Abstract
Background: Cancer-related fatigue (CRF) often co-occurs with sleep disturbance and is one of the most pervasive toxicities resulting from cancer and its treatment. We and other investigators have previously reported that yoga therapy can improve sleep quality in cancer patients and survivors. No nationwide multicenter phase III randomized controlled trial (RCT) has investigated whether yoga therapy improves CRF or whether improvements in sleep mediate the effect of yoga on CRF. We examined the effect of a standardized, 4-week, yoga therapy program (Yoga for Cancer Survivors [YOCAS]) on CRF and whether YOCAS-induced changes in sleep mediated changes in CRF among survivors. Study Design and Methods: Four hundred ten cancer survivors were recruited to a nationwide multicenter phase III RCT comparing the effect of YOCAS to standard survivorship care on CRF and examining the mediating effects of changes in sleep, stemming from yoga, on changes in CRF. CRF was assessed by the Multidimensional Fatigue Symptom Inventory. Sleep was assessed via the Pittsburgh Sleep Quality Index. Between- and within-group intervention effects on CRF were assessed by analysis of covariance and 2-tailed t test, respectively. Path analysis was used to evaluate mediation. Results: YOCAS participants demonstrated significantly greater improvements in CRF compared with participants in standard survivorship care at post-intervention (P < .01). Improvements in overall sleep quality and reductions in daytime dysfunction (eg, excessive napping) resulting from yoga significantly mediated the effect of yoga on CRF (22% and 37%, respectively, both P < .01). Conclusions: YOCAS is effective for treating CRF among cancer survivors; 22% to 37% of the improvements in CRF from yoga therapy result from improvements in sleep quality and daytime dysfunction. Oncologists should consider prescribing yoga to cancer survivors for treating CRF and sleep disturbance.
Keywords: cancer-related fatigue, sleep, yoga, mediation, randomized controlled trial
Introduction
The majority of cancer patients, up to 99%, experience cancer-related fatigue (CRF) during primary treatments, and approximately a third of these patients will continue to experience moderate to severe CRF as survivors for months and years after treatment.1-9 CRF is characterized by unusual decreased physical and mental energy and increased need for rest; however, it is not directly correlated to recent physical exertion and cannot be alleviated by simply sleeping or resting.10-15 CRF reduces survivors’ physical ability and motivation for performing essential daily activities such as cleaning the house, walking, climbing stairs, and engaging in social activities.6,16 Importantly, due to the lack of physical and mental energy, CRF prevents some survivors from completing post-primary medical treatments (eg, hormonal and biologic therapies, medical follow-ups). These noxious CRF effects negatively affect survivors’ recovery by impairing physical and mental function, interfering with recovery and resumption of normal life activities, and reducing overall survival.6,16-18
Cancer-related sleep disturbance is also commonly experienced by survivors. Fifty-one percent to 90% of cancer survivors experience some form of sleep disturbance such as having difficulty falling asleep and staying asleep, early and frequent awakenings, and excessive daytime sleepiness that leads to excessive daytime dysfunction such as excessive napping.19-22 Sleep disturbance in cancer survivors negatively influences cancer biology, daily physical and mental function, recovery and resumption of daily activities, and overall quality of life.23-25
CRF and sleep disturbance are highly correlated and co-occur.3,4,9,22,26-31 Because these 2 toxicities often co-occur, it is hypothesized that the sleep disturbance stemming from cancer and its treatments contributes to the CRF experienced by patients. Practitioners and patients often believe that improvements in sleep will automatically lead to improvements in CRF. While this may be true in part, it is plausible that CRF is influenced via multiple mechanistic pathways and this is why it is not possible to simply sleep or rest CRF away.
In a phase III randomized control trial (RCT), we demonstrated that cancer survivors who participated in the 4-week standardized Yoga for Cancer Survivors (YOCAS) program significantly improved overall and subjective sleep quality and reduced daytime dysfunction (eg, excessive napping) and sleep medication use compared with standard survivorship care alone.32 A number of small phase I/II studies suggest that yoga is an effective treatment for CRF.33-41 To our knowledge, no nationwide, multicenter, phase III RCT has tested whether yoga is an effective treatment for CRF or whether improvements in sleep resulting from yoga are responsible for all or part of yoga-induced improvements in CRF among cancer survivors. Therefore, the purpose of this study is to report 2 secondary outcomes from our phase III RCT: (1) the effect of YOCAS on CRF and (2) whether YOCAS-induced changes in sleep mediated changes in CRF.
Methods
Study Design
We conducted a nationwide, multicenter, phase III RCT via the University of Rochester Cancer Center (URCC) National Cancer Institute (NCI) Community Oncology Research Program (NCORP) Research Base. Participants were recruited from 12 NCORP community oncology practices through direct contact by study coordinators at clinic visits across the United States. This study received approval from the institutional review boards at the University of Rochester and each of the participating NCORP community oncology practices. Study coordinators recruited participants in cohorts of 20 to 30 and obtained written informed consent. Study participants were registered and randomly assigned to 1 of 2 interventions: (1) standard survivorship care plus the 4-week YOCAS intervention or (2) standard survivorship care alone. Randomization was stratified by gender and pre-intervention sleep disturbance (≤5 or >5 on an 11-point Symptom Inventory scale, with 0 = no sleep disturbance and 10 = worst possible sleep disturbance). A computer-generated random numbers table (blocks of 2, allocation ratio of 1:1) was used to determine intervention assignment. After study coordinators completed participant registration, they received an email containing the random allocation generated by the website. For the analyses performed in the current study, the principal investigator was blinded to the allocation.
Participants
Inclusion criteria included the following: (1) diagnosis of any type of cancer; (2) completion of primary treatment 2 to 24 months prior to enrollment; (3) persistent sleep disturbance (≥3 on 11-point Symptom Inventory scale, with 0 = no sleep disturbance and 10 = worst possible sleep disturbance); (4) age ≥21 years; and (5) ability to read and understand English. Participants were excluded if they (1) regularly practiced yoga within 3 months before enrollment; (2) were diagnosed with sleep apnea or metastatic cancer; and (3) were currently receiving primary cancer treatments such as surgery, chemotherapy, or radiation therapy.
Yoga Intervention
The standardized YOCAS program, designed by researchers at the University of Rochester Medical Center, was used for the yoga therapy intervention. Each YOCAS session consisted of breathing exercises, physical alignment postures, and mindfulness exercises. The breathing exercises included slow, controlled, diaphragmatic breaths, and breathing coordinated with movement. The physical alignment postures included 16 Gentle Hatha and Restorative yoga poses, including seated, standing, transitional, and supine poses. The mindfulness exercises included meditation, visualization, and affirmation.32 The intensity of YOCAS was low to moderate.42
Each YOCAS session lasted 75 minutes and was delivered twice per week for 4 weeks. YOCAS sessions were conducted in a group setting of 10 to 15 participants, delivered by Yoga Alliance–registered instructors in community-based sites (eg, community centers, cancer centers, yoga studios). To ensure the standardization, quality, and fidelity of the yoga intervention, all instructors were required to take a 2-hour standardized training session provided by the URCC research team. A detailed YOCAS manual and DVD were also provided to instructors for delivering standardized yoga classes. The study coordinator at each NCORP community oncology practice performed a random, independent observation of YOCAS sessions in order to verify the content was delivered as planned.
Standard Survivorship Care Intervention
Participants in the standard survivorship care intervention continued with follow-up care from their oncologists and primary care providers. They received the same amount of time and attention from study staff during the assessments, minus the contact time associated with yoga attendance. Participants in the standard survivorship care intervention were offered the opportunity to participate in the YOCAS program after completing the study.
Measures
Race and ethnicity information was collected using the NCI Cancer Therapy Reporting Program criteria for clinical trials. Participants identified themselves as 1 of 3 racial categories: white, African American, or Other. Additional demographic and clinical information was collected using study-specific forms and participants’ medical records to confirm eligibility as well as for descriptive reporting purposes. Participants were instructed to continue their routine activities and not engage in any new yoga or exercise during the study in order to prevent yoga and exercise contamination. Adherence and compliance were monitored by daily diaries and yoga session attendance records. Adverse events were monitored using NCI Common Terminology Criteria for Adverse Events and reported to the URCC Data Safety Monitoring Committee.
Cancer-Related Fatigue
CRF was measured using the short form of the Multidimensional Fatigue Symptom Inventory (MFSI)43,44 at pre- and post-intervention. MFSI is a 30-item self-report and validated fatigue instrument. Each item is rated on a 5-point scale from 0 = not at all to 4 = extremely. MFSI has a total CRF score and 5 subdomain scores: general, physical, emotional, mental, and vigor subdomains of CRF.
Sleep
Sleep quality was evaluated using the Pittsburgh Sleep Quality Index (PSQI) at pre- and post-intervention. PSQI includes an overall sleep quality score and subdomain scores calculated from 7 sleep components including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, sleep medication use, and daytime dysfunction (eg, excessive napping).45
Statistical Analyses
Of the 410 participants, 328 provided fully evaluable pre- and post-intervention CRF data. The sample size of 328 participants (YOCAS, n = 168; standard survivorship care, n = 160) provided 90% power to detect the effect size of 0.31 in between-group differences in Total MFSI scores assuming a correlation coefficient of 0.5 between pre- and post-intervention assessments at a significance level of 5% with a 2-sided F test using analysis of covariance (ANCOVA).
Between-group differences for demographic and pre-intervention clinical data were examined with a 2-tailed t test for continuous variables and a χ2 test for categorical variables. ANCOVA, with intervention condition as the group factor and pre-intervention CRF as the covariate, was used to examine the between-group intervention effect on CRF at post-intervention. An effect size was calculated using between-group ANCOVA estimates in MFSI scores divided by the standard deviation of pre-intervention MFSI scores. The within-group intervention effect on CRF was assessed by a 2-tailed paired t test. ANCOVA and t tests were performed with SAS 9.4 (SAS Institute Inc, Cary, NC).
The mediation analyses were conducted only among participants who provided complete sleep and CRF data at pre- and post-intervention for the PSQI overall score and all subdomain scores (all N = 321). Because our mediation analyses included 4 to 7 fewer participants on the PSQI subdomains of subjective sleep quality, sleep medication use, and daytime dysfunction compared with the number of participants included in our original published analyses,32 we reevaluated between-group intervention effects on subjective sleep quality, sleep medication use, and daytime dysfunction in the current study.
The mediational relationships were evaluated using path analysis on change scores in overall PSQI, subjective sleep quality, sleep medication use, and daytime dysfunction with Total MFSI as the dependent variable to derive path coefficients. Each mediation model included 3 paths: (1) a direct path between the intervention condition (YOCAS vs standard survivorship care) and CRF; (2) a path between the intervention condition and 1 of the 4 sleep outcomes; and (3) a path between the sleep outcome and CRF.46-48 Mediation analysis (using R 3.5.1) was assessed using nonparametric bootstrap 95% confidence intervals (CIs) for the indirect path coefficient from the intervention condition to CRF on each sleep outcome. Because both sleep quality and CRF were assessed at the same time, we conducted χ2 goodness of fit tests to determine whether sleep quality mediated CRF or vice versa, using MPlus, version 7.4 (Muthén & Muthén, Los Angeles, CA). Statistical significance was determined for P ≤ .05.
Reported results were based on complete case analyses because analyses revealed that missing data were missing completely at random,49 and no substantive differences were shown between analyses with complete cases versus estimated data with multiple imputations (using fully conditional specification method SAS PROC MI:FCS).50 Analyses followed the intent-to-treat principle51,52 by keeping participants in the arm they were randomized to for complete case analyses regardless of whether they participated in yoga or not and by utilizing data from all participants (N = 410) for multiple imputations.50
Results
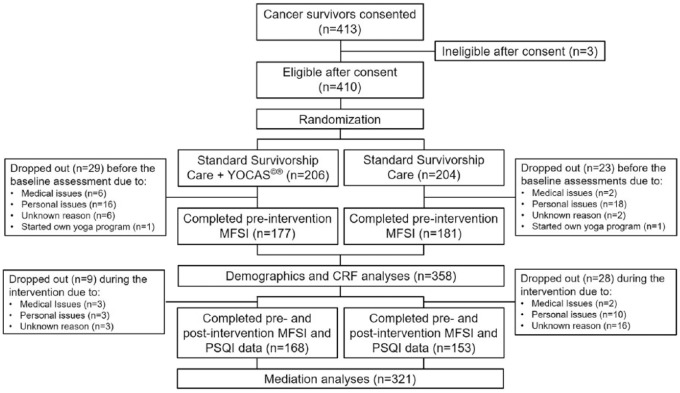
Four hundred thirteen cancer survivors were consented from 12 NCORP affiliate sites. Three survivors were found ineligible after consent. A total of 410 eligible participants were randomly assigned to YOCAS (n = 206) or the standard survivorship care (n = 204) intervention. Among the 410 participants, 358 participants provided complete pre-intervention CRF data, 328 participants provided complete pre- and post-intervention CRF data, and 321 participants provided complete pre- and post-intervention CRF and sleep data. Thirty-eight participants (18%) from the YOCAS intervention dropped out due to medical (n = 9), personal (n = 19), or unknown reasons (n = 9), and 1 participant started an additional yoga program. Fifty-one participants (25%) from the standard survivorship care intervention dropped out due to medical (n = 4), personal (n = 28), or unknown reasons (n = 18), and 1 participant started their own yoga practice. The proportion of dropout was not significantly different between YOCAS and standard survivorship care interventions (P = .12). Figure 1 shows the CONSORT diagram.
Figure 1.
CONSORT diagram.
Abbreviations: YOCAS, Yoga for Cancer Survivors; MFSI, Multidimensional Fatigue Symptom Inventory; PSQI, Pittsburgh Sleep Quality Index.
Demographics and Characteristics of Study Participants
Table 1 shows the baseline characteristics of the 358 participants (YOCAS, n = 177; standard survivorship care, n = 181) who provided complete pre-intervention CRF data. The majority of study participants were female (96%), white (93%), married or in a committed relationship (73%), having some college or higher education (82%), employed (82%), and breast cancer (77%) survivors. On average, participants were 54.3 ± 10.2 years old and had completed their surgery, chemotherapy, and radiation therapy 15.6 ± 8.2 months prior to enrolling in this study. There were no significant group differences with regard to participants’ demographics and characteristics. There were no study-related adverse events.32
Table 1.
Demographics and Characteristics of Study Participants.
| Characteristics | Total (N = 358) | YOCAS (n = 177) | Standard Survivorship Care (n = 181) |
|---|---|---|---|
| Age, years, mean ± SD | 54.3 ± 10.2 | 55.0 ± 11.1 | 53.7 ± 9.3 |
| Gender, n (%) | |||
| Female | 344 (96) | 169 (95) | 175 (97) |
| Male | 14 (4) | 8 (5) | 6 (3) |
| Race/ethnicity, n (%) | |||
| White | 334 (93) | 170 (96) | 164 (91) |
| African American | 21 (6) | 6 (3) | 15 (8) |
| Other | 3 (1) | 1 (1) | 2 (1) |
| Marital status, n (%) | |||
| Married or committed relationship | 261 (73) | 126 (71) | 137 (75) |
| Separated/divorced | 52 (15) | 25 (14) | 27 (15) |
| Widowed | 15 (4) | 11 (6) | 4 (2) |
| Single | 30 (8) | 15 (9) | 15 (8) |
| Education, n (%) | |||
| Some college or higher | 292 (82) | 147 (83) | 145 (80) |
| High school graduate | 63 (18) | 28 (16) | 35 (19) |
| Less than a high school education | 3 (1) | 2 (1) | 1 (1) |
| Currently employed, n (%) | 292 (82) | 150 (85) | 142 (79) |
| Cancer type, n (%) | |||
| Breast | 275 (77) | 133 (75) | 142 (79) |
| Hematologic | 25 (7) | 13 (7) | 12 (7) |
| Alimentary | 21 (6) | 7 (4) | 14 (8) |
| Gynecologic | 16 (5) | 10 (6) | 6 (3) |
| Other | 21 (6) | 14 (8) | 7 (4) |
| Cancer stage, n (%) | |||
| Stage 0 | 17 (5) | 9 (5) | 8 (4) |
| Stage I | 127 (36) | 56 (32) | 71 (39) |
| Stage II | 122 (34) | 62 (35) | 60 (33) |
| Stage III | 57 (16) | 28 (16) | 29 (16) |
| Stage IV | 10 (3) | 7 (4) | 3 (2) |
| Unknown | 25 (7) | 15 (9) | 10 (6) |
| Previous treatment, n (%) | |||
| Surgery | 324 (91) | 160 (90) | 164 (91) |
| Chemotherapy | 255 (71) | 129 (73) | 126 (70) |
| Radiation therapy | 239 (67) | 119 (67) | 120 (66) |
| Hormonal therapy | 26 (7) | 12 (7) | 14 (8) |
| Current hormonal therapy, n (%) | 185 (52) | 84 (48) | 101 (56) |
| Time since the completion of treatment, months, mean ± SD | 15.6 ± 8.2 | 14.9 ± 6.8 | 16.4 ± 9.4 |
Abbreviation: YOCAS, Yoga for Cancer Survivors.
Adherence and Compliance
Participants in YOCAS intervention attended an average of 6.5 of the 8 prescribed sessions and completed 1 additional session of yoga per week on their own. All yoga sessions were of moderate intensity with an average perceived exertion rating of 3.4 on a 0 to 10 scale (0 = Nothing at all to 10 = Very, very strong). Participants in the YOCAS arm averaged practicing yoga 182 minutes each week for a total average of 728 minutes of yoga over the 4-week intervention period—128 minutes more than the prescribed 600 minutes of yoga (75 minutes/session × 8 sessions). Yoga contamination in the standard survivorship care arm was insignificant; 7 participants reported an average of 20 minutes of yoga practice weekly during the intervention.
Cancer-Related Fatigue
CRF: Total MFSI
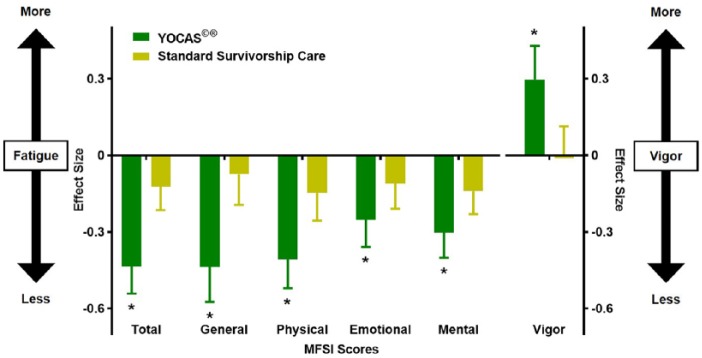
ANCOVA results, controlling for pre-intervention CRF, showed that participants in the YOCAS intervention, compared with those receiving standard survivorship care, had significantly greater improvements in CRF (−6.8 ± 1.4, P < .01) at post-intervention (Table 2 and Figure 2). Follow-up t tests revealed that YOCAS participants demonstrated significant improvements in CRF from pre- to post-intervention (−9.5 ± 1.2, P < .01), but standard survivorship care participants did not (Table 2).
Table 2.
Within- and Between-Group Differences in Total and Subdomains of Cancer-Related Fatiguea.
| MFSI (Mean ± SE) | YOCAS | Standard Survivorship Care | YOCAS Versus Standard Survivorship Care | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Intervention (n = 177) | Post-Intervention (n = 168) | Within-Group Difference | P | Pre-Intervention (n = 181) | Post-Intervention (n = 160) | Within-Group Difference | P | Between-Group Difference | P | Between-Group Effect Size (95% CI) | |
| General | 11.4 ± 0.5 | 8.6 ± 0.4 | −2.7 ± 0.4 | <0.01 | 11.1 ± 0.5 | 10.4 ± 0.5 | −0.4 ± 0.4 | 0.31 | −2.1 ± 0.5 | <0.01 | −0.34 (−0.50 to −0.18) |
| Physical | 7.3 ± 0.4 | 4.9 ± 0.4 | −2.3 ± 0.3 | <0.01 | 7.6 ± 0.4 | 6.7 ± 0.4 | −0.8 ± 0.3 | 0.10 | −1.6 ± 0.4 | <0.01 | −0.28 (−0.42 to −0.15) |
| Emotional | 7.0 ± 0.4 | 5.3 ± 0.4 | −1.5 ± 0.3 | <0.01 | 7.5 ± 0.4 | 6.6 ± 0.4 | −0.7 ± 0.3 | 0.12 | −1.0 ± 0.4 | <0.01 | −0.17 (−0.29 to −0.04) |
| Mental | 8.2 ± 0.4 | 6.2 ± 0.4 | −1.7 ± 0.3 | <0.01 | 8.0 ± 0.4 | 7.2 ± 0.4 | −0.8 ± 0.3 | 0.15 | −0.9 ± 0.3 | <0.01 | −0.17 (−0.28 to −0.05) |
| Vigor | 10.2 ± 0.4 | 11.6 ± 0.4 | 1.5 ± 0.3 | <0.01 | 10.5 ± 0.4 | 10.6 ± 0.4 | 0.0 ± 0.3 | 0.78 | 1.3 ± 0.4 | <0.01 | 0.27 (0.11 to 0.43) |
| Total MFSI | 23.6 ± 1.6 | 13.4 ± 1.6 | −9.5 ± 1.2 | <0.01 | 23.8 ± 1.7 | 20.2 ± 1.6 | −2.7 ± 1.0 | 0.13 | −6.8 ± 1.4 | <0.01 | −0.31 (−0.44 to −0.18) |
Abbreviations: YOCAS, Yoga for Cancer Survivors; MFSI, Multidimensional Fatigue Symptom Inventory; SE, standard error; CI, confidence interval.
Each MFSI subdomain score ranges from 0 to 24. The total MFSI score ranges from −24 to 96. Higher scores indicate worse fatigue.
Figure 2.
Changes in cancer-related fatigue (*P ≤ .05).
Abbreviations: YOCAS, Yoga for Cancer Survivors; MFSI, Multidimensional Fatigue Symptom Inventory.
CRF: General, Physical, Emotional, Mental, and Vigor Subdomains
ANCOVA results, controlling for pre-intervention levels, showed that participants in the YOCAS intervention, compared with those receiving standard survivorship care, demonstrated significantly greater improvements in general (−2.1 ± 0.5, P < .01), physical (−1.6 ± 0.4, P < .01), emotional (−1.0 ± 0.4, P < .01), and mental (−0.9 ± 0.3, P < .01) fatigue, and had significantly higher vigor (1.3 ± 0.4, P < .01) at post-intervention (Table 2 and Figure 2). Follow-up t tests revealed that YOCAS participants demonstrated significant improvements in general (−2.7 ± 0.4, P < .01), physical (−2.3 ± 0.3, P < .01), emotional (−1.5 ± 0.3, P < .01), mental (−1.7 ± 0.3, P < .01), and vigor (1.5 ± 0.3, P < .01) subdomains from pre- to post-intervention, but standard survivorship care participants did not (Table 2).
Sleep: Overall PSQI Score and Subdomain Scores (Subjective Sleep Quality, Sleep Medication Use, and Daytime Dysfunction)
ANCOVA results, controlling for pre-intervention levels, showed that participants in the YOCAS intervention, compared with those receiving standard survivorship care, demonstrated significantly greater improvements in overall sleep quality (−0.8 ± 0.3, P < .01), subjective sleep quality (−0.1 ± 0.1, P = .05), and daytime dysfunction (−0.2 ± 0.1, P < .01) at post-intervention. YOCAS participants also demonstrated a statistical trend for greater reductions in sleep medication use compared with control participants (−0.2 ± 0.1, P = .09) at post-intervention (Table 3). These results mirror the primary results we previously published.32
Table 3.
Between-Group Differences in Overall Sleep Quality, Subjective Sleep Quality, Sleep Medication Use, and Daytime Dysfunctiona.
| PSQI (Mean ± SE) | YOCAS Versus Standard Survivorship Care | ||
|---|---|---|---|
| Between-Group Difference | P | Between-Group Effect Size (95% CI) | |
| Subjective sleep quality | −0.1 ± 0.1 | .05 | −0.19 (−0.39 to 0.00) |
| Sleep medication use | −0.2 ± 0.1 | .09 | −0.13 (−0.30 to 0.02) |
| Daytime dysfunction | −0.2 ± 0.1 | <.01 | −0.37 (−0.56 to −0.18) |
| Overall PSQI | −0.8 ± 0.3 | <.01 | −0.24 (−0.41 to −0.06) |
Abbreviations: YOCAS, Yoga for Cancer Survivors; PSQI, Pittsburgh Sleep Quality Index; SE, standard error; CI, confidence interval.
Each PSQI subdomain score ranges from 0 to 3. The overall PSQI score ranges from 0 to 21. Higher scores indicate worse sleep quality.
Mediation Effect of Yoga-Induced Changes in Sleep on CRF
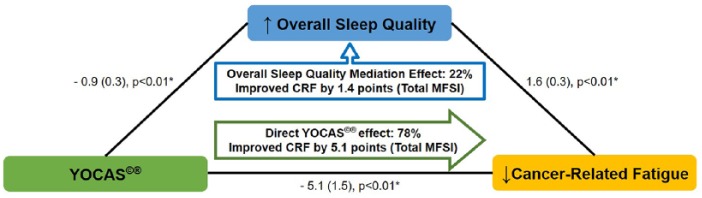
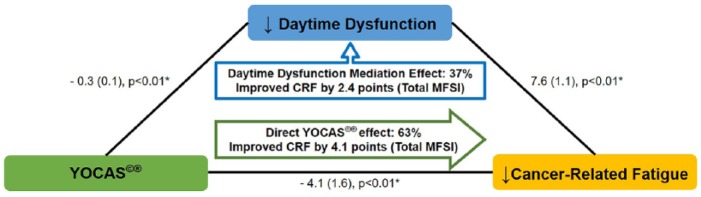
Chi-square goodness of fit tests suggest changes in sleep-mediated changes in CRF (P = .90, better fit) but not vice versa (P = .03, lack of fit). Path results suggest that the additional improvements in CRF from combining YOCAS with standard survivorship care versus standard survivorship care alone were mediated by changes in sleep (P < .01). Results suggest that the total effect of YOCAS plus standard survivorship care on CRF (−6.5) included a mediating effect whereby 22% (95% CI = 7% to 54%) of the improvements in CRF were attributed to improvements in overall sleep quality (−1.4, P < .01; Figure 3). Results also suggest that the total effect of YOCAS plus standard survivorship care on CRF included a mediating effect whereby 37% (95% CI = 23% to 81%) of the improvements in CRF were attributed to improvements in daytime dysfunction, a subdomain of the overall sleep quality score (−2.4, P < .01; Figure 4). Results suggest that no other sleep subdomains contributed to the mediation effects of changes in sleep on changes in CRF (all P > .05).
Figure 3.
Mediational effect of overall sleep quality on cancer-related fatigue (data are presented as regression coefficient [standard error]; *P ≤ .05).
Abbreviations: CRF, cancer-related fatigue; MFSI, Multidimensional Fatigue Symptom Inventory; YOCAS, Yoga for Cancer Survivors.
Figure 4.
Mediational effect of daytime dysfunction on cancer-related fatigue (data are presented as regression coefficient [standard error]; *P ≤ .05).
Abbreviations: CRF, cancer-related fatigue; MFSI, Multidimensional Fatigue Symptom Inventory; YOCAS, Yoga for Cancer Survivors.
Discussion
To our knowledge, this is the first nationwide, phase III, multicenter, RCT demonstrating that a standardized, 4-week, yoga intervention (YOCAS) significantly improves CRF and all subdomains of CRF including general, physical, emotional, and mental fatigue, and vigor in cancer survivors and that improvements in overall sleep quality and daytime dysfunction partially mediate the effect of YOCAS on CRF. Our findings suggest that gentle Hatha and Restorative-based yoga therapy performed 2 to 3 times per week at a moderate intensity over 4 weeks (728 minutes total) is effective for treating CRF in cancer survivors. Results also suggest that 22% of the YOCAS effects on CRF were attributed by improving overall sleep quality. More specifically, we found that 37% of the YOCAS effect on CRF was due to reducing daytime dysfunction (eg, less daytime napping and sleepiness, more daytime activity and energy).53,54 This mediation effect of reductions in daytime dysfunction on improvements in CRF was also supported by the fact that participants reported more vigor (or felt more energized) after participating in YOCAS compared with receiving standard survivorship care alone. Since no other sleep subdomains was found contributing to the mediation effects of changes in sleep on changes in CRF, improvements in daytime dysfunction stemming from YOCAS contributed the most effect to the mediational relationship of overall sleep quality and CRF.
These results suggest that yoga may improve overall sleep quality, mainly by improving daytime dysfunction, and, ultimately, CRF not by simply increasing the amount of time a cancer survivor sleeps, but rather by helping cancer survivors nap less, feel less sleepy, feel more energized, and be more active during the day. In turn, these improvements in daytime dysfunction may reduce sleep inertia and create a priming effect for nighttime sleep so that survivors sleep better at night leading to improved sleep quality and less CRF. One of the distinguishing characteristics of CRF is that it cannot be mitigated by simply resting or sleeping more. Existing CRF treatment guidelines suggest that cancer survivors limit daytime sleeping and increase daytime physical activity.10,11,13,14,55,56 Our results suggest that behavioral interventions, like YOCAS, are effective at treating impaired sleep and CRF among cancer survivors precisely because they reduce daytime napping and sleepiness and improve daytime energy and activity, not because they lead to increased total sleep time.
Despite these novel and positive findings, our results need to be interpreted with limitations in mind. Limitations of the current secondary analyses include the following: (1) The majority of study participants were highly educated white female breast cancers survivors, thereby limiting the generalizability of the results. (2) The study design did not compare yoga with an active behavioral placebo controlling for specific and nonspecific characteristics. Therefore, we cannot assess whether the improvements in daytime dysfunction were specific to yoga. (3) No biophysiological data were collected making it impossible to further understand the etiological and pathophysiological mechanisms of sleep or CRF. (4) The study design only included 2 assessment time points, therefore mediational relationships cannot be examined temporally. (5) The dose of YOCAS yoga might be challenging to some cancer patients with medical, physical, financial, or environmental difficulties. However, compliance to our yoga intervention was good (81%), suggesting that YOCAS yoga is feasible for many cancer survivors. (6) No follow-up assessments were done to assess the sustainability of the yoga effect on sleep or CRF.
Conclusions
Our findings suggest that 4 weeks of YOCAS can help alleviate CRF in cancer survivors and between 22% and 37% of the improvements in CRF resulting from yoga therapy are mediated by improvements in sleep, specifically less daytime dysfunction (eg, less daytime napping and sleepiness, increased daytime energy and activity) and better overall sleep quality. Oncologists should consider prescribing yoga to cancer survivors for treating CRF, especially survivors who report both impaired sleep and CRF. Future phase II/III RCTs need to (1) test yoga therapy among diverse cancer patients and survivor populations and caregivers; (2) compare yoga therapy to known effective treatments for CRF such as exercise, psychosocial interventions, and pharmaceuticals11; (3) collect and analyze biophysiological data to increase knowledge regarding the etiology and pathophysiology of CRF; (4) add intermediate and follow-up assessments to evaluate the mediational relationships and the sustainability of yoga effects on CRF; (5) study the dose-response relationships that can help identify the minimal dose required to improve CRF and sleep disturbances; and (6) combine yoga with other potential dietary approaches such as high-fiber diet, functional foods, juices, and nutraceuticals to elicit greater reductions in CRF.
Acknowledgments
The authors acknowledge and thank the URMC PEAK Human Performance Lab, especially Michelle Porto, Ann Colasurdo, and Adam Szczupakowski, for their help with this project. We also acknowledge and thank Dr Amber Kleckner for editorial assistance, all of the participating NCORP affiliates and cancer survivors, and all of the URCC NCORP Research Base faculty and staff.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Cancer Institute (U10CA037420, U10CA037420 with supplemental funding from the Office of Cancer Complementary and Alternative Medicine, UG1CA189961, UG1CA189961 with supplemental funding from the Division of Cancer Prevention, R01CA181064, R25CA102618, K07CA168886, K07CA168911, K07CA190529, and K07CA221931).
Clinical Trial Information: ClinicalTrials.gov: NCT00397930
ORCID iDs: Po-Ju Lin  https://orcid.org/0000-0003-0362-1120
https://orcid.org/0000-0003-0362-1120
Kah Poh Loh  https://orcid.org/0000-0002-6978-0418
https://orcid.org/0000-0002-6978-0418
References
- 1. Patrick DL, Ferketich SL, Frame PS, et al. National Institutes of Health State-of-the-Science Conference Statement: Symptom Management in Cancer: Pain, Depression, and Fatigue, July 15-17, 2002. J Natl Cancer Inst. 2003;95:1110-1117. [DOI] [PubMed] [Google Scholar]
- 2. Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: results from a cross-sectional national survey in the U.S. Support Care Cancer. 2008;16:791-801. [DOI] [PubMed] [Google Scholar]
- 3. Kuhnt S, Ernst J, Singer S, et al. Fatigue in cancer survivors—prevalence and correlates. Onkologie. 2009;32:312-317. [DOI] [PubMed] [Google Scholar]
- 4. Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. Eur J Cancer. 2002;38:27-43. [DOI] [PubMed] [Google Scholar]
- 5. Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;(32):40-50. [DOI] [PubMed] [Google Scholar]
- 6. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(suppl 1):4-10. [DOI] [PubMed] [Google Scholar]
- 7. Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106:751-758. [DOI] [PubMed] [Google Scholar]
- 8. Servaes P, Gielissen MF, Verhagen S, Bleijenberg G. The course of severe fatigue in disease-free breast cancer patients: a longitudinal study. Psychooncology. 2007;16:787-795. [DOI] [PubMed] [Google Scholar]
- 9. Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743-753. [DOI] [PubMed] [Google Scholar]
- 10. Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw. 2015;13:1012-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barsevick AM, Irwin MR, Hinds P, et al. Recommendations for high-priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst. 2013;105:1432-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32:1840-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howell D, Keller-Olaman S, Oliver TK, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol. 2013;20:e233-e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitchell SA, Hoffman AJ, Clark JC, et al. Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following treatment. Clin J Oncol Nurs. 2014;18(suppl):38-58. [DOI] [PubMed] [Google Scholar]
- 16. Barsevick AM, Cleeland CS, Manning DC, et al. ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. J Pain Symptom Manage. 2010;39:1086-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353-360. [DOI] [PubMed] [Google Scholar]
- 18. Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer. 2010;116:5740-5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24:583-590. [DOI] [PubMed] [Google Scholar]
- 20. Schultz PN, Klein MJ, Beck ML, Stava C, Sellin RV. Breast cancer: relationship between menopausal symptoms, physiologic health effects of cancer treatment and physical constraints on quality of life in long-term survivors. J Clin Nurs. 2005;14:204-211. [DOI] [PubMed] [Google Scholar]
- 21. Otte JL, Carpenter JS, Russell KM, Bigatti S, Champion VL. Prevalence, severity, and correlates of sleep-wake disturbances in long-term breast cancer survivors. J Pain Symptom Manage. 2010;39:535-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carpenter JS, Elam JL, Ridner SH, Carney PH, Cherry GJ, Cucullu HL. Sleep, fatigue, and depressive symptoms in breast cancer survivors and matched healthy women experiencing hot flashes. Oncol Nurs Forum. 2004;31:591-598. [DOI] [PubMed] [Google Scholar]
- 23. Redeker NS, Pigeon WR, Boudreau EA. Incorporating measures of sleep quality into cancer studies. Support Care Cancer. 2015;23:1145-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Howell D, Oliver TK, Keller-Olaman S, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol. 2014;25:791-800. [DOI] [PubMed] [Google Scholar]
- 25. Palesh O, Aldridge-Gerry A, Zeitzer JM, et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37:837-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roscoe JA, Kaufman ME, Matteson-Rusby SE, et al. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12(suppl 1):35-42. [DOI] [PubMed] [Google Scholar]
- 27. Stepanski EJ, Walker MS, Schwartzberg LS, Blakely LJ, Ong JC, Houts AC. The relation of trouble sleeping, depressed mood, pain, and fatigue in patients with cancer. J Clin Sleep Med. 2009;5:132-136. [PMC free article] [PubMed] [Google Scholar]
- 28. Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45:384-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Donovan KA, Jacobsen PB. Fatigue, depression, and insomnia: evidence for a symptom cluster in cancer. Semin Oncol Nurs. 2007;23:127-135. [DOI] [PubMed] [Google Scholar]
- 30. Beck SL, Dudley WN, Barsevick A. Pain, sleep disturbance, and fatigue in patients with cancer: using a mediation model to test a symptom cluster. Oncol Nurs Forum. 2005;32:542. [DOI] [PubMed] [Google Scholar]
- 31. Berger AM, Farr L. The influence of daytime inactivity and nighttime restlessness on cancer-related fatigue. Oncol Nurs Forum. 1999;26:1663-1671. [PubMed] [Google Scholar]
- 32. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31:3233-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Banasik J, Williams H, Haberman M, Blank SE, Bendel R. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. J Am Acad Nurse Pract. 2011;23:135-142. [DOI] [PubMed] [Google Scholar]
- 34. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118:3766-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carson JW, Carson KM, Porter LS, Keefe FJ, Seewaldt VL. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Support Care Cancer. 2009;17:1301-1309. [DOI] [PubMed] [Google Scholar]
- 36. Danhauer SC, Mihalko SL, Russell GB, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psychooncology. 2009;18:360-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kiecolt-Glaser JK, Bennett JM, Andridge R, et al. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2014;32:1040-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Littman AJ, Bertram LC, Ceballos R, et al. Randomized controlled pilot trial of yoga in overweight and obese breast cancer survivors: effects on quality of life and anthropometric measures. Support Care Cancer. 2012;20:267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stan DL, Croghan KA, Croghan IT, et al. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support Care Cancer. 2016;24:4005-4015. [DOI] [PubMed] [Google Scholar]
- 40. Lin PJ, Peppone LJ, Janelsins MC, et al. Yoga for the management of cancer treatment-related toxicities. Curr Oncol Rep. 2018;20:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kleckner IR, Dunne RF, Asare M, et al. Exercise for toxicity management in cancer—a narrative review. Oncol Hematol Rev. 2018;14:28-37. [PMC free article] [PubMed] [Google Scholar]
- 42. Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575-1581. [DOI] [PubMed] [Google Scholar]
- 43. Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27:14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143-152. [DOI] [PubMed] [Google Scholar]
- 45. Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage. 2004;27:140-148. [DOI] [PubMed] [Google Scholar]
- 46. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173-1182. [DOI] [PubMed] [Google Scholar]
- 47. Kraemer HC. Messages for clinicians: moderators and mediators of treatment outcome in randomized clinical trials. Am J Psychiatry. 2016;173:672-679. [DOI] [PubMed] [Google Scholar]
- 48. Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59:877-883. [DOI] [PubMed] [Google Scholar]
- 49. Jamshidian M, Jalal S, HJansen C. MissMech: an R package for testing homoscedasticity, multivariate normality, and missing completely at random (MCAR). J Stat Softw. 2014;56:1-31. [Google Scholar]
- 50. Van Buuren S, Brand JPL, Groothuis-Oudshoorn CGM, Rubin DB. Fully conditional specification in multivariate imputation. J Stat Comput Simul. 2006;76:1049-1064. [Google Scholar]
- 51. Piantadosi S. Clinical Trials: A Methodologic Perspective. 2nd ed. Hoboken, NJ: Wiley; 2005. [Google Scholar]
- 52. Fisher LD, Dixon DO, Herson J, Frankowski RK, Hearron MS, Peace KE. Intention to treat in clinical trials. In: Peace KE, ed. Statistical Issues in Drug Research and Development. New York, NY: Marcel Dekker; 1990:331-350. [Google Scholar]
- 53. Chen HM, Clark AP, Tsai LM, Liu Y, Wu LM, Wu SJ. Excessive daytime sleepiness in Taiwanese people with heart failure. J Nurs Res. 2013;21:39-48. [DOI] [PubMed] [Google Scholar]
- 54. Vena C, Parker K, Allen R, Bliwise D, Jain S, Kimble L. Sleep-wake disturbances and quality of life in patients with advanced lung cancer. Oncol Nurs Forum. 2006;33:761-769. [DOI] [PubMed] [Google Scholar]
- 55. Physical Activity Guidelines Advisory Committee Report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev. 2009;67:114-120. [DOI] [PubMed] [Google Scholar]
- 56. Mustian KM, Lin PJ, Loh KP, Kleckner IR. Fatigue. In: Feuerstein M, Nekhlyudov L, eds. Handbook of Cancer Survivorship. 2nd ed. Berlin, Germany: Springer; 2018:129-144. [Google Scholar]