Abstract
We report on the application of radon inhalation therapy to patients with 4 types of cancer: colon, uterine, lung, and liver cell. The radon treatments were given to improve the efficacy of chemotherapy and were potent in all 4 cases. Marker values decreased and disease symptoms were alleviated. We include a lengthy discussion on the mechanism that may be responsible for the observed results. While employing the radon generator to treat the patient with hepatocellular carcinoma, we discovered that a concentration of 6 MBq/m3 was very effective, while 1 MBq/m3 was marginal. This implies different, and rather high, radon concentration thresholds for the treatment of different types of cancer. The evidence from these 4 cases suggests that radon inhalation may be beneficial against various cancer types as an important adjuvant therapy to conventional chemotherapy and for local high-dose radiotherapy, which would address the problem of distant metastasis. A previous case report on 2 patients with advanced breast cancer, who refused chemotherapy or radiotherapy, indicates that radon may be effective as a primary therapy for cancer. Clinical trials should be carried out to determine the best radon concentrations for treatment of other types of cancer, at different stages of progression.
Keywords: radon therapy, colon uterine lung liver cancer, breast cancer, primary or adjuvant treatment, optimum radon concentration, radiation hormesis
Introduction
Our previous article on the radiation treatment of cancer using α-emitting nuclides pointed out the advantages of targeted internal therapy with short-lived α-emitters.1 It also discussed non-targeted, low-dose radiotherapy and its mechanism of action, the stimulation or inhibition of many genes, depending on the radiation dose or the dose rate. The effect is called radiation hormesis.1 About three quarters of human tissue is water, so ionizing radiation induces reactive oxygen species (ROS), which have very important effects. Both ROS and direct α-particle hits damage biomolecules. However, they also send signals to many adaptive biological protection systems that function against the effects of radiogenic and nonradiogenic toxins and also attack pathogens. These vital systems prevent, repair, and remove DNA damage and other biomolecular damages that are produced endogenously at a very high rate by the very abundant ROS associated with aerobic metabolism. Low-dose stimulation of many protection systems, which include very important immune system, results in many important beneficial effects, among them, a lower risk of cancer.2,3
Radon has been used for many years to treat various diseases, such as low back pain, high blood pressure, and cancer, in radon/radium spas, such as Misasa Onsen Izumi (Tottori) and Tamagawa Onsen (Akita) in Japan. Clinical studies are underway at Okayama University Hospital, Misasa Medical Center. Diseases treated with radon are ROS-related diseases such as arteriosclerosis, osteoarthritis, and bronchial asthma. Patients generally stay for 40 minutes, every 2 days, in a room maintained at 42°C temperature, 90% humidity, and 2000 Bq/m3 radon concentration.4-6 Several years ago, we constructed radon rooms, with conditions close to those in Europe and tried radon therapy on a patient with ulcerative colitis, diagnosed as inoperable.7
Our previous article reported the cases of 2 patients with advanced breast cancer who refused conventional chemotherapy and radiotherapy.1 Their recovery after receiving radon inhalation therapy suggests that radon may be effective as the primary therapy for other types of cancers. One patient inhaled radon from our α-Radiorespiro-Rn radon generator and the other was treated in a radon hormesis room.1
In this article, we report its application as an adjuvant therapy for 4 other types of cancer: colon, uterine, lung, and liver cell. The 4 patients requested radon after receiving conventional chemotherapy or high-dose radiotherapy. An acceptable recovery was observed in all 4 cases—somewhat surprising because conventional anticancer drugs produce harsh side effects, which suppress immunity. The radon treatments appear to have caused very strong stimulation that countered these effects and induced powerful action against the cancerous cells. In view of the difficulty of producing special short-lived radionuclides for targeted α-emitting therapies and the ease of generating ubiquitous radon gas, provision of radon inhalation therapy against cancer is very appealing.
Methods of Delivering Radon Inhalation Therapy
Radon Hormesis Room
As previously described,8 the therapy room was designed to reproduce the conditions of a natural radon health spa. Supplied by Lead & Company, Yokohama, Japan, the room has walls that contain natural uranium ore. The average γ-radiation dose rate inside is 11 μGy per hour, and the average concentration of radon in the room air is 200 000 Bq/m3, as measured using a TRACERLAB Alpha-Scint-1 monitor.
α-Radiorespiro-Rn Generator
The radon generator is made from very simple parts and housed in a small cabinet.1 A layer of high-grade uranium ore particles, about 4 mm in size, is covered by 2.5 L of water in a 16-L tank. The concentration of radon in the air above the water can be adjusted over the range from about 1 to 10 MBq/m3, as prescribed by the therapist. The patient inhales through the suction tube using a special respirator, for the specified time.1
Defining the Radiation Exposure From Radon Therapy
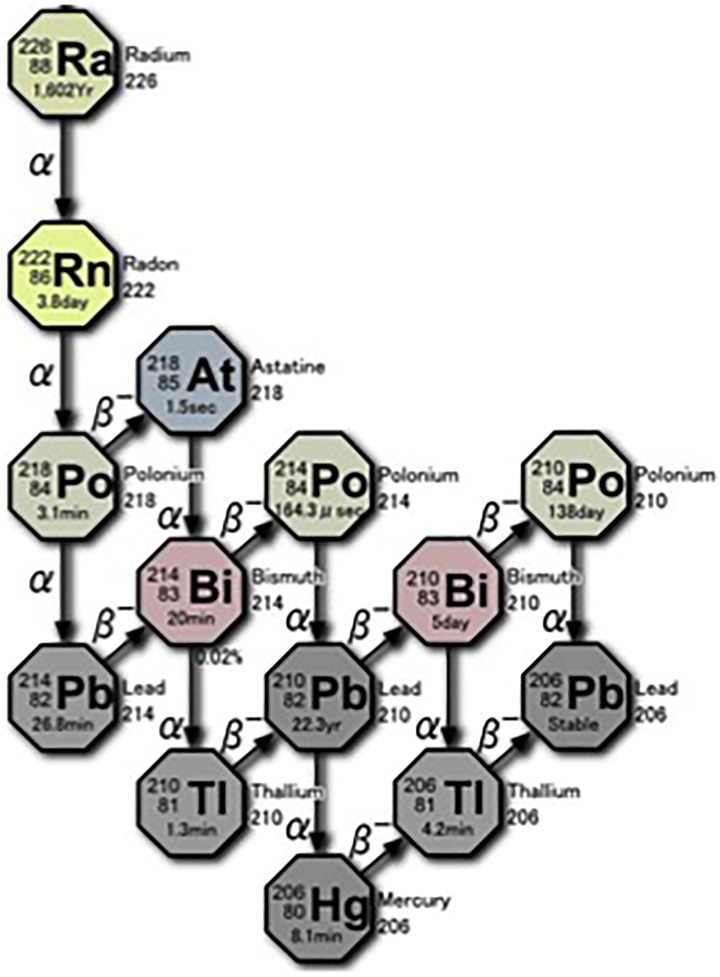
As shown in Figure 1, the radioactive decay of radium-226 (present in natural uranium ore) is the source of radon-222 gas. Each decay pathway of radon releases 4 α-particles (each with energy of about 5 MeV). Also released are 4 β-particles and their associated γ-rays. Most of inhaled radon is exhaled, but a small amount of gas and its decay products (progeny) adhere to the mucosa of the trachea and the lung surface. Some are taken up by alveolar epithelial cells and transferred into the bloodstream together with oxygen. After 2 weeks, the gas (3.8-day half-life) almost disappears. There is no reported evidence of adverse health effects from this therapy and no significant long-term accumulation of radionuclides in any specific tissue. The patient also receives a low dose from the γ-radiation emitted from the walls of the radon room.
Figure 1.
Radioactive decay chain of 222Rn.
The absorbed dose (Gy) from a treatment is complicated to calculate, and the mechanisms by which the different radiations produce health effects are very complex. As mentioned in the introduction section, there are direct hits on biomolecules (including DNA) in the lungs and throughout the body. Water molecules are ionized. Various ROS, mainly hydroxyl radicals, and hydrogen peroxide are formed. Cells are damaged and signals are sent, which stimulate many of the body’s natural protection systems (>150 genes), to remedy the radiation-induced damage.2,3
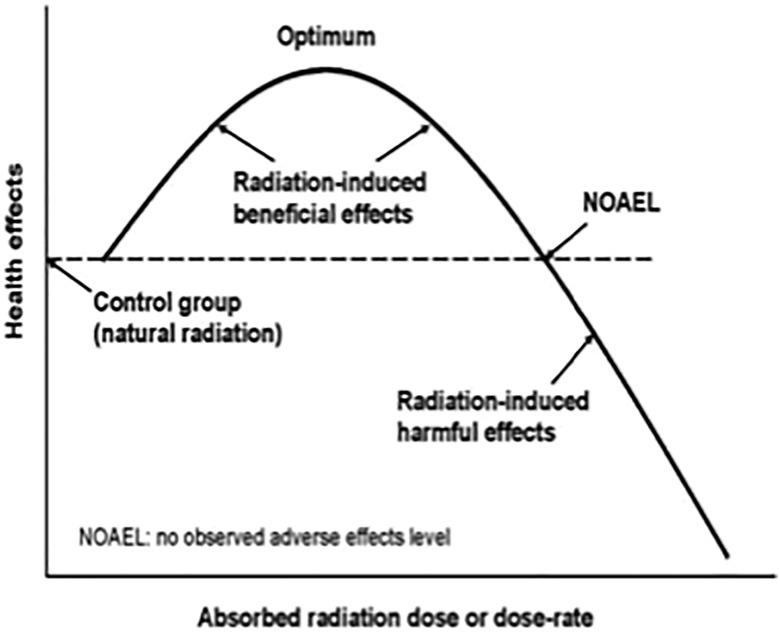
The protective systems, which normally cope with the endogenous oxidative stress and the effects of toxins, injuries, diseases, and so on, begin to function much more intensely if an exposure of radon and/or low-dose γ-radiation occurs in the hormetic dose and dose-rate range (Figure 2). This results in very important beneficial effects. In this article, we express the dose received as simply the radon concentration and duration of each treatment, recognizing that each patient inhales air at a different rate (L/min).
Figure 2.
Idealized hormetic dose–response model.
Figure 2 is an idealized graph of the hormetic dose–response, showing lower and upper thresholds and optimum levels of radiation dose and dose rate, for observed beneficial effects. Treatments are repeated for a period of weeks, with the expectation of eventually achieving a degree of lasting relief from the symptoms of the disease. The number of weeks necessary to reach an acceptable level of relief will depend on the genetics of the patient, the disease, its severity, the dose and duration of each treatment, and the number of treatments per week.
The extreme health scare about radiation-induced cancer that was started in the late 1950s has persisted for more than 60 years. The authorities that regulate the uses of ionizing radiation have been ignoring many successful medical treatments to cure diseases with moderate doses of radiation.9 However, the recent evidence of a rather high-dose threshold for onset of radiation-induced leukemia in humans and the recent evidence of a high dose-rate threshold for lifelong exposure of dogs to γ-radiation and α-radiation suggest that radon therapy does not present health risks.10-12
Results of Radon Treatments: 4 Case Reports
Two Cancer Cases Using Radon-Room Therapy
Colorectal cancer
The patient, a 70-year-old man, had been slightly feverish in December 2017. He visited a hospital in Tokyo on January 1, 2018, and was scheduled for comprehensive tests. The tests on January 22 revealed tumor marker carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), and cancer antigen 125 (CA125) values that were extremely high: 650 U/mL, 150 ng/mL, and 48.4 U/mL, respectively. Furthermore, endoscopic examination of the large intestine revealed severe tumor progression and inflammation; the endoscope could not be inserted. His illness was diagnosed as suspected stage IVB colon cancer, peritoneal dissemination with bone metastasis. He was informed that his life expectancy was 2 to 3 months. Immediately after this examination, he received instillation of the anticancer drugs Xeloda (capecitabine, Chugai Pharm. Co., Ltd, Tokyo, Japan) and Avastin (bevacizumab, Chugai Pharm. Co., Ltd, Tokyo, Japan).
Since all of these markers decreased, but were well above their normal values, the patient decided to begin radon therapy on March 9, 2018, concurrently with chemotherapy. He inhaled air with a radon concentration of 200 000 Bq/m3 for 40 minutes, twice a day for the first 2 months, and then 4 times a week from May 2 onward. By May 16, the values of his tumor markers had dramatically improved (Table 1). He stopped taking all cancer drugs from May 16 onward, as the markers continued to decline. Unfortunately, it is not clear whether or not the decrease in the marker values is due to radon therapy. The markers have remained within their normal ranges, as of March 22, 2019. Meanwhile, his stamina has improved to the extent that he has been able to walk slowly.
Table 1.
Change of Marker During Radon-Room Therapy of Patient With Colorectal Cancer.a
| Marker | Normal Valueb | January 22, 2018 | February 21, 2018 | March 14, 2018 | April 4, 2018 | May 16, 2018 | July 18, 2018 |
|---|---|---|---|---|---|---|---|
| CA19-9 (U/mL) | ≤37.0 | 650.0 | 132.8 | 51.5 | 31.6 | 23.0 | 31.9 |
| CEA (ng/mL) | ≤5.0 | 150.0 | 31.3 | 8.5 | 6.5 | 4.3 | 3.9 |
| CA125 (U/mL) | ≤35.0 | 48.4 | 24.4 | 16.8 | 14.7 | 17.6 | 20.2 |
Abbreviations: CA19-9, carbohydrate antigen 19-9; CA125, cancer antigen 125; CEA, carcinoembryonic antigen.
aThe marker values were measured at a medical laboratory in the Komagome Hospital, Tokyo.
bAs recommended by the Japanese medical community.
Uterine cancer
The patient was bleeding from the uterus on February 16, 2011. At the health clinic, she was diagnosed with uterine body cancer, stage I, and underwent surgery on March 20. The diagnosis changed to stage IIB, and combination chemotherapy paclitaxel/carboplatin (PC) was started on May 2. Cancer was not evident in the diagnostic imaging that was carried out on September 25, so the patient was discharged from the hospital. However, this cancer was observed a year later, on September 13, 2012, as metastasis to the lung (an 8-mm tumor in the right upper lobe). She received PC therapy again, which was terminated on February 4, 2013. Then hyperthermia therapy was provided until September 11, 2013, but tumor shrinkage was not observed. On the contrary, the tumor was slightly larger. After the patient received more hyperthermia and immunotherapy, a reduction in the tumor size was observed in December 2014. However, 3 months later, the tumor size increased.
The patient became aware that radon therapy might be an effective remedy, so she stopped immunotherapy and hyperthermia and began receiving a radon treatment daily from April 7 until May 2, 2015. From May 8 onward, she received radon therapy at a frequency of once for every 2 days. Examination of the June 10 computed tomography (CT) images revealed necrosis of the tumor metastasis in the right upper lobe and shrinkage of a tumor in left upper lobe. In the standard X-ray image recorded on September 9, 2015, both left and right lobular metastatic cancers were scaled down. The radon therapy is still ongoing. Unfortunately, there is no specific tumor marker for uterine body cancer.
Two Cancer Cases Using α-Radiorespiro-Rn Therapy
Radon gas is taken in mainly by pulmonary respiration, and most of the radon is soon exhaled. However, the radioactive decay products and a small fraction of the inhaled radon adhere to the mucous membrane of the trachea and the lung surface. From there, a fraction of the radionuclides enter the blood. To improve the delivery of radon therapy, we developed our α-Radiorespiro-Rn generator. It can supply radon gas at a high concentration, from about 1 to 10 MBq/m3, directly into the lungs.
Lung cancer
The patient, a 44-year-old man, smoked about 10 cigarettes a day from age 20 until age 30. Since then, he did not smoke any cigarettes. From the summer of 2016, he felt uncomfortable in his abdomen and lungs, and visited a nearby clinic. Following his physician’s referral on October 3, 2016, he underwent a careful examination at a Tokyo hospital. The tests resulted in the diagnosis of stage IV right middle- and lower-lobe lung cancer, pleurisy, peritoneal cancer, and micro brain metastasis. The lung cancer had metastasized to the pleura, the peritoneum, and the brain.
His detailed course of treatment follows in chronological order. On October 14, 2016, he took Picibanil (OK-432, antineoplastic agent, Chugai Pharm. Co., Ltd., Tokyo, Japan). On October 19, he began taking 150-mg tablets of Tarceva (molecular targeted drug, Chugai Pharm. Co., Ltd., Tokyo, Japan), and Avastin instillation was started at a frequency of once every 3 weeks. On January 5, 2017, the dose of Tarceva was reduced to 100 mg because of the decreasing tumor marker. However, the tumor marker rose again, and pleural effusion increased as well, in July. On August 30, 2017, the positive test of T790M/L858 R (gene mutation) was detected from pleural effusion. This led to the cessation of Tarceva drip. Instead of Tarceva, Tagrisso (anticancer drug, AstraZeneca Co., Osaka, Japan) drip was started on September 19.
On November 28, the concentration of D-dimer (determined by a blood test) was sharply elevated, resulting in urgent hospitalization for suspicion of lower extremity thrombosis. On December 26, the cancer metastasized to the brain and suddenly became a strabismus by pressing on the optic nerve. On January 5, 2018, right back pain and right water nephropathy were observed, and on January 8, the patient was hospitalized urgently with a severe pain in the right hip. On the next day, a stenosis was found in the bladder transition area of the urethra; a catheter was inserted. Stenosis due to retroperitoneal dissemination was suspected. An MRI examination on January 9 revealed the metastasis to the skull base diagonal platform (Figure 3). After a few days, he received radiotherapy.
Figure 3.
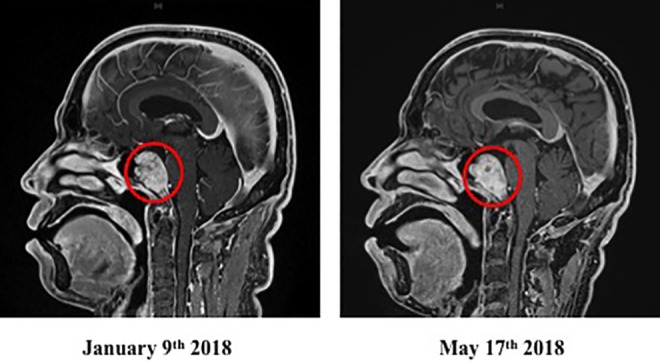
Brain MRI image, January 9 and May 17, 2018, of patient with metastatic lung cancer after α-Radiorespiro-Rn therapy. Inhalation 40 minutes, 3 times/week, 2 MBq/m3 radon concentration. Red circle indicates site of metastatic lung cancer to skull base diagonal platform.
He then visited a radon facility in Osaka on January 17. The treatment was inhalation for 40 minutes, 3 times a week from a α-Radiorespiro-Rn generator. The radon concentration was 2 MBq/m3. During this therapy, he received heparin therapy to bring down the D-dimer level, and an intravenous drip of the anticancer drug Tagrisso. On April 18, an improvement of the tumor markers was observed (Table 2). The brain MRI examination on May 17, 2018, confirmed that the brain metastasis of lung cancer, which is the primary cancer, disappeared (Figure 4), and indicated that the pleural effusion was present, but the other diagnostic results showed no deterioration. The patient still receives radon treatments 3 times a week.
Table 2.
Change of Marker During α-Radiorespiro-Rn Therapy of Patient with Lung Cancer.a
| Marker | Normal Valueb | January 19, 2018 | February 28, 2018 | March 28, 2018 | April 18, 2018 |
|---|---|---|---|---|---|
| CA19-9 (U/mL) | ≤37.0 | 146 | 145 | 78 | 62 |
| SCC (ng/mL) | ≤1.50 | 1.6 | 1.1 | 1.2 | 1.1 |
| NSE (ng/mL) | ≤16.3 | 28.9 | 17.4 | 12.3 | 12.5 |
| CEA (ng/mL) | ≤5.0 | 9.2 | 12.5 | 7.2 | 5.6 |
Abbreviations: CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; NSE, neuron-specific enolase; SCC, squamous cell carcinoma.
aThe marker values were measured at a medical laboratory in the Osaka International Cancer.
bAs recommended by the Japanese medical community.
Figure 4.
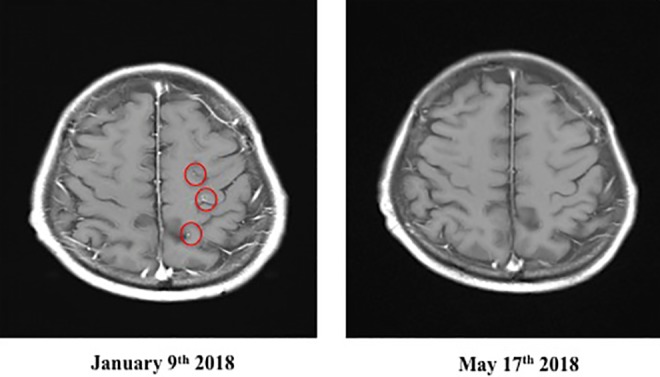
Brain MRI Image, January 9 and May 17, 2018, of patient with metastatic lung cancer after α-Radiorespiro-Rn therapy. Inhalation 40 minutes, 3 times/week, 2 MBq/m3 concentration. Red circles indicate sites of metastatic lung cancer to brain.
Hepatocellular cancer
On March 13, 2018, during a medical examination at a clinic in Tokyo, the male patient was informed that there was a shadow around his lungs. On the next day, he received a CT-scan diagnosis at the National Center for Global Health and Medicine (NCGM) in Tokyo. The images revealed thrombus in blood vessels of the liver, lungs, and heart. He was diagnosed to be in a very serious condition—stage IVB hepatocellular carcinoma. On March 22, he visited a different radiology imaging department, at the Tokyo University Hospital, for a second opinion. He was hospitalized at the NCGM on the afternoon of the same day. He received a catheter treatment on the morning of March 23, followed by 25 treatments of X-rays (2 Gy-dose fractions) between March 26 and April 27. The patient left the hospital on April 28. Subsequently, he received no further treatments at the hospital, only a monthly blood examination.
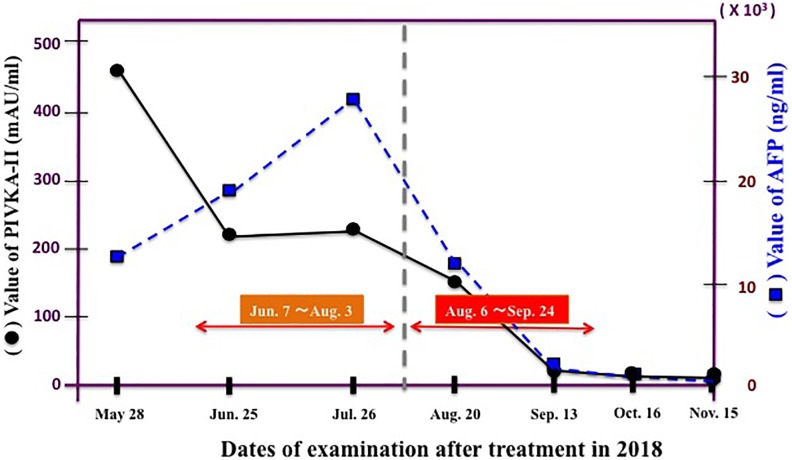
The patient visited a radon therapy facility on May 28 and received treatments from June 7 until August 3, once every 2 days (25 times in total). The concentration of radon from a α-Radiorespiro-Rn generator during this period was 1 MBq/m3. On July 31, he received blood tests and a CT examination. The results indicated the disappearance of ascites, reduction of cardiac thrombus, and a slight contraction of the liver region. However, a part of the lung remained somewhat enlarged. Regarding the cancer marker α-fetoprotein (AFP) and the marker prothrombin induced by vitamin K absence-II (PIVKA-II), these values were both only slightly reduced after starting radon treatment, and no significant decrease was achieved by the radon therapy.
The radon concentration was increased 6-fold to 6 MBq/m3, and 22 treatments were delivered from August 6 until September 22, once every 2 days. On September 13, soon after the start of these treatments, the blood markers decreased dramatically (Figure 5). Of these, the change in hepatocellular carcinoma marker PIVKA-II was particularly prominent: 449 mAU/mL on May 28, 223 mAU/mL on June 25, 231 mAU/mL on July 26, 150 mAU/mL on August 20, 19 mAU/mL on September 13, 12 mAU/mL on October 16, 19 mAU/mL on November 15, and 19 mAU/mL on December 6, 2018. The normal value has been maintained from December 6 until now. The value of the AFP marker, which was 12 651 ng/mL on May 28, decreased to 1519 ng/mL on September 13, 202 ng/mL on October 16, and 96 ng/mL on November 15, 2018. The CT image on November 29 showed that the tumor size had decreased compared to what it was on July 26, 2018 (Figure 6). The treatments were stopped when hepatocellular carcinoma was not seen, even on the positron emission tomography image recorded on December 21, 2018.
Figure 5.
Tumor markers of hepatocellular cancer patient during α-Radiorespiro-Rn therapy. AFP indicates α-fetoprotein; PIVKA-II, prothrombin induced by vitamin K absence-II.
Figure 6.
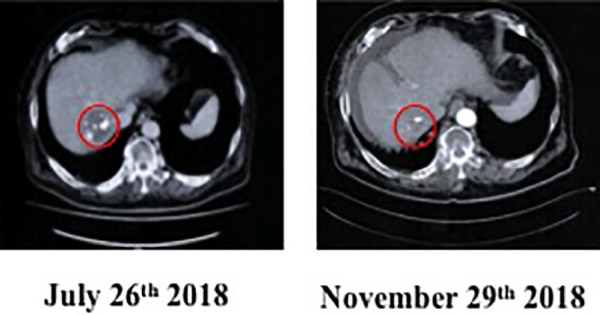
Computed tomography image, Jul 26 and Nov 29, 2018, of patient with hepatocellular cancer after α-Radiorespiro-Rn therapy.
From this experience, we conclude that there is an optimum radon concentration for treating hepatocellular cancer. A radon concentration of about 6 MBq/m3 is effective; 1 MBq/m3 is inadequate. It is unlikely that 6 MBq/m3 is effective for all cancer types and stages. Clinical trials will be necessary to establish the effective protocol for each cancer type.
Discussion
Antitumor immunity eliminates cancer cells by coordinating cellular immunity and humoral immunity. Previous studies reveal that high-dose ionizing radiation subdues antitumor immunity and promotes the development of cancer, whereas low-dose radiation enhances antitumor immune mechanisms thereby suppressing cancer.13-18 The cancer suppressive effect of low-level radiation is also supported by the epidemiological studies, which show that the cancer mortality rates, in elevated natural radiation areas in Brazil, China, India (Kerala state), the United States, and Japan (Misasa Hot Springs), are lower than the cancer mortality rates in average natural radiation areas.19-23 Similar evidence has been reported in other studies on humans and animals.24-26
Stimulation of anticancer immunity has been reported after exposing mice to low doses of radiation.17,27 In basic experiments using our animals, retardation of tumor growth rate and suppression of metastasis by low-dose X-rays or gamma rays have been shown in various kinds of mice with different lineages. The evidence correlated well with the enhancement of immunity.18,28,29 Low-dose irradiation increases NK activity, antibody-dependent cellular cytotoxic (ADCC) activity of splenocytes, and the activities of cell-surface molecules, such as IL-2 receptors and immune system signaling molecules, as well as other anticancer factors.13 As a result of examining the correlation between glutathione concentration, which is an endogenous antioxidant induced by low-dose γ-rays, and NK activity/ADCC activity, and cultured splenocytes obtained from normal mice, the NK and ADCC activities were shown to increase in a dose-dependent manner when the precursor necessary for glutathione synthesis was added. It is believed that this effect is due, at least in part, to an increase in these cellular immunities.30 These results support the view that low-dose irradiation activates antitumor immunity in the body via induction of glutathione synthesis.
In addition, we investigated the effect of repeated 500 mGy γ-irradiation on the Th1/Th2 immune balance in mice with Ehrlich solid tumors.31 The tumor growth is delayed, and the immune balance was shifted to Th1, suggesting enhanced cellular immunity. The increased IL-12 production in macrophages by the irradiation further supports Th1-shifted immunity. These findings support the enhancement of the natural protections against cancer by the fractioned irradiation.
Our past research findings demonstrate that α-emitting radon suppresses inflammation and stimulates immunity.1,7,8,32 Inhibition of inflammation can be inferred from the observed suppression of inflammation-related diseases. In addition, the stimulation of antitumor immunity can be inferred from the observed suppression of cancer metastasis.
We also examined the effect of consuming water saturated with radon on cancer metastasis, which we attributed to its stimulation of the natural defense mechanisms against cancer. In a study, we injected B16 melanoma cells into the tail vein of 2 groups of mice. In the group that drank radon water, the number of metastatic colonies in their lungs was significantly lower (P < .005) than those in the lungs of the control group.33 This result suggested a radon-induced beneficial effect on the immune system (antitumor immunity).
Using our α-Radiorespiro-Rn generator, we provided life-saving treatments to an advanced breast cancer patient with brain metastasis.1 She had refused conventional chemotherapy and radiotherapy. After receiving private therapy for 2 years, her cancer progressed significantly. She then chose radon treatments as the primary therapy. Some improvement was observed after 3 months, and after 6 months, her recovery was dramatic. A second patient with advanced breast cancer also achieved remission after 1 year of treatments in a radon room.1
Following these encouraging results, treating 2 patients with advanced breast cancer, we agreed to deliver radon therapy to 4 patients, with different types of cancer, as described in the above case reports. Radon therapy on the 4 different cancers, lung, colorectal, uterine body, and hepatocellular, resulted in an efficient improvement. Therefore, we believe it is reasonable to expect a favorable outcome for any patient with any cancer. Unlike our 2 breast cancer patients who received primary therapy, these 4 patients received adjuvant therapy after chemotherapy with anticancer drugs or after radiotherapy with high-dose radiation. This suggests that a significant benefit could be gained by employing radon therapy as an adjuvant therapy to remove residual cancer cells, after cancer treatment with chemotherapy or radiotherapy.
The abscopal effect is a phenomenon in which an untreated tumor, which metastasized to a different site, temporarily or permanently regresses (due to the circulation of CD8 T cells between the tumor sites) after treatment of the primary or the metastatic tumor by stereotactic radiotherapy.34,35 It is possible that the abscopal effect contributed to the improvement of the 2 patients with lung and hepatocellular cancer in the radon therapy. Further studies are needed to determine the role of this effect.
Other applications of radon for cancer are conceivable, such as an ischemic pretreatment for chemotherapy or radiotherapy. Chemotherapy using doxorubicin or the like always needs to control side effects, such as heart and renal toxicity. Recently, it was revealed in an animal study that doxorubicin cardiotoxicity can be ameliorated by pretreatment with low-dose radiation (75 mGy X-rays). The authors consider inhibition of DOX-induced cell death and apoptosis signaling, via mitochondrial-dependent oxidative stress, to be a mechanism to protect cardiac tissue.36 Furthermore, it has been reported in an animal study that low-dose X-ray irradiation with 300 mGy can restore ischemic hind limb perfusion by inducing the expression of angiogenesis promoting genes to increase the density of capillaries and collaterals.37 In clinical practice, it was reported that the 5-year survival rate of patients treated with topical radiation therapy (2 Gy, 5 times per week for 6 weeks) alone was 65%, whereas local radiation therapy with pretreatment by low-dose whole-body irradiation (150 mGy, twice a week for a total of 5 weeks) showed a survival rate of 84% (P < .05). In these patients, the percentage of peripheral blood CD4+ helper T lymphocytes was significantly increased.38 Summarizing the above, radon therapy may be used for various cancer treatments not only as a primary therapy but also as an adjuvant therapy and in combination therapies with conventional chemotherapy and radiotherapy.
The optimal protocol for radon treatments is an unsettled issue. It is necessary to know the right dose (concentration) for suppressing cancer with good efficiency and also the threshold for the onset of harmful side effects due to the α-radiation. We have the capability to measure the therapeutic effect of this therapy using our newly developed α-Radiorespiro-Rn generator, which can be adjusted to provide radon in air, at a concentration from 1 to 10 MBq/m3. Briefly, we treated our hepatocellular patient with 1 MBq/m3 of radon for 40 minutes, every 2 days for 2 months (25 times in total), but no notable decreases in the markers (PIVKA-II and AFP) were observed. Then, we increased the radon concentration to 6 MBq/m3 and continued the same therapy for another 2 months (22 times in total). Surprisingly, a dramatic decline in both cancer markers was observed immediately after this treatment. To date, no adverse side effects have emerged, not even minor ones from this radon treatment protocol.
Our clinician was asked how many cancer patients have been treated with radon therapy and what were the outcomes. He indicated that it is premature to offer this therapy because the treatment parameters for successful outcomes are uncertain. Few people are aware of this option, and patients who ask are evaluated for their need. Every cancer patient who has been treated with radon therapy showed at least some improvement. Clinical trials are needed.
How can we consider the biological effects of radiation in the low-dose range? We have reviewed reports on dose-dependent effects of radiation on living organisms in both in vitro and in vivo animal experimental systems.39 Based on the research, we concluded that DNA double strand breaks (DSBs), as an indicator of radiation impairment, are observed with γ-ray/X-ray exposures of 1 to 500 mGy. On the other hand, stress response genes such as DNA damage repair and radiation damage protective substances, such as antioxidants, are induced at doses lower than the DSB dose of 10 to 50 mGy. From these reports, the harmful effects (biomolecular damage) and the beneficial effects (adaptive responses: repair, expression of protective genes, etc) appear to be induced at the same time, regardless of the radiation dose to organisms. Restoration/defense is favored in the low-dose range.
Research is necessary to identify the genetic factors that influence individual sensitivity to radiation-induced cancer. Based on the hormetic dose–response model (reflected by the idealized curve in Figure 2), we could expect individuals who are more radiation-sensitive than average to benefit more in the low-dose hormetic range and suffer more in the range above the threshold for harm.
Accepted radiation risk assessment is based on the linear no threshold dose–response model. It assumes that the risk of cancer death is proportional to the accumulation of radiation-induced mutations with no threshold. However, there is much evidence for the immune suppression model, which predicts that the risk of cancer death will increase when the immune system is suppressed. Low doses of ionizing radiation stimulate immunity.15,18,28,38,40,41
We previously explored the relationship between induction of protective factors against radiation in organisms and the dose in a detailed manner by using animals and cultured cells. We found that these are induced by α-rays at doses greater than 100 mGy in animal cells, reaching a peak at around 500 mGy; significant induction of intracellular antioxidant heme oxygenase-1 occurred in mouse macrophage-like RAW264.7 cultured cells at 100 mGy or more.42 In addition, it is already clear that a dose of 500 mGy does not cause the oxidative damage to the cells, based on the results of functional ATP release from irradiated mouse B-16 melanoma cells that we measured 10 years ago.43 Furthermore, the nuclear translocation of the cell membrane epidermal growth factor receptor, which plays a pivotal role in cell survival, was expressed from 50 mGy in human lung cancer A549 cultured cells.44 In various models of autoimmune diseases, a remarkable inhibitory effect was also induced by 500 mGy irradiation.45-49 The optimal dose for γ-irradiation obtained with animals is around 500 mGy. Studies will be needed to determine the radon exposure that induces the same effect as this dose.
Recently published human evidence of a dose threshold at about 1100 mGy for radiation-induced leukemia raises the question of whether there are thresholds, higher than 1100 mGy, for the radiation induction of cancer in tissues/organs, whose cells are generally less radiation-sensitive than the blood-forming stem cells.10,11 There is also recently published evidence of a dose-rate threshold at about 600 mGy per year for life-span reduction of dogs exposed lifelong to γ-radiation.12 This evidence suggests that fears of a risk of adverse health effects from therapies that employ low doses of ionizing radiation are unwarranted.
Conclusions
We provided a series of radon treatments over many weeks to 4 patients who have different types of cancer, all at an advanced stage. These adjuvant treatments appear to have improved the efficacy of the conventional chemotherapy and radiotherapy that they received before seeking radon therapy. This article reports on the 4 cases. These patients obtained significant, lasting relief from symptoms of lung cancer, colorectal cancer, uterine body cancer, and hepatocellular cancer—an apparent benefit from radon gas inhalation and from low-level γ-radiation in the radon room. Our previous article reported on 2 cases of patients who recovered from advanced breast cancer after receiving primary radon therapy; they had refused the options chemotherapy and radiotherapy.1
Based on these 6 cases, radon therapy appears to be a promising treatment modality for different kinds of cancer, either as a primary therapy or as an adjuvant therapy for conventional chemotherapy and/or local, high-dose radiotherapy.
Our experience in treating a patient with liver cell cancer indicates that a radon concentration of 6 MBq/m3 was necessary to activate immune protection against this type of cancer. This suggests the existence of different, and rather high, radon concentration thresholds for the treatment of different types of cancer. Clinical trials should be carried out to understand the characteristics of this potentially important method of managing cancer and to learn how to employ it optimally.
Acknowledgments
The authors would like to thank our patients for granting permission to report their cases. The authors would like to express their deep gratitude to the reviewers for providing many excellent comments and suggestions, which significantly improved the quality of this article.
Footnotes
Declaration of Conflicting Interests: The author(s) declare that there are no potential conflicts of interests with respect to the report, the authorship, and/or the publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Shuji Kojima  https://orcid.org/0000-0001-6944-9250
https://orcid.org/0000-0001-6944-9250
References
- 1. Kojima S, Cuttler JM, Shimura N, Koga H, Murata A, Kawashima A. Present and future prospects of radiation therapy using α-emitting nuclides. DoseResponse. 2018;16(1):1–8. doi:10.1177/1559325817747387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feinendegen LE, Cuttler JM. Biological effects from low doses and dose rates of ionizing radiation: science in the service of protecting humans, a synopsis. Health Phys. 2018;114(6):623–626. [DOI] [PubMed] [Google Scholar]
- 3. Feinendegen LE, Pollycove M, Neumann RD. Low-dose cancer risk modeling must recognize up-regulation of protection. DoseResponse. 2010;8(2):227–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mitsunobu F, Yamaoka K, Kojima S, et al. Elevation of antioxidant enzymes in the clinical effects of radon and thermal therapy for bronchial asthma. J Radiat Res. 2003;44(2):95–99. [DOI] [PubMed] [Google Scholar]
- 5. Yamaoka K, Mitsunobu F, Hanamoto K, et al. Biochemical comparison between radon effects and thermal effects on humans in radon hot spring therapy. J Radiat Res. 2004;45(1):83–88. [DOI] [PubMed] [Google Scholar]
- 6. Kataoka T. Study of anti-oxidative effects and anti-inflammatory effects in mice due to low-dose X-irradiation or radon inhalation. J Radiat Res. 2013;54(4):587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kojima S, Tsukimoto M, Shimura N, Koga H, Murata A, Takara T. Treatment of cancer and inflammation with low-dose ionizing radiation: three case reports. DoseResponse. 2017;15(1):1–7. doi:10.1177/1559325817697531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kojima S, Thukimoto M, Cuttler JM, et al. Recovery from rheumatoid arthritis following 15 months of therapy with low doses of ionizing radiation: a case report. DoseResponse. 2018;16(3):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calabrese EJ, Dhawan G, Kapoor R, Kozumbo WJ. Radiotherapy treatment of human inflammatory diseases and conditions: optimal dose. Hum Exper Toxicol. 2019;38(5):1–11. [DOI] [PubMed] [Google Scholar]
- 10. Cuttler JM. Evidence of a dose threshold for radiation-induced leukemia. DoseResponse. 2018;16(4):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cuttler JM. Evidence of dose threshold for radiation-induced leukemia: absorbed dose and uncertainty. DoseResponse. 2019;17(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cuttler JM, Feinendegen LE, Socol Y. Evidence of a dose-rate threshold for life span reduction of dogs exposed lifelong to γ-radiation. DoseResponse. 2018;16(4):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farooque A, Mathur R, Verma A, et al. Low-dose radiation therapy of cancer: role of immune enhancement. Expert Rev Anticancer Ther. 2011;11(5):791–802. [DOI] [PubMed] [Google Scholar]
- 14. Hosoi Y, Sakamoto K. Suppressive effect of low dose total body irradiation on lung metastasis: dose dependency and effective period. Radiother Oncol. 1993;26(2):177–179. [DOI] [PubMed] [Google Scholar]
- 15. Liu SZ. Nonlinear dose-response relationship in the immune system following exposure to ionizing radiation: mechanisms and implications. Nonlinearity Biol Toxicol Med. 2003;1(1):71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ren H, Shen J, Tomiyama-Miyaji C, et al. Augmentation of innate immunity by low-dose irradiation. Cell Immunol. 2006;244(1):50–56. [DOI] [PubMed] [Google Scholar]
- 17. Ina Y, Tanooka H, Yamada T, Sakai K. Suppression of thymic lymphoma induction by life-long low-dose-rate irradiation accompanied by immune activation in C57BL/6 mice. Radiat Res. 2005;163(2):153–158. [DOI] [PubMed] [Google Scholar]
- 18. Liu SZ. Cancer control related to stimulation of immunity by low-dose radiation. DoseResponse. 2007;5(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mifune M, Sobue T, Arimoto H, Komoto Y, Tanooka H. Cancer mortality survey in a spa area (Misasa, Japan) with a high radon background. Jpn J Cancer Res. 1992;83(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei LX, Zha YR, Tao ZF, He W, Chen D, Yuan Y. Epidemiological investigation of radiological effects in high background radiation areas of Yangjiang, China. J Radiat Res (Tokyo). 1990;31(1):119–136. [PubMed] [Google Scholar]
- 21. Nambi KS, Soman SD. Environmental radiation and cancer in India. Health Phys. 1987;52(5):653–657. [DOI] [PubMed] [Google Scholar]
- 22. Fornalski KW, Dobrzyn´ski L. The cancer mortality in high natural radiation areas in Poland. DoseResponse. 2012;10(4):541–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghiassi-Nejad M, Zakeri F, Assaei RG, Kariminia A. Long-term immune cytogenetic effects of high level natural radiation on Ramsar inhabitants in Iran. J Environ Radioact. 2014;74(1-3):107–116. [DOI] [PubMed] [Google Scholar]
- 24. Miller AB, Howe GR, Sherman GJ, et al. Mortality from breast cancer after irradiation during fluoroscopic examinations in patients being treated for tuberculosis. N Engl J Med. 1989;321(19):1285–1289. [DOI] [PubMed] [Google Scholar]
- 25. Kendall GM, Muirhead CR, MacGibbon BH, et al. Mortality and occupational exposure to radiation: first analysis of the national registry for radiation workers. BMJ. 1992;304(6821):220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu SZ, Cai L, Sun SQ. Induction of a cytogenetic adaptive response by exposure of rabbits to very low dose-rate γ-radiation. Int J Radiat Biol. 1992;62(2):187–190. [DOI] [PubMed] [Google Scholar]
- 27. Liu R, Xiong S, Zhang L, Chu Y. Enhancement of antitumor immunity by low-dose total body irradiations associated with selectively decreasing the proportion and number of T regulatory cells. Cell Mol Immunol. 2010;7(7):157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janiak MK, Wincenciak M, Cheda A, Nowosielska EM, Calabrese EJ. Cancer immunotherapy: how low-level ionizing radiation can play a key role. Cancer Immunol Immunother. 2017;66(7):819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scott BR. Small radiation doses enhance natural barriers to Cancer. J Am Phys Surg. 2017;22(4):105–110. [Google Scholar]
- 30. Kojima S, Nakayama K, Ishida H. Low dose γ-rays activate immune function via induction of glutathione and delay tumor growth. J Radiat Res. 2004;45(1):33–39. [DOI] [PubMed] [Google Scholar]
- 31. Hayase H, Ohshima Y, Takahashi M, Kojima S. The enhancement of Th1 immunity and the suppression tumor growth by low-dose γ-radiation. Int J Low Radiation. 2013;5(4):275–298. [Google Scholar]
- 32. Kojima S, Cuttler JM, Shimura N, Koga H, Murata A, Kawashima A. Radon therapy for autoimmune diseases pemphigus and diabetes: 2 case reports. DoseResponse. 2019;17(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takahashi M, Kojima S. Suppression of atopic dermatitis and tumor metastasis in mice by small amounts of radon. Radiat Res.2006;165(3):337–342. [DOI] [PubMed] [Google Scholar]
- 34. Rees GJ, Ross CM. Abscopal regression following radiotherapy for adenocarcinoma. Br J Radiol. 1983;56(661):63–66. [DOI] [PubMed] [Google Scholar]
- 35. Wersäll PJ, Henric Blomgren H, Pavel Pisa P, Ingmar Lax I, Kälkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncologica. 2006;45(4):493–497. [DOI] [PubMed] [Google Scholar]
- 36. Jiang X, Hong Y, Zhao DI, et al. Low dose radiation prevents doxorubicin-induced cardiotoxicity. Ongotarget. 2018;9(1):332–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ministro A, de Oliveira P, Nunes RJ, et al. Low-dose ionizing radiation induces therapeutic neovascularization in pre-clinical model of hindlimb ischemia. CardiovascRes. 2017;113(7):783–794. [DOI] [PubMed] [Google Scholar]
- 38. Sakamoto K. Radiobiological basis for cancer therapy by total or half-body irradiation. Nonlinearity Biol Toxicol Med. 2004;2(4):293–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shimura N, Kojima S. The lowest radiation dose having molecular changes in the living body. DoseResponse. 2018;16(2):1559325818777326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pollycove M. Radiobiological basis of low-dose irradiation in prevention and therapy of cancer. DoseResponse. 2007;5(1):26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Doss M. Changing the paradigm of cancer screening, prevention and treatment. DoseResponse. 2016;14(4):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsukimoto M, Tamaishi N, Homma T, Kojim S. Low-dose gamma-ray irradiation induces translocation of Nef2 into nuclear in mouse macrophage RAW264.7 cells. J Radiat Res. 2010;51(3):349–353. [DOI] [PubMed] [Google Scholar]
- 43. Ohshima Y, Tsukimoto M, Takenouchi T, et al. Gamma-irradiation induces P2X7 receptor-dependent ATP release from B16 melanoma cells. Biochem Biophys Acta. 2010;1800(1):40–46. [DOI] [PubMed] [Google Scholar]
- 44. Tamaishi N, Tsukimoto M, Kitami A, Kojima A. P2Y6 receptors and ADAM17 mediate low-dose gamma-ray irradiation-induced focus formation (activation) of EGF receptor. Radiat Res. 2011;175(2):193–200. [DOI] [PubMed] [Google Scholar]
- 45. Tanaka T, Tago F, Fang SP, Shimura N, Kojima S. Repeated 0.5 Gy gamma-ray irradiation attenuates autoimmune manifestation in MRL-lpr/lpr mice. Int J Radait Biol. 2005;81(10):731–740. [DOI] [PubMed] [Google Scholar]
- 46. Tago F, Tsukimoto M, Nakatsukasa H, Kojima S. Repeated 0.5-Gy gamma irradiation attenuates autoimmune disease in MRL-lpr/lpr mice with suppression of CD3+CD4-CD8-B220+ T-cell proliferation and with up-regulation of CD4+CD25+Foxp3+ regulatory T cells. Radiat Res. 2008;169(1):59–66. [DOI] [PubMed] [Google Scholar]
- 47. Nakatsukasa H, Tsukimoto M, Ohshima Y, Tago F, Masada A, Kojima S. Suppressing effect of low-dose gamma-ray irradiation on collagen-induced arthritis. J Radiat Res. 2008;49(4):381–389. [DOI] [PubMed] [Google Scholar]
- 48. Nakatsukasa H, Tsukimoto M, Tokunaga A, Kojima S. Repeated gamma-ray irradiation attenuates collagen-induced arthritis via up-regulation of regulatory T cells, but not by damaging lymphocytes directly. Radiat Res. 2010;174(3):313–324. [DOI] [PubMed] [Google Scholar]
- 49. Tsukimoto M, Nakatsukasa K, Sugawara K, Yamashita Y, Kojima S. Repeated 0.5-Gy γ irradiation attenuates experimental autoimmune encephalomyelitis with up-regulation of regulatory T cells and suppression of IL-17 production. Radiat Res. 2008;170(4):429–436. [DOI] [PubMed] [Google Scholar]