Abstract
The association of folinate salts with 5-fluorouracil (5-FU) represents a gold standard in the treatment of many cancers. In several clinical trials, the simultaneous administration of calcium–folinic acid (Ca-FA) and the prolonged infusion of 5-FU resulted in a better clinical response compared with fluoropyrimidine alone and 5-FU bolus. However, the simultaneous infusion of 5-FU and Ca-FA mixed in the same infusion pump is hindered by the crystallization of calcium salts, which eventually leads to catheter obstruction and damage. The sodium salt of leucovorin-disodium levofolinate (Na-Lv) is a novel molecule with a pharmacological profile similar to Ca-FA. Owing to its higher solubility, it can be safely mixed with 5-FU in a single pump without the risk of precipitation and catheter occlusion. The efficacy and safety of Na-Lv have been widely examined in preclinical and clinical phase II studies in combination with various schedules of 5-FU and in several cancer types. PubMed, EMBASE, SCOPUS and Web of Science databases were searched from inception to November 2018 to retrieve available published phase I and II series, including Western patients. Compared with Ca-FA, Na-Lv shows a more favourable efficacy and toxicity profile in terms of overall response rate, progression-free survival, time to progression and occurrence of severe adverse events. Moreover, it allows treatment time to be shortened, decreasing the number of required human resources for drug administration and limiting the occurrence of catheter damage.
Keywords: 5-fluorouracil, calcium-folinic acid, concomitant infusion, disodium levofolinate, solubility, synergism
Introduction
Since the beginning of its development, 5-fluorouracil (5-FU) has played a central role in the therapy of many solid cancers and is still a common therapeutic option especially in metastatic and adjuvant setting of colorectal and gastric tumours.1,2
5-FU is an antimetabolite, acting during the S-phase of the cell cycle.1 It exerts its anticancer effects through the inhibition of thymidylate synthase (TS) and the incorporation of its metabolites into RNA and DNA, thus inducing to cell death.1
Initially, 5-FU was administered alone as an intravenous bolus or as a short-time infusion; however, it became soon clear that the association with folinic acid compounds strengthened its anticancer activity and allowed dose escalation.3 Indeed, the concomitant presence of folate with 5-FU persistently inhibits the TS catalytic activity, leading to a consistent slowdown of DNA synthesis and tumour cell replication.
Since 1986, when the advantages of combining calcium–folinic acid (Ca-FA) with 5-FU were described for the first time, the association of these compounds has become a gold standard in cancer treatment.4,5 In 1991, a 24-hour simultaneous infusion of 5-FU and Ca-FA was proposed as a novel administration schedule.6 The prolonged 5-FU infusion in combination with Ca-FA allowed increasing the 5-FU dose up to 2600 mg/m2 weekly.7 This schedule achieved a better clinical response compared with both fluoropyrimidine alone and 5-FU bolus, and it was favourable even as a second-line treatment after failure of conventional 5-FU bolus.7–10 These advantages may be explained by the different mechanism of action observed between bolus and combination: 5-FU bolus affects cancer cells by interfering with RNA metabolism, whereas the combination with Ca-FA prolongs the inhibition of TS activity, thus improving both effectiveness and capability to overcome tumour resistance against 5-FU.10,11
In the first trial, 5-FU and Ca-FA were mixed for a 24-hour infusion in a single pump; however, during the simultaneous infusion, Ca-FA and 5-FU precipitated as calcium carbonate and clogged catheters. Therefore, the single pump system was early replaced by two separate pumps, one for each compound, to avoid catheter clogging.6,11,12 In the later trials, the schedule was further modified as a sequential administration of a 2-hour infusion of Ca-FA followed by 5-FU administered first as a bolus injection, then as a 24-hour infusion.3 The sequential administration of Ca-FA followed by 5’-FU has become the standard in clinical practice, even though it no longer achieved the promising results of the original schedule.2,5
Progress in drug development lead to the formulation of disodium levofolinate (Na-Lv), a compound with similar pharmacological properties to Ca-FA,3,13 but with higher solubility. Na-Lv can be safely mixed with 5-FU in a single pump without the risk of precipitation and catheter occlusion13; in addition, it can shorten the administration time for the two drugs, requiring less human resources compared with sequential administration, it reduces discomfort for patients and increases their compliance to treatment.14 The availability of Na-Lv had offered also the opportunity to re-test the administration of the original schedule proposed by Ardalan6 in clinical trials, obtaining favourable results and toxicity overlapping to that previously reported for combination of 5-FU and Ca-FA.10,11,15–24
Methods
Electronic databases including PubMed, EMBASE, SCOPUS and Web of Science were searched from inception to November 2018.
The term used were ‘disodium folinate’ OR ‘sodium folinate’ OR ‘disodium folinic acid’ OR ‘sodium folinic acid’.
All original articles and previous reviews were evaluated for relevant references. Available phase I and II published series including Western patients were evaluated. Only papers published in English language were selected.
Results
Pharmacology, mechanism of action and pharmacokinetics of disodium levofolinate
Pharmacological properties
Folinic acid, also known as citrovorum factor, is the 5-formyl derivative of tetrahydrofolic acid. Levofolinic acid, sometimes also called levoleucovorin, with the systematic name N-[4-({[(6S)-2-amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydro-6pteridinyl]methyl}amino) benzoyl]-L-glutamic acid is the biologically active L-isomer of the racemic folinic acid. It is involved in various metabolic processes, including amino acid metabolism, purine synthesis and pyrimidine nucleotide synthesis.25 In 2008, the FDA approved L-isomer of folinic acid (as levofolinic acid) for pharmacological use.26
Within cells, levofolinic acid is readily converted into other reduced folic acid derivatives, such as tetrahydrofolate.26 Folinic acid was found to re-activate the dihydrofolate reductase itself even in the presence of dihydrofolate reductase antagonists, such as methotrexate.27 Although the mechanism is still not clear, the polyglutamylation of methotrexate and dihydrofolate in malignant cells plays a significant role in selective re-activation of dihydrofolate reductase by folinic acid.28
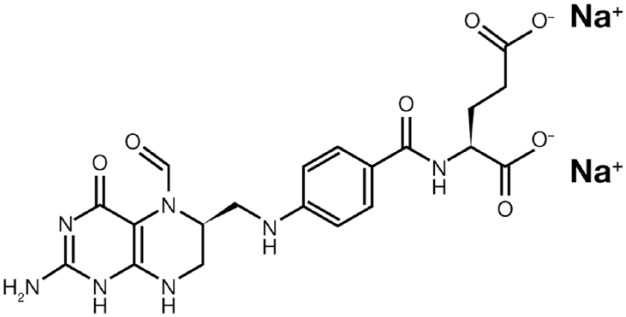
Na-Lv with the chemical formula Na2C20H21N7O7 (Figure 1) is a novel formulation of folinic acid,11,15 used in combination with 5-FU in chemotherapy treatments of solid tumours.3,11,15–17,20,21 Na-Lv is also indicated to diminish the toxicity and counteract the action of folic acid antagonists, such as methotrexate, in cytotoxic therapy and overdose.29,30
Figure 1.
Chemical two-dimensional structure of disodium levofolinate.
Pharmacodynamics
5-FU, alone or in combination, is the most predominant and effective chemotherapeutic agent for the treatment of solid cancers.31 5-FU binds TS and causes its irreversible inhibition, thus blocking DNA synthesis.32 In combination with Na-Lv, 5-FU covalently binds TS, and forms a ternary complex, including TS, 2’-deoxy-uridine-5’-mono-phosphate, and [6R]-5,10-methylene-tetrahydrofolate.33–35 This results in a persistent blockade of TS with enhanced inhibition of DNA biosynthesis.3,32,34
Pharmacokinetics and metabolism
Since peer-reviewed publications about Na-Lv pharmacokinetics in humans are not available, data in this section are mostly based on the information of the Electronic Medicines Compendium internet pages.30 After intravenous administration of Na-Lv, plasma levels of levofolinic acid and 5-methyl-tetrahydrofolic acid peak. In the blood, about 27% of levofolinic acid is bound to proteins and its half-life is 0.5 h. Levofolinic acid is quickly metabolized in the liver to 5-methyl-tetrahydrofolic acid which has a half-life of 6.5 h. This conversion is probably not dependent on the presence of dihydrofolate reductase. Furthermore, nearly 20% of the intravenously administrated levofolinic acid is secreted in the urine, without being metabolized. The clearance rate for levofolinic acid is about 205 ml/min.30
Preclinical evidence and clinical studies
Disodium and calcium formulations of levofolinate are bioequivalent in terms of plasma concentration and half-life. However, Na-Lv can be simultaneous administrated with 5-FU, owing to the highest solubility.3,11,15 The differences between sequential and simultaneous administration of 5-FU with either Ca-FA or Na-Lv have been investigated in human colon cancer cells and in xenograft nude mice.3 In vitro data indicated that the simultaneous administration of both Ca-FA and Na-Lv with 5-FU enhanced the antiproliferative effect of 5-FU and induced apoptosis in cancer cells.3 However, a remarkable difference between the two folinate salts was described: Na-Lv and 5-FU showed a synergistic action in both 24-hour and 72-hour simultaneous administration, whereas Ca-FA had just an additive effect to 5-FU activity.3 Surprisingly, the sequential infusion of Na-Lv or Ca-FA and 5-FU had an antagonistic action.3 This opposite effect was mainly due to different regulation on gene expression: simultaneous combination of Na-Lv and 5-FU inhibited the expression of TS gene, whereas sequential administration resulted in a reduced expression of the soluble carrier 19A member 1 gene, the major folate transporter in mammalian cells.3,36 Low levels of folate transporter reduced intracellular folate concentration and impaired the stability of ternary complex that blocks TS.3,33,34 In vivo experiments confirmed the impact of simultaneous administration of Na-Lv and 5-FU to strengthen antitumor activity3 and, consistently, a low expression of the soluble carrier 19A member 1 gene correlated to poor response to Na-Lv and 5-FU.36
Na-Lv was investigated in several phase I and II trials in different cancer subtypes.10,11,15–24 Nine prospective phase I and II studies using Na-Lv have been conducted so far (Table 1), all including patients affected by gastrointestinal cancers. Four of them were randomized studies.11,15,16,24
Table 1.
Overview of clinical trials.
| Reference | Cancer type | Participants (N) | Treatment | Phase | Line | Results (efficacy) | Toxicities G3–G4 (%) |
|---|---|---|---|---|---|---|---|
| Hartung et al.11 | mCRC | 51 | 5-FU (2600 mg/m2 c.i. 24 h, weekly) + Na-Lv (500 mg/m2) dissolved in single pump for 6 weeks, q8w | II random | I | mOS 16.5 mo mTTP 8.5 mo ORR 37% |
Diarrhoea 25 Stomatitis 8 Cardiac toxicity 4 |
| Kuhfhahl et al.15 | mCRC | 42 | 5-FU (2600 mg/m2 c.i. 24 h weekly), Na-Lv (500 mg/m2) c.i. 6w, q8w | II | II | mOS 14.7 mo mTTP 5.7 mo ORR 10% |
Diarrhoea 19 Hand–foot syndrome 12 Stomatitis 7 |
| Gnad-Vogt et al.19 | mGC | 27 | 5-FU (2000 mg/m2 weekly) + Na-Lv (500 mg/m2) d 1,8, 15, 22, 29, 36 + L-doxo (20 mg/m2) d 1,29 + MMC (7 mg/m2) bolus d 8,36 | I | Any | mOS 14.7 mo mTTP 8.1 mo ORR 47% |
Anaemia 12 Leukopenia 8 Febrile neutropenia 4 Thrombo 12 Nausea 15 Diarrhoea 8 Mucositis .4 Haemolytic-uremic syndrome 4 |
| Hofheinz et al.21 | mGC/PC/BT/ unknown origin |
27 | 5-FU (2000 mg/m2 c.i. 24 h, weekly) + Na-Lv (500 mg/m2) dissolved in single pump for 6 weeks + MMC (7 mg/m2) bolus d 8,36 + L-doxo (15-25-30-35 mg/m2) d 1,29 | I | I–II–III | mOS GC (I line) NR mOS PC (II/III line) 6.5 mo ORR 24% |
With L-doxo 20 mg/m2
Leukopenia 12 Thrombocytopenia 6 Nausea/vomiting 29 Diarrhoea 18 Stomatitis/mucositis 12 |
| Wolff et al.23 | mEC | 25 | AIO regimen | II | I | mOS 13.6 mo mTTP 6.6 mo ORR 33% |
Leukopenia 33 Febrile neutropenia 19 Thrombocytopenia 7 Diarrhoea 12 Nausea 4 Vomiting 4 Thromboembolism 8 Hyponatraemia 8 |
| Moehler et al.20 | mGC | 49 | Cetuximab (400 mg/m2, then 250 mg/ m2) + irinotecan (80 mg/m2) + Na-Lv (200 mg/m2) and 5-FU (1500 mg/m2) c.i. 24 h d 1,8,15,22,29,36 of a 50-day cycle | II | I | mOS 16.5 mo mPFS 9.0 mo ORR 46% DCR 79% |
Diarrhoea 15 Skin toxicity 8 Infection 8 Allergy 6 |
| Bleiberg et al.16 | mCRC | 57 | Arm A: Ca-FOLFIRI or Ca-FOLFOX Arm B: Na-FOLFIRI or Na-FOLFOX |
II random | I | mOS 11.9 mo (Arm A) versus 22.9 mo (Arm B), p = 0.02 mPFS 8.0 mo (Arm A) versus 11.5 mo (Arm B), p = NS ORR 55% (Arm A) versus 35% (Arm B), p = 0.02 |
Arm A: Neutropenia 14 Nausea 7 Arm B: Neutropenia 18 Diarrhoea 7 |
| Moehler et al.18 | mGC | 91 | Arm A: Na-FOLFIRI +SUNITINIB* Arm B: Na-FOLFIRI+PLACEBO *(starting dose 25 mg/day orally for 4 weeks, q 6 weeks ) |
II random | II or III | mOS 10.4 mo (Arm A) versus 8.9 mo (Arm B) p = 0.21 one sided mPFS 3.5 mo (Arm A) versus 3.3 mo (Arm B) p = 0.66 |
Arm A: Neutropenia 56 Leukopenia 20 Nausea 7 Vomiting 7 Arm B: Neutropenia 27 Leukopenia 16 Diarrhoea 13 Fatigue 9 Pain 9 |
| Wein et al.24 | mCRC | 59 | AIO regimen | II random | II | mPFS 4.2 mo mOS 25 mo ORR 10% |
Leukopenia 5 Thrombocytopenia 2 Diarrhoea 14 |
AIO, ArbeitsgemeinschaftInternistischeOnkologie; BT, biliary tract cancer; Ca.LV, calcium levofolinate; c.i., continuous infusion; CRC, colorectal cancer; d, days; DCR, disease control rate; EC, oesophageal cancer; FA, folinic acid; G, grade; GC, gastric cancer; L-doxo, liposomal doxorubicin; m, metastatic; mCRC, metastatic colorectal cancer; MMC, mitomycin-C; mo, months; mOS, median overall survival; mPFS, median progression-free survival; mTTP, median time to progression; Na-Lv, disodium levofolinate; ORR, overall response rate; PC, pancreatic cancer; PFS, progression-free survival; pts, patients, q, every; q8w, every 8 weeks; w, weeks; 5-FU, 5-fluorouracil;
Ca FOLFIRI: Irinotecan 180 mg/mq g1, CA/LV 400 mg/mq g1, 5-FU bolus 400 mg/mq g1, 5-FU c.i. 3000 mg/mq g1 over 46 h every 14 days.
Ca FOLFOX: Oxaliplatin 100 mg/mq g1, CA/LV 400 mg/mq g1, 5-FU bolus 400 mg/mq g1, 5-FU c.i. 3000 mg/mq g1 over 46 h every 14 days.
Na FOLFIRI: Irinotecan 180 mg/mq g1, NA-Lv 400 mg/mq g1, 5-FU bolus 400 mg/mq g1, 5-FU c.i. 3000 mg/mq g1 over 46 h every 14 days.
Na FOLFOX: Oxaliplatin 100 mg/mq g1, NA-Lv 400 mg/mq g1, 5-FU bolus 400 mg/mq g1, 5-FU c.i. 3000 mg/mq g1 over 46 h every 14 days.
AIO regimen: irinotecan (80 mg/m²) as 1-hour infusion followed by 5-FU (2000 mg/m²) combined with FA (500 mg/m²) as 24-hour infusion (d1, 8, 15, 22, 29, 36, qd 57).
In 51 patients with metastatic colorectal cancer (mCRC), the therapeutic efficacy of a 24-hour infusion with 5-FU and Na-Lv was compared with Ardalan’s schedule in the first-line setting.11 All patients received 5-FU (2600 mg/m2) and Na-Lv (500 mg/m2) combined in a single infusion pump, applied once a week for 24 h over 6 weeks with a 2-week rest (8-week cycle) until disease progression.11 The time to progression was 8.5 months, longer than that previously reported in similar populations (6–7 months).36–38
The same regimen was investigated as a second-line therapy in 42 patients with mCRC, first-line treated with 5-FU bolus.15 Na-Lv at dose of 500 mg/m2 was mixed in the same portable pump to 2600 mg/m2 5-FU as intravenous infusion over 24 h, once a week in an 8-week cycle.15 More than 100 cycles were administered without any catheter occlusion, demonstrating a full feasibility of simultaneous infusion.15 The overall response rate (ORR = 9%) was in the range of comparable regimens that use sequential administration of Ca-FA and 5-FU, whereas median overall survival (mOS = 14.7 months) was more favourable than that reported in previous second line trials.15,36–38
A further phase II study compared the efficacy and safety of simultaneous infusion of Na-Lv and 5-FU versus sequential administration of Ca-FA, followed by 5-FU combined with irinotecan or oxaliplatin in previously untreated mCRC patients.16 Both treatments achieved similar ORR and median progression-free survival (mPFS), with a favourable trend for Na-Lv arm; the median overall survival (mOS) was significantly longer in the Na-Lv arm (11.9 months versus 22.9 months, p = 0.02), probably due to an unidentified bias in patients management during second-line treatment or follow-up.16 In the last few years, the infusion of Na-Lv with 5-FU has been largely tested in association with other chemotherapeutic and molecular-target agents, showing efficacy and safety profile overlapping with those observed with Ca-FA.17–24
Treatment with Na-Lv at 500 mg/m2 dose in association with 5-FU 2000 mg/m2 in continuous infusion over 24 h once weekly for 6 weeks was compared with oral capecitabine in the treatment of advanced breast cancer.17 In a retrospective series, mPFS was 8.6 months and mOS 18.5 months. The toxicity profile was better with Na-Lv and 5-FU; no dose-limiting adverse events (AEs) typical of capecitabine, such as diarrhoea or hand–foot syndrome, were reported.17 Na-Lv and 5-FU at the same doses were associated with irinotecan at 80 mg/m2 (1-hour infusion) and administered on days 1, 8, 15, 22, 29 and 36 in an 8-week cycle. This schedule (AIO regimen) was administered to patients with advanced gastroesophageal adenocarcinoma.22 In 76 patients, a mPFS of 5.3 months and mOS of 11.2 months were retrospectively determined; 16 patients had a downstaging in the disease extension and underwent a secondary resection (for metastatic disease) reaching a significant mOS of 23.7 months. The treatment had tolerable toxicity: diarrhoea (17%) and infections (12%) were the most frequent grade 3/4 (G 3/4) AEs.22
The AIO regimen in association with irinotecan was tested in advanced and pretreated mCRC (after failure of the AIO regimen combined to oxaliplatin) and in first-line treatment of squamous cell carcinoma of the esophagus.23,24 This treatment showed good efficacy with a high ORR (33%) in oesophageal disease,23 and a positive tolerability profile.23 Among AEs of grade 3/4, higher hematologic toxicity was seen in oesophageal cancer (leukopenia 33%, febrile neutropenia 19%),23 whereas the incidence of diarrhoea was similar (12 and 14%).24
Recently, Na-Lv, 5-FU and irinotecan were combined with biological agents. FOLFIRI and sunitinib were compared with FOLFIRI and placebo in the setting of pretreated and advanced stomach or lower oesophagus cancer.18 The experimental arm showed a trend towards longer mOS (19.4 versus 8.9 months for FOLFIRI and placebo, one sided p = 0.21). However, grade 3/4 hematologic AEs were considerable, with high incidence of both neutropenia (56%) and leukopenia (20%).18 In chemo-naïve patients with advanced gastroesophageal cancer, FOLFIRI in addition to cetuximab was mainly effective in disease control (ORR 46%, DCR 79%), with few AEs being mostly diarrhoea (15%) and skin toxicity (8%).20 Two more studies evaluated different combinations of doxorubicin, mitomycin C, Na-Lv and 5-FU in gastrointestinal cancers, with a nonoptimal toxicity profile (Table 1).19,21
Taken together, the results of the efficacy analysis accounted for the noninferiority of Na-Lv with a favourable efficacy trend for Na-Lv. In addition, the simultaneous infusion of Na-Lv and 5-FU appears to be the most convenient and cost-saving approach, shortening the treatment time for outpatients in ambulatory care units and decreasing the possibility of catheter dysfunction and damage.14
Safety and tolerability
To date, no preclinical toxicity trials with Na-Lv have been performed in addition to acute and local tolerance studies. Nevertheless, some information can be retrieved from in vitro studies, preclinical studies in mice and phase II trials.29
In mice, simultaneous administration of 5-FU and Na-Lv showed a good toxicity profile and only a progressive weight loss was reported. Both simultaneous combination of Ca-FA with increasing doses of 5-FU (50 mg/kg, 100 mg/kg, 150 mg/kg, respectively) and sequential combination with 100 mg/kg 5-FU resulted in high-grade toxicity.3
In 51 mCRC patients treated with concomitant administration of 24-hour 5-FU and the Na-Lv, gastrointestinal toxicity was the most frequently reported AE, with grade 3/4 diarrhoea occurring in 21.5% of patients.11 No severe grade of haematological toxicity was described and just a negligible percentage of patients required dose reductions.11 Overall, the toxicity profile was similar to that observed with Ca-FA/5-FU schedules in previous trials.11 Overlapping results, in terms of safety, were reported in a later phase II clinical trial, administering the same regimen to mCRC patients in the second-line setting.15
In a phase II trial, enrolling 57 patients with mCRC who received 5-FU/Na-Lv or 5-FU/Ca-FA, nausea, diarrhoea and fatigue were the most frequent AEs described in both arms. AEs were of grade 1–2 in most cases; neutropenia was the most frequent grade 3–4 AE, occurring in 14% and 18% of patients in Ca-FA and Na-Lv arms, respectively.16 Although not statistically significant, a higher number of patients in Na-Lv arm did not experience grade 3–4 AEs and fewer patients had more than two AEs of severe grade, compared with the Ca-FA arm.16
In several recent trials, 5-FU and Na-Lv were tested in various associations with other drugs in colon, oesophageal, gastric and breast cancer with data of safety concordant with those reported in the studies mentioned above.17–24 None of these trials described the occurrence of catheter occlusion or damage.11,15–24
In conclusion, Na-Lv simultaneously administered with 5-FU appears as safe in many chemotherapeutic regimens; it mainly increases gastrointestinal and hematologic toxicity caused by 5-FU, with a favourable toxicity trend compared with Ca-FA.
Discussion
Since the early 1990s, preclinical evidence indicated that a prolonged exposure of 5-FU to folinic acid is synergistic and strengthens the cytotoxic activity of 5-FU, resulting in an increased efficacy against cancer proliferation.1,2,4,5–11 It soon became clear that the simultaneous administration of these compounds was optimal to persistently block TS and, consequently, cell replication.5–11 However, the translation of this preclinical evidence into clinical practice with the concomitant administration of 5-FU and Ca-FA in the same infusion pump was hindered by catheter obstruction due to the crystallization and precipitation of calcium salts.3,6,12 Since then, a 1- or 2-hour infusion of Ca-FA followed by 5-FU administration has become standard in the clinical setting owing to its better feasibility.2,3,5
Na-Lv is a new compound with high solubility and stability that could be administered concomitantly with 5-FU in continuous infusion.3,13,14 The combination of 5-FU with Na-Lv enhances the formation of a covalently bound ternary complex constituted by 5’-FdUMP, TS and folate, which results in persistent TS blockade and inhibition of DNA synthesis.3,30–33 This drug can be safely administered mixed with 5-FU in the same pump, without precipitating or damaging the catheter.3,13,14 In addition to confirm its strong rationale, recent phase II studies described the effectiveness and safety of this new molecule in the clinical practice, evaluating its concomitant administration with 5-FU in several chemotherapeutic regimens, in metastatic colon, gastric, oesophageal and breast cancer patients.11,15–24 Taken together, these trials showed comparable results between Ca-FA and Na-Lv, with a more favourable efficacy and toxicity profile for the latter, in terms of ORR, mPFS, TTP and the occurrence of severe AEs.11,15–24
The use of intravenous 5-FU instead of oral capecitabine is more suitable in certain subgroups of patients, as experienced in clinical practice.17 In particular, for the elderly or patients with comorbidities who require frequent drug intake, adherence to oral treatment with capecitabine may be scarce and detrimental for quality of life. Moreover, the intravenous regimen with 5-FU demonstrates a partly favourable toxicity compared with oral capecitabine, reducing the incidence of diarrhoea and hand–foot syndrome. Interestingly, most women prefer intravenous administration of 5-FU rather than oral intake of chemotherapy, as recently emerged from a large meta-analysis conducted in ovarian cancer patients.39 Combination of disodium levofolinate with 5-FU in a 24-h infusion represents a safe and comfortable method of administration, alternative to capecitabine, especially in the case of nonadherence to an oral therapy.17
Furthermore, the simultaneous administration of Na-Lv and 5-FU allows the time of treatment to be shortened and this may be particularly important for schedules (e.g. the commonly used FOLFOX-4) which last 2 days, with a Ca-FA and 5-FU bolus infusion on day 2. With Na-Lv, these schedules may be modified, and infusions may be limited to a single day, cutting human resources for drug infusion, limiting the occurrence of catheter damage and reducing patient’s discomfort.13,14 Hence, Na-Lv appears to be the most convenient and cost-saving approach and its use in clinical practice is recommended.
Conclusions
Na-Lv showed a reassuring toxicity profile and a trend towards higher efficacy with respect to the comparator Ca-FA when used in combination with 5-FU. Then, it may represent a possible new standard of therapy when a 5-FU-based treatment is administered. Further studies, including phase III and larger series, are warranted to clearly assess its profile of toxicity and efficacy.
Acknowledgments
Authors would like to thank Medac Pharma for supporting literature search and open access publication, with an unrestricted fund. Thanks to Content Ed Net and Elisa Sala, PhD, for linguistic revision, paper formatting and submission work.
Footnotes
Author contribution: RM, HJC, TL, CE, PF, PR, BS, TG and GM contributed to design of the work, drafted the article and approved the final version.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Content Ed Net and Elisa Sala, PhD for linguistic revision, paper formatting and submission work; these activities were supported by Medac Pharma.
Contributor Information
Margherita Ratti, Oncology Unit, Oncology Department, ASST of Cremona, Hospital of Cremona, Italy.
Jens Claus Hahne, Division of Molecular Pathology, The Institute for Cancer Research, Sutton, UK.
Laura Toppo, Oncology Unit, Dept Medicine, Hospital of Voghera, Italy.
Emanuela Castelli, Pharmacy Unit, ASST of Bergamo Ovest, Treviglio, Italy.
Fausto Petrelli, Oncology Unit, Oncology Department, ASST of Bergamo Ovest, Treviglio, Italy.
Rodolfo Passalacqua, Oncology Unit, Oncology Department, ASST of Cremona, Hospital of Cremona, Italy.
Sandro Barni, Oncology Unit, Oncology Department, ASST of Bergamo Ovest, Treviglio, Italy.
Gianluca Tomasello, Oncology Unit, Oncology Department, ASST of Cremona, Hospital of Cremona, Italy.
Michele Ghidini, Oncology Unit, Oncology Department, ASST of Cremona, Hospital of Cremona, Viale Concordia, 1, Cremona CR, 26100, Italy.
References
- 1. Longley DB, Harkin P, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 2003; 3: 330–338. [DOI] [PubMed] [Google Scholar]
- 2. Pardini B, Kumar R, Naccarati A, et al. 5-Fluorouracil-based chemotherapy for colorectal cancer and MTHFR/MTRR genotypes. Br J Clin Pharmacol 2011; 72: 162–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Di Paolo A, Orlandi P, Di Desidero T, et al. Simultaneous, but not consecutive, combination with folinate salts potentiates 5-fluorouracil antitumor activity in vitro and in vivo. Oncol Res 2017; 25: 1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Machover D, Goldschmidt E, Chollet P, et al. Treatment of advanced colorectal and gastric adenocarcinomas with 5-fluorouracil and high dose folinic acid. J Clin Oncol 1986; 4: 685–696. [DOI] [PubMed] [Google Scholar]
- 5. Leslie A, Steele RJC. Management of colorectal cancer. Postgrad Med J 2002; 78: 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ardalan B, Chua L, Tian EM. A phase II study of weekly 24-hour infusion with high-dose fluorouracil with leucovorin in colorectal carcinoma. J Clin Oncol 1991; 9: 625–630. [DOI] [PubMed] [Google Scholar]
- 7. Lokich JJ, Ahlgren JD, Gullo JJ, et al. Prospective randomised comparison of continuous infusion fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma: a mid-Atlantic oncology programme study. J Clin Oncol 1989; 7: 425–432. [DOI] [PubMed] [Google Scholar]
- 8. Schmoll HJ, Kohne CH, Lorenz M, et al. Weekly 24h infusion of high-dose (HD) 5-Fluorouracil with or without folinic acid (FA) vs. Bolus 5-FU/FA (NCCTG/Mayo) in advanced colorectal cancer (CRC): a randomized phase III study of the EORTC GOTCCG and the AIO. Proc Am Soc Clin Oncol 2000; 19: 935. [Google Scholar]
- 9. Jager E, Klein O, Wachter B, et al. High-dose 5-fluorouracil (5-FU) and folinic acid in advanced colorectal cancer resistant to standard dose 5-FU treatment: result of a phase II study. Eur J Cancer 1995; 31: 1717. [DOI] [PubMed] [Google Scholar]
- 10. Hartmann JT, Köhne CH, Schmoll HJ, et al. Is continuous 24-hour infusion of 5-fluorouracil plus high-dose folinic acid effective in patients with progressive or recurrent colorectal cancer? A phase II study. Oncology 1998; 55: 320–325. [DOI] [PubMed] [Google Scholar]
- 11. Hartung G, Hofheinz RD, Wein A, et al. Phase II study of a weekly 24-hour infusion with 5-fluorouracil and simultaneous sodium-folinic acid in the first-line treatment of metastatic colorectal cancer. Onkologie 2001; 24: 457–462. [DOI] [PubMed] [Google Scholar]
- 12. Bruch HR, Esser M. Catheter occlusion by calcium carbonate during simultaneous infusion of 5-FU and calcium folinate. Onkologie 2003; 26: 469–472. [DOI] [PubMed] [Google Scholar]
- 13. Galanti L, Lebitasy MP, Hecq JD, et al. Long-term stability of 5-Fluorouracil in 0.9% sodium chloride after freezing, microwave thawing, and refrigeration. Can J Hosp Pharm 2009; 62: 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bourget P, Moriceau A, Amin A, et al. Stability of irinotecan and sodium levofolinate admixtures in polyolefin bags: clinical and nursing considerations. Eur J Hosp Pharm Sci Pract 2011; 17: 66–69. [Google Scholar]
- 15. Kuhfahl J, Steinbrecher C, Wagner T, et al. Second-line treatment of advanced colorectal cancer with a weekly simultaneous 24-hour infusion of 5-fluorouracil and sodium-folinate: a multicentre phase II trial. Onkologie 2004; 27: 449–454. [DOI] [PubMed] [Google Scholar]
- 16. Bleiberg H, Vandebroek A, Deleu I, et al. A phase II randomized study of combined infusional leucovorin sodium and 5-FU versus the leucovorin calcium followed by 5-FU both in combination with irinotecan or oxaliplatin in patients with metastatic colorectal cancer. Acta Gastroenterol Belg 2012; 75: 14–21. [PubMed] [Google Scholar]
- 17. Terjung A, Kummer S, Friedrich M. Simultaneous 24 h infusion of high-dose 5-fluorouracil and sodium-folinate as alternative to capecitabine in advanced breast cancer. Anticancer Res 2014; 34: 7233–7238. [PubMed] [Google Scholar]
- 18. Moehler M, Gepfner-Tuma I, Maderer A. Sunitinib added to FOLFIRI versus FOLFIRI in patients with chemorefractory advanced adenocarcinoma of the stomach or lower esophagus: a randomized, placebo-controlled phase II AIO trial with serum biomarker program. BMC Cancer 2016; 16: 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gnad-Vogt SU, Hofheinz RD, Saussele S. Pegylated liposomal doxorubicin and mitomycin C in combination with infusional 5-fluorouracil and sodium folinic acid in the treatment of advanced gastric cancer: results of a phase II trial. Anticancer Drugs 2005; 16: 435–440. [DOI] [PubMed] [Google Scholar]
- 20. Moehler M, Mueller A, Trarbach T. Cetuximab with irinotecan, folinic acid and 5-fluorouracil as first-line treatment in advanced gastroesophageal cancer: a prospective multi-center biomarker-oriented phase II study. Ann Oncol 2011; 22: 1358–1366. [DOI] [PubMed] [Google Scholar]
- 21. Hofheinz RD, Willer A, Weisser A. Pegylated liposomal doxorubicin in combination with mitomycin C, infusional 5-fluorouracil and sodium folinic acid. A phase-I-study in patients with upper gastrointestinal cancer. Br J Cancer 2004; 90: 1893–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koucky K, Wein A, Konturek PC. Palliative first-line therapy with weekly high-dose 5-fluorouracil and sodium folinic acid as a 24-hour infusion (AIO regimen) combined with weekly irinotecan in patients with metastatic adenocarcinoma of the stomach or esophagogastric junction followed by secondary metastatic resection after downsizing. Med Sci Monit 2011; 17: CR248–CR258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolff K, Wein A, Reulbach U, et al. Weekly high-dose 5-fluorouracil as a 24-h infusion and sodium folinic acid (AIO regimen) plus irinotecan in patients with locally advanced non resectable and metastatic adenocarcinoma or squamous cell carcinoma of the oesophagus: a phase II trial. Anticancer Drugs 2009; 20: 165–173. [DOI] [PubMed] [Google Scholar]
- 24. Wein A, Siebler J, Wolff K, et al. Weekly high-dose 5-fluorouracil as 24-hour infusion combined with sodium folinic acid (AIO regimen) plus irinotecan in second-line and sequential therapy of metastatic colorectal cancer (CRC). Anticancer Res 2017; 37: 3771–3779. [DOI] [PubMed] [Google Scholar]
- 25. Konno M, Asai A, Kawamoto K, et al. The one-carbon metabolism pathway highlights therapeutic targets for gastrointestinal cancer. Int J Oncol 2017; 50: 1057–1063. [DOI] [PubMed] [Google Scholar]
- 26. Tuan V, Chuang G, Suno M. Levoleucovorin as replacement for leucovorin in cancer treatment. Ann Pharmacother 2012; 46: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 27. Lambie DG, Johnson RH. Drugs and folate metabolism. Drugs 1985; 30: 145–155. [DOI] [PubMed] [Google Scholar]
- 28. Goldman ID, Matherly LH. Biochemical factors in the selectivity of leucovorin rescue: selective inhibition of leucovorin reactivation of dihydrofolate reductase and leucovorin utilization in purine and pyrimidine biosynthesis by methotrexate and dihydrofolate polyglutamates. NCI Monogr 1987; (5): 17–26. [PubMed] [Google Scholar]
- 29. Diddens H, Teufel T, Niethammer D. High-dose methotrexate therapy with leucovorin rescue – In vitro investigations on human osteosarcoma cell-lines. Cancer Chem Pharm 1987; 20: 128–132. [DOI] [PubMed] [Google Scholar]
- 30. eMC. https://www.medicines.org.uk/emc/product/6373 (accessed May 2019).
- 31. Matsusaka S, Lenz HJ. Pharmacogenomics of fluorouracil -based chemotherapy toxicity. Exp Opin Drug Metab Toxicol 2015; 11: 811–821. [DOI] [PubMed] [Google Scholar]
- 32. Danenberg PV, Gustavsson B, Johnston P, et al. Folates as adjuvants to anticancer agents: chemical rationale and mechanism of action. Crit Rev Oncol Hematol 2016; 106: 118–131. [DOI] [PubMed] [Google Scholar]
- 33. Drake JC, Voeller DM, Allegra CJ, et al. The effect of dose and interval between 5-fluorouracil and leucovorin on the formation of thymidylate synthase ternary complex in human cancer cells. Br J Cancer 1995; 71: 1145–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilson PM, Danenberg PV, Johnston PG, et al. Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat Rev Clin Oncol 2014; 11: 282–298. [DOI] [PubMed] [Google Scholar]
- 35. Hou Z, Matherly LH. Biology of the major facilitative folate transporters SLC19A1 and SLC46A1. Curr Top Membr 2014; 73: 175–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Odin E, Sonden A, Gustavsson B, et al. Expression of folate pathway genes in stage III colorectal cancer correlates with recurrence status following adjuvant bolus 5-FU-based chemotherapy. Mol Med 2015; 21: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weh HJ, Wilke HJ, Dierlamm J, et al. Weekly therapy with folinic acid and high-dose 5-Fluorouracil 24-hour infusion in pretreated patients with metastatic colorectal carcinoma. A multicenter study by the Association of Medical Oncology of the German Cancer Society (AIO). Ann Oncol 1994; 5: 233–237. [DOI] [PubMed] [Google Scholar]
- 38. Stoffregegen C, Zurborn KH, Boehme V, et al. Weekly high-dose 5-fluorouracil 24-hour infusion and intermediate dose folinic acid bolus in metastatic colorectal cancer. Onkologie 1996; 19: 410–414. [Google Scholar]
- 39. Chekerov R, Harter P, Fuxius S, et al. Preference of elderly patients’ to oral or intravenous chemotherapy in heavily pre-treated recurrent ovarian cancer: final results of a prospective multicenter trial. Gynecol Oncol Res Pract 2017; 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]