Abstract
Background:
Dementia prevalence is increasing worldwide, and dementia is frequently comorbid with depression during its disease course. Additionally, safety concerns are rising regarding the prescription of psychotropic agents to patients with dementia. Thus, our study assessed the influence of prescribing antidepressants in dementia with depression on mortality risk, and the differences between classes of antidepressants.
Methods:
This study was a population-based retrospective cohort study that utilized the National Health Insurance (NHI) medical claims data on mental illness in Taiwan between 1998 and 2013. We identified 25,890 cases of newly diagnosed dementia with depression and divided them into two groups: antidepressant users and nonusers. All-cause mortality between the two groups and the effects of different antidepressants were analyzed.
Results:
Antidepressants reduced all-cause mortality in patients with dementia and depression after adjusting for all covariates. Furthermore, the effect was significant when antidepressant exposure was more than 168 cumulative defined daily dosages, and most classes of antidepressants had this protective effect.
Conclusions:
Antidepressant treatment showed significant protective effects in all-cause mortality for patients with dementia and depression. Most classes of antidepressants were effective, especially with longer treatment duration or higher dosage.
Keywords: antidepressants, dementia, depression, longitudinal study, mortality
Background
Dementia prevalence is increasing worldwide, with more than 80 million people estimated to be affected by dementia by 2040.1 A dementia study estimated a prevalence of 3.8% across mainland China, Hong Kong, and Taiwan.2 Additionally, dementia is frequently comorbid with depression, and approximately 50% of patients with dementia have suffered from depressive episodes during its disease course.3 Studies have indicated that depression in later life results in poor prognosis and increased all-cause mortality.4–7 Another study revealed increased all-cause mortality in those who had depression at baseline before incident dementia.8
Recently, concern has been raised about the safety of prescribing psychotropic agents, especially to elderly patients.9–11 An increased risk of mortality from prescribing antipsychotics in patients with dementia has been observed for many years.11,12 However, the safety of prescribing antidepressants to patients with dementia remains debatable. Some studies have reported increased mortality,10,11 but another showed a protective effect.13 Dementia is frequently comorbid with depression, and the risk of antidepressants in treating dementia with depression has not been fully investigated.
We investigated the influence of antidepressants in treating dementia comorbid with depression regarding all-cause mortality and analyzed any differences between antidepressants. Thus, the aims of our study were to investigate the risk of mortality in prescribing antidepressants for patients with dementia and depression, and to explore if any difference exists in protective effect among classes of antidepressants.
Methods
Patient selection
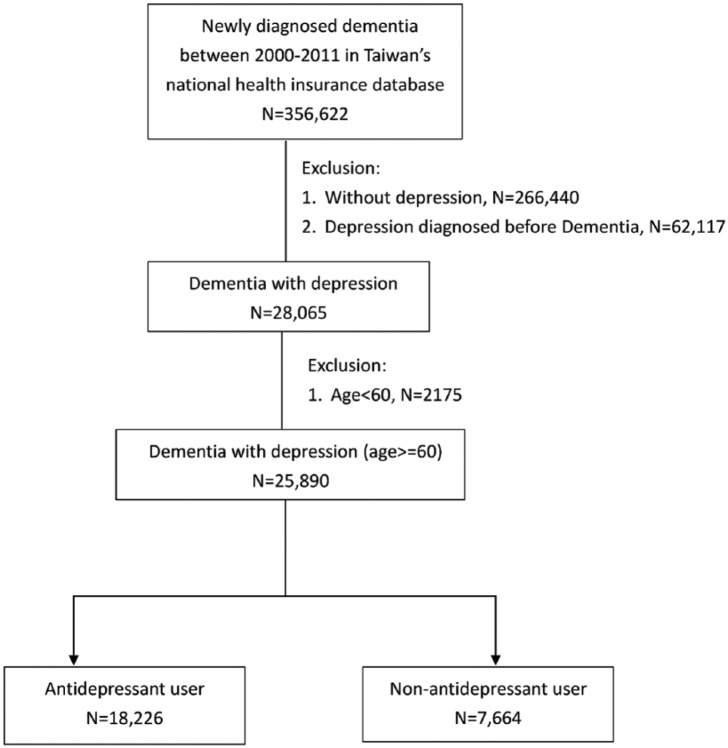
This study obtained the approval of the Institutional Review Board (IRB) from Chang Gung Memorial Hospital (IRB No:2017017141B1). The IRB committee received full accreditation from the Association for the Accreditation of Human Research Protection Programs in 2018. This study utilized a deidentified medical dataset, and the IRB waived the requirement of obtaining informed consent. Figure 1 shows the procedure of participant selection.
Figure 1.
Study selection protocol.
The data were extracted from the Taiwan National Health Insurance Research Dataset (NHIRD) of medical claims that were registered from 1998 to 2013. The National Health Insurance (NHI) is a compulsory universal health care program for Taiwanese residents and was established in 1995 its dataset covers nearly the entire population. The dataset comprises patient medical information, including demographics, outpatient visits, outpatient diagnoses, admission dates, discharge diagnoses, and prescriptions. The diagnosis system used by the NHI is the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM).14
In step 1, we recruited those who were newly diagnosed with dementia based on ICD-9-CM code (290.X, 294.1X, 331.0) between 2000 and 2011; those who were diagnosed with dementia before 2000 were excluded. There were 356,622 cases after step 1. In step 2, those who received depression diagnoses (296.2X, 296.3X, 300.4, 311) after the diagnosis of dementia were defined as the study participants. We excluded those without depression diagnoses during 2000 to 2011 and those whose depression was diagnosed before dementia. After step 2, 28,065 cases remained. In step 3, we excluded those diagnosed with dementia at an age younger than 60 years. The index date was defined as the date of incident diagnosis of depression. Finally, 25,890 patients with dementia who subsequently experienced depression were recruited, and we divided them into two groups for further analysis based on antidepressant use. We used the data from only between 2000 and 2011 (with follow-ups through 2013), because the data from 1998 to 2000 were used to determine whether the diagnosis of dementia was new, and whether the patient had depression before the dementia diagnosis. Specifically, without previous diagnostic data, we could not determine whether a person diagnosed with dementia in the 1998 data was newly diagnosed. Additionally, we could not determine whether a patient had dementia or depression first using 1998 data. Therefore, we assumed that if a person did not have a diagnosis of dementia from 1998 to 2000, a diagnosis of dementia given after the year of 2001 indicated newly diagnosed dementia. Additionally, if a person with dementia diagnosed in year 2001 had a diagnosis of depression between 1998 and 2000, we removed that person because of the possibility that depression came before dementia. Apart from the initial year’s data, we could analyze data through 2011 (and follow-up to 2013) because the released dataset was from 1998 to 2013. As a result, follow-up results after 2013 were not available.
Antidepressants and other covariates
The antidepressants prescribed to the study participants were identified using the Anatomical Therapeutic Chemical (ATC) Classification System of the World Health Organization. Prescription dosage was operationalized by cumulative defined daily dosage (cDDD),15 and we classified it into three different exposures: <28 cDDDs, 28–168 cDDDs, and >168 cDDDs. Antidepressant nonusers were defined as <28 cDDDs.
Other covariates we collected comprised area of residence; annual income; severity of depression; antidepressants use within 1 year before index date; general physical conditions as assessed using the Charlson comorbidity index (CCI);16 and other comorbidities, including hypertension, diabetes, malignancy, coronary artery disease, heart failure, end stage renal disease (ESRD), and chronic obstructive pulmonary disease (COPD), that were recorded before the index date. Those who received depressive diagnostic codes of 296.2X and 296.3X were defined as major depression, and those of 300.4 and 311 were defined as minor depression, respectively. All diagnoses of dementia, depression, and other physical comorbidities in our study were operationalized as having an inpatient diagnosis or at least three medical records of outpatient care within 1 year. Antidementia drug prescriptions, including acetylcholine esterase inhibitors (AChEIs) and memantine, were also recorded as covariates.
Statistical analysis
Sociodemographic data, severity of depression, CCI, antidepressant use, comorbidities, and antidepressants exposure before the index date were compared between patients with and without antidepressant prescriptions using Pearson’s chi-square test for categorical variables. In addition, we also displayed effect size to show the magnitude of effect.17 The primary outcome event was defined as all-cause mortality. Mortality risk was analyzed using the chi-square test. Additionally, Kaplan-Meier survival curves by different cDDD of antidepressants exposure were compared using log-rank test. The time from index date until death or until the end of 2013 was calculated as time to event. Cox proportional hazards models were performed to calculate the hazard ratio (HR) with 95% confidence intervals (CIs). The covariates included gender, age, urbanization, income, and year of index date. The cumulative effects of prescribing antidepressants were calculated according to cDDD. Those who were still alive or ceased follow-up before the end of 2013 were defined as censoring data. In order to avoid immortal time bias, Cox models were analyzed using time-dependent covariates.
In order to examine the consistency between antidepressants use and mortality risk, several sensitivity analyses were performed by adding other potential confounders, including severity of depression, antidepressants use within 1 year before index date, different comorbidities, CCI, and antidementia drugs. Consequently, we examined the outcome stratified by age, gender, severity of depression, and antidepressants use within 1 year before index date to test the potential effects of modifiers. All data were analyzed using SAS 9.4 software (SAS institute Inc., Cary, NC, USA).
Results
Characteristics of participants
Through selection in accordance with the protocol in the Figure 1, we identified 25,890 cases of newly diagnosed dementia with incident depression. Of these, 18,226 cases were defined as antidepressant users (cDDD ⩾28), and 7664 cases were defined as antidepressant nonusers (cDDD <28). Demographic data, clinical variables, severity of depression, underlying diseases, dementia treatment, and antidepressants use within 1 year before index date were compared between the two groups; the comparisons are displayed in Table 1. Antidepressant users were younger and had higher income, lower CCI, fewer comorbidities (including heart failure, ESRD, and COPD), more severe in depression, and more exposure to antidementia drugs (p < .0001). All-cause mortality was significantly lower in antidepressant users than in nonusers (p < .0001).
Table 1.
Demographic data and clinical characteristics of all patients with dementia and incident depression.
| Variables | Antidepressant user (N = 18,226) |
Antidepressant nonuser (N = 7664) |
Chi-square test | df | p value | Cramer’s Va | ||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| Severity of depression | 509.6268 | 1 | <0.0001 | 0.1403 | ||||
| Major depression | 5286 | 29.0 | 1202 | 15.7 | ||||
| Minor depression | 12,940 | 71.0 | 6462 | 84.3 | ||||
| AD use within 1 year before index date | 2940.7494 | 1 | <0.0001 | 0.3370 | ||||
| Yes | 10,727 | 58.9 | 1684 | 22.0 | ||||
| No | 7499 | 41.1 | 5980 | 78.0 | ||||
| Gender | 1.5728 | 1 | 0.2098 | 0.0078 | ||||
| Male | 8171 | 44.8 | 3501 | 45.7 | ||||
| Female | 10,055 | 55.2 | 4163 | 54.3 | ||||
| Age (year) | 164.8553 | 1 | <0.0001 | 0.0798 | ||||
| 60–80 | 11,676 | 64.1 | 4258 | 55.6 | ||||
| >80 | 6550 | 35.9 | 3406 | 44.4 | ||||
| Urbanization level | 179.5746 | 3 | <0.0001 | 0.0833 | ||||
| 1 (City) | 3613 | 19.8 | 1543 | 20.1 | ||||
| 2 | 5156 | 28.3 | 2426 | 31.7 | ||||
| 3 | 2364 | 13.0 | 1316 | 17.2 | ||||
| 4 (Villages) | 7093 | 38.9 | 2379 | 31.0 | ||||
| Income | 55.1474 | 3 | <0.0001 | 0.0462 | ||||
| 0 | 5456 | 29.9 | 2295 | 30.0 | ||||
| 1–15,840 | 4236 | 23.2 | 1714 | 22.4 | ||||
| 15,841–25,000 | 6936 | 38.1 | 3167 | 41.3 | ||||
| >25,000 | 1598 | 8.8 | 488 | 6.4 | ||||
| Charlson comorbidity Index | 8.8535 | 2 | 0.0120 | 0.0185 | ||||
| 0–2 | 1972 | 10.8 | 769 | 10.0 | ||||
| 3–5 | 6769 | 37.1 | 2759 | 36.0 | ||||
| ⩾6 | 9485 | 52.0 | 4136 | 54.0 | ||||
| Comorbidities | ||||||||
| Coronary artery disease | 0.5983 | 1 | 0.4392 | 0.0048 | ||||
| Yes | 8738 | 47.9 | 3634 | 47.4 | ||||
| No | 9488 | 52.1 | 4030 | 52.6 | ||||
| Heart failure | 19.1702 | 1 | <0.0001 | 0.0272 | ||||
| Yes | 3123 | 17.1 | 1488 | 19.4 | ||||
| No | 15,103 | 82.9 | 6176 | 80.6 | ||||
| End stage renal disease | 6.8896 | 1 | 0.0087 | 0.0163 | ||||
| Yes | 245 | 1.3 | 136 | 1.8 | ||||
| No | 17,981 | 98.7 | 7528 | 98.2 | ||||
| Chronic obstructive pulmonary | 30.4784 | 1 | <0.0001 | 0.0343 | ||||
| Yes | 7396 | 40.6 | 3394 | 44.3 | ||||
| No | 10,830 | 59.4 | 4270 | 55.7 | ||||
| Malignancy | 3.1336 | 1 | 0.0767 | 0.0110 | ||||
| Yes | 1248 | 6.9 | 572 | 7.5 | ||||
| No | 16,978 | 93.2 | 7092 | 92.5 | ||||
| Diabetes | 0.0070 | 1 | 0.9332 | 0.0005 | ||||
| Yes | 6392 | 35.1 | 2692 | 35.1 | ||||
| No | 11,834 | 64.9 | 4972 | 64.9 | ||||
| Hypertension | 0.6201 | 1 | 0.4310 | 0.0049 | ||||
| Yes | 14,195 | 77.9 | 6003 | 78.3 | ||||
| No | 4031 | 22.1 | 1661 | 21.7 | ||||
| Anti-dementia drugs | ||||||||
| Donepezil | 67.6332 | 1 | <0.0001 | 0.0511 | ||||
| Yes | 2125 | 11.7 | 629 | 8.2 | ||||
| No | 16,101 | 88.3 | 7035 | 91.8 | ||||
| Rivastigmine | 66.5535 | 1 | <0.0001 | 0.0507 | ||||
| Yes | 1602 | 8.8 | 444 | 5.8 | ||||
| No | 16,624 | 91.2 | 7220 | 94.2 | ||||
| Galantamine | 17.0465 | 1 | <0.0001 | 0.0257 | ||||
| Yes | 390 | 2.1 | 105 | 1.4 | ||||
| No | 17,836 | 97.9 | 7559 | 98.6 | ||||
| Memantine | 56.3735 | 1 | <0.0001 | 0.0467 | ||||
| Yes | 583 | 3.2 | 118 | 1.5 | ||||
| No | 17,643 | 96.8 | 7546 | 98.5 | ||||
| Death | 33.9066 | 1 | <0.0001 | 0.0362 | ||||
| Yes | 6339 | 34.8 | 2957 | 38.6 | ||||
ADs: Antidepressants; df: degrees of freedom.
Interpretation of Cramer’s V: (1) in df = 1, small: 0.10, medium: 0.30, large: 0.50; (2) in df = 2, small: 0.07, medium: 0.21, large: 0.35; (3) in df = 3, small: 0.06, medium: 0.17, large: 0.29.
Associations of antidepressant prescription and clinical variables with all-cause mortality
In the adjusted Cox regression (Table 2), all-cause mortality revealed no differences when cDDDs were between 28 and 167 when compared with cDDDs <28, but it was significantly lower if cDDDs were ⩾168 (HR: 0.65, 95% CI: 0.62–0.68, p < .0001). In sensitivity analysis, mortality risk was similar when including other potential confounders. Additionally, the protective effect of antidepressants was still significant in the subgroup analysis when the data was stratified according to gender, age, severity of depression and the antidepressants use within 1 year before index date. Furthermore, the result showed the consistency that using antidepressants still could reduce mortality risk in time-dependent model (Table 3). In Figure 2, the mortality of different cDDDs was illustrated by Kaplan-Meier survival curve. The result was identical and the log-rank test also revealed statistical significance (p < .001).
Table 2.
Adjusted hazard ratios of all-cause mortality associated with antidepressants use during the follow-up period in all recruited patients.
| Variables | 28–167 cDDD |
⩾168 cDDD |
||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |||
| Main modela | 0.98 | 0.93 | 1.03 | 0.3307 | 0.65 | 0.62 | 0.69 | <0.0001 |
| Additional covariatesb | ||||||||
| Main model + Severity of depression | 0.97 | 0.92 | 1.02 | 0.2917 | 0.65 | 0.62 | 0.68 | <0.0001 |
| Main model + ADs use within 1 year before Index date | 0.93 | 0.88 | 0.98 | 0.0074 | 0.61 | 0.58 | 0.64 | <0.0001 |
| Main model + CCI | 0.97 | 0.92 | 1.02 | 0.2287 | 0.66 | 0.62 | 0.69 | <0.0001 |
| Main model + Coronary artery disease | 0.97 | 0.92 | 1.02 | 0.2847 | 0.65 | 0.62 | 0.68 | <0.0001 |
| Main model + Heart failure | 0.98 | 0.93 | 1.03 | 0.3990 | 0.66 | 0.63 | 0.69 | <0.0001 |
| Main model + End stage renal disease | 0.98 | 0.93 | 1.03 | 0.3532 | 0.65 | 0.62 | 0.69 | <0.0001 |
| Main model + Chronic obstructive pulmonary disease | 0.98 | 0.93 | 1.03 | 0.3320 | 0.66 | 0.63 | 0.69 | <0.0001 |
| Main model + Malignancy | 0.97 | 0.92 | 1.02 | 0.2156 | 0.65 | 0.62 | 0.68 | <0.0001 |
| Main model + Diabetes | 0.97 | 0.92 | 1.02 | 0.2515 | 0.65 | 0.62 | 0.69 | <0.0001 |
| Main model + Hypertension | 0.98 | 0.93 | 1.03 | 0.3284 | 0.65 | 0.62 | 0.68 | <0.0001 |
| Main model + Donepezil | 0.98 | 0.93 | 1.03 | 0.3856 | 0.66 | 0.63 | 0.69 | <0.0001 |
| Main model + Rivastigmine | 0.98 | 0.93 | 1.03 | 0.3852 | 0.66 | 0.63 | 0.69 | <0.0001 |
| Main model + Galantamine | 0.98 | 0.93 | 1.03 | 0.3499 | 0.65 | 0.62 | 0.69 | <0.0001 |
| Main model + Memantine | 0.98 | 0.93 | 1.03 | 0.3467 | 0.66 | 0.63 | 0.69 | <0.0001 |
| Subgroup effects | ||||||||
| Gender | ||||||||
| Male | 0.96 | 0.90 | 1.03 | 0.2914 | 0.64 | 0.60 | 0.68 | <0.0001 |
| Female | 0.99 | 0.92 | 1.07 | 0.8169 | 0.67 | 0.62 | 0.72 | <0.0001 |
| Age (year) | ||||||||
| 60–80 | 0.99 | 0.92 | 1.06 | 0.7021 | 0.63 | 0.59 | 0.67 | <0.0001 |
| >80 | 0.96 | 0.89 | 1.03 | 0.2580 | 0.68 | 0.63 | 0.73 | <0.0001 |
| Severity of depression | ||||||||
| Major depression | 0.92 | 0.83 | 1.03 | 0.1557 | 0.56 | 0.51 | 0.62 | <0.0001 |
| Minor depression | 0.98 | 0.92 | 1.04 | 0.4046 | 0.68 | 0.64 | 0.72 | <0.0001 |
| ADs use within 1 year before Index date | ||||||||
| Yes | 0.94 | 0.86 | 1.03 | 0.1835 | 0.60 | 0.55 | 0.66 | <0.0001 |
| No | 0.92 | 0.86 | 0.98 | 0.0138 | 0.61 | 0.57 | 0.66 | <0.0001 |
Main model was adjusted for gender, age, urbanization, income, and year of index date.
The models were adjusted for covariates in the main model as well as each additional listed covariate.
cDDD: cumulative defined daily dosage; HR: hazard ratio; CI: confidence interval; ADs: antidepressants; CCI: Charlson comorbidity index.
Table 3.
Antidepressant prescription and all-cause mortality analyzed by time-dependent model.
| Variables | Crude |
Adjusteda |
||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |||
| Antidepressants | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| ⩾28 cDDD | 0.89 | 0.85 | 0.93 | <0.0001 | 0.89 | 0.85 | 0.93 | <0.0001 |
Adjusted for gender, age, urbanization, income, and year of index date.
cDDD, cumulative defined daily dosage; HR, hazard ratio; CI, confidence interval.
Figure 2.
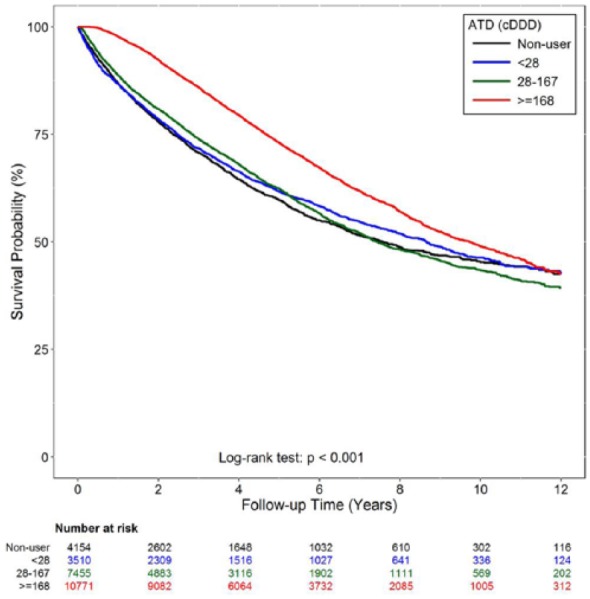
Survival curve of by different cumulative defined daily dosage (cDDD) of antidepressants use during follow-up period.
Prescription of different antidepressants and all-cause mortality
Cox regression was conducted to evaluate the effect of the exposure of different antidepressants on all-cause mortality in patients with dementia and depression. After adjusting for all covariates, prescription of most antidepressants was not associated with a difference in mortality in participants with cDDDs 28–167 when compared with those with cDDDs <28 (Table 4). However, mortality significantly decreased in those with cDDDs >168. Among antidepressant classes, selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), norepinephrine dopamine reuptake inhibitor (NDRI), mirtazapine, tricyclic/tetracyclic antidepressants, serotonin antagonist and reuptake inhibitor (SARI), and monoamine oxidative inhibitors (MAOIs) consistently showed protective effects against mortality. Furthermore, most classes of antidepressants revealed the similar trend of protective effects as well when analyzed by time-dependent model (Table 5).
Table 4.
Antidepressant prescription and all-cause mortality in patients with dementia and incident depression.
| Variables | Crude |
Adjusteda |
||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |||
| SSRI | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| 28–167 cDDD | 1.08 | 1.03 | 1.14 | 0.0023 | 1.05 | 1.00 | 1.11 | 0.0688 |
| ⩾168 cDDD | 0.78 | 0.74 | 0.82 | <.0001 | 0.74 | 0.70 | 0.78 | <.0001 |
| SNRI | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| 28–167 cDDD | 0.93 | 0.84 | 1.03 | 0.1611 | 0.95 | 0.86 | 1.05 | 0.2910 |
| ⩾168 cDDD | 0.85 | 0.78 | 0.94 | 0.0010 | 0.86 | 0.78 | 0.94 | 0.0016 |
| NDRI | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| 28–167 cDDD | 0.89 | 0.77 | 1.02 | 0.0938 | 0.94 | 0.82 | 1.09 | 0.4100 |
| ⩾168 cDDD | 0.43 | 0.33 | 0.56 | <.0001 | 0.46 | 0.35 | 0.60 | <.0001 |
| Mirtazapine | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| 28–167 cDDD | 1.05 | 0.94 | 1.16 | 0.4044 | 1.02 | 0.92 | 1.14 | 0.6963 |
| ⩾168 cDDD | 0.61 | 0.54 | 0.70 | <.0001 | 0.64 | 0.56 | 0.73 | <.0001 |
| Tricyclic/tetracyclic ADs | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| 28–167 cDDD | 0.69 | 0.63 | 0.75 | <.0001 | 0.77 | 0.70 | 0.83 | <.0001 |
| ⩾168 cDDD | 0.56 | 0.48 | 0.65 | <.0001 | 0.63 | 0.55 | 0.73 | <.0001 |
| SARI | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| 28–167 cDDD | 0.81 | 0.75 | 0.87 | <.0001 | 0.83 | 0.78 | 0.90 | <.0001 |
| ⩾168 cDDD | 0.53 | 0.46 | 0.61 | <.0001 | 0.56 | 0.49 | 0.64 | <.0001 |
| MAOI, selective | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| 28–167 cDDD | 0.79 | 0.68 | 0.91 | 0.0008 | 0.92 | 0.80 | 1.06 | 0.2602 |
| ⩾168 cDDD | 0.70 | 0.60 | 0.82 | <.0001 | 0.75 | 0.64 | 0.88 | 0.0005 |
Adjusted for all covariates including all kinds of antidepressants, gender, age, urbanization, income, and year of index date.
cDDD: cumulative defined daily dosage; SSRI: selective serotonin reuptake inhibitors; SNRI: serotonin norepinephrine reuptake inhibitor; NDRI: norepinephrine dopamine reuptake inhibitor; SARI: Serotonin antagonist and reuptake inhibitor; MAOIs: monoamine oxidative inhibitor; ADs: antidepressants.
Table 5.
Different antidepressant prescription and all-cause mortality analyzed by time-dependent model.
| Variables | Crude |
Adjusteda |
||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |||
| SSRI | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| ⩾28 cDDD | 0.95 | 0.90 | 0.99 | 0.0289 | 0.92 | 0.88 | 0.97 | 0.0019 |
| SNRI | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| ⩾28 cDDD | 0.96 | 0.86 | 1.06 | 0.3828 | 0.93 | 0.84 | 1.04 | 0.2007 |
| NDRI | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| ⩾28 cDDD | 0.73 | 0.60 | 0.89 | 0.0016 | 0.76 | 0.63 | 0.92 | 0.0057 |
| Mirtazapine | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| ⩾28 cDDD | 1.03 | 0.92 | 1.15 | 0.6442 | 1.02 | 0.91 | 1.14 | 0.7560 |
| Tricyclic/tetracyclic ADs | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| ⩾28 cDDD | 0.81 | 0.72 | 0.92 | 0.0008 | 0.84 | 0.75 | 0.95 | 0.0064 |
| SARI | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| ⩾28 cDDD | 0.90 | 0.81 | 0.99 | 0.0319 | 0.88 | 0.80 | 0.97 | 0.0136 |
| MAOI, selective | ||||||||
| <28 cDDD | 1.00 | Reference | 1.00 | Reference | ||||
| ⩾28 cDDD | 0.69 | 0.57 | 0.84 | 0.0002 | 0.71 | 0.58 | 0.86 | 0.0007 |
Adjusted for all covariates including all kinds of antidepressants, gender, age, urbanization, income, and year of index date.
cDDD: cumulative defined daily dosage; SSRI: selective serotonin reuptake inhibitors; SNRI: serotonin norepinephrine reuptake inhibitor; NDRI: norepinephrine dopamine reuptake inhibitor; SARI: Serotonin antagonist and reuptake inhibitor; TCA: tricyclic antidepressant; MAOIs: monoamine oxidative inhibitor; Ads: antidepressants.
Discussion
Our study indicated that antidepressant treatment significantly reduced mortality risk in patients with dementia and depression, especially with higher cDDDs. Furthermore, most antidepressant classes (i.e., SSRIs, SNRIs, NDRI, mirtazapine, tricyclic/tetracyclic antidepressants, SARI, and MAOIs) were effective at decreasing mortality.
The influence of depression in increasing mortality has been reported in studies on both the general population and elderly patients.4–7, 18,19 A meta-analysis of 293 studies showed a relative risk of 1.52 of mortality among participants with depression.18 A 10-year prospective cohort study conducted in community elderly in the United States indicated that depression, especially when combined with low self-rating of health, strongly predicted mortality.7 Another 10-year prospective study conducted in Amsterdam revealed that chronic depression was associated with higher mortality.19 Additionally, studies have found that more severe depression is linked to higher mortality.19,20 However, few studies have focused on the effects of depression on all-cause mortality in patients with dementia. Lara et al.21 recruited individuals aged >65 years to investigate the relationship between Alzheimer dementia and depression. They assessed the depressive symptoms at baseline, and the results indicated that depression was significantly associated with higher mortality in the overall sample and in those with incident Alzheimer dementia.8 In our study, the mortality in patients with dementia and depression was approximately 35.9%, which was almost triple the mortality in the matched controls without dementia and depression (~12.5%). The result was similar to that of an aforementioned study that associated dementia with mortality. However, in our further analysis, antidepressant use in the control group led to higher mortality than nonuse did (18.1% in antidepressant users and 12.2% in antidepressant nonusers), implying that antidepressant prescription to older adults requires careful consideration of the costs and benefits.
We propose some mechanisms for the association of depression and increased mortality. First, depressed patients may be prone to unhealthier lifestyles (e.g., smoking, alcohol drinking, unfavorable diet, insufficient outdoor activity) that may result in inadequate physical condition.21 Moreover, they engage in infrequent medical treatment irrespective of their physical condition.20 Many biological pathways play roles in the pathophysiology of depression.3,22,23 Increased inflammatory cytokines, alteration of the hypothalamic–pituitary–adrenal (HPA) axis, neurotrophin deficiency, reduced heart-rate variability, and increased catecholamine levels are all biological mechanisms that might lead to cardiovascular events and mortality.22,24 Our study showed antidepressant treatment could decrease mortality, likely because antidepressants alleviate depressive symptoms and attenuate the effect of depression on mortality. Similar studies have showed the protective effects against mortality in patients with late-life depression.25,26 Notably, the major limitations of these studies were that they recruited later-life patients with depression instead of patients with dementia and depression. Some double-blinded clinical trials have demonstrated improvement after antidepressant treatment for patients with dementia and depression.27–30 However, these studies have analyzed only the efficacy of antidepressants and ignored mortality outcomes. Clinical studies specifically investigating the mortality risk of antidepressant treatment in patients with dementia and depression are scant. In the future, further well-designed clinical investigation is warranted to replicate results suggesting the protective effects of antidepressants.
In terms of the medical care system in Taiwan, patients can visit a specialist in the hospital directly without a referral from general practitioners. Accordingly, most patients with depression were diagnosed and treated by psychiatrists (including geropsychiatrists) or neurologists. All of these doctors were well trained in this field; therefore, we believed that our results were not influenced by different specialties among these doctors.
Our study revealed that antidepressant exposure reduced mortality among patients with dementia and depression by 4%; additionally, SSRIs, SNRIs, NDRIs, mirtazapine, and MAOIs all exhibited a protective effect in the Cox regression model. The effect was significant if the prescription exceeded 168 cDDDs. In addition, most antidepressants showed the protective effect in time-dependent model, except for SNRIs and mirtazapine. In clinical practice, SSRIs are the most commonly prescribed medications. However, SSRIs might be switched to other classes of antidepressants, such as SNRIs or mirtazapine, if patients showed poor response. In this circumstance, it implied that their depression was relatively difficult to treat. On the other hand, the side effects (e.g., nausea in SNRIs and somnolence or dizziness in mirtazapine) might be prominent,31 which may subsequently result in poor drug compliance. Regarding the possible mechanism of protective effect of the antidepressants we proposed above, depression in patients taking SNRIs and mirtazapine might still be persistent, i.e., the protective effect was not observed. Furthermore, comparing to the Cox regression model, the time-dependent model is more conservative, and therefore our study results should be interpreted with caution. Accordingly, in clinical application, long treatment duration and high dosage are warranted when treating patients with dementia and depression. A study on the Swedish Prescribed Drug Register dataset showed a similar result that antidepressant exposure for more than 3 years consecutively before dementia diagnosis can significantly lower mortality risk.13 Another study focused on the association of mortality risk and psychotropic prescription in a nursing home population with cognitive impairment or dementia diagnoses.26 The results showed a protective effect of antidepressant treatment with appropriate indication, optimal dosage, and a treatment duration of >90 days. These findings suggest the importance of correct diagnosis and treatment. We emphasize the necessity of adherence and maintenance of therapy to confer the survival benefit. Depressive symptoms could be part of the behavior and psychological symptoms of dementia (BPSD) and were associated with increased mortality.32 Antidepressants can alleviate depression in BPSD and may reduce the risk of mortality.33,34 Other possibilities are that antidepressants can reduce HPA axis activity, increase levels of brain-derived neurotropic factor, or promote neurogenesis and neuron plasticity.35,36
Our study was limited by factors affecting all similar medical dataset studies. First, drug adherence and the exact exposure dosage and duration were impossible to assess. Second, smoking, lifestyle, nutrition, and exercise habits may affect mortality, but this information was unavailable in the dataset. Third, the diagnosis of depression was through clinical diagnosis by using the ICD-9-CM without confirmation through a structural diagnostic interview. Fourth, our study was not a randomized controlled trial. Therefore, our results should be interpreted cautiously.
Our study also has a number of strengths. This was a large population-based cohort study that was representative of all cases of dementia with depression in Taiwan. Additionally, we investigated the topic of mortality risk, which is rarely addressed when prescribing antidepressants, using a respective cohort study design because it might take many years to observe outcomes. Moreover, we conducted sensitivity analyses by stratification to clarify the potential confounders, and the results showed no significant changes in HRs between different models.
Conclusion
Antidepressant treatment can reduce mortality risk in patients with dementia and depression, especially at higher cDDDs. For clinical physicians treating patients with dementia and depression, sufficient dosage and duration are warranted to provide the benefit of decreased mortality risk.
Acknowledgments
The authors would like to thank Health Information and Epidemiology Laboratory of Chang Gung Memorial Hospital, Chiayi Branch, for its assistance in data analysis and Wallace Academic Editing for editing the manuscript.
Footnotes
Author contribution: J-AS and C-CC contributed equally. J-AS and C-CC initiated the study and wrote the first draft of the manuscript. K-JC and Y-HY analyzed the data. C-YL and H-MW interpreted the data, and C-YL critically revised the manuscript. All authors read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Chang Gung Memorial Hospital (grant number CFRPG6J0011).
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The datasets generated and analyzed in this study are not publicly available because of patient privacy and ethical concerns. They are available from the corresponding author upon reasonable request.
ORCID iD: Chung-Ying Lin  https://orcid.org/0000-0002-2129-4242
https://orcid.org/0000-0002-2129-4242
Contributor Information
Jian-An Su, Department of Psychiatry, Chang Gung Medical Foundation, Chiayi Chang Gung Memorial Hospital, Chiayi School of Medicine, Chang Gung University, Taoyuan Department of Nursing, Chang Gung Institute of Technology, Taoyuan, Taiwan.
Chih-Cheng Chang, Department of Psychiatry, Chi Mei Medical Center, Tainan; Department of Health Psychology, Chang Jung Christian University, Tainan, Taiwan.
Hsuan-Min Wang, Department of Psychiatry, Chang Gung Medical Foundation, Chiayi Chang Gung Memorial Hospital, Chiayi, Taiwan.
Ko-Jung Chen, Health Information and Epidemiology Laboratory, Chang Gung Memorial Hospital, Chiayi, Taiwan.
Yao-Hsu Yang, Health Information and Epidemiology Laboratory, Chang Gung Memorial Hospital, Chiayi; Department of Traditional Chinese Medicine, Chiayi Chang Gung Memorial Hospital, Chiayi; School of Traditional Chinese Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan.
Chung-Ying Lin, Department of Rehabilitation Sciences, Faculty of Health and Social Sciences, The Hong Kong Polytechnic University, Hung Hom, Hong Kong.
References
- 1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005; 366: 2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu YT, Lee HY, Norton S, et al. Prevalence studies of dementia in mainland China, Hong Kong and Taiwan: a systematic review and meta-analysis. PLoS One 2013; 8: e66252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chi S, Yu JT, Tan MS, et al. Depression in Alzheimer’s disease: epidemiology, mechanisms, and management. J Alzheimers Dis 2014; 42: 739–755. [DOI] [PubMed] [Google Scholar]
- 4. Diniz BS, Reynolds CF, Butters MA, et al. The effect of gender, age, and symptom severity in late-life depression on the risk of all-cause mortality: the Bambui Cohort Study of Aging. Depress Anxiety 2014; 31: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ho C, Jin A, Nyunt MS, et al. Mortality rates in major and subthreshold depression: 10-year follow-up of a Singaporean population cohort of older adults. Postgrad Med 2016; 128: 642–647. [DOI] [PubMed] [Google Scholar]
- 6. Sun W, Schooling CM, Chan WM, et al. The association between depressive symptoms and mortality among Chinese elderly: a Hong Kong cohort study. J Gerontol A Biol Sci Med Sci 2011; 66: 459–466. [DOI] [PubMed] [Google Scholar]
- 7. Ganguli M, Dodge HH, Mulsant BH. Rates and predictors of mortality in an aging, rural, community-based cohort: the role of depression. Arch Gen Psychiatry 2002; 59: 1046–1052. [DOI] [PubMed] [Google Scholar]
- 8. Lara E, Haro JM, Tang MX, et al. Exploring the excess mortality due to depressive symptoms in a community-based sample: the role of Alzheimer’s Disease. J Affect Disord 2016; 202: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kripke DF, Klauber MR, Wingard DL, et al. Mortality hazard associated with prescription hypnotics. Biol Psychiatry 1998; 43: 687–693. [DOI] [PubMed] [Google Scholar]
- 10. Jennum P, Baandrup L, Ibsen R, et al. Increased all-cause mortality with use of psychotropic medication in dementia patients and controls: a population-based register study. Eur Neuropsychopharmacol 2015; 25: 1906–1913. [DOI] [PubMed] [Google Scholar]
- 11. Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry 2015; 72: 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294: 1934–1943. [DOI] [PubMed] [Google Scholar]
- 13. Enache D, Fereshtehnejad SM, Kareholt I, et al. Antidepressants and mortality risk in a dementia cohort: data from SveDem, the Swedish Dementia Registry. Acta Psychiatr Scand 2016; 134: 430–440. [DOI] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. International Classification of Disease Ninth Revision Clinical Modification, ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/ICD9-CM/2011 (2001, accessed 27 April 2019).
- 15. WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index, https://www.whocc.no/atc_ddd_index/ (2019, accessed 27 April 2019).
- 16. D’Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med 1993; 32: 382–387. [PubMed] [Google Scholar]
- 17. Kim HY. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor Dent Endod 2017; 42: 152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cuijpers P, Vogelzangs N, Twisk J, et al. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry 2014; 171: 453–462. [DOI] [PubMed] [Google Scholar]
- 19. Schoevers RA, Geerlings MI, Deeg DJ, et al. Depression and excess mortality: evidence for a dose response relation in community living elderly. Int J Geriatr Psychiatry 2009; 24: 169–176. [DOI] [PubMed] [Google Scholar]
- 20. Schulz R, Beach SR, Ives DG, et al. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med 2000; 160: 1761–1768. [DOI] [PubMed] [Google Scholar]
- 21. van Gool CH, Kempen GI, Penninx BW, et al. Relationship between changes in depressive symptoms and unhealthy lifestyles in late middle aged and older persons: results from the Longitudinal Aging Study Amsterdam. Age Ageing 2003; 32: 81–87. [DOI] [PubMed] [Google Scholar]
- 22. Elderon L, Whooley MA. Depression and cardiovascular disease. Prog Cardiovasc Dis 2013; 55: 511–523. [DOI] [PubMed] [Google Scholar]
- 23. Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci 2009; 34: 4–20. [PMC free article] [PubMed] [Google Scholar]
- 24. Hare DL, Toukhsati SR, Johansson P, et al. Depression and cardiovascular disease: a clinical review. Eur Heart J 2014; 35: 1365–1372. [DOI] [PubMed] [Google Scholar]
- 25. Gallo JJ, Morales KH, Bogner HR, et al. Long term effect of depression care management on mortality in older adults: follow-up of cluster randomized clinical trial in primary care. BMJ 2013; 346: f2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wei YJ, Simoni-Wastila L, Zuckerman IH, et al. Quality of psychopharmacological medication prescribing and mortality in Medicare beneficiaries in nursing homes. J Am Geriatr Soc 2014; 62: 1490–1504. [DOI] [PubMed] [Google Scholar]
- 27. Karlsson I, Godderis J, Augusto De, Mendonça Lima C, et al. A randomised, double-blind comparison of the efficacy and safety of citalopram compared to mianserin in elderly, depressed patients with or without mild to moderate dementia. Int J Geriatr Psychiatry 2000; 15: 295–305. [DOI] [PubMed] [Google Scholar]
- 28. Nyth AL, Gottfries CG, Lyby K, et al. A controlled multicenter clinical study of citalopram and placebo in elderly depressed patients with and without concomitant dementia. Acta Psychiatr Scand 1992; 86: 138–145. [DOI] [PubMed] [Google Scholar]
- 29. Petracca G, Tesón A, Chemerinski E, et al. A double-blind placebo-controlled study of clomipramine in depressed patients with Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 1996; 8: 270–275. [DOI] [PubMed] [Google Scholar]
- 30. Roth M, Mountjoy CQ, Amrein R. Moclobemide in elderly patients with cognitive decline and depression: an international double-blind, placebo-controlled trial. Br J Psychiatry 1996; 168: 149–157. [DOI] [PubMed] [Google Scholar]
- 31. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacological Treatments. Can J Psychiatry 2016; 61: 540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park JE, Lee JY, Suh GH, et al. Mortality rates and predictors in community-dwelling elderly individuals with cognitive impairment: an eight-year follow-up after initial assessment. Int Psychogeriatr 2014; 26: 1295–1304. [DOI] [PubMed] [Google Scholar]
- 33. Bergh S, Selbaek G, Engedal K. Discontinuation of antidepressants in people with dementia and neuropsychiatric symptoms (DESEP study): double blind, randomised, parallel group, placebo controlled trial. BMJ 2012; 344: e1566. [DOI] [PubMed] [Google Scholar]
- 34. Huang TY, Wei YJ, Moyo P, et al. Treated behavioral symptoms and mortality in medicare beneficiaries in nursing homes with Alzheimer’s Disease and related dementias. J Am Geriatr Soc 2015; 63: 1757–1765. [DOI] [PubMed] [Google Scholar]
- 35. Alboni S, van Dijk RM, Poggini S, et al. Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment. Mol Psychiatry 2017; 22: 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matrisciano F, Bonaccorso S, Ricciardi A, et al. Changes in BDNF serum levels in patients with major depression disorder (MDD) after 6 months treatment with sertraline, escitalopram, or venlafaxine. J Psychiatr Res 2009; 43: 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]