Abstract
Increased concentrations of interleukin 1 (IL-1) in the cerebrospinal fluid and serum of patients with Alzheimer disease (AD) reduced phagocytic capacity point to an inflammatory activation of mononuclear phagocytes in AD. Interleukin 1 receptors (IL-1R) and the macrophage scavenger receptor I (MSRI) are important players in IL-1 signaling and phagocytosis. In 20 patients with AD and 17 controls, IL-1RI, IL-1RII, and MSRI were assessed on peripheral blood mononuclear cells by flow cytometry. IL-1β, soluble IL-1 receptors, and IL-1R antagonist (IL-1Ra) were measured by enzyme-linked immunosorbent assay. The fraction of IL-1RI+ monocytes was increased by 10% and the expression of MSRI was reduced by 12% in AD. A 3.6% increased fraction of IL-1RI+ lymphocytes was accompanied by a 6.1% reduced expression of IL-1RII. The IL-1RI on monocytes and lymphocytes discriminated patients with AD with an accuracy of 0.79 and 0.75, respectively. The IL-1Ra was elevated in AD. Changes in the expression of IL-1 receptors and MSRI on peripheral blood cells fit to the concept of a proinflammatory state of the peripheral immune system. However, the observed differences are not strong enough to suggest their application as biomarkers for AD.
Keywords: Alzheimer disease, dementia, neurodegeneration, neuroinflammation, biomarker, IL-1RI, MSRI
Introduction
The search for peripheral diagnostic markers of Alzheimer disease (AD) is one of the most important issues in the field. Besides amyloidosis and the deposition of neurofibrillary tangles, neuroinflammation is an increasingly recognized feature of AD.1,2 This neuroinflammation is characterized by activated microglia, increased production of cytokines, and migration of blood-derived monocytes into the brain.3-5 The phenotype of monocyte-derived macrophages and microglia in brains of patients with AD is postulated to be specific.6 A systemic immune response accompanies the inflammatory processes in AD brains.7,8 Although a meta-analysis indicates elevated levels of interleukin 1 (IL-1) and other cytokines in plasma of patients with AD, measurements are heterogeneous and the intraindividual variance impedes their utilization as diagnostic markers in AD.7 As an alternative, changes of peripheral immune cells were suggested to be such a marker.9,10
Interleukin 1 is a key mediator of neuroinflammation and one of the most extensively studied molecules in the blood of patients with AD.11 Interleukin 1 is mainly secreted by activated monocytes/macrophages with a proinflammatory phenotype.12 This phenotype goes along with changes in the expression of surface receptors, such as IL-1 receptors and the macrophage scavenger receptor I (MSRI).
There are several types of IL-1 receptors. The IL-1RI is the receptor propagating the proinflammatory signal, while the IL-1RII is a decoy receptor, binding IL-1 without initiating an intracellular response.13 The expression of the IL-1RI is increased on monocytes with a proinflammatory phenotype whereas IL-1RII is decreased.12 Both receptors can be processed by an extracellular protease and released into the serum as soluble IL-1 receptors (sIL-1RI, sIL-1RII). Soluble IL-1 receptors act as decoy receptors and, together with the IL-1R antagonist (IL-1Ra), have anti-inflammatory properties.14 Blocking the IL-1R1 with an antibody rescued cognition and attenuated tau pathology via reduced activity of nuclear factor-κB (NF-κB) in a mouse model of AD.15
One of the most important receptors for phagocytosis is the scavenger receptor MSRI, also termed Scara1, SR-A, or CD204. The MSRI seems to be involved in the phagocytosis of amyloid-β (Aβ) peptides and plaques.16,17 Its expression on monocytes/macrophages with a proinflammatory phenotype is decreased.12 In a murine model of AD, Hickman et al found a lower expression of MSRI and other scavenger receptors in later stages of AD, while concentrations of inflammatory cytokines were shown to be increased.18
As changes in the expression of the MSRI and the IL-1 cytokine family were seen in AD and since the IL-1 and macrophage scavenger system influence each other, mutually via NF-κB, they seem to be very promising markers of the systemic inflammation in AD and potentially suitable as a peripheral diagnostic marker to support the diagnosis.19,20
Within this study, we are testing, if AD leads to measurable changes in the expression of the IL-1 receptors and MSRI on peripheral blood mononuclear cells (PBMC) and whether or not this feature can be used to support the diagnosis of AD.
Materials and Methods
Study Population
The patients were recruited in the memory clinic of the Department of Psychiatry and Psychotherapy in Erlangen, Germany. The study protocol was approved by the ethical committee (Nr. 3987) of the University hospital Erlangen. All participants provided informed written consent. The participants underwent a physical, neurological, psychiatric, and a neuropsychological examination according to the Consortium to establish a Registry for Alzheimer disease battery test.21 Diagnosis was supported by a brain magnetic resonance imaging scan, Hexamethylpropylenaminooxim-single photon emission computed tomogrphy (HMPAO-SPECT), and the measurement of Aβ1-40, Aβ1-42, total tau (t-tau), and phospho-tau (p-tau) in cerebrospinal fluid. Neurochemical Dementia Diagnostics analyses were performed with the enzyme-linked immunosorbent assay (ELISA) from IBL International (Hamburg, Germany; Aβ1-40) and Fujirebio Europe (former Innogenetics, Gent, Belgium; Aβ1-42, t-tau, and p-tau181). Based on the International Working Grpup 2 (IWG-2) criteria, study participants were categorized into 2 groups, those with subjective cognitive complaints but without clinical or biomarker evidence for AD or an inflammatory neurological disease (symptomatic controls, SC) and those with AD.22,23 In accordance with the revised A/T/N categories suggested by Jack et al., patients in the control group are negative for amyloid, tau, or neurodegeneration (A-/T-/N-) whereas patients in the AD cohort are positive for all 3 biomarkers.24 Patients with clinical signs of infection, increased C-reactive protein (CRP), or blood leukocyte count as well as those with a chronic inflammatory or malignant disease were excluded from the study.
Flow Cytometry
Multiparameter flow cytometry was used to analyze PBMC. Cells were obtained by cubital vein puncture in ethylenediaminetetraacetic acid (EDTA) tubes and processed within 20 minutes by density-gradient centrifugation using Ficoll solution (Biochrom, Berlin, Germany). The cells were resuspended in phosphate buffer saline (PBS) with 2 mM EDTA to avoid monocyte adherence. Staining was performed for 30 minutes at 4°C with fluorochrome-conjugated antibodies CD4-FITC (clone EDU-2), CD8-PE (clone MEM-31), CD14-PECy5 (clone 18D11), CD19-APC (clone LT-19; all Immunotools, Friesoythe, Germany), MSRI-PE (clone 351615), IL-1RII-FITC (clone 34141), and IL-1RI-PE (FAB269; all R&D Systems, Minneapolis). All measurements were performed with cells obtained by a one and only single blood draw regarding each patient.
Measurements were performed on a CyFlowSpace (Partec, Görlitz, Germany) and data were analyzed with the Kaluza 2.1 software package (Beckman & Coulter, Krefeld, Germany). The gating strategy is shown in Supplementary Figure 1. At this stage, the experimenter was blinded for the diagnosis of the patients. The expression of CD4, CD8, CD14, and CD19 was determined in terms of a general immunophenotyping and to exclude systemic immunological or hematooncological diseases (1 patient suffering of chronic lymphocytic leukemia was detected and excluded). The same experiments showed a purity of >95% of monocytes within the monocyte gate defined by forward-and-side scatter.
Enzyme-Linked Immunosorbent Assay
The plasma levels of sIL-1RI, sIL-1RII, and IL-1Ra were measured by ELISA Duoset systems (R&D Systems) in the same samples used for the flow cytometry measurements described above. No plasma samples were available for 3 SCs and 1 patient with AD. Optimal working concentrations for the capture-/detection-antibodies (R&D Systems) and the dilution of the plasma samples were established. The dilutions of the samples were necessary to avoid matrix effects and were titrated individually for each assay to yield optimal spike recovery. Coating of the 96-well plates with the capture antibodies (sIL-1RI: 4 µg/mL, sIL-1RII: 1 µg/mL, IL-1Ra: 5 µg/mL) diluted in PBS was performed overnight at room temperature. Between each incubation, wells were washed 3 times with PBS/0.05% Tween-100 (Sigma-Aldrich, Munich, Germany). Wells were blocked with reagent diluent (1% bovine serum albumin [BSA; Sigma-Aldrich] in PBS), for 1 hour at room temperature. Then, 100 µL of plasma, diluted with PBS (diluting factors: sIL-1RI 1:16, sIL-1RII 1:8, IL-1Ra 1:8), calibration standards and blanks were added in duplicates and incubated at room temperature for 2 hours. Detection antibodies were diluted in 1% BSA/PBS (sIL-1RI: 20 ng/mL, sIL-1RII: 50 ng/mL, IL-1Ra: 50 ng/mL) and incubated for 2 hours at room temperature. Before analysis, streptavidin-horseradish peroxidase and substrate solution (H2O2 and tetramethylbenzidine, both R&D Systems) were added and the reaction was stopped with 2 N H2SO4 (Sigma-Aldrich). Absorption at 450 nm was read by a Benchmark microplate reader (Bio-Rad, Hercules, California) and analyzed by microplate manager 5.2 (Bio-Rad) without the knowledge of the clinical diagnosis. To allow a statistical evaluation, measurements below the lower limit of quantification were arbitrarily set to the lower limit of quantification. The lower limit of quantification was defined as the lowest concentration in the standard curve with a signal-to-noise ratio of 2, multiplied with the dilution of the respective samples (IL1-RI: 60 pg/mL, IL1-RII: 250 pg/mL, IL1Ra: 115 pg/mL).
To analyze the concentration of IL-1β, a commercially available ELISA assay (IL-1β Quantikine, R&D Systems) was used according to the manufacturer’s protocol.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 6 (GraphPad Software Inc, La Jolla, California) and SPSS 21 (IBM Deutschland, Ehningen, Germany). As some of the data did not follow a Gaussian distribution, nonparametric statistics using the 2-sided Mann-Whitney U test for the comparison of 2 samples and the Spearman correlation coefficient for correlation analysis were applied. All results are presented as median [interquartile range]. The diagnostic performance of the analysis was evaluated by receiver operating characteristic curves (ROC) and the resulting area under the curve (AUC). To adjust the results for age, linear regression analysis was performed. Results were supposed to be significant, when the P value was lower than .05. A P value between .05 and .10 is reported as a trend. All data sets analyzed within this study can be made available upon request.
Results
Patient Characteristics
Patients included in the study all approached our memory clinic because of cognitive complaints. As a control group, the SC is composed of patients with depression, suspected vascular disease, or mild cognitive impairment not due to AD. Patients with AD include A+/T+/N+ individuals with mild cognitive impairment (MCI) and dementia (Supplementary Figure 2). As the patients were recruited consecutively, an exact matching in terms of age and sex was not possible. As a consequence, the SC were younger. No differences between the groups were found in terms of CRP or leukocyte counts and in the basic immune phenotyping with CD4, CD8, and CD14 (Table 1). No P values were calculated for Aβ1-40, Aβ1-42, t-tau, and p-tau, as the study population was selected according to these values.
Table 1.
Patient Characteristics.a
| Symptomatic Controls (SC) | Alzheimer disease (AD) | |
|---|---|---|
| N (female) | 17 (10) | 20 (11) |
| Age | 63.6 [60.6-71.3] | 73.9 [66.3-76.1]b |
| MMSE | 29 [27-30] | 25 [23-28]c |
| Aβ 40 (ng/mL) | 15.4 [12.3-18.9] | 19.7 [16.7-23.5] |
| Aβ 42 (pg/mL) | 1262 [969-1419] | 764 [622-905] |
| Aβ ratio | 0.08 [0.08-0.10] | 0.04 [0.03-0.05] |
| t-Tau (pg/mL) | 221 [158-250] | 526 [434-596] |
| p-Tau (pg/mL) | 41.6 [29.8-56.5] | 98.6 [79.8-114.5] |
| Leukocyte counts (103/µL) | 6.4 [5.4-7.5] | 7.3 [6.0-8.4] |
| CRP (mg/L) | 2.5 [0.9-5.3] | 2.6 [0.9-5.3] |
| CD4+ (%) | 46.4 [40.9-52.0] | 44.8 [38.3-56.3] |
| CD8+ (%) | 30.8 [27.6-42.0] | 28.6 [24.8-33.9] |
| CD4/CD8 | 1.6 [1.0-1.9] | 1.6 [1.2-2.1] |
| CD14+ (%) | 21.8 [18.8- 27.4] | 25.9 [18.4-31.1] |
Abbreviations: CRP, C-reactive protein; MMSE, Mini-Mental Status Examination.
aPatient characteristics are presented as median [interquartile range]. SC include patients with depression, suspected vascular disease, or A-/T-/N- mild cognitive impairment. AD comprises patients with MCI or dementia due to Alzheimer disease. Aβ levels and tau proteins were measured in CSF, leukocyte counts, CRP, and the percentage of CD4+, CD8+, and CD14+ leukocytes were assessed in blood. Differences were calculated with the nonparametric Mann-Whitney U test. No statistical difference was calculated for the Aβ and tau levels, as they were used to define the groups.
bP < .05.
cP < .01.
Enhanced IL-1RI and Reduced MSRI Expression on Peripheral Monocytes in AD
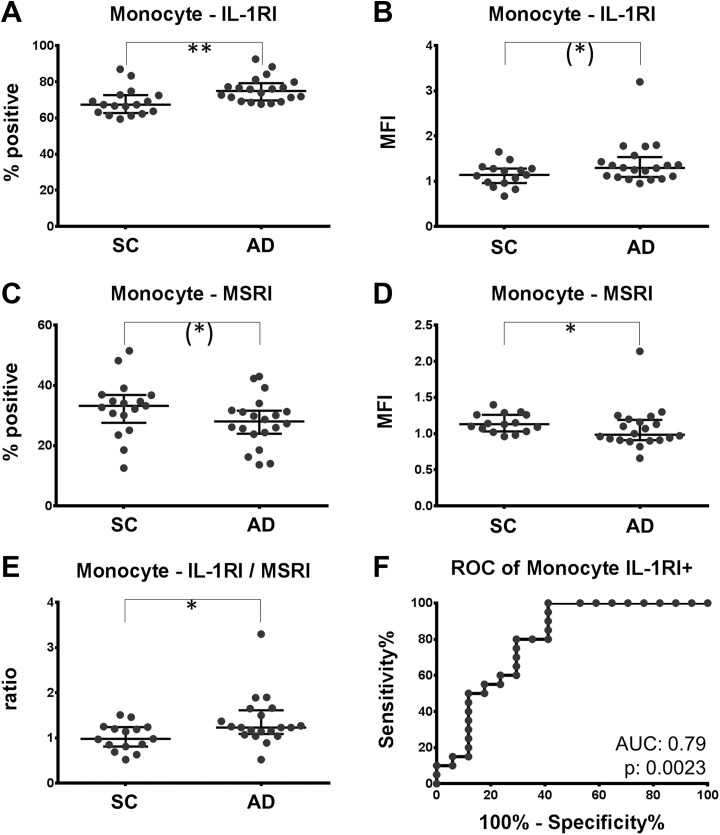
Surface analysis of IL-1 receptors and MSRI by flow cytometry indicated that the fraction of monocytes staining positive for IL-1-RI was increased by 10% in AD (67.4% [62.8-72.7] vs 74.9% [69.7-79.2]; P = .0017; Figure 1A). Likewise, there was a trend of an increased mean fluorescence intensity (MFI) of IL-1RI on all monocytes by 14% in AD (1.14 [0.96-1.28] vs 1.30 [1.10-1.54]; P = .07; Figure 1B). In contrast, the MFI of the MSRI was reduced by 12% (1.13 [1.03-1.26] vs 0.99 [0.91-1.19]; P = .047; Figure 1D) and the fraction staining positive for the MSRI tended to be lower in AD (33.24 [27.6-36.9] vs 28.0 [24.0-31.6]; P = .07; Figure 1C). The ratio of IL-1RI (MFI) and MSRI (MFI), being an indicator of the inflammatory properties of monocytes, was consequently increased by 26% in AD (0.98 [0.81-1.24] vs 1.23 [0.1.09-1.61]; P = .023; Figure 1E). No change was found for the IL-1RII on monocytes. Receiver operating characteristic (ROC) analysis indicated that the fraction of IL-1RI-positive monocytes separated patients with AD from nondemented controls with a diagnostic accuracy of 79% (AUC 0.79; P = .0023; Figure 1F). For a cutoff value of 70% IL-1RI-positive monocytes, AD can be diagnosed with a sensitivity and specificity of 80.0% and 70.6%, respectively. Within the AD group, neither a difference between patients with dementia and those with MCI was found nor a correlation with the Mini-Mental Status Examination (MMSE; (Table 2). Also, no correlation with the Aβ40, Aβ42, Aβ ratio, tau, or p-tau concentrations were detected (Table 2). As the SC group was younger than the AD group, the influence of age on the measurements was evaluated. No correlations between age and IL-1RI or MSRI were found. Only an intermediate and marginally significant correlation of the IL-1RI/MSRI ratio with age was found. Additionally, the differences between the groups remain significant, even if matching the age by removing the 5 oldest patients with AD from the analysis. To further show that the age difference between the study groups did not confound the results, a linear regression analysis was performed for the IL-1RI+ and MSRI (MFI) versus age (Figure 2A, C, and E). For the linear regression analysis of MSRI, one outlier, identified by the Grubbs test, had to be removed from the AD group. For both parameters and their ratio, the slope of the regression line was not significantly different between AD and SC. However, the elevation of the line differed for IL-1RI with a P value of .046, for the MRSI with a P value of .054, and for the ratio with a P value of .080. In other words, in linear regression models with age and diagnosis as independent variables, and MSRI, IL-1RI as well as the IL-1RI/MSRI ratio as dependent variables, the contribution of age to the final models was not significant. However, the diagnosis contributed with P values of .05 for MSRI (MFI), 0.046 for IL-1RI+, and 0.08 for the IL-1RI/MSRI ratio.
Figure 1.
Expression of IL-1RI is increased; expression of MSRI is decreased on monocytes of patients with AD. PBMC of patients with AD and SC was labeled with antibodies directed against IL-1RI and MSRI. A and C, Fraction of monocytes staining positive for IL-1RI and MSRI. B and D, The mean fluorescence intensity (MFI) of the IL-1RI and the MSRI on monocytes are depicted. The ratio of IL-1RI (MFI)/MSRI (MFI) comparing the 2 populations is plotted in (E). F, The receiver operating characteristic curve (ROC) for the discrimination of patients with and without AD by the fraction of IL-1RI-positive monocytes. Trend: *P < .10; significance: *P < .05; **P < .01. AD indicates Alzheimer disease; IL-1RI, interleukin 1 receptor subtype I; MSRI, macrophage scavenger receptor I; PBMC, peripheral blood mononuclear cell; SC, symptomatic controls.
Table 2.
Correlations Between the Expression of Leukocyte Receptors and Soluble IL-1 Receptor–Associated Mediators With Established AD Biomarkers, Age, and MMSE.a
| Age (SC) | Age (AD) | MMSE (AD) | Aβ 40 (AD) | Aβ 42 (AD) | Aβ Ratio (AD) | Tau (AD) | p-Tau (AD) | |
|---|---|---|---|---|---|---|---|---|
| Monocyte IL-1RI+ | 0.40 | 0.32 | −0.16 | −0.19 | −0.15 | 0.08 | 0.15 | 0.29 |
| Monocyte IL-1RI (MFI) | 0.05 | 0.34 | 0.11 | −0.19 | −0.41 | −0.05 | −0.20 | 0.03 |
| Monocyte MSRI+ | 0.04 | −0.26 | 0.34 | 0.00 | 0.20 | 0.43 | −0.11 | 0.06 |
| Monocyte MSRI (MFI) | 0.01 | −0.16 | 0.22 | −0.04 | −0.04 | 0.08 | −0.11 | −0.09 |
| Monocyte IL-RI/MSRI | 0.04 | 0.45 b | −0.10 | −0.19 | −0.26 | −0.06 | −0.01 | 0.16 |
| Lymphocyte IL-1RI+ | 0.46 | −0.11 | 0.35 | 0.33 | −0.04 | −0.55 b | −0.28 | −0.33 |
| Lymphocyte IL-1RI (MFI) | −0.37 | −0.13 | 0.34 | 0.08 | −0.24 | −0,34 | −0,44 | −0,37 |
| Lymphocyte IL-1RII+ | 0.34 | −0,19 | 0.07 | 0.22 | −0.03 | −0.15 | −0.02 | −0.33 |
| Lymphocyte IL-1RII (MFI) | 0.39 | 0.00 | 0.06 | 0.01 | −0.27 | −0.25 | −0.18 | −0.28 |
| Lymphocyte IL-1RI/IL-1RII | 0.02 | −0.15 | 0.63 c | 0.26 | 0.11 | −0.22 | −0.38 | −0.34 |
| IL-1β (plasma) | 0.14 | 0.02 | −0.01 | −0.17 | −0.31 | −0.16 | 0.06 | 0.12 |
| sIL-1RI (plasma) | −0.32 | −0.02 | 0.23 | 0.16 | 0.11 | −0.06 | −0.01 | −0.13 |
| sIL-1RII (plasma) | −0.29 | −0.26 | 0.27 | −0.06 | 0.19 | 0.23 | −0.36 | −0.39 |
| IL-1Ra (plasma) | N/A | −0.04 | 0.12 | 0.29 | 0.32 | 0.00 | −0.33 | −0.35 |
Abbreviations: AD, Alzheimer disease; IL-1RI, interleukin 1 receptor subtype I; IL-1RII indicates interleukin 1 receptor subtype II; IL-1RII, interleukin 1 receptor subtype II; MFI, mean fluorescence intensity; MMSE, Mini-Mental Status Examination; MSRI, macrophage scavenger receptor I; SC, symptomatic controls; sIL-1RI, soluble interleukin 1 receptor subtype I; sIL-1RII, soluble interleukin 1 receptor subtype II.
aCorrelations were calculated separately for patients with Alzheimer disease (AD) and symptomatic controls (SC) with the nonparametric Spearman test. For each pair of analytes, Spearman ρ is presented at the respective position in the correlation matrix. For IL-1Ra, a correlation with age could not be calculated as all values were below the lower limit of quantification and were set to 115 pg/mL as indicated. Significant correlations are written in bold letters.
bP < .05.
cP < .001.
Figure 2.
Regression of IL-1RI, MSRI, and the IL-1RI/MSRI ratio in monocytes as well as IL-1RI, IL-1RII, and the IL-1RI/IL-1RII ratio on lymphocytes with age. All parameters are plotted versus age and linear regression lines were calculated separately for patients with AD (filled circles and continuous line) and symptomatic controls (empty circles, broken line). For none of the parameters, slopes differed significantly. The probability that the elevations of the 2 regression lines are identical is indicated as P value in the lower right corner of the diagram. AD indicates Alzheimer disease; IL-1RI, interleukin 1 receptor subtype I; IL-1RII, interleukin 1 receptor subtype II; MSRI, macrophage scavenger receptor I.
Elevated IL-1RI Expression and Reduced IL-1RII Expression on Lymphocytes in AD
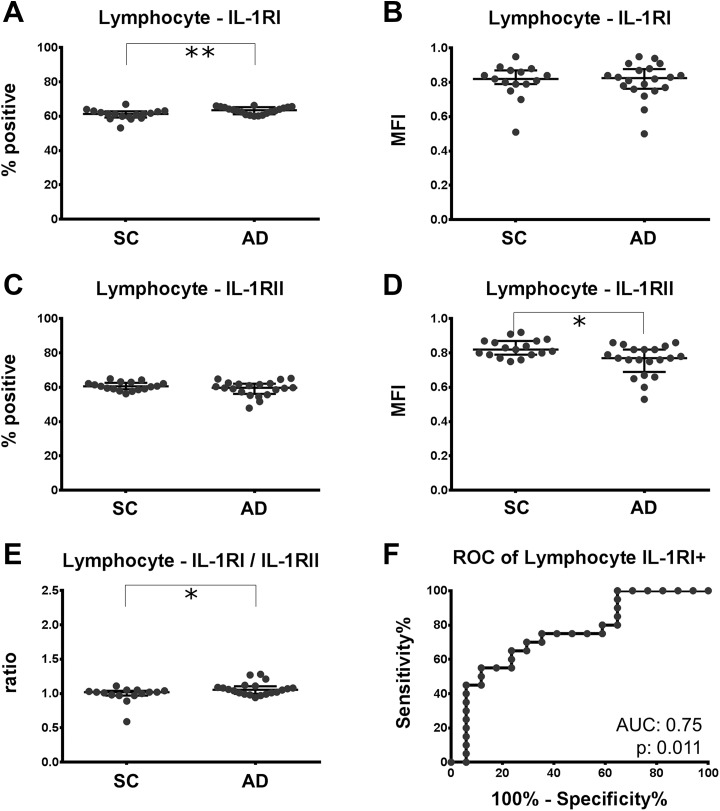
Among lymphocytes of patients with AD, the number of cells being positive for the IL-1RI was increased by 3.6% (61.3% [59.2-62.9] vs 63.5% [61.2-65.3]; P = .0097; Figure 3A). No change was observed concerning the MFI of IL-1RI on lymphocytes (Figure 3B). However, the MFI of the decoy receptor, IL-1RII, was reduced by 6.1% (0.82 [0.79-0.87] vs 0.77 [0.69-0.82]; P = .010) in patients with AD (Figure 3D) and the ratio of IL-1RI (MFI)/IL-1RII (MFI) was 3.9% higher in patients with AD than in the nondemented controls (1.02 [0.97-1.04] vs 1.06 [0.97-1.04]; P = 0.045; Figure 3E). Patients with AD with MCI had slightly higher IL-1RI/IL-1RII ratios than those with AD dementia (1.08 [1.05-1.12] vs 1.01 [0.98-1.06]; P = .021). Also, an intermediate positive correlation of the IL-1RI/IL-1RII ratio with the MMSE was found (ρ = .63, P = .003; Table 2). Receiver operating characteristic (ROC) analysis indicated that the fraction of IL-1RI-positive lymphocytes effectively discriminates AD from SC (AUC 0.75; P = .011, Figure 3F). For a cutoff value of 62% IL-1RI-positive lymphocytes, AD can be diagnosed with a sensitivity and specificity of 70.0% and 70.6%, respectively. No correlation of the lymphocyte IL-1 receptor expression with age was found, and the results remained robust when the 5 oldest patients with AD were removed from the analysis. Additionally, for the IL-1RI+ and IL-1RII (MFI) values, a linear regression versus age was calculated to exclude a confounding effect of the age difference between the groups (Figure 2). For both parameters and their ratio, the elevations but not the slopes differed significantly (IL-1RI+: P = .027; IL-1RII (MFI): P = .027; IL-1RI/IL-1RII: P = .024). This means, in the regression models with age and diagnosis as independent variables, and IL-1RI+, IL-1RI (MFI), and the IL1RI/IL1RII ratio as dependent variables, the contribution of age to the final models was not significant. The diagnosis contributed with P values of .027 for IL-1RI+, .026 for IL-1RII (MFI), and .024 for the IL-1RI/IL-1RII ratio, indicating a difference between the groups that is independent of age.
Figure 3.
Expression of IL-1RI is increased and expression of IL-RII is decreased on lymphocytes of patients with AD. PBMC of patients with AD and SC was labeled with antibodies directed against IL-1RI and IL-1RII. A, C, Fraction of lymphocytes staining positive for IL-1RI and IL1-RII. B, D, The mean fluorescence intensity (MFI) of IL-1RI and IL-1RII on lymphocytes. The ratio of IL-1RI/IL-1RII comparing the 2 populations is plotted in (E). F, The receiver operating characteristic curve (ROC) for the discrimination of patients with or without AD by the fraction of IL-1RI-positive lymphocytes. Trend: *P < .10; significance: *P < .05; **P < .01. AD indicates Alzheimer disease; IL-1RI, interleukin 1 receptor subtype I; IL-1RII indicates interleukin 1 receptor subtype II; IL-1RII, interleukin 1 receptor subtype II; MSRI, macrophage scavenger receptor I; PBMC, peripheral blood mononuclear cell; SC, symptomatic controls.
Increased Concentrations of Soluble IL-1RI and IL-1Ra in AD
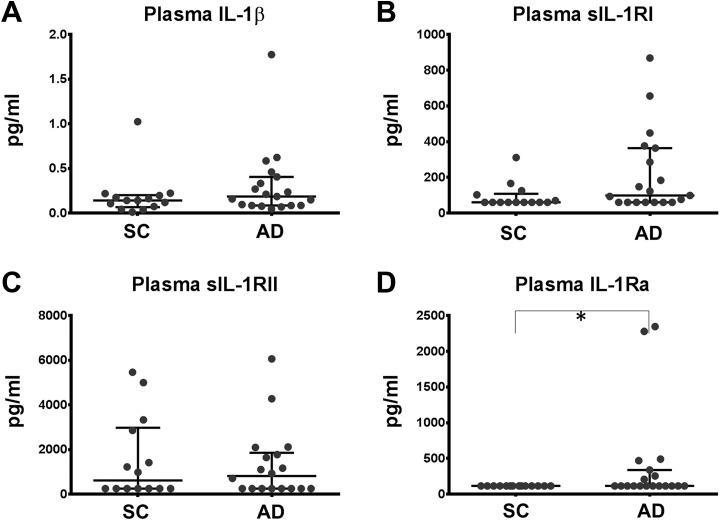
The ELISA was used to quantify IL-1β, sIL-1RI, sIL-1RII, and IL-1Ra in plasma samples of the same patients indicated above. For each assay, all samples were run in duplicates on one plate. Coefficients of variation were below 20%. To allow statistical evaluation of the data, measurements below the limit of quantification were set to the lower limit of quantification. The lower limit of quantification was 0.156 pg/mL for IL-1β, 60 pg/mL for sIL-1RI, 250 pg/mL for IL-1RII, and 115 pg/mL for IL-1Ra. For sIL-RI, 7 of the 19 AD samples and 8 of the 14 control samples were below the lower limit of quantification. For sIL1-RII, there were 8/19 AD samples and 7/14 control samples, for IL-1Ra 12/19 AD samples and 14/14 control samples below the lower limit of quantification.
Levels of IL-1β tended to be increased in AD. In accordance with the above-reported increased expression of cell-bound IL-1RI, not significantly increased levels of sIL-1RI were observed in AD. No change was observed for sIL-1RII. The IL-1Ra was not detectable in any patient of the control group, while 7 patients in AD group had measurements above the limit of detection. Thereby, concentrations of the IL-1Ra were significantly increased in AD (Table 3 and Figure 4). The concentration of sIL-1RII was negatively correlated with the expression of IL-1RI (rs: −0.55; P = .019) on monocytes, reflecting its anti-inflammatory properties. Likewise, the concentration of IL-1Ra was correlated with the expression of the MSRI (rs: 0.48; P = .036). No correlations of these parameters with established biomarkers, MMSE, or age were found (Table 2).
Table 3.
Concentrations of IL-1β, sIL-1RI, sIL-1RII, and IL-1Ra in Plasma of Patients With AD.a
| SC, n = 14 | AD, n = 19 | P Value | |
|---|---|---|---|
| IL-1β (pg/mL) | 0.141 [0.065-0.202] | 0.185 [0.084-0.405] | .209 |
| sIL-1RI (pg/mL) | 60 [60.0-107.9] | 98.4 [60.0-364.1] | .067 |
| sIL-1RII (pg/mL) | 615.7 [250-2977] | 816.1 [250-1853] | .952 |
| IL-1Ra (pg/mL) | 115.0 [115.0-115.0] | 115.0 [115.0-338.4] | .022 |
Abbreviations: AD, Alzheimer disease; IL-1β, interleukin 1β; IL-1Ra, interleukin 1 receptor antagonist; SC, symptomatic controls; sIL-1RI, soluble interleukin 1 receptor subtype I; sIL-1RII, soluble interleukin 1 receptor subtype II.
aResults are depicted as median [interquartile range]. Measurements below the lower limit of detection were arbitrarily set to the lower limit of quantification. Significance of differences is calculated with the nonparametric Mann-Whitney U test.
Figure 4.
Concentrations of IL-1β, sIL-1RI, sIL-1RII, and IL-1Ra in plasma of patients with AD. Levels of IL-1β, sIL-1RI, sIL-1RII, and IL-1Ra were quantified by ELISA in plasma. Results are depicted as median [interquartile ranges]. Significance was calculated with the nonparametric Mann-Whitney U test. Values below the lower limit of quantification were set to the lower limit of quantification (IL-1RI: 60 pg/mL, IL-1RII: 250 pg/mL, IL-1Ra: 115 pg/mL). *P < .05. AD indicates Alzheimer disease; IL-1Ra, interleukin 1 receptor antagonist; IL-1RI, interleukin 1 receptor subtype I; IL-1RII indicates interleukin 1 receptor subtype II; IL-1RII, interleukin 1 receptor subtype II; IL-1Ra, interleukin 1 receptor antagonist; SC, symptomatic controls; sIL-1RI, soluble interleukin 1 receptor subtype I; sIL-1RII, soluble interleukin 1 receptor subtype II.
Discussion
As signs of a proinflammatory phenotype of peripheral blood cells in AD, we found moderately elevated IL-1RI and reduced MSRI expression on monocytes as well as slightly elevated IL-1RI and reduced IL-1RII on blood lymphocytes. The fraction of IL-1RI-positive monocytes and lymphocytes separated patients with AD from SC with an AUC of 0.79 and 0.75, respectively. Changes in the expression of these markers were accompanied by elevated concentrations of IL-1Ra in plasma of patients with AD as measured by ELISA.
Although these results support our initial hypothesis, several limitations of the study must be acknowledged. First, the mean age in our control sample is 10 years lower than in the AD group. This difference occurred because of the continuous inclusion of the patients in our memory clinic. It is not surprising that younger patients referring to the memory clinic are more likely to be non-demented than the older ones are. However, when controlling for age with a regression analysis, the results were mainly confirmed. Only for the MSRI and the IL-1RI/MSRI ratio in monocytes, the regression analysis indicated a trend rather than a significant difference. Second, due to the small sample size, the power of this study was rather low. As plasma was not available for all the patients the sample size is even lower for the ELISA assays, leading to a missing significance in most of the tests. Third, measurements by flow cytometry are hard to standardize, especially when performed over a longer period of time. Thereby, the rather large variances can be explained.
One of the major strengths of this study is the use of SC as comparison group. Patients referring to a memory clinic are almost never asymptomatic and healthy. They suffer from all different kinds of neurodegenerative and non-neurodegenerative brain diseases. Therefore, from a clinician point of view, biomarkers differentiating other diseases from AD are of special interest. As the diagnoses in both study groups were made according to the biomarker based on A/T/N criteria, the diagnostic accuracy can still be considered to be very high. However, the missing correlation of the A/T/N parameters with our measures of the inflammatory process indicates once more that they are highly valuable trait markers but are weak as a surrogate for the state of the disease.
To monitor the systemic effects of the neuroinflammation in AD, one of the most intensively studied molecules in the blood is IL-1β.25 Fifty percent of the reports reviewed by Brosseron in 2014 show a significant upregulation of IL-1β in the blood of patients with AD, whereas the other half describe slightly elevated, statistically nonsignificant values.25 So our finding of slightly elevated IL-1β in the plasma of patients with AD is consistent with previous studies, indicating that IL-1β is elevated in AD. Among other effects, IL-1β signaling leads to increased expression of IL-1RI.26,27
We observed an increased fraction of IL-1RI expressing lymphocytes together with a reduced surface expression of IL-1RII. This was accompanied by an increased expression of IL-1RI and reduced expression of MSRI on monocytes. Although the magnitude of the differences is modest, both findings fit perfectly into the model of a systemic inflammatory status in AD, as the IL-1RI propagates a proinflammatory signal, while the IL-1RII is a decoy receptor limiting the activity of IL-1.14 Interestingly, the lymphocyte IL-1R1/IL-1RII ratio is positively correlated with the MMSE in patients with AD. Also, patients with MCI due to AD had a higher IL-1RI/IL-1RII ratio than those with dementia. This indicates that the proinflammatory shift in lymphocytes measured with the IL-1RI/IL-1RII ratio is especially high in the beginning of the disease. The cultivation of blood peripheral monocytes in the presence of lipopolysaccharide also increased the expression of IL-1RI, while reducing the number of IL-1RII receptors per cell.28 In analogy to the polarization of T cells, mononuclear phagocytes were divided into a M1 and M2 subgroup.12 Although this categorization is outdated, M1 and M2 macrophages can still be regarded as two extremes of a spectrum. M1 macrophages prototypically express high levels of IL-1RI, low levels of MSRI and secrete proinflammatory cytokines, whereas M2 monocytic cells express large amounts of IL-1RII and secrete anti-inflammatory mediators.12 Consequently, our finding of elevated IL-1RI and decreased MSRI on monocytes in AD indicates a polarization toward a proinflammatory phenotype.
Elevated levels of sIL-1RI and IL-1Ra indicate a simultaneous counterregulation to limit the inflammatory process. Secretion of the IL-1Ra and release of sIL-1RI are known to be induced by the same processes that trigger IL-1β and by IL-1β itself.26,29 During acute infections, the secretion of IL-1β precedes that of IL-1Ra.30 In chronic inflammatory processes, such as AD, a simultaneous increase in levels of IL-1β and IL-1Ra is the consequence. However, as detailed above, several problems and shortcomings of the ELISA measurements limit its validity. The low sample size, large interindividual variations, and, in several individuals, concentrations below the limit of detection increased the risk of false-negative results. Although the presence of more severe infections was excluded by measurement of CRP and leukocyte counts, the possibility remains that subclinical infections are responsible for these problems.
The reduced expression of MSRI on monocytes of patients with AD matches the finding of reduced phagocytic capacity of macrophages in AD.31 The MSRI is one of the main receptors for phagocytosis and seems to be involved in the clearance of Aβ-peptides/Aβ-plaques.16,17,32 Unfortunately, amyloid deposits trigger a proinflammatory polarization, which is associated with secretion of IL-1β and reduced expression of MSRI.18 Thereby, the presence of amyloid deposits activates mononuclear cells and, at the same time, prevents them from an effective phagocytosis.18 Furthermore, IL-1 stimulates the expression of the amyloid precursor protein, thus increasing the amyloid turnover.33 That is why our findings seem to be the consequence of a chronically activated immune system by amyloid deposits. In this case, MSRI as well as IL-1RI would be easy accessible markers that indicate the systemic proinflammatory state of monocytes as an additional aspect of AD pathophysiology.
With an AUC of 0.79 for the fraction of IL-1RI-positive monocytes and an AUC of 0.75 for lymphocytes, both parameters are not applicable as diagnostic biomarkers for AD. So far, several blood-derived biomarkers have been suggested. Among other were Aβ peptides, vascular cell adhesion molecule 1, ceramide, neurofilament light chain, and several cytokines.34-37 However, the diagnostic accuracy of those markers was also not yet sufficient or required the measurement of panels. While our markers are currently not suitable as diagnostic biomarkers, they may still support the existing diagnostic procedure by adding information on systemic inflammation as an additional aspect of AD pathophysiology. Therefore, a replication of our results in an age-matched sample using additional readouts such as messenger RNA expression is needed. It will also be interesting to see if these markers that reflect the dysregulation within the IL-1 system are exclusively altered in AD. As anti-inflammatory approaches to treat AD are under way, these markers may serve as a proximal readout to monitor the effect of these treatments.
Conclusion
In summary, the increased fraction of IL-1RI expressing monocytes and lymphocytes, together with the reduced surface expression of MSRI and IL-1RII, supports the hypothesis of a systemic inflammation in AD. However, the magnitude of the observed changes is too weak to allow an application as biomarkers.
Supplemental Material
Supplemental_Material for Analysis of Surface Levels of IL-1 Receptors and Macrophage Scavenger Receptor I in Peripheral Immune Cells of Patients With Alzheimer Disease by Philipp Spitzer, Johannes Weinbeer, Martin Herrmann, Timo Jan Oberstein, Mateja Condic, Piotr Lewczuk, Johannes Kornhuber and Juan Manuel Maler in Journal of Geriatric Psychiatry and Neurology
Footnotes
Authors’ Note: PS, JW, and JMM designed the study and drafted the manuscript. PS, JK, TO, PL, and JMM investigated the patients and collected the samples. PS, JW, TO, and MC carried out the experiments. MH, TO, JK, and JMM supervised and substantially supported the acquisition of data by providing vast experience. Statistics were carried out by PS, JW, TO, and JMM. All authors reviewed the manuscript critically and provided constructive comments to improve the quality of the manuscript. All authors read and approved the final manuscript.
Philipp Spitzer and Johannes Weinbeer contributed equally to this study. The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PL received consultation and lectures honoraria from Innogenetics, IBL International, and AJ Roboscreen.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by grants from the Interdisciplinary Center for Clinical Research (IZKF), Erlangen; PL is supported by the German Bundesministerium für Bildung und Forschung (grant 01ED1203D) within the BIOMARKAPD Project of the JPND. The research leading to these results has also received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement nr. 115372, resources of which are composed of financial contribution from the European Union’s Seventh Framework Program (FP7/2007-2013) and EFPIA companies’ in kind contribution. The present work was performed in fulfillment of the requirements for obtaining the degree “Dr. med” of JW.
ORCID iD: Philipp Spitzer  https://orcid.org/0000-0001-9555-605X
https://orcid.org/0000-0001-9555-605X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16(6):358–372. [DOI] [PubMed] [Google Scholar]
- 3. Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49(4):489–502. [DOI] [PubMed] [Google Scholar]
- 4. Patel NS, Paris D, Mathura V, Quadros AN, Crawford FC, Mullan MJ. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer’s disease. J Neuroinflamm. 2005;2(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stewart CR, Stuart LM, Wilkinson K, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11(2):155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martin E, Boucher C, Fontaine B, Delarasse C. Distinct inflammatory phenotypes of microglia and monocyte-derived macrophages in Alzheimer’s disease models: effects of aging and amyloid pathology. Aging Cell. 2017;16(1):27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68(10):930–941. [DOI] [PubMed] [Google Scholar]
- 8. Britschgi M, Wyss-Coray T. Systemic and acquired immune responses in Alzheimer’s disease. Int Rev Neurobiol. 2007;82:205–233. [DOI] [PubMed] [Google Scholar]
- 9. Mietelska-Porowska A, Wojda U. T Lymphocytes and inflammatory mediators in the interplay between brain and blood in Alzheimer’s disease: potential pools of new biomarkers. J Immunol Res. 2017. doi:10.1155/2017/4626540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wojda U. Alzheimer’s disease lymphocytes: potential for biomarkers? Biomark Med. 2016;10(1):1–4. [DOI] [PubMed] [Google Scholar]
- 11. Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78(2):151–156. [DOI] [PubMed] [Google Scholar]
- 12. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. [DOI] [PubMed] [Google Scholar]
- 13. Boraschi D, Tagliabue A. The interleukin-1 receptor family. Semin Immunol. 2013;25(6):394–407. [DOI] [PubMed] [Google Scholar]
- 14. Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39(6):1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kitazawa M, Cheng D, Tsukamoto MR, et al. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal beta-catenin pathway function in an Alzheimer’s disease model. J Immunol. 2011;187(12):6539–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382(6593):716–719. [DOI] [PubMed] [Google Scholar]
- 17. Frenkel D, Wilkinson K, Zhao L, et al. Scara1 deficiency impairs clearance of soluble amyloid-beta by mononuclear phagocytes and accelerates Alzheimer’s-like disease progression. Nat Commun. 2013;4:2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28(33):8354–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev. 2008;226:10–18. [DOI] [PubMed] [Google Scholar]
- 20. Godoy B, Murgas P, Tichauer J, Von Bernhardi R. Scavenger receptor class A ligands induce secretion of IL1beta and exert a modulatory effect on the inflammatory activation of astrocytes in culture. J Neuroimmunol. 2012;251(1-2):6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to establish a registry for Alzheimer’s disease (CERAD) clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacol Bull. 1988;24(4):641–652. [PubMed] [Google Scholar]
- 22. Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–629. [DOI] [PubMed] [Google Scholar]
- 23. Teunissen CE, Tumani H, Engelborghs S, Mollenhauer B. Biobanking of CSF. international standardization to optimize biomarker development. Clin Biochem. 2014;47(4-5):288–292. [DOI] [PubMed] [Google Scholar]
- 24. Jack CR, Jr, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brosseron F, Krauthausen M, Kummer M, Heneka MT. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: a comparative overview. Mol Neurobiol. 2014;50(2):534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watkins LR, Hansen MK, Nguyen KT, Lee JE, Maier SF. Dynamic regulation of the proinflammatory cytokine, interleukin-1beta: molecular biology for non-molecular biologists. Life Sci. 1999;65(5):449–481. [DOI] [PubMed] [Google Scholar]
- 27. Prieto GA, Snigdha S, Baglietto-Vargas D, et al. Synapse-specific IL-1 receptor subunit reconfiguration augments vulnerability to IL-1beta in the aged hippocampus. Proc Natl Acad Sci U S A. 2015;112(36):E5078–E5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vasilyev FF, Lopatnikova JA, Sennikov SV. Optimized flow cytometry protocol for analysis of surface expression of interleukin-1 receptor types I and II. Cytotechnology. 2013;65(5):795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bartfai T, Sanchez-Alavez M, Andell-Jonsson S, et al. Interleukin-1 system in CNS stress: seizures, fever, and neurotrauma. Ann N Y Acad Sci. 2007;1113:173–177. [DOI] [PubMed] [Google Scholar]
- 30. Spulber S, Bartfai T, Schultzberg M. IL-1/IL-1ra balance in the brain revisited – evidence from transgenic mouse models. Brain Behav Immun. 2009;23(5):573–579. [DOI] [PubMed] [Google Scholar]
- 31. Fiala M, Lin J, Ringman J, et al. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2005;7(3):221–232; discussion 255-262. [DOI] [PubMed] [Google Scholar]
- 32. Czirr E, Wyss-Coray T. The immunology of neurodegeneration. J Clin Invest. 2012;122(4):1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldgaber D, Harris HW, Hla T, et al. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci U S A. 1989;86(19):7606–7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F. Plasma amyloid-beta as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol. 2012;69(7):824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mielke MM, Bandaru VV, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging. 2010;31(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zuliani G, Cavalieri M, Galvani M, et al. Markers of endothelial dysfunction in older subjects with late onset Alzheimer’s disease or vascular dementia. J Neurol Sci. 2008;272(1-2):164–170. [DOI] [PubMed] [Google Scholar]
- 37. Gaiottino J, Norgren N, Dobson R, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One. 2013;8(9):e75091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_Material for Analysis of Surface Levels of IL-1 Receptors and Macrophage Scavenger Receptor I in Peripheral Immune Cells of Patients With Alzheimer Disease by Philipp Spitzer, Johannes Weinbeer, Martin Herrmann, Timo Jan Oberstein, Mateja Condic, Piotr Lewczuk, Johannes Kornhuber and Juan Manuel Maler in Journal of Geriatric Psychiatry and Neurology