Abstract
Breast carcinosarcoma is an extremely rare, clinically aggressive tumor, and no standard treatment has been established. We report about a 34-year-old woman presenting with a 2.5-cm-sized carcinosarcoma in her right breast. She presented to our hospital for examination of this mass. Ultrasonography showed a hypoechoic mass with partially irregular margins. Fine-needle aspiration cytology indicated malignancy. No enlarged lymph nodes or distant metastases were detected. We diagnosed right breast cancer and performed partial mastectomy, sentinel lymph node biopsy, and latissimus dorsi muscle flap transfer. Histological findings revealed that the tumor consisted of a mixture of an epithelial component and a mesenchymal component. The final diagnosis was carcinosarcoma. After undergoing adjuvant chemotherapy and radiotherapy, the patient has had no recurrence, and her cosmesis is maintained. Clinical data of carcinosarcoma are insufficient. Breast conservation and reconstruction for carcinosarcoma may be suitable as local treatments; however, the most appropriate treatment method has not been established.
Keywords: Carcinosarcoma, breast-conserving surgery, reconstruction
Introduction
Breast carcinosarcoma is an extremely rare tumor, accounting for 0.08%–0.2% of all breast malignancies.1 This tumor comprises a carcinoma element and a sarcoma-like element; however, its clinical behavior may differ from both carcinomas and sarcomas. No standard treatment has been established, and the prognosis is poorer than that of other metaplastic carcinoma types. The cumulative 5-year survival rate is 49%.2 Here, we report our experience with a case of breast carcinosarcoma, including clinical and pathological findings.
Case
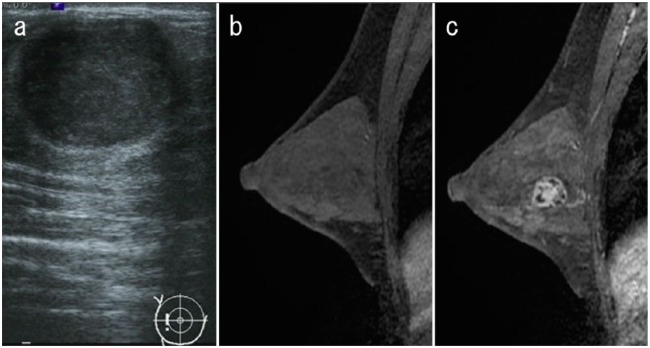
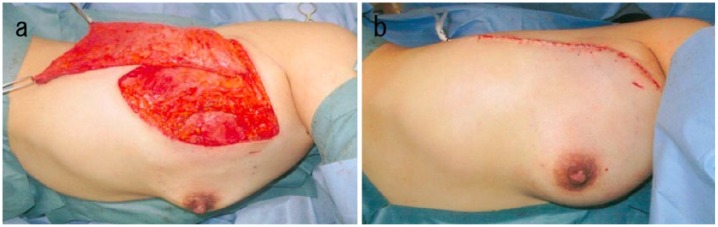
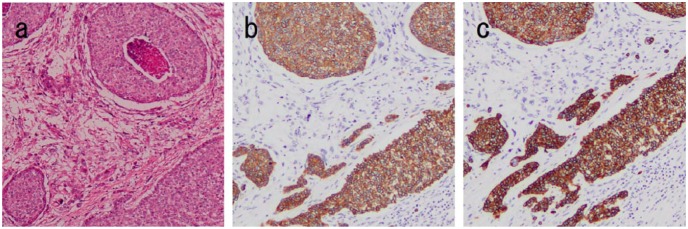
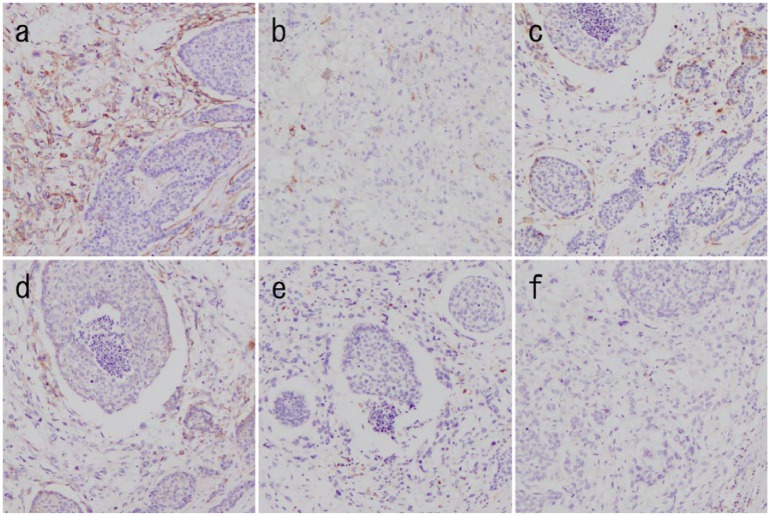
A 34-year-old woman was admitted to our hospital owing to a mass in the right breast that she had noted a month prior. She had no family history of cancer or sarcoma. She was breastfeeding, therefore, she did not undergo mammography. Physical examination showed a firm, well-defined, oval mass in the upper outer quadrant of the right breast. Ultrasonography showed a circumscribed hypoechoic mass measuring 23 × 19 × 17 mm3 that was oval in shape, with a parallel orientation and posterior acoustic enhancement (Figure 1(a)). Enhanced breast magnetic resonance imaging (MRI) revealed an oval mass with enhancing internal septation, an early peak, and a delayed washout pattern (Figure 1(b) and (c)). Fine-needle aspiration cytology of the mass revealed epithelial cells with nuclear atypia, indicating malignancy. Computed tomography (CT) did not reveal axially enlarged lymph nodes or distant metastasis. We performed partial mastectomy, sentinel lymph node biopsy, and latissimus dorsi muscle flap transfer (Figure 2(a) and (b)). Macroscopically, the inside of the tumor was necrotic or bleeding. Microscopically, there was a sarcoma component with necrosis or bleeding in the tumor center, with an invasive carcinoma wrapped around it. A boundary line between the carcinoma and the sarcoma clearly divided the epithelial component and interstitial component. The epithelial element was mitotic and comprised atypical cells composed of glandular formation. The mesenchymal element contained sheets of spindle-shaped cells (Figure 3(a)). No metastasis was found in the sentinel lymph nodes. Immunohistochemically, the epithelial element was positive for cytokeratins (AE1/3 and MNF-116) (Figure 3(b) and (c)) but negative for myoepithelial markers (smooth muscle actin (SMA) and calponin). The mesenchymal element was positive for SMA, h-caldesmon, calponin, and HHF-35 and negative for AE1/3, MNF-116, S-100, and desmin (Figure 4(a)–(f)). We diagnosed the patient with carcinosarcoma comprising tubular carcinoma and leiomyosarcoma. The epithelial element was negative for the estrogen receptor and HER2 and positive for the progesterone receptor. The Ki-67 expression was 75.7%.
Figure 1.
Imaging findings. (a) Ultrasonography showed a circumscribed hypoechoic mass. (b) Magnetic resonance imaging (MRI) before enhancement did not show details of the tumor. (c) Enhanced MRI showing a mass with enhancing internal septation, early peak.
Figure 2.
Breast reconstruction. (a) Creating a latissimus dorsi flap after partial mastectomy. (b) State at the time of the end of surgery.
Figure 3.
Pathological findings (magnification × 100). (a) The specimen shows proliferation of both epithelial and mesenchymal components (hematoxylin and eosin staining). The tumor is positive for (b) AE1/3 staining and (c) MNF-116 staining.
Figure 4.
Pathological findings (magnification × 100). The tumor is positive for (a) smooth music actin, (b) h-caldesmon, (c) calponin, and (d) HHF-35 staining and negative for (e) s-100 and (f) desmin staining.
Although the possibility of hereditary breast cancer was considered, she did not wish to undergo genetic counseling. The patient received adjuvant radiotherapy (50 Gy) and chemotherapy with 5-fluorouracil, epirubicin, and cyclophosphamide, followed by weekly paclitaxel. She also received hormonal therapy with tamoxifen, but she stopped after 2 years because of abnormal vaginal bleeding. She is doing well 5 years after surgery, with no recurrence.
Discussion
The definition of true carcinosarcoma is a biphasic malignant tumor with a carcinomatous and mesenchymal component, without a transitional zone between the two components.3Breast carcinosarcoma is classified as metaplastic carcinoma, along with squamous cell carcinoma; adenocarcinoma with spindle cell proliferation; adenosquamous, including mucoepidermoid carcinoma, and mixed epithelial and mesenchymal carcinoma. Mixed epithelial and mesenchymal carcinoma subtypes include carcinoma with chondroid metaplasia, carcinoma with osseous metaplasia, and carcinosarcoma.4 The origin of breast carcinosarcoma is controversial; however, recent studies have reported that carcinosarcoma is derived from single stem cells that become myoepithelial cells with biphasic differentiation.5–7
Carcinosarcoma usually forms firm, well-defined, solid, nodular tumors. Some examinations show benign imaging features such as a round shape and circumscribed margins.3 Even in our case, these morphological features were similar. The tumor margin was nearly smooth, and at first glance, it appeared to be fibroadenoma. We were able to diagnose malignant cells using needle cytology; however, there are some reports that state it is difficult to diagnose depending on the proportion of the tumor component.8,9 Therefore, the biopsy site should be carefully considered.
Breast carcinosarcomas are usually negative for hormone receptors and HER2 and are therefore classified as triple-negative breast cancer. They are also poorly differentiated and tend to have a high Ki-67 proliferation index.10 In our case, only PgR was positive; however, it is unclear whether it affected a good prognosis in the same way as invasive cancer.
A standard treatment strategy for breast carcinosarcoma has not yet been established, and current treatment strategies are similar to those for invasive ductal carcinoma. Modified radical mastectomy, with or without axillary dissection, is generally performed because it is an efficient and practical treatment for carcinosarcoma, except for patients with advanced stage disease.11 Several studies have reported about modified radical mastectomy for carcinosarcoma, with many cases having a tumor size of >2 cm at diagnosis.11–13 Several cases of breast-conserving surgery for tumor carcinosarcoma with small tumor size have also been reported.14,15 Dave et al.16 retrospectively reviewed 43 patients with metaplastic breast cancer and found that the 5-year local recurrence-free, disease-free, and overall survival rates were not significantly different between patients who underwent modified radical mastectomy and those who underwent breast-conserving surgery. Moreover, they suggested that radiation is effective for metaplastic breast cancer as an adjuvant therapy, which is similar to that commonly reported for invasive cancer. These previous reports suggest that making a negative excision margin, regardless of the surgical procedure type, is important, and if it is an early diagnosis, it is possible to safely perform breast-conserving surgery. We diagnosed general invasive breast duct carcinoma before surgery and there was hope for cosmesis from the patient; therefore, we performed breast-conserving surgery and autologous tissue reconstruction. Five years after surgery, no local recurrence has occurred, and cosmesis is maintained. Although carcinosarcoma is considered an aggressive tumor, our case suggests that depending on the tumor size, treatments that maintain tolerability can be selected.
Both hematogenous and lymphogenous metastases have been reported, and the most common site of distant metastasis is the lung, followed by the bone and liver. Axillary lymph node metastasis occurs in 26% of cases and can arise from either the carcinomatous or sarcomatous component.2 Therefore, sentinel node biopsy or axillary dissection is suggested. In contrast, various chemotherapy treatments for carcinosarcoma have been performed, with no reports of any notable effects. Taxane- and anthracycline-based regimens for invasive ductal carcinoma have been commonly used; however, their effects are limited. Rayson et al.17 examined 13 patients with distant metastasis of metaplastic carcinoma who received various chemotherapy regimens, among whom only 1 showed a partial response to adriamycin. Chen et al.18 reported that of 11 patients with advanced stage (T3–4) tumors who received neoadjuvant chemotherapy with paclitaxel or fluorouracil, 2 showed a partial response. These reports suggest that anthracycline and taxane chemotherapy have a partial therapeutic effect on tumors. Furthermore, these agents are used as adjuvant therapy for hormone receptor-negative invasive ductal carcinoma; therefore, we used these drugs. We conduct CT examinations every year for our patient, and no evidence of distant metastasis has been found 5 years after surgery. We are continuing careful follow-up of the patient.
Conclusion
Breast carcinosarcoma is rare, and current knowledge of this disease is insufficient. As local treatments, conserving therapy and reconstruction may also be selected. More studies are required to establish an appropriate treatment strategy, in particular, more reports on systemic therapy and surveillance are needed.
Acknowledgments
The authors thank Editage (www.editage.jp) for English language editing.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Hirofumi Kanaizumi  https://orcid.org/0000-0001-8364-5493
https://orcid.org/0000-0001-8364-5493
References
- 1. Rosai J. Special techniques in surgical pathology. In: Rosai J. (ed.) Rosai and Ackerman’s surgical pathology. 9th ed. St. Louis, MO: Mosby, 2004, pp. 1810–1812. [Google Scholar]
- 2. Wargotz ES, Norris HJ. Metaplastic carcinomas of the breast. III. Carcinosarcoma. Cancer 1989; 64: 1490–1499. [DOI] [PubMed] [Google Scholar]
- 3. Fletcher C. Tumors of the upper respiratory tract. In: Fletcher C. (ed.) Diagnostic histopathology of tumors. 4th ed. London: Churchill Livingstone, 2007, pp. 1100–1102. [Google Scholar]
- 4. Cakir A, Gonul II, Uluoglu O. Metaplastic breast carcinomas and their relationship with basal-like phenotype. Turk Patoloji Derg 2012; 28: 134–141. [DOI] [PubMed] [Google Scholar]
- 5. Rosen PP. Carcinoma with metaplasia. In: Rosen PP. (ed.) Rosen’s breast pathology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2016, pp. 425–454. [Google Scholar]
- 6. Zhuang Z, Lininger RA, Man YG, et al. Identical clonality of both components of mammary carcinosarcoma with differential loss of heterozygosity. Mod Pathol 1997; 10: 354–362. [PubMed] [Google Scholar]
- 7. Teixeira MR, Qvist H, Bohler PJ, et al. Cytogenetic analysis shows that carcinosarcomas of the breast are of monoclonal origin. Genes Chromosomes Cancer 1998; 22: 145–151. [PubMed] [Google Scholar]
- 8. Yang YF, Liu J, Fang ZY, et al. Clinical features and prognosis of 25 cases of breast carcinosarcoma. Zhonghua Zhong Liu Za Zhi 2012; 34: 620–623. [DOI] [PubMed] [Google Scholar]
- 9. Tian W, Xu D. Diagnosis and management of multiple carcinosarcoma of the breast in a young Chinese patient. Breast Care (Basel) 2012; 7: 147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hennessy BT, Giordano S, Broglio K, et al. Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann Oncol 2006; 17: 605–613. [DOI] [PubMed] [Google Scholar]
- 11. Tokudome N, Sakamoto G, Sakai T, et al. A case of carcinosarcoma of the breast. Breast Cancer 2005; 12: 149–153. [DOI] [PubMed] [Google Scholar]
- 12. Wada H, Enomoto T, Tsujimoto M, et al. Carcinosarcoma of the breast: molecular-biological study for analysis of histogenesis. Hum Pathol 1998; 29: 1324–1328. [DOI] [PubMed] [Google Scholar]
- 13. Laky D, Penciu M. Mammary carcinosarcoma with osteochondrosarcomatous differentiations. Rom J Morphol Embryol 1993; 39: 149–152. [PubMed] [Google Scholar]
- 14. Esbah O, Turkoz FP, Turker I, et al. Metaplastic breast carcinoma: case series and review of the literature. Asian Pac J Cancer Prev 2012; 13: 4645–4649. [DOI] [PubMed] [Google Scholar]
- 15. Haruki T, Maeta H, Sawazumi Y, et al. Biphasic metaplastic sarcomatoid carcinoma of the breast: report of a case. Breast Cancer 2009; 16: 229–233. [DOI] [PubMed] [Google Scholar]
- 16. Dave G, Cosmatos H, Do T, et al. Metaplastic carcinoma of the breast: a retrospective review. Int J Radiat Oncol Biol Phys 2006; 64: 771–775. [DOI] [PubMed] [Google Scholar]
- 17. Rayson D, Adjei AA, Suman VJ, et al. Metaplastic breast cancer: prognosis and response to systemic therapy. Ann Oncol 1999; 10: 413–419. [DOI] [PubMed] [Google Scholar]
- 18. Chen IC, Lin C, Huang CS, et al. Lack of efficacy to systemic chemotherapy for treatment of metaplastic carcinoma of the breast in the modern era. Breast Cancer Res Treat 2011; 130: 345–351. [DOI] [PubMed] [Google Scholar]