < .001; I2 = 0%). Increased CSF anandamide levels were found at any stage of psychosis (prodromal, first-episode, and multiepisode) (eResults 1 in the Supplement) in individuals who were antipsychotic-free and antipsychotic-naive and current users or nonusers of cannabis.
Figure 2. Forest Plot of Standardized Mean Difference (SMD) in Cerebrospinal Fluid Anandamide Levels in Patients and Healthy Controls.
Weights obtained from random-effects analysis. Horizontal lines represent 95% CIs. The arrow indicates that the upper limit of the CI for that study is equal or superior to the upper limit of the SMD indicated at the bottom of the graph. The diamond shows the overall pooled SMD. The vertical line is the line of no effect, representing no difference between healthy controls and schizophrenia.
None of the studies into CSF anandamide levels in psychosis reported data on clinically stable patients. Thus, describing data on the basis of illness phase was not possible.
Nine studies24,33,34,35,36,37,38,39,47 reported data on levels of anandamide in the blood, including 344 patients and 411 healthy controls. One study35 was excluded from the quantitative synthesis because it analyzed anandamide levels in whole blood, a method believed to produce heterogeneous results, as compared with plasma and serum extraction.24,33 Across the remaining 8 studies, significantly higher concentrations of anandamide were found in the blood of patients compared with controls (SMD, 0.55; 95% CI, 0.05-1.04; P = .03), with evidence of substantial heterogeneity (I2 = 89.6%) (Figure 3). Sequentially removing single studies consistently reduced heterogeneity, with the results remaining statistically significant (SMD, 0.33; 95% CI, 0.11-0.55; P = .003; I2 = 31.1%). This result is in line with findings in the only study excluded from the quantitative synthesis, which showed a substantial increase in whole-blood anandamide levels of patients with acute psychosis compared with controls.35 The overall pooled estimate noted the increased anandamide levels in blood of patients compared with controls, but when the studies were divided according to the a priori–identified clinical subgroups, none of the subgroups substantially differed from the controls. However, larger SMDs were found when individuals with multiepisode and acute illness were compared with controls (eResults 2 in the Supplement), suggesting that the overall findings might have been driven by these 2 clinical subgroups. Anandamide levels in the CSF and blood did not correlate in any of the studies that reported data on both body compartments.24,33,34,47
Figure 3. Forest Plot of Standardized Mean Difference (SMD) in Blood Anandamide Levels in Patients and Healthy Controls.
Weights obtained from random-effects analysis. Horizontal lines represent 95% CIs. The arrow indicates that the upper limit of the CI for that study is equal or superior to the upper limit of the SMD indicated at the bottom of the graph. The diamond shows the overall pooled SMD. The vertical line is the line of no effect, representing no difference between healthy controls and schizophrenia.
Five studies reported data on CB1 receptor expression on peripheral immune cells and whole blood of patients compared with healthy controls.40,41,42,43,44 Owing to the lack of extractable data, a meta-analytic synthesis was performed by including only 3 of these 5 studies.41,43,44 Across the 3 studies, involving 88 patients and 179 controls, a significantly higher expression of CB1 receptors was observed in patients compared with healthy controls (SMD, 0.57; 95% CI, 0.31-0.84; P < .001; I2 = 0%) (Figure 4). These 3 studies were all conducted on patients with multiepisode psychosis and included mixed samples of patients who were medicated or unmedicated, clinically stable or acutely ill, and current or not-current cannabis users. The 2 studies not included in the quantitative synthesis reported mixed findings: one showed increased CB142 expression in patients compared with controls, and the other reported no differences.40
Figure 4. Forest Plot of Standardized Mean Difference (SMD) in Type 1 Cannabinoid Receptor Expression in Blood of Patients and Healthy Controls.
Weights obtained from random-effects analysis. Horizontal lines represent 95% CIs. The arrow indicates that the upper limit of the CI for that study is equal or superior to the upper limit of the SMD indicated at the bottom of the graph. The diamond shows the overall pooled SMD. The vertical line is the line of no effect, representing no difference between healthy controls and schizophrenia.
Owing to the lack of extractable data, performing a quantitative synthesis on CB2 receptor and ECS expression levels in patients compared with controls was not possible. Five studies reported data on CB2 receptor expression on peripheral immune cells of patients compared with healthy controls,31,40,41,42,43 with mixed findings: 2 studies indicated increased expression in patients,41,42 and 3 described no differences31,42,43 (Table). Three studies reported data on ECS-degrading enzymes on peripheral immune cells of patients compared with controls: 2 studies31,35 reported increased expression of degrading enzymes, whereas 1 study indicated no differences.43 Two studies31,43 reported data on ECS-synthesizing enzymes on peripheral immune cells of patients compared with controls. One described reduced expression in patients,31 and one described no differences.43
Table. Main Characteristics and Summary of Findings in Schizophrenia.
| Source | Participants | % Female | Tissue of the ECS | AP Medication Use | Illness Stage | Illness Phase | Cannabis Use | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Leweke et al,32 1999 | n = 10 with SCZ; n = 11 HC | SCZ: 30.1%; HC: not reported | CSF | n = 3 taking AP; n = 7 AP free | SCZ | Acutely ill, defined as “BPRS total scores 49-73” | (1) Participants with comorbid substance abuse or dependence excluded; (2) n = 3 with SCZ reported intermittent use of cannabis; (3) No correlation between cannabis use and AEA levels | (1) ↑ AEA in CSF of participants with SCZ vs HC |
| De Marchi et al,35 2003 | n = 12 with SCZ; n = 20 HC | SCZ: 25.0%; HC: not reported | Whole blood; PBMC | n = 12 AP free (baseline); n = 5 taking olanzapine (participants in remission [at least 50% pretreatment to posttreatment reduction in BPRS score]) | SCZ | Acutely ill, defined as “acute phase of their schizophrenic illness” | (1) Participants reporting cannabis use in the month preceding the study excluded | (1) ↑ AEA in blood of participants with SCZ vs HC; (2) ↑ FAAH in PBMC of participants with SCZ vs HC; (3) Remission (at least 50% pretreatment to posttreatment reduction in BPRS score) of psychotic symptoms associated with ↓ AEA in whole blood; ↓ CB2 and ↓ FAAH in PBMC |
| Giuffrida et al,33 2004 | n = 47 with FEP; n = 71 with SCZ; n = 84 HC | FEP: 27.7%; SCZ: 19.7%; HC: 62.4% | Serum; CSF | Among participants with FEP or SCZ: n = 47 AP free; n = 37 taking typical AP; n = 35 taking atypical AP | FEP or SCZ | Acutely ill inpatients | (1) Participants positive to urine drug screen (including cannabis) excluded; (2) Measured self-reported lifetime cannabis use; (3) No association between cannabis use and primary end points of the study | (1) ↑ AEA in CSF of AP-naïve participants with FEP vs HC; (2) ↑ AEA in CSF of participants with SCZ taking atypical AP vs HC; (3) = AEA in CSF of participants with SCZ taking typical AP vs HC; (4) = AEA in serum of participants with FEP/SCZ vs HC; (5) Inverse correlation between levels of AEA in CSF and negative symptoms in participants with FEP or SCZ |
| Leweke et al,24 2007 | n = 44 with FEP; n = 81 HC | FEP: 39.1%; HC: 44.4% | Serum; CSF | All participants with FEP were AP naive | FEP | Acutely ill inpatients | (1) Participants divided in groups based on lifetime cannabis use: HF-FEP = 19; LF-FEP = 25; LF-HC: 55; HF-HC: 26 (HF: lifetime cannabis use more than 20 lifetimes; LF: lifetime cannabis use ≤5 lifetimes); (2) Participants with a comorbid substance abuse or dependence excluded; (3) No association between cannabis use and primary end points of the study | (1) ↑ AEA in CSF of participants with LF-FEP vs HC; (2) AEA in CSF of participants with HF-FEP vs HC; (3) Inverse correlation between levels of AEA in CSF and negative symptoms in participants with HF-FEP and LF-FEP; (4) Inverse correlation between levels of AEA in CSF and positive symptoms in participants with LF-FEP; (5) = AEA in serum of participants with FEP vs HC |
| Potvin et al,36 2008 | n = 29 with SCZ+SUD; n = 17 HC | SCZ-SUD: 24.0%; HC: 16.0% | Plasma | Mixed typical and atypical APs | SCZ | Clinically stable, defined as “not acutely ill or not hospitalized” | (1) All participants had a comorbid SUD; n = 18/29 with cannabis SUD | (1) ↑ AEA in plasma in participants with SCZ+SUD vs HC |
| Koethe et al,45 2009 | n = 27 with prodromal SCZ; n = 81 HC | Prodromal SCZ: 30.2%; HC: 44.3% | Serum; CSF | AP free | Prodromal SCZ | Not applicable | (1) No cannabis use within 6 wk prior to the study; (2) Participants positive to urine drug screen excluded; (3) Lifetime cannabis use considered; HF-prodromal = 16; LF-prodromal = 11 (HF: lifetime cannabis use more than 20 lifetimes; LF: lifetime cannabis use ≤5 lifetimes); (4) No association between cannabis use and primary end points of the study | (1) ↑ AEA in CSF of participants with prodromal SCZ vs HC; (2) = AEA in serum of participants with prodromal SCZ vs HC; (3) Participants with prodromal SCZ with high values of AEA in CSF (split on median value) had delayed psychotic onset; (4) In participants with prodromal SCZ, inverse correlation between levels of AEA in CSF and “cognitive factor” as measured by PANSS |
| Desfossés et al,37 2012 | n = 23 with SCZ; n = 38 with SUD; n = 27 HC | SCZ: 32.0%; SUD: 34.2%; HC: 40.7% | Plasma | Among participants with FEP or SCZ: n = 4 AP free; n = 14 taking atypical AP; n = 5 taking typical AP | SCZ | Clinically stable, defined as “patients excluded if total score on PANSS<65; inpatients excluded” | (1) In SCZ, exclusion of any SUD (including cannabis); (2) In SUD, 20 had cannabis SUD | (1) ↑ AEA in plasma of participants with SUD vs HC; (2) AEA in plasma of participants with SCZ vs HC |
| Leweke et al,11 2012 | n = 42 with SCZ | SCZ: 31.2% | Serum | Randomized clinical trial: amisulpride vs cannabidiol | SCZ | Acutely ill, defined as “BPRS>36; and BPRS-thought content factor ≥12” | (1) Participants with a comorbid substance abuse or dependence excluded; (2) Participants positive to drug screening excluded | (1) Treatment with cannabidiol ↑ AEA in plasma of participants with SCZ; (2) Posttreatment ↑ AEA in plasma of participants with SCZ positively associated with improvement in psychotic symptoms |
| Bioque et al,31 2013 | n = 95 with FEP; n = 90 HC | FEP: 28.0%; HC: 28.1% | PBMC | n = 18 AP-naïve; n = 77 taking typical and atypical APs (mean CPZ equivalents = 357.33 mg/d) | FEP | Mixed: not clearly stated | (1) Comorbid diagnosis of substance abuse or dependence used to divide the sample in FEP-CAN+ = 46; FEP-CAN- = 49; (CAN+ = comorbid diagnosis of cannabis abuse or dependence over at least 12 consecutive months during their lifetime); (2) Among participants with FEP, FEP-CAN+ showed more severe ECS abnormalities | (1) In participants with FEP vs HC: ↓ CB2 (which was no longer significant after adjusting for confounding factors); ↓ NAPE, ↓ DGL (synthesizing enzymes); ↑ MGL; and ↑ FAAH (degrading enzymes); (2) In participants with FEP, ↑ FAAH in males vs females |
| Ferretjans et al,40 2014 | n = 53 with SCZ; n = 22 HC | SCZ: 45.3%; HC: 57.1% | PBMC | Mixed typical and atypical APs (mean CPZ equivalents = 344.22 mg/d) | FEP or SCZ | Clinically stable, defined as “PANSS positive score of ≤19 (and <4 in any item of the positive scale)” | (1) Self-report of no cannabis use in the month preceding the start of study | (1) ↓ CB2 in participants with SCZ vs HC (which was no longer significant after adjusting for confounding factors); (2) = CB1 in participants with SCZ vs HC; (3) in participants with SCZ, inverse association between CB1, CB2 expression, and overall cognitive performances |
| Bioque et al,46 2016 | n = 73 with FEP; n = 67 HC | FEP: 28.8%; HC: 31.4% | PBMC | n = 16 AP-naive; n = 57 taking typical and atypical AP | FEP | Mixed: not clearly stated | (1) Comorbid diagnosis of substance abuse or dependence used to divide the sample in FEP-CAN+ = 33; FEP-CAN- = 35; (2) Correlation analyses corrected for cannabis use; (3) No association between cannabis use and primary end points of the study | (1) In participants with FEP, decreased expression of ECS-synthesizing enzymes and increased expression of degrading enzymes associated with poorer cognitive performances |
| Chase et al,41 2016 | n = 35 with SCZ; n = 35 HC | SCZ: 49.0%; HC: 51.1% | PBMC | n = 3 AP-free; n = 3 taking typical AP; n = 29 taking atypical AP | SCZ | Mixed: n = 15 inpatients, and n = 20 outpatients | (1) Participants with comorbid cannabis abuse or dependence or cannabis use in the last 12 mo were excluded | (1) ↑ CB1, ↑ CB2 in participants with SCZ vs HC; (2) In participants with SCZ, inverse correlation between CB1 and CB2 expression and performances specific to cognitive domains (attention, processing speed, working memory) |
| de Campos-Carli et al,42 2017 | n = 55 with SCZ; n = 48 HC | SCZ: 50.0%; HC: 46.0% | PBMC | n = 16 taking atypical AP; n = 39 taking typical AP (mean CPZ equivalents = 358.00 mg/d) | SCZ | Clinically stable outpatients | (1) Participants with self-report of cannabis dependence/use excluded | (1) ↑ CB1, ↑ CB2 in participants with SCZ vs HC |
| D’Addario et al,43 2017 | n = 25 with SCZ; n = 34 HC | SCZ: 44.1%; HC: 55.9% | PBMC | Among participants with SCZ: n = 14 taking atypical AP; n = 11 taking typical AP (mean CPZ equivalents = 506.12 mg/d) | SCZ | Mixed | (1) Participants with a comorbid substance abuse in the last 2 mo excluded | (1) ↑ CB1 in participants with SCZ vs HC; (2) = CB2, = NAPE and DGL, and = FAAH and MGL in participants with SCZ vs HC |
| Reuter et al,34 2017 | n = 28 with FEP; n = 81 HC | FEP: 21.4%; HC: 45.6% | Serum; CSF | All participants were AP-naive | FEP | Acutely ill inpatients only | (1) Investigated lifetime cannabis use; (2) No association between cannabis use and primary end points of the study | (1) ↑ AEA in CSF of participants with FEP vs HC; (2) In participants with FEP, positive association between serum levels of AEA and cognitive performances (measure of higher visual information processing); (3) In participants with FEP, no correlation between AEA in CSF and serum; (4)= AEA in participants with FEPvs HC |
| Koethe et al,39 2018 | Twin pairs discordant for disease status: n = 50 twins with SCZ; n = 14 twins with BD; n = 16 HC twins | SCZ: 44.0%; BD: 85.7%; HC: 37.5% | Plasma | Among participants with SCZ, mixed typical and atypical APs not reported in detail | SCZ | Clinically stable | (1) n = 13 twin pairs reported use of cannabis in past, 0 met DSM-III-R criteria for abuse or dependence; (2) Participants positive to urine drug screening excluded | (1) ↑ AEA in plasma of twins with SCZ vs HC twins; (2) = AEA in plasma of affected vs nonaffected twins with SCZ; (3) ↑ AEA in plasma of BD twins vs HC; (4) ↓ AEA in plasma of nonaffected twins with SCZ who will later develop the disorder |
| Moretti et al,44 2018 | n = 162 with SCZ; n = 94 HC | SCZ: 35.9%; HC: 43.6% | PBMC | Mixed typical and atypical APs not reported in detail | SCZ | Clinically stable chronic outpatients | Not reported | (1) ↑ CB1 in participants with SCZ vs HC |
| Wang et al,38 2018 | n = 115 with SCZ; n = 88 HC | SCZ: 55.7%; HC: 65.7% | Serum | n = 27 AP-naive; n = 128 AP-free | FEP and SCZ | Acutely ill inpatient | Not reported | (1) ↑ AEA in serum of participants with SCZ vs HC; (2) = AEA in serum of participants with FEP vs SCZ; (3) ↓ AEA in serum of participants with SCZ after 8 wk of TAU |
Abbreviations: AEA, anandamide; AP, antipsychotic; BD, bipolar disorders; BPRS, Brief Psychiatric Rating Scale; CAN, cannabinoid; CB1 and CB2, types 1 and 2 cannabinoid receptors; CPZ, chlorpromazine; CSF, cerebrospinal fluid; DGL, diacylglycerol lipase; ECS, endocannabinoid system; FAAH, fatty acid amide hydrolase; FEP, first-episode psychosis; HC, healthy controls; HF, high frequency; LF, low frequency; MGL, monoacylglycerol lipase; NAPE, N-acyl phosphatidylethanolamine phospholipase; PANSS, Positive and Negative Syndrome Scale; PBMC, peripheral blood mononuclear cells; SCZ, schizophrenia; SUD, substance use disorder; TAU; treatment as usual; ↑, increase; ↓, decrease; =, no difference.
A downregulating association of cannabis use with CB2 expression in peripheral immune cells was suggested by 1 study.31 However, most studies on peripheral ECS receptors and enzymes reported no association between cannabis use and their findings.
In people with psychosis, the association between severity of positive and negative symptoms, cognitive performances, and ECS tone in the blood and CSF was investigated by 7 studies.24,31,33,34,40,41,46,47 Severity of positive and negative symptoms was associated with (1) decreased anandamide levels in CSF24,33 and (2) increased expression of CB1 and CB2 receptors in peripheral blood mononuclear cells (PBMC).41 Poor cognitive performances were associated with (1) lower anandamide levels in CSF,47 (2) lower anandamide levels in serum,34 (3) higher expression of CB1 and CB2 receptors in PBMC,40,41 and (4) reduced expression of ECS-synthesizing enzymes and increased expression of ECS-degrading enzymes in PBMC.46
Only 2 studies focused on the modifications of the ECS in the context of response to treatment as defined by standardized criteria.11,35 De Marchi et al35 showed that remission of symptoms after 3 months of treatment as usual was associated with a substantial reduction in whole-blood anandamide levels and in messenger RNA levels of fatty acid amide hydrolase and CB2 in PBMC of patients with schizophrenia. Leweke et al11 conducted a 1-month randomized clinical trial in 2012 to compare the efficacy of amisulpride compared with CBD in inpatients with schizophrenia. Cannabidiol induced a pretreatment to posttreatment increase in serum anandamide levels, which was associated with amelioration in positive symptoms.
Discussion
To our knowledge, this is the first systematic review and meta-analysis of the clinical relevance of blood and CSF markers of the ECS in psychotic illness. The main findings were of increased anandamide levels in the CSF and blood and increased CB1 expression in peripheral immune cells of people with psychotic illness, compared with healthy controls. Higher ECS tone was found at an early stage of illness and in antipsychotic free/naive individuals, had an inverse association with symptoms severity and normalized following successful treatment.
In the whole-group analysis, anandamide in the CSF of patients with schizophrenia was elevated, in comparison to controls (Figure 2). This elevation was found in every stage of psychosis, from first episode to longstanding illness, and was independent of antipsychotic treatment and current cannabis use. Taken together, these findings suggest that anandamide in the CSF might represent a diagnostic marker of schizophrenia and that engagement of the ECS in the brain is protective in schizophrenia.48 Increased anandamide levels in the CSF were associated with reduced severity of both positive and negative symptoms and better cognitive performances in patients. Mechanistically, anandamide release in the brain may provide retrograde inhibition of the mesolimbic hyperdopaminergic state, resulting in reduced positive symptoms.2 The advantage for negative symptoms and cognition may be associated with the regulation of ECS mediators on the excitatory-inhibitory balance in the central nervous system, mediated by N-methyl-D-aspartate49 (Figure 1).
In the whole-group analysis, anandamide in the blood of patients with schizophrenia was elevated, in comparison to controls. None of the a priori–identified clinical subgroups showed significant differences in blood anandamide levels when compared with controls. However, the largest differences were found between patients with multiepisode and acute illness and healthy controls, suggesting that the findings in the overall group may have been driven by the elevation of anandamide concentration in these 2 clinical categories.
Blood levels of anandamide are associated with a number of peripheral sources, including inflammation, activation of the hypothalamic pituitary adrenal axis,50 modifications of the gut microbiome,51 alterations of metabolic functions,52 and medications.33 These peripheral sources may be associated with the increased concentration of anandamide seen in those with more chronic illness and in those with acute psychotic episodes.
The stage- and phase-related increase in serum anandamide does not seem to be associated with antipsychotic medications. Antipsychotic medications seem to downregulate anandamide blood levels (eResults 2 in the Supplement).
These peripheral variables in blood anandamide levels are likely associated with a lack of direct correlation between CSF and blood anandamide levels, as reported in 4 studies. Furthermore, the anandamide-hydrolyzing enzyme fatty acid amide hydrolase is highly expressed in cellular components of the blood-brain barrier,1 providing another possible factor in the discrepancies between peripheral and central anandamide findings.
However, as suggested by correlational analyses, peripheral anandamide levels might still have relevance for central brain processes. Serum anandamide levels and other blood markers of the ECS, such as the expression of cannabinoid receptors, have been associated with the severity of cognitive deficits in patients with schizophrenia. As suggested by Reuter and colleagues,34 the association between peripheral ECS markers and cognition might be the direct frontal regions draining directly into the bloodstream through the dural venous sinuses.34 Furthermore, as shown by 2 long-term studies by Koethe and colleagues,39,47 the engagement of ECS in the periphery is associated with reduced risk of developing schizophrenia in high-risk individuals. These findings further support the hypothesis32 that the activation of the ECS in the periphery is clinically relevant for psychotic illnesses.
Two studies of treatment response have shown that both olanzapine and CBD (a potential ECS enhancer) are associated with reduced anandamide levels in people with schizophrenia.11,35 This finding suggests that both drugs have a comparable action on the ECS, perhaps by reducing the need for the protective feedback operated by the ECS (olanzapine) or by potentiating its outcomes (CBD).
This meta-analytic synthesis showed an increase in CB1 receptors expression in the blood of patients compared with controls. Activation of CB1 has both anti-inflammatory and neuromodulatory outcomes.51 As for anandamide, different research groups have hypothesized that the increase in cannabinoid receptors in the periphery of patients with schizophrenia might represent a compensatory mechanism toward increased inflammation.41,43 A chronic proinflammatory status has been shown to characterize psychotic illnesses, independent of the phase or stage of the disorder,53,54,55 and is believed to be associated with long-term brain structure and functionality as well as symptom severity and cognitive performance.56,57 Exploring the association of stage, phase, current cannabis use, or antipsychotic medications with CB1 receptor expression was not possible because of the limited number of studies available.
Methodologic Considerations
Different methodologies and clinical confounders may be factors in the moderate or high heterogeneity observed. Some studies did not control for relevant variables, such as cannabis use38,44 (Table). Studies into blood levels of anandamide used different biological samples (whole blood,35 plasma,36,37 and serum24,33,34,39,47). In addition, the specific methods in each of the laboratories used to measure anandamide levels or expression of ECS are likely to vary considerably between studies, including the equipment used (eg, immunoassays, bioassays). Different assay procedures may yield different results, and certain platforms for assessment might be more sensitive than others. However, the use of random-effects models in the current study accounted for such between-study heterogeneity.
Shedding light on ECS activity in these disorders is particularly relevant given that exogenous cannabinoids are currently being tested as a novel therapeutic approach in multicentered randomized clinical trials (OPTiMiSE study).58,59,60 Evidence of the modifications of ECS tone in prodromal states of psychosis is limited but encouraging. If results are confirmed, enhancing ECS tone (eg, using CBD) in individuals with prodromal psychosis could represent a new therapeutic strategy for psychosis prevention,59,60 as suggested by a recent study.60
Limitations
This systematic review and meta-analysis has limitations. First, most of the clinical subgroup descriptive analyses of anandamide in the CSF and blood were based on a small number of studies. Furthermore, the definition of cannabis use was not always provided in studies, and the noncurrent cannabis user group included individuals who used cannabis in the past, a variable that is known to be associated with ECS functions. Evidence suggests a nonlinear relationship between cannabis use and psychotic illness,10,61 which is likely associated with the complex interaction between endogenous ECS mediators and THC, a partial agonist at the CB1 receptors. Future studies need to use standardized measures to assess cannabis use to clarify the association of cannabis use with blood and CSF markers of the ECS.
Second, all of the studies into anandamide levels in the CSF of patients with schizophrenia were from a single research group and included patients and healthy controls from the same geographic area, likely limiting the generalizability of these findings. Analyses targeting a broader population need to be performed.
Third, despite our efforts to contact all of the authors to retrieve data, this meta-analysis on CB1 receptors in schizophrenia is missing information from 2 studies, and providing a quantitative synthesis of the findings on CB2 receptors and ECS enzymes was not possible. For a similar reason, in the stage-related clinical subgroup descriptive analysis of blood anandamide findings, Wang and colleagues38 was categorized among the multiepisode studies, but it actually included a minority of first-episode psychosis (23.5%).
Fourth, data on ECS prognostic properties are limited and should be further explored by future studies. Future studies should also clarify the diagnostic specificity of these ECS abnormalities, both within psychotic diagnoses and across psychiatric disorders.
Conclusions
This study highlights the diagnostic and prognostic potential of measuring ECS tone in the CSF and blood of patients with psychotic illnesses. Understanding ECS activity in these disorders is relevant owing to the ongoing trials on exogenous cannabinoids as a therapeutic approach.
eMethods 1. MOOSE Checklist for Meta-analyses of Observational Studies
eMethods 2. Research Protocol
eMethods 3. AMSTAR-2 Checklist
eFigure. PRISMA Flow Diagram
eResults 1. AEA Levels in the CSF: Stage, Antipsychotic Medications, Cannabis Use
eResults 2. AEA Levels in the Blood: Stage, Phase, Antipsychotic Medications, Cannabis Use
eTable 1. Excluded Studies and Reasons for Exclusion
eTable 2. Risk of Bias
References
- 1.Battista N, Di Tommaso M, Bari M, Maccarrone M. The endocannabinoid system: an overview. Front Behav Neurosci. 2012;6:9. doi: 10.3389/fnbeh.2012.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alger BE. Getting high on the endocannabinoid system. Cerebrum. 2013;2013:14. [PMC free article] [PubMed] [Google Scholar]
- 3.Lupica CR, Hu Y, Devinsky O, Hoffman AF. Cannabinoids as hippocampal network administrators. Neuropharmacology. 2017;124:25-37. doi: 10.1016/j.neuropharm.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 4.Pava MJ, Makriyannis A, Lovinger DM Endocannabinoid signaling regulates sleep stability. PLoS One 2016;11(3):e0152473. doi: 10.1371/journal.pone.0152473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aronne LJ, Pagotto U, Foster GD, Davis SN. The endocannabinoid system as a target for obesity treatment. Clin Cornerstone. 2008;9(1):52-64; discussion 65-66. doi: 10.1016/S1098-3597(08)60028-9 [DOI] [PubMed] [Google Scholar]
- 6.Katchan V, David P, Shoenfeld Y. Cannabinoids and autoimmune diseases: a systematic review. Autoimmun Rev. 2016;15(6):513-528. doi: 10.1016/j.autrev.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 7.Kuepper R, Ceccarini J, Lataster J, et al. Delta-9-tetrahydrocannabinol-induced dopamine release as a function of psychosis risk: 18F-fallypride positron emission tomography study. PLoS One. 2013;8(7):e70378. doi: 10.1371/journal.pone.0070378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farkas I, Kalló I, Deli L, et al. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology. 2010;151(12):5818-5829. doi: 10.1210/en.2010-0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherif M, Radhakrishnan R, D’Souza DC, Ranganathan M. Human laboratory studies on cannabinoids and psychosis. Biol Psychiatry. 2016;79(7):526-538. doi: 10.1016/j.biopsych.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharyya S, Falkenberg I, Martin-Santos R, et al. Cannabinoid modulation of functional connectivity within regions processing attentional salience. Neuropsychopharmacology. 2015;40(6):1343-1352. doi: 10.1038/npp.2014.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94-e94. doi: 10.1038/tp.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henquet C, Rosa A, Krabbendam L, et al. An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology. 2006;31(12):2748-2757. doi: 10.1038/sj.npp.1301197 [DOI] [PubMed] [Google Scholar]
- 13.Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21. doi: 10.1017/S0033291702006402 [DOI] [PubMed] [Google Scholar]
- 14.van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol. 2002;156(4):319-327. doi: 10.1093/aje/kwf043 [DOI] [PubMed] [Google Scholar]
- 15.Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to pot - a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54. doi: 10.3389/fpsyt.2014.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932. doi: 10.1007/s00213-018-4885-9 [DOI] [PubMed] [Google Scholar]
- 17.McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231. doi: 10.1176/appi.ajp.2017.17030325 [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117. doi: 10.1001/jamapsychiatry.2018.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price C, Hemmingsson T, Lewis G, Zammit S, Allebeck P. Cannabis and suicide: longitudinal study. Br J Psychiatry. 2009;195(6):492-497. doi: 10.1192/bjp.bp.109.065227 [DOI] [PubMed] [Google Scholar]
- 20.Kotin J, Post RM, Goodwin FK. 9-tetrahydrocannabinol in depressed patients. Arch Gen Psychiatry. 1973;28(3):345-348. doi: 10.1001/archpsyc.1973.01750330041007 [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto S, Ohba H, Nishiyama S, et al. Subanesthetic doses of ketamine transiently decrease serotonin transporter activity: a PET study in conscious monkeys. Neuropsychopharmacology. 2013;38(13):2666-2674. doi: 10.1038/npp.2013.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin LL, Smith DJ. Ketamine inhibits serotonin synthesis and metabolism in vivo. Neuropharmacology. 1982;21(2):119-125. doi: 10.1016/0028-3908(82)90150-2 [DOI] [PubMed] [Google Scholar]
- 23.Koethe D, Gerth CW, Schreiber D, et al. The endocannabinoid anandamide in CSF is related to the patterns of cannabis use in first-episode schizophrenia. Biol Psychiatry. 2005;57(8):186S. 202057 [Google Scholar]
- 24.Leweke FM, Giuffrida A, Koethe D, et al. Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: impact of cannabis use. Schizophr Res. 2007;94(1-3):29-36. doi: 10.1016/j.schres.2007.04.025 [DOI] [PubMed] [Google Scholar]
- 25.Lazary J, Eszlari N, Juhasz G, Bagdy G. Genetically reduced FAAH activity may be a risk for the development of anxiety and depression in persons with repetitive childhood trauma. Eur Neuropsychopharmacol. 2016;26(6):1020-1028. doi: 10.1016/j.euroneuro.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. doi: 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morton RW, Murphy KT, McKellar SR, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376-384. doi: 10.1136/BJSPORTS-2017-097608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper H, Hedges LV, Valentine JC. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. New York, NY: Russell Sage Foundation; 2009. https://www.scholars.northwestern.edu/en/publications/the-handbook-of-research-synthesis-and-meta-analysis-2nd-edition. Accessed December 9, 2018.
- 30.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bioque M, García-Bueno B, Macdowell KS, et al. ; FLAMM-PEPs study—Centro de Investigacio´n Biome´dica en Red de Salud Mental . Peripheral endocannabinoid system dysregulation in first-episode psychosis. Neuropsychopharmacology. 2013;38(13):2568-2577. doi: 10.1038/npp.2013.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport. 1999;10(8):1665-1669. doi: 10.1097/00001756-199906030-00008 [DOI] [PubMed] [Google Scholar]
- 33.Giuffrida A, Leweke FM, Gerth CW, et al. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29(11):2108-2114. doi: 10.1038/sj.npp.1300558 [DOI] [PubMed] [Google Scholar]
- 34.Reuter AR, Bumb JM, Mueller JK, et al. Association of anandamide with altered binocular depth inversion illusion in schizophrenia. World J Biol Psychiatry. 2017;18(6):483-488. doi: 10.1080/15622975.2016.1246750 [DOI] [PubMed] [Google Scholar]
- 35.De Marchi N, De Petrocellis L, Orlando P, Daniele F, Fezza F, Di Marzo V. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2003;2:5. doi: 10.1186/1476-511X-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potvin S, Kouassi E, Lipp O, et al. Endogenous cannabinoids in patients with schizophrenia and substance use disorder during quetiapine therapy. J Psychopharmacol. 2008;22(3):262-269. doi: 10.1177/0269881107083816 [DOI] [PubMed] [Google Scholar]
- 37.Desfossés J, Stip E, Bentaleb LA, et al. Plasma endocannabinoid alterations in individuals with substance use disorder are dependent on the “mirror effect” of schizophrenia. Front Psychiatry. 2012;3:85. doi: 10.3389/fpsyt.2012.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D, Sun X, Yan J, et al. Alterations of eicosanoids and related mediators in patients with schizophrenia. J Psychiatr Res. 2018;102:168-178. doi: 10.1016/j.jpsychires.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 39.Koethe D, Pahlisch F, Hellmich M, et al. Familial abnormalities of endocannabinoid signaling in schizophrenia. World J Biol Psychiatry. 2018;(March):1-9. [DOI] [PubMed] [Google Scholar]
- 40.Ferretjans R, de Campos SM, Ribeiro-Santos R, et al. Cognitive performance and peripheral endocannabinoid system receptor expression in schizophrenia. Schizophr Res. 2014;156(2-3):254-260. doi: 10.1016/j.schres.2014.04.028 [DOI] [PubMed] [Google Scholar]
- 41.Chase KA, Feiner B, Rosen C, Gavin DP, Sharma RP. Characterization of peripheral cannabinoid receptor expression and clinical correlates in schizophrenia. Psychiatry Res. 2016;245:346-353. doi: 10.1016/j.psychres.2016.08.055 [DOI] [PubMed] [Google Scholar]
- 42.de Campos-Carli SM, Araújo MS, de Oliveira Silveira AC, et al. Cannabinoid receptors on peripheral leukocytes from patients with schizophrenia: evidence for defective immunomodulatory mechanisms. J Psychiatr Res. 2017;87:44-52. doi: 10.1016/j.jpsychires.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 43.D’Addario C, Micale V, Di Bartolomeo M, et al. A preliminary study of endocannabinoid system regulation in psychosis: distinct alterations of CNR1 promoter DNA methylation in patients with schizophrenia. Schizophr Res. 2017;188:132-140. doi: 10.1016/j.schres.2017.01.022 [DOI] [PubMed] [Google Scholar]
- 44.Moretti PN, Ota VK, Gouvea ES, et al. Accessing gene expression in treatment-resistant schizophrenia. Mol Neurobiol. 2018;55(8):7000-7008. doi: 10.1007/s12035-018-0876-4 [DOI] [PubMed] [Google Scholar]
- 45.Koethe D, Giuffrida A, Schreiber D, et al. Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br J Psychiatry. 2009;194(4):371-372. doi: 10.1192/bjp.bp.108.053843 [DOI] [PubMed] [Google Scholar]
- 46.Bioque M, Cabrera B, García-Bueno B, et al. ; FLAMM-PEPs Study - Centro de Investigación Biomédica en Red de Salud Mental . Dysregulated peripheral endocannabinoid system signaling is associated with cognitive deficits in first-episode psychosis. J Psychiatr Res. 2016;75:14-21. doi: 10.1016/j.jpsychires.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 47.Koethe D, Giuffrida A, Schreiber D, et al. Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. [published correction appears in Br J Psychiatry. 2011;198(6):495]. Br J Psychiatry. 2009;194(4):371-372. doi: 10.1192/bjp.bp.108.053843 [DOI] [PubMed] [Google Scholar]
- 48.Leweke FM, Giuffrida A, Gerth CW, et al. Endogenous cannabinoids and their neurobiological and functional role in schizophrenia spectrum disorders. Biol Psychiatry. 2004;55(8):102S-103S. 202111 [Google Scholar]
- 49.Gupta S, Cahill JD, Ranganathan M, Correll CU. The endocannabinoid system and schizophrenia: links to the underlying pathophysiology and to novel treatment approaches. J Clin Psychiatry. 2014;75(3):285-287. doi: 10.4088/JCP.14ac08982 [DOI] [PubMed] [Google Scholar]
- 50.Hillard CJ, Beatka M, Sarvaideo J. Endocannabinoid signaling and the hypothalamic-pituitary-adrenal axis. Compr Physiol. 2016;7(1):1-15. doi: 10.1002/cphy.c160005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mestre L, Carrillo-Salinas FJ, Mecha M, Feliú A, Guaza C. Gut microbiota, cannabinoid system and neuroimmune interactions: new perspectives in multiple sclerosis. Biochem Pharmacol. 2018;157(August):51-66. doi: 10.1016/j.bcp.2018.08.037 [DOI] [PubMed] [Google Scholar]
- 52.Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8(5):585-589. doi: 10.1038/nn1457 [DOI] [PubMed] [Google Scholar]
- 53.Salviati M, Bersani FS, Macrì F, et al. Capgras-like syndrome in a patient with an acute urinary tract infection. Neuropsychiatr Dis Treat. 2013;9(1):139-142. doi: 10.2147/NDT.S39077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dickerson F, Stallings C, Origoni A, et al. Inflammatory markers in recent onset psychosis and chronic schizophrenia. Schizophr Bull. 2016;42(1):134-141. doi: 10.1093/schbul/sbv108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liemburg EJ, Nolte IM, Klein HC, Knegtering H, Knegtering H; PHAMOUS investigators . Relation of inflammatory markers with symptoms of psychotic disorders: a large cohort study. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:89-94. doi: 10.1016/j.pnpbp.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 56.Mostaid MS, Dimitrakopoulos S, Wannan C, et al. An interleukin-1 beta (IL1B) haplotype linked with psychosis transition is associated with IL1B gene expression and brain structure. Schizophr Res. 2019;204:201-205. doi: 10.1016/j.schres.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 57.Minichino A, Ando’ A, Francesconi M, Salatino A, Delle Chiaie R, Cadenhead K. Investigating the link between drug-naive first episode psychoses (FEPs), weight gain abnormalities and brain structural damages: relevance and implications for therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:9-22. doi: 10.1016/j.pnpbp.2017.03.020 [DOI] [PubMed] [Google Scholar]
- 58.Leucht S, Winter-van Rossum I, Heres S, et al. The optimization of treatment and management of schizophrenia in Europe (OPTiMiSE) trial: rationale for its methodology and a review of the effectiveness of switching antipsychotics. Schizophr Bull. 2015;41(3):549-558. doi: 10.1093/schbul/sbv019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mandolini GM, Lazzaretti M, Pigoni A, Oldani L, Delvecchio G, Brambilla P. Pharmacological properties of cannabidiol in the treatment of psychiatric disorders: a critical overview. Epidemiol Psychiatr Sci. 2018;27(4):327-335. doi: 10.1017/S2045796018000239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davies C, Cipriani A, Ioannidis JPA, et al. Lack of evidence to favor specific preventive interventions in psychosis: a network meta-analysis. World Psychiatry. 2018;17(2):196-209. doi: 10.1002/wps.20526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhattacharyya S, Fusar-Poli P, Borgwardt S, et al. Modulation of mediotemporal and ventrostriatal function in humans by Δ9-tetrahydrocannabinol: a neural basis for the effects of cannabis sativa on learning and psychosis. Arch Gen Psychiatry. 2009;66(4):442-451. doi: 10.1001/archgenpsychiatry.2009.17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. MOOSE Checklist for Meta-analyses of Observational Studies
eMethods 2. Research Protocol
eMethods 3. AMSTAR-2 Checklist
eFigure. PRISMA Flow Diagram
eResults 1. AEA Levels in the CSF: Stage, Antipsychotic Medications, Cannabis Use
eResults 2. AEA Levels in the Blood: Stage, Phase, Antipsychotic Medications, Cannabis Use
eTable 1. Excluded Studies and Reasons for Exclusion
eTable 2. Risk of Bias